Abstract
Human immunodeficiency virus type 1 (HIV-1) vaccines that elicit protective antibody responses at mucosal sites would be highly desirable. Here, we report that intramuscular immunization of candidate HIV-1 vaccine vectors and purified Env proteins elicited potent and durable humoral immune responses in colorectal mucosa in rhesus monkeys. The kinetics, isotypes, functionality, and epitope specificity of these mucosal antibody responses were similar to those of peripheral responses in serum. These data suggest a close immunological relationship between mucosal and systemic antibody responses following vaccination in primates.
TEXT
Human mucosal surfaces represent the major portal of entry for human immunodeficiency virus type 1 (HIV-1) (1). Thus, a prophylactic vaccine will likely need to elicit protective antibody responses at mucosal sites of virus exposure. However, mucosal humoral immune responses following vaccination are poorly characterized. The RV144 clinical trial suggested that vaccine-elicited HIV-1 envelope (Env)-specific humoral immune responses may have contributed to the partial protection observed for vaccinees (2), but mucosal immune responses were not assessed in that trial. In contrast, previous studies have shown that immunization with peptide, DNA, protein, or attenuated bacterial or viral vector-based vaccines through parental or mucosal routes may elicit antigen-specific humoral immune responses at mucosal sites in mice, nonhuman primates, and humans (3–7). However, the characteristics, functionality, and epitope specificity of vaccine-elicited mucosal antibody responses have not been fully explored. Moreover, whether mucosal antibody responses reflect distinct populations compared with those for peripheral antibody responses remains to be determined. We therefore assessed the magnitude, durability, isotype, neutralizing activity, and epitope specificity of mucosal and peripheral antibody responses in rhesus monkeys elicited by adenovirus (Ad) vector-based and protein-based HIV-1 vaccine candidates.
We first collected blood and colorectal mucosal secretions using Weck-Cel sponges from 8 healthy adult rhesus monkeys. Using sera and mucosal secretions eluted from Weck-Cel sponges (8), we assessed the amount of total IgG and IgA (monkey IgG/IgA enzyme-linked immunosorbent assay [ELISA] kit; Alpha Diagnostic). The average volume of eluates from 8 unused sponges was used as the elution buffer volume, and a dilution factor was calculated according to the volume of eluate for each sample: dilution factor = experiment sponge eluate volume/(experiment sponge eluate volume − unused sponge eluate volume). The dilution factor was used to calculate total IgG and IgA as well as titers of antigen-specific IgG and IgA. As expected, we found that the amount of IgG in serum was significantly higher than that of IgA (P = 0.0039; paired t test), whereas the amount of IgA in colorectal mucosal secretions was significantly higher than that of IgG (P = 0.0337; paired t test) (Fig. 1A). Nevertheless, the total amounts of both IgG and IgA in mucosal secretions were substantially lower than those found in serum. To confirm that the Igs collected from mucosal sites actually represented mucosal antibodies, we assessed mucosal and serum IgA for the IgA α-chain (α-specific responses) and IgA secretory component (SC-specific responses). The α-specific responses represent both monomeric and polymeric IgA, whereas SC-specific IgA is only found in secretory IgA (sIgA) in mucosal secretions (7, 9). Serum samples showed high α-specific IgA and no detectable SC-specific IgA, as expected. In contrast, mucosal secretions showed both α-specific and SC-specific IgA (Fig. 1B). SC-specific anti-IgA antibody proved specific for sIgA, with minimal cross-reactivity to monomeric and polymeric IgA (Fig. 1C). These results confirm that the IgA from mucosal secretions was largely sIgA and not serum contamination.
FIG 1.
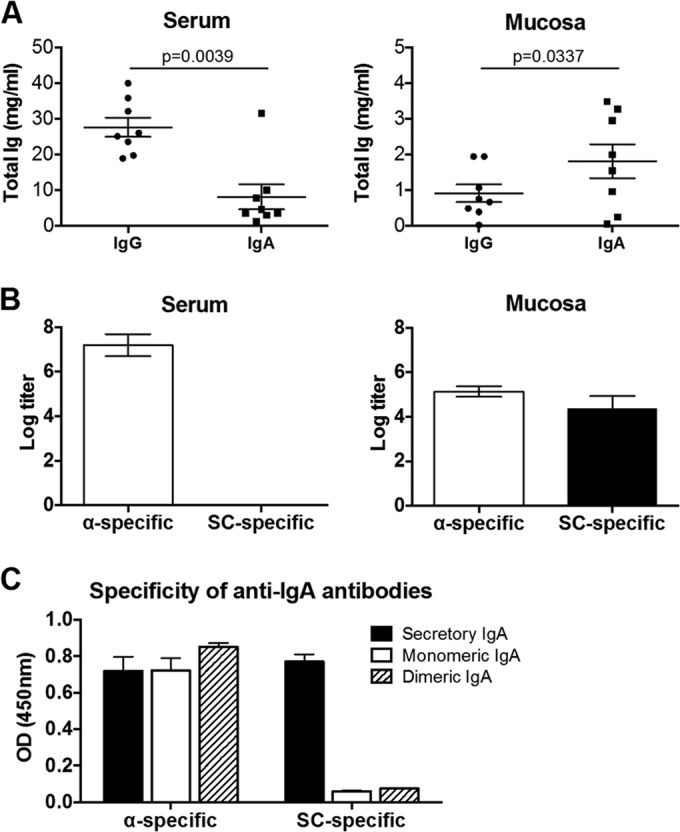
Total mucosal IgG and IgA in rhesus monkeys. Sera and colorectal mucosal secretions were collected from 8 healthy adult rhesus monkeys. (A) The amount of total IgG and IgA was determined by quantitative ELISA. (B) The amount of serum and mucosal IgA containing the α-chain (α-specific) or the secretory component (SC-specific) was also determined. Means and standard deviations (SD) of endpoint titers are shown. (C) Responses of α-specific and SC-specific anti-IgA antibodies to recombinant IgA monomer and polymer, as well as the sIgA standard, were determined by ELISA. Means and SD of the optical density (OD; 450 nm) from 4 replicates are shown.
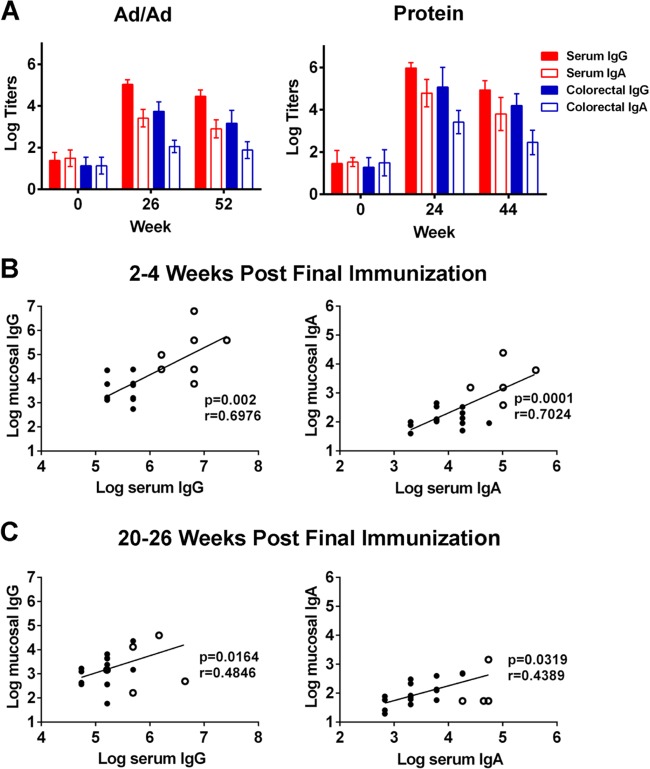
We next assessed Env-specific IgG and IgA responses in colorectal secretions and sera from 24 rhesus monkeys immunized with candidate HIV-1 vaccines. Sixteen adult rhesus monkeys were immunized intramuscularly (i.m.) with 2 × 1010 viral particles of adenovirus serotype 35 (Ad35) at week 0 and 2 × 1010 viral particles of Ad26 (10) at week 24 (Ad/Ad). Both Ad vectors encoded simian immunodeficiency virus SIVSME543 Env-Gag-Pol antigens (11). Eight additional adult rhesus monkeys were immunized i.m. with 0.25 mg recombinant HIV-1 clade C CZA97.012 Env gp140 (12) with adjuvant at weeks 0, 4, 8, 12, 16, and 20. IgG and IgA responses specific to SIV Env (SIVmac251 gp120; Immune Technology Corp.) and HIV-1 clade C CZA97.012 Env gp140 (12) were determined for both sera and colorectal mucosal secretions by ELISA 2 to 4 weeks and 20 to 24 weeks following the final immunization. Responses were defined as positive if the absorbance was greater than the mean plus 3 standard deviations of the absorbance of negative controls. The cutoff absorbance was generally 0.05 for IgG responses and 0.1 for IgA responses. Both Ad/Ad and protein immunization elicited high titers of Env-specific IgG and IgA responses in sera and mucosal secretions at the peak time point (P < 0.01; paired t test), and these responses declined by approximately 0.5 log (range, 0.17 to 0.57 log; median, 0.54) by 20 to 24 weeks following the final immunization (Fig. 2A). Consistent with prior reports (5), mucosal antibody titers were 1 to 2 logs lower than those found in serum. Moreover, Env-specific IgG titers were approximately 1.5 logs (range, 1.29 to 1.69 logs; median, 1.59) higher than Env-specific IgA titers in both serum and mucosal secretions. Env-specific IgA in mucosal secretions but not in serum exhibited SC-specific responses (data not shown), consistent with the data shown in Fig. 1B. Env-specific mucosal IgG and IgA responses correlated with Env-specific systemic IgG and IgA responses at both the peak time point (P = 0.002 for IgG; P = 0.0001 for IgA) and later time points (P = 0.02 for IgG; P = 0.03 for IgA) (Fig. 2B and C), suggesting that intramuscular immunization of Ad-vectored and protein HIV-1 candidate vaccines elicited immunologically coordinated antibody responses in the periphery and at mucosal sites.
FIG 2.
Vaccine-elicited mucosal antibody responses in rhesus monkeys. Rhesus monkeys were immunized i.m. with Ad35 (at week 0) and Ad26 (at week 24) encoding SIV Gag-Env-Pol (Ad/Ad; n = 16 monkeys) or recombinant HIV-1 Env protein trimer at weeks 0, 4, 8, 12, 16, and 20 (Protein; n = 8). (A) Env-specific IgG and IgA responses were determined for both serum and colorectal mucosal secretions by ELISA at baseline and 2 to 4 weeks and 20 to 26 weeks after the final immunization. Means and standard deviations of endpoint ELISA titers are shown. Correlations between Env-specific IgG (left) and IgA (right) responses in sera and mucosal secretions at (B) 2 to 4 weeks and (C) 20 to 26 weeks after the final immunization were analyzed using Spearman rank-correlation tests. Filled circles, monkeys from the Ad/Ad group; open circles, monkeys from the protein group.
Follow-up studies of RV144 suggested that vaccine-elicited Env-specific IgG3 titers may correlate with protective efficacy (13, 14). Rhesus IgG1, IgG2, IgG3, and IgG4 sequences are 88.0% to 90.1% identical to the corresponding human IgG subclasses, although our understanding of the biology of rhesus IgG subclasses remains incomplete (15). To dissect subclasses of Env-specific IgG responses elicited in rhesus monkeys, we performed ELISAs for 6 monkeys from each group using secondary antibodies specific for rhesus IgG1 and IgG3 (kindly provided by K. Reimann, NIH Nonhuman Primate Reagent Resource). Both Ad/Ad and protein vaccines elicited IgG1 and IgG3 responses in serum and mucosal secretions. The Ad vectors elicited comparable titers of IgG3 and IgG1 responses in both sera and mucosal secretions, whereas the protein vaccine elicited approximately 1 log higher titers of IgG3 than IgG1 (Fig. 3A and B), although mucosal responses were lower than serum responses for both subclasses. The anti-IgG1 and anti-IgG3 monoclonal antibodies exhibited minimal cross-reactivity to the other rhesus IgG subclasses (Fig. 3E).
FIG 3.
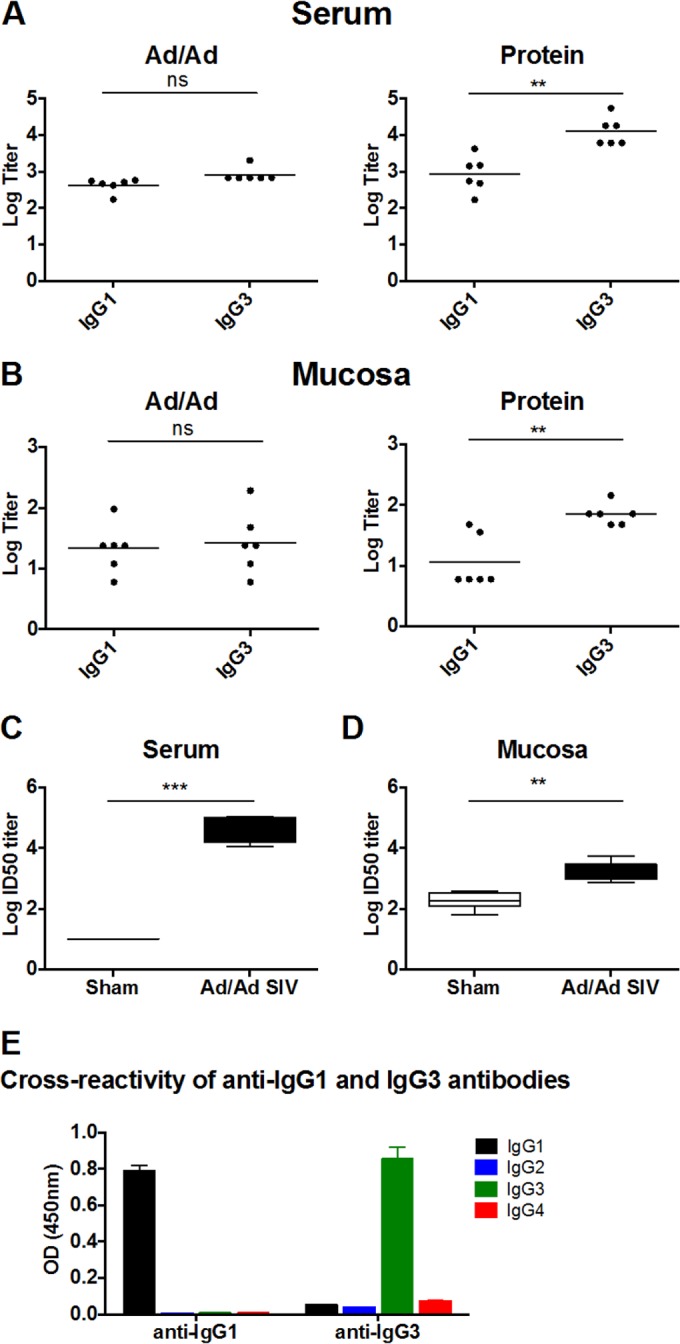
IgG subclasses and neutralizing activity of vaccine-elicited mucosal antibodies. (A) Env-specific IgG1 and IgG3 titers were determined for sera from Ad/Ad (left)- and protein (right)-immunized monkeys by ELISA. (B) Env-specific IgG1 and IgG3 titers were also determined for mucosal secretions. ns, not significant; *, P < 0.05; **, P < 0.01 (paired t test). (C) Pseudovirus neutralizing assays were performed using tissue culture-adapted strain SIVmac251.15. The 50% infective dose (ID50) titers were determined for sera from monkeys immunized with Ad/Ad at 2 weeks after the final immunization. (D) ID50 titers were also determined for mucosal secretions from these monkeys. Samples from sham-immunized monkeys were used as controls. **, P < 0.01; ***, P < 0.001 (unpaired t test). (E) Responses of anti-IgG1 and anti-IgG3 monoclonal antibodies to recombinant rhesus IgG1, IgG2, IgG3, and IgG4 were determined by ELISA. Means and SD of the OD (450 nm) from 6 replicates are shown.
To determine if vaccine-elicited mucosal antibodies were functional, we performed neutralization assays using mucosal secretions collected at 2 weeks after the final immunization from monkeys that received the Ad/Ad vaccines compared with eight additional control monkeys that received a sham vaccine. Mucosal secretions and sera from vaccinated monkeys exhibited significantly greater neutralizing activity against the TCLA strain of SIVmac251.15 compared to that of the sham group (P < 0.001 for sera and P < 0.01 for mucosal secretions; unpaired t test) (Fig. 3C and D). Thus, these vaccine vectors elicited functional neutralizing antibodies in colorectal secretions, although titers in mucosal secretions were around 2 logs lower than those found in serum. These data suggest that IgG isotypes and functionality of vaccine-elicited, Env-specific mucosal antibody responses are similar to those of antibodies in peripheral blood, despite the overall predominance of IgA in mucosal secretions.
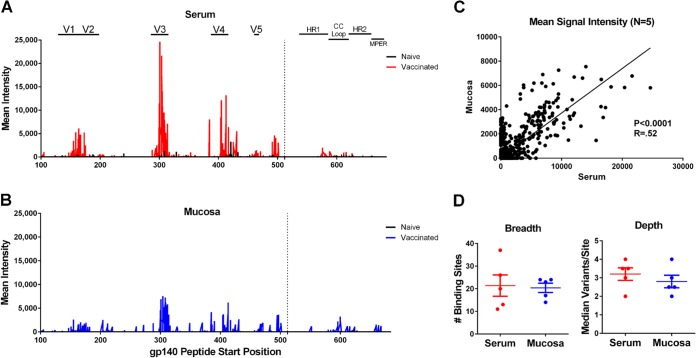
To determine if vaccine-elicited antibodies at mucosal sites had epitope specificities similar to those of vaccine-elicited antibodies in serum, we assessed paired mucosal and serum samples for Env-specific IgG linear epitope profiling from 5 monkeys immunized with the Env protein using peptide microarrays (JPT Peptide Technology). As a negative control, we also evaluated mucosal and serum samples from two naive unvaccinated monkeys, the results of which proved negligible (16, 17). Microarrays consisted of 3 identical subarrays containing 3,882 linear Env peptides covering 57% of global HIV-1 Env sequences in the Los Alamos National Laboratory database (18). Mucosal and serum samples were incubated with microarrays and a labeled secondary antibody, and the signal intensity (SI) of peptide binding was measured by a Genepix 4300A scanner. The threshold value used to define a minimum positive signal was calculated for each slide using the computational tool rapmad (robust alignment of peptide microarray data) (19) and was established as 5 standard deviations above the mean of the noise distribution. To calculate the breadth of antibody responses, we evaluated the number of Env peptide responses for each animal, and we aligned the reactive peptide sequences to eliminate overlap. If any reactive peptide sequences shared 5 or more amino acids, we assumed that the peptides were recognized by the same antigen-binding site on a single antibody; these overlapping sequences were conservatively defined as a single positive “binding site.” If the first and last overlapping peptide in a string of overlapping peptides shared 4 or fewer amino acids, we assumed that the peptides were recognized by a minimum of two antibody binding sites. To calculate the depth of antibody responses, we evaluated the overlapping sequences of each binding site and determined the number of unique sequence variations for each binding site. We then calculated the median number of variations/binding site for each animal.
The pattern of IgG binding to linear Env peptides appeared strikingly similar between serum and mucosal samples, with both serum and mucosal antibodies binding predominantly to V3 linear peptides, followed by V1/V2 and V4 peptides (Fig. 4A and B). Within compartments, there was some variability between animals in the binding to V1/V2 peptides, but V3 binding was universal (data not shown). For individual monkeys, a mean of 97% of serum responses overlapped by ≥5 amino acids with mucosal responses, whereas a mean of 81% of mucosal responses overlapped by ≥5 amino acids with serum responses. There was a significant positive correlation between the mean signal intensity of mucosal and serum IgG peptide binding (P < 0.0001, Spearman rank-correlation test) (Fig. 4C). Of note, both mucosal and serum IgG from protein-immunized monkeys showed binding to peptides within the V1/V2 region of Env (positions 120 to 204) (2). In addition, there was no difference between the breadth and depth of IgG binding to linear Env peptides between the mucosal and serum compartments (Fig. 4D). These results suggest that Env-specific mucosal and serum IgG generally share similar epitope specificities.
FIG 4.
Mucosal and serum IgG binding to linear Env peptides by peptide microarrays. IgG binding to 3,882 linear Env peptides was assessed by peptide microarrays using serum and mucosal secretions from 5 monkeys immunized with the Env protein vaccine 16 weeks after the final immunization as well as 2 naive control monkeys. (A) Mean signal intensity of binding is plotted by the peptide gp140 start position for serum. (B) Mean signal intensity of binding is plotted for mucosal samples. (C) Mean signal intensity of mucosal peptide binding is plotted against mean signal intensity of serum peptide binding for the 5 immunized monkeys. (D) The breadth (number of binding sites) and depth (median number of epitope variants/binding site) for each immunized monkey are plotted for serum and mucosal samples. Mean responses and standard errors of the means (SEM) are depicted.
HIV-1 infects humans primarily through mucosal surfaces, and therefore it is likely that a prophylactic vaccine would elicit protective antibodies both at mucosal surfaces and in the systemic circulation. For optimal induction of mucosal immune responses, several studies have suggested that vaccines should be administered through mucosal routes (3, 20). However, other studies have demonstrated that vaccines given parentally can also induce mucosal antibody responses in certain settings (21, 22). A comparative evaluation of intranasal (i.n.) and parental (i.m.) immunization of an HIV-1 peptide-based immunogen with adjuvant in cynomolgus monkeys showed that antibody responses at the nasal and genital mucosa were highest in animals immunized parentally (23). Individual mucosal compartments also display differences with respect to antibody isotypes and densities, as well as origins of cells involved in innate and adaptive immunity.
In this study, we demonstrate that intramuscular immunization with both Ad-vectored and protein-based candidate HIV-1 vaccines elicited potent and durable Env-specific antibody responses in colorectal mucosa and that the kinetics, isotype, functionality, and epitope specificity of mucosal antibodies generally mirror those found in serum. These data suggest that vaccine-elicited peripheral and mucosal humoral immune responses are likely immunologically coordinated. The remarkable degree of similarity also raises the possibility that mucosal and peripheral antibodies elicited by vaccination may originate from the common B cell populations, although a detailed study of mucosal B cells is beyond the scope of this study. Previous studies suggest that mucosal IgA is largely synthesized locally and transported through epithelial cells into the lumen (24). The degree to which mucosal IgG and IgA elicited by vaccination reflects peripheral versus local B cells requires additional investigation.
ACKNOWLEDGMENTS
We thank G. Tomaras, P. Kozlowski, M. Beck, J. Kramer, G. Russo, J. Nkolola, and L. Parenteau for their generous advice and assistance.
We acknowledge support from National Institutes of Health grants AI078526, AI084794, AI095985, AI096040 (D.H.B.), and AI060354 (K.E.S.) and the Ragon Institute of MGH, MIT, and Harvard (D.H.B.).
The authors report no financial conflicts of interest.
Footnotes
Published ahead of print 10 September 2014
REFERENCES
- 1.Hladik F, McElrath MJ. 2008. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8:447–457. 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286. 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devito C, Zuber B, Schroder U, Benthin R, Okuda K, Broliden K, Wahren B, Hinkula J. 2004. Intranasal HIV-1-gp160-DNA/gp41 peptide prime-boost immunization regimen in mice results in long-term HIV-1 neutralizing humoral mucosal and systemic immunity. J. Immunol. 173:7078–7089. 10.4049/jimmunol.173.11.7078. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira AF, Cardoso SA, Almeida FBDR, de Oliveira LL, Pitondo-Silva A, Soares SG, Hanna ES. 2012. Oral immunization with attenuated Salmonella vaccine expressing Escherichia coli O157:H7 intimin gamma triggers both systemic and mucosal humoral immunity in mice. Microbiol. Immunol. 56:513–522. 10.1111/j.1348-0421.2012.00477.x. [DOI] [PubMed] [Google Scholar]
- 5.Buffa V, Klein K, Fischetti L, Shattock RJ. 2012. Evaluation of TLR agonists as potential mucosal adjuvants for HIV gp140 and tetanus toxoid in mice. PLoS One 7:e50529. 10.1371/journal.pone.0050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, Neutra MR. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169:566–574. 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 7.Patterson LJ, Kuate S, Daltabuit-Test M, Li Q, Xiao P, McKinnon K, DiPasquale J, Cristillo A, Venzon D, Haase A, Robert-Guroff M. 2012. Replicating adenovirus-simian immunodeficiency virus (SIV) vectors efficiently prime SIV-specific systemic and mucosal immune responses by targeting myeloid dendritic cells and persisting in rectal macrophages, regardless of immunization route. Clin. Vaccine Immunol. 19:629–637. 10.1128/CVI.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. 2000. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J. Acquir. Immune Defic. Syndr. 24:297–309. 10.1097/00042560-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. 1995. Molecular and cellular aspects of the secretory immunoglobulin system. APMIS 103:1–19. 10.1111/j.1699-0463.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 10.Abbink P, Lemckert AAC, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJE, Barouch DH. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654–4663. 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, Sanmiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nkolola JP, Peng H, Settembre EC, Freeman M, Grandpre LE, Devoy C, Lynch DM, La Porte A, Simmons NL, Bradley R, Montefiori DC, Seaman MS, Chen B, Barouch DH. 2010. Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs. J. Virol. 84:3270–3279. 10.1128/JVI.02252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast A-S, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. 2014. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med. 6:228ra38–228ra38. 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 14.Yates NL, Liao HX, Fong Y, DeCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang Z-Y, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci. Transl. Med. 6:228ra39. 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scinicariello F, Engleman CN, Jayashankar L, McClure HM, Attanasio R. 2004. Rhesus macaque antibody molecules: sequences and heterogeneity of alpha and gamma constant regions. Immunology 111:66–74. 10.1111/j.1365-2567.2004.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahtman T, Jernberg A, Mahdavifar S, Zerweck J, Schutkowski M, Maeurer M, Reilly M. 2007. Validation of peptide epitope microarray experiments and extraction of quality data. J. Immunol. Methods 328:1–13. 10.1016/j.jim.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Masch A, Zerweck J, Reimer U, Wenschuh H, Schutkowski M. 2010. Antibody signatures defined by high-content peptide microarray analysis. Methods Mol. Biol. 669:161–172. 10.1007/978-1-60761-845-4_13. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson KE, Pawlowski N, Hoegen Von P, Reimer U, Rosenberg ES, Barouch DH. HIV-1 antibody epitope mapping of individuals treated with antiretroviral therapy during acute/early HIV-1-infection, poster P03.70 LB AIDS Vaccine Conference, Barcelona, Spain, 7 to 10 October 2013 http://www.vaccineenterprise.org/conference/2013/sites/default/files/AIDS-Vaccine-2013-Abstract-Book.pdf. [Google Scholar]
- 19.Renard BY, Löwer M, Kühne Y, Reimer U, Rothermel A, Türeci O, Castle JC, Sahin U. 2011. rapmad: robust analysis of peptide microarray data. BMC Bioinformatics 12:324. 10.1186/1471-2105-12-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mapletoft JW, Latimer L, Babiuk LA, Littel-van den Hurk SVD. 2010. Intranasal immunization of mice with a bovine respiratory syncytial virus vaccine induces superior immunity and protection compared to those by subcutaneous delivery or combinations of intranasal and subcutaneous prime-boost strategies. Clin. Vaccine Immunol. 17:23–35. 10.1128/CVI.00250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoji M, Katayama K, Tachibana M, Tomita K, Sakurai F, Kawabata K, Mizuguchi H. 2012. Intramuscular DNA immunization with in vivo electroporation induces antigen-specific cellular and humoral immune responses in both systemic and gut-mucosal compartments. Vaccine 30:7278–7285. 10.1016/j.vaccine.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 22.Krause A, Whu WZ, Xu Y, Joh J, Crystal RG, Worgall S. 2011. Protective anti-Pseudomonas aeruginosa humoral and cellular mucosal immunity by AdC7-mediated expression of the P. aeruginosa protein OprF. Vaccine 29:2131–2139. 10.1016/j.vaccine.2010.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egan MA, Chong SY, Hagen M, Megati S, Schadeck EB, Piacente P, Ma B-J, Montefiori DC, Haynes BF, Israel ZR, Eldridge JH, Staats HF. 2004. A comparative evaluation of nasal and parenteral vaccine adjuvants to elicit systemic and mucosal HIV-1 peptide-specific humoral immune responses in cynomolgus macaques. Vaccine 22:3774–3788. 10.1016/j.vaccine.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Mestecky J, Moro I, Kerr MA, Woof JM. 2005. Chapter 9, Mucosal immunoglobulins, p 153–182 In Mestecky J, Lamm ME, McGhee JR, Bienenstock J, Mayer L, Strober W. (ed), Mucosal immunology, 3rd ed. Academic Press, Burlington, MA. [Google Scholar]