ABSTRACT
HIV-1 Nef and Vpu are thought to optimize virus replication in the infected host, at least in part via their ability to interfere with vesicular host cell trafficking. Despite the use of distinct molecular mechanisms, Nef and Vpu share specificity for some molecules such as CD4 and major histocompatibility complex class I (MHC-I), while disruption of intracellular transport of the host cell restriction factor CD317/tetherin represents a specialized activity of Vpu not exerted by HIV-1 Nef. To establish a profile of host cell receptors whose intracellular transport is affected by Nef, Vpu, or both, we comprehensively analyzed the effect of these accessory viral proteins on cell surface receptor levels on A3.01 T lymphocytes. Thirty-six out of 105 detectable receptors were significantly downregulated by HIV-1 Nef, revealing a previously unappreciated scope with which HIV-1 Nef remodels the cell surface of infected cells. Remarkably, the effects of HIV-1 Vpu on host cell receptor exposure largely matched those of HIV-1 Nef in breadth and specificity (32 of 105, all also targeted by Nef), even though the magnitude was generally less pronounced. Of particular note, cell surface exposure of all members of the tetraspanin (TSPAN) protein family analyzed was reduced by both Nef and Vpu, and the viral proteins triggered the enrichment of TSPANs in a perinuclear area of the cell. While Vpu displayed significant colocalization and physical association with TSPANs, interactions of Nef with TSPANs were less robust. TSPANs thus emerge as a major target of deregulation in host cell vesicular transport by HIV-1 Nef and Vpu. The conservation of this activity in two independent accessory proteins suggests its importance for the spread of HIV-1 in the infected host.
IMPORTANCE In this paper, we define that HIV-1 Nef and Vpu display a surprising functional overlap and affect the cell surface exposure of a previously unexpected breadth of cellular receptors. Our analyses furthermore identify the tetraspanin protein family as a previously unrecognized target of Nef and Vpu activity. These findings have implications for the interpretation of effects detected for these accessory gene products on individual host cell receptors and illustrate the coevolution of Nef and Vpu function.
INTRODUCTION
One of the features that distinguish primate lentiviruses (human immunodeficiency viruses [HIVs] and simian immunodeficiency viruses [SIVs]) from less complex retroviruses is the fact that they encode so-called “accessory” gene products. These proteins, comprising Vif, Vpr, Vpu, and Nef in the case of HIV-1, are dispensable for virus replication in vitro but play essential roles for efficient viral spread, maintenance, and pathogenicity in vivo (1). The acquisition of these additional viral factors appears to enable HIV-1 to cope with complex host defense mechanisms, such as innate and adaptive virus-specific immune responses or intrinsic resistance factors. It emerges that the strong need for escape mechanisms provoked the acquisition and evolution of these genes (reviewed in references 1 – 3). In addition, the multifunctionality of the accessory proteins enables the virus to manipulate host cell machineries at multiple steps in a way to deregulate and exploit them toward its own propagation.
Functions described for HIV-1 Nef and Vpu display some overlap, as they share the ability to reduce the density of receptors such as CD4, major histocompatibility complex class I (MHC-I), CD1d, and poliovirus receptor (PVR) at the surface of infected cells (4 – 10; reviewed in references 1 and 11). This is particularly remarkable since both proteins are fully divergent with regard to their amino acid sequence, the presence of functional motifs, domain organization, and even membrane topology (see below). This functional redundancy most likely is the result of coevolution of both genes. Vpu is a characteristic feature of HIV-1 and some related SIVs but is not encoded by other primate lentiviruses. It has been proposed that the vpu gene was acquired by a common simian ancestor that, by several recombination and cross-species transmission events, gave rise to Vpu-containing viruses found in chimpanzees, gorillas, and humans (reviewed in reference 3). In contrast, nef is present in all primate lentiviral genomes. Interestingly, in most non-Vpu-containing viruses, Nef was shown to harbor some of the additional host modulatory functions that were otherwise “overtaken” by Vpu, such as the counteraction of the antiviral restriction factor CD317/tetherin (12, 13) (see below).
The 25- to 35-kDa myristoylated Nef protein of HIV-1 is abundantly expressed early in the HIV-1 life cycle to promote HIV-1 replication and clinical progression to AIDS in infected individuals (14 – 17). HIV-1 Nef exerts its multiple activities by acting as a protein adaptor to a plethora of host cell factors, allowing the viral protein to subvert cellular trafficking and signaling machineries (18, 19). Nef-mediated subversion of intracellular trafficking leads to profound alterations in the density of receptors on the surface of HIV-infected cells. Identified as the first target of this Nef activity, the viral entry receptor CD4 is efficiently downmodulated from the cell surface by the viral protein (4), an effect linked to the prevention of superinfection of target cells and also to the ability of Nef to enhance the infectivity of HIV-1 particles (20, 21). Similarly, functions of Nef in the evasion of host immune responses are also coupled to its ability to alter the receptor density on the surface of infected host cells, as Nef prevents the recognition and lysis of infected cells by cytotoxic T lymphocytes (CTLs) by selective downmodulation of HLA-A and -B from the host cell surface (6, 22, 23). Simultaneously, Nef protects infected cells from natural killer (NK) cell lysis (24) by sparing HLA-C from downregulation and by reducing cell surface levels of the lipid antigen presentation molecule CD1d (8) and several ligands of activating NK cell receptors, such as PVR, MICA, ULBP2, and NKp44L (10, 25, 26).
Nef also hampers MHC-II-mediated antigen presentation via enrichment of the invariant chain (Ii) (27) and downregulation of the costimulatory molecules CD80 and CD86 from the cell surface (28). In addition, Nef broadly affects chemokine receptor surface expression, perturbing the host immune system by altering the responsiveness to chemokine attractants and contributing to the prevention of superinfection (29 – 32).
In addition to facilitating immune evasion of infected cells by modulation of cell surface receptor trafficking, Nef alters T cell receptor (TCR) signaling to modulate basal levels of T cell activation and the responsiveness of cells to TCR stimulation (reviewed in reference 33). These activities presumably optimize viral spread by balancing between unfavorable, premature, activation-induced cell death and specific lymphocyte activation required for efficient HIV-1 replication. This is achieved via a block of early TCR-triggered signaling events such as actin remodeling, tyrosine phosphorylation, and the formation of signaling microclusters (34 – 36). Simultaneously, a specific TCR-distal signaling cascade is initiated by the Src family kinase Lck, which is retargeted by Nef from the plasma membrane (PM) to recycling endosomes (REs) and the trans-Golgi network (TGN) (35, 37, 38). Using the same mechanism for the inhibition of host cell actin dynamics as that used during TCR signaling, Nef also impairs the motility of HIV-1-infected T lymphocytes (39 – 42).
Significantly fewer activities are currently described for HIV-1 Vpu, a 16-kDa integral membrane protein expressed at late stages of the viral life cycle, than for Nef. Similar to Nef, the removal of CD4 and MHC-I molecules from the infected host cell surface is a cardinal function of Vpu (5, 7), and Vpu was reported to reduce cell surface levels of CD1d (9). Recent investigations assigned Vpu an additional role in NK cell-mediated antiviral immunity by downregulation of NK T and B cell antigen (NTBa) and PVR, both ligands for activating NK cell receptors, from the host cell surface (10, 43). Vpu also exerts ion channel activity, the biological relevance of which remains to be determined (44, 45). Since the discovery of CD317/BST-2/tetherin as a potent cellular restriction factor that prevents the release of infectious HIV-1, antagonism of this restriction is considered the most important biological activity of Vpu (46, 47). While the precise mechanism by which Vpu antagonizes CD317 remains to be elucidated, most current models predict that this activity is related to the ability of Vpu to downregulate surface levels of CD317 on virus-producing cells by interference with anterograde transport of the restriction factor following biosynthesis or internalization from the cell surface (48 – 51).
Together, these observations characterize Nef and Vpu as highly multifunctional proteins, the effects of which are in large part directly linked to their ability to modulate host cell surface receptor exposure. Mechanistic analyses indicate that Nef typically acts to affect receptor transport routes both from as well as to the PM (reviewed in references 11 and 52), while Vpu preferentially acts to suppress PM delivery of newly synthesized or recycling cargo (9, 48, 50, 53, 54). Irrespective of the specific transport step affected and the molecular mechanism used, Nef and Vpu are thought to target receptors by physical association with the cytoplasmic tail or the transmembrane domain of the receptor, respectively (direct connector model) (55 – 59). With the number of host cell receptors reported to be affected by Nef and/or Vpu steadily increasing, however, it is becoming unclear how the specificity of these viral proteins for individual receptors can be achieved. Furthermore, this suggests that we may not yet appreciate the full scope of receptor modulation by Nef and Vpu. Finally, it is unclear whether the overlap between the functions of Nef and Vpu is limited to activities related to receptor modulation. To address these issues, we conducted a comparative analysis of the biological activities of Nef and Vpu. We found that their effect on host cell surface receptor exposure is far broader than previously thought and identified tetraspanins (TSPANs) as a major target for downmodulation. Furthermore, we conclude that while Nef and Vpu display remarkable similarity in their profiles of targeted surface receptors, Nef activities unrelated to receptor modulation are not shared by Vpu.
MATERIALS AND METHODS
Cells, plasmids, and reagents.
A3.01 and Jurkat CCR7 T cells and peripheral blood mononuclear cells (PBMCs) were cultivated in RPMI 1640 plus GlutaMAX-I supplemented with 10% fetal calf serum and 1% penicillin-streptomycin (all from Invitrogen). 293T and TZM-bl cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with reagents identical to those described above for RPMI medium. The expression constructs for green fluorescent protein (GFP) and monomeric red fluorescent protein (mRFP) fusion proteins of HIV-1SF2 wild-type (wt) and mutant Nef proteins were described previously (34, 60, 61). The plasmid encoding SIVmac239 Nef.YFP (yellow fluorescent protein) was described previously (62). HIV-1SF2 Nef.myc and Lck.RFP were described previously (38). pcDNA-Vphu, expressing a codon-optimized, Rev-independent HIV-1NL4-3 Vpu protein (63), was provided by Klaus Strebel. Generation of the plasmid encoding the corresponding Vpu.GFP fusion protein was described previously (64). Point-mutated GFP-tagged Vpu and Nef variants were generated by site-directed mutagenesis. GFP-tagged Vpu with the replaced transmembrane domain of the vesicular stomatitis virus G protein (TM VSVG) was created by introducing PCR-amplified Vpu TM VSVG (template vector generously provided by Juan Bonifacino) into the peGFP-N1 expression vector. The Vpu.mCherry expression plasmid was created by introducing PCR-amplified codon-optimized Vpu into the pmCherry-N1 vector (Clontech) by using the EcoRI and BamHI restriction enzymes. Expression plasmids for N18src.GFP (GFP fusion protein of the first 18 amino acids of Src) and pDisplay.YFP (kindly provided by B. Müller, Heidelberg) were described previously (41, 65).
Proviral plasmids pHIV-1NL4-3 wt, pHIV-1NL4-3 Vpu stop (ΔVpu), pHIV-1NL4-3 Nef stop (ΔNef), and pHIV-1NL4-3 Vpu stop Nef stop (ΔΔ) and their internal ribosomal entry site (IRES).GFP-encoding variants (pBR.HIV-1NL4-3.IRES.GFP) were kindly provided by Frank Kirchhoff. Expression constructs for C-terminally enhanced YFP (eYFP)-tagged TSPANs were created either by using the Gateway cloning system by ligation of the TSPANs into the pDEST-ctYFP expression vector or by introducing PCR products encoding different TSPANs into the peYFP-N1 expression vector by using NheI and HindIII restriction enzymes.
The following antibodies and reagents were used: the Lyoplate human cell surface marker screening panel antibody collection (BD Biosciences); mouse anti-human CD3 (HIT3a), mouse anti-human CD37 (M-B371), mouse anti-human CD53 (HI29), mouse anti-human CD63 (H5C6), mouse anti-human CD81 (JS-81), allophycocyanin (APC)-conjugated mouse anti-human CD4 (RPA-T4), and anti-HLA-ABC (G46-2.6) antibodies (all from BD Biosciences); mouse anti-CD317 (eBioscience); mouse anti-Lck (3A5) (Santa Cruz); polyclonal anti-Vpu (Biozol); sheep anti-Nef (kind gift from Mark Harris) (66); monoclonal mouse anti-c-Myc (Santa Cruz); rat anti-GFP (Chromothek); sheep anti-HIV-1 p24CA antiserum (from Barbara Müller); rabbit anti-p42/p44 mitogen-activated protein kinase (MAPK) (Erk1/2) (Cell Signaling Technology); sheep anti-TGN46 (AbD Serotec); rabbit anti-protein disulfide isomerase (anti-PDi) (endoplasmic reticulum [ER] marker) (H-160; Santa Cruz), mouse CM1 recognizing β-COP (coatomer marker) (from Britta Brügger); and goat anti-EEA1 (early endosome marker) (C-15; Santa Cruz). Secondary fluorescent antibodies were obtained from Molecular Probes or BD Biosciences, and a protease inhibitor cocktail was purchased from Sigma-Aldrich. Phalloidin-TRITC (tetramethyl rhodamine isocyanate) was obtained from Sigma, and stroma-derived factor 1α (SDF-1α) was obtained from Immunotools.
Virus production and infection.
Virus stocks were generated by transfection (via Metafectene [Biontex] or JetPEI [Biomol]) of proviral HIV-1 plasmids into 293T cells. At 3 days posttransfection, culture supernatants were harvested, and virus stocks were concentrated (or not) via 20% sucrose cushion ultracentrifugation (24,000 rpm for 2 h). The HIV-1 p24 antigen concentration was determined by a p24 antigen enzyme-linked immunosorbent assay (ELISA).
PBMCs were isolated from fresh buffy coats via Ficoll (Ficoll-Paque Plus; GE Healthcare) gradient centrifugation essentially as described previously (67). For enrichment of CD4+ T cells, the RosetteSep human CD4+ T cell enrichment cocktail (Stemcell Technologies) was applied according to the manufacturer's instructions. Cells were stimulated with phytohemagglutinin (PHA) (5 μg/ml; Sigma) and interleukin-2 (IL-2) (100 U/ml; Biomol) for 3 days. A total of 3 × 106 cells were infected with 1 to 2 μg p24-containing virus stock in Retronectin (TaKaRa Bio, MoBiTec)-coated 24-well plates via spin infection (800 rpm for 1.5 h). Cells were kept in IL-2 for 3 days before being subjected to surface staining as described below for A3.01 cells. After fixation (2% paraformaldehyde [PFA] in phosphate-buffered saline [PBS] for 1.5 h), cells were investigated by flow cytometry.
Surface-exposed receptor levels.
In order to quantify the surface expression levels of a variety of cell surface markers in the presence of Nef or Vpu, the complete set (242 antibodies) of the Lyoplate human cell surface marker screening panel (BD) was applied for cell surface staining of A3.01 cells. A total of 5 × 106 cells were transfected with 18 μg plasmid DNA for Nef.GFP or Vpu.GFP fusion proteins via electroporation (950 μF, 300 V) (GenePulser; Bio-Rad). At 48 h posttransfection, ∼5 × 105 cells in 50 μl fluorescence-activated cell sorter (FACS) buffer (0.5% fetal calf serum [FCS] plus 0.09% NaN3 in PBS) were stained with 0.125 μg of each antibody in 96-well plates for 45 min on ice. After being washed with FACS buffer, cells were incubated with 100 μl (0.125 μg) of the diluted provided secondary antibodies (Alexa Fluor647-conjugated goat anti-mouse Ig and goat anti-rat Ig) for 30 to 45 min on ice.
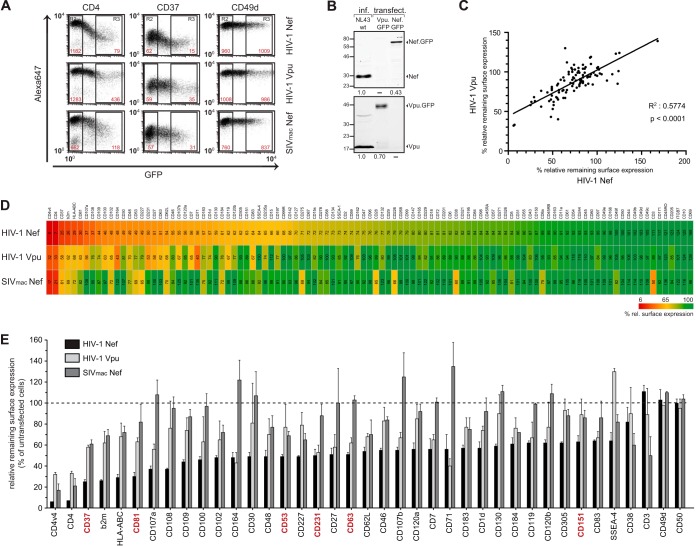
For selected surface receptors (CD37, CD53, CD63, CD81, and CD317), 5 × 105 to 1 × 106 cells in 50 μl FACS buffer were stained with 0.5 μg of primary antibody (0.1 to 0.25 μg for CD317), followed by staining with secondary goat anti-mouse Alexa Fluor600 (Invitrogen) or Alexa Fluor647 (BD Biosciences) antibodies. Staining against CD4 and HLA-ABC was assessed by APC-conjugated antibodies in a single step. The relative cell surface expression of the respective receptors was analyzed by flow cytometry (FACSCalibur with BD CellQuest Pro 4.0.2 software [BD Pharmingen] and Cyflogic 1.2.1 free analysis software). Within one sample, the receptor levels (y-axis geometric mean [YGeoMean]) on medium- to high-GFP-expressing cells (gate R3) (Fig. 1) were compared to levels on non-GFP-expressing cells (gate R2), as described previously (30). Data were processed with Microsoft Office Excel 2007 (heat map) and GraphPad Prism5 software.
FIG 1.
Lentiviral Nef and Vpu share the ability to downregulate a multiplicity of markers from the cell surface. A3.01 cells were transiently transfected with expression plasmids for GFP fusion proteins for HIV-1 Nef, HIV-1 Vpu, and SIVmac239 Nef (YFP fusion protein) and stained 48 h later with the individual antibodies in the Lyoplate human cell surface marker screening panel (BD). Surface expression of the respective markers was investigated by flow cytometry. (A) Representative flow cytometry dot plots of gated living cells. The MFI (YGeoMean) of untransfected (gate R2) and medium- to high-GFP-expressing (gate R3) cells is indicated in red for the respective surface receptor. (B) Western blot analysis of A3.01 CD4 T cells infected with wt HIV-1 or transiently transfected with expression plasmids for GFP fusion proteins for HIV-1 Nef or HIV-1 Vpu. A total of 1 × 105 infected or transfected cells were lysed and subjected to Western blotting for expression analysis of Nef (top) and Vpu (bottom). Intensities of the Nef and Vpu bands were quantified by using ImageStudioLite software (Li-Cor). Numbers below the panels indicate Nef and Vpu expression levels relative to that observed for cells infected with wt HIV-1, which was arbitrarily set to 1.0. (C) Correlation analysis of the cell surface receptor downregulation activity of HIV-1 Vpu and HIV-1 Nef. (D) Heat map diagram of the relative remaining surface expression (percent) of the individual markers screened for. Red indicates strong downregulation, yellow indicates medium downregulation, and green indicates no downregulation from the cell surface. The factors are ordered according to the identified surface levels upon HIV-1 Nef expression. The numbers represent mean values of the MFI ratio of transfected to untransfected cells (R3/R2) from three independent experiments. b2m, β2 microglobulin; ITGB7, integrin β7 (see also Table S1 in the supplemental material). (E) Diagram of all receptors showing <65% relative cell surface expression upon expression of HIV-1 Nef or Vpu or SIVmac Nef and two unaffected surface receptors (CD49d and CD50). Values are the arithmetic means and standard deviations from three independent experiments. Highlighted in red are members of the tetraspanin (TSPAN) family.
Immunofluorescence microscopy.
293T cells growing on cover glasses were transfected with mCherry, Vpu.mCherry, or Nef.RFP expression constructs together with different YFP-tagged TSPAN-encoding vectors. At 24 h posttransfection, the cells were fixed with 4% PFA, mounted in Mowiol, and analyzed with a Zeiss LSM510 confocal microscope with a 100× Plan-Apo objective lens. Images were recorded with Zeiss LSM5 proprietary software and processed with Adobe Photoshop 6.0. For detection of different subcellular compartments, 293T cells expressing either mCherry, Vpu.mCherry, or Nef.RFP together with the different YFP-tagged TSPANs were fixed and permeabilized for 2 min with 0.1% Triton X-100 in PBS, blocked for 1 h with 1% bovine serum albumin (BSA) in PBS, and stained with appropriate primary and secondary antibodies for detection of different subcellular compartments (TGN, ER, coatomer, or early endosomes). These cells were then analyzed by confocal microscopy as described above.
For Lck accumulation studies, microscope cover glasses (Marienfeld) were coated with a 0.01% poly-l-lysine (Sigma) solution for 1 h at room temperature. One day after transfection with the indicated expression plasmids (via electroporation) (see above), A3.01 T cells were plated onto the glasses (3 × 105 cells/cover glass) and fixed after 10 min at 37°C with PBS–3% PFA for 15 to 30 min. For indirect immunofluorescence of endogenous Lck, cells were permeabilized with 0.1% Triton X-100 for 2 min and blocked with PBS–1% BSA for 30 min. The primary mouse anti-Lck antibody (1:50) was applied for 2 h, followed by secondary goat anti-mouse Alexa Fluor568 for 1 h. After washing with PBS, cover glasses were mounted in LinMount (Linearis); in the case of cotransfection analyses, cells were mounted directly after cell fixation. Images were taken by using a 100× oil immersion objective lens (Ultra View VoX spinning-disk confocal system; PerkinElmer) and processed by using Adobe Photoshop. Image quantification was performed as described recently (38).
Virion infectivity assays.
TZM-bl cells (1 × 105 cells/well) were seeded into 12-well plates 1 day before Lipofectamine 2000 (Invitrogen) transfection of proviral DNA (1 μg of wt or vpu- and nef-deleted variants). At 2 days posttransfection, culture supernatants were investigated for particle release by a p24 ELISA and infectivity. For the latter, 25 μl of the culture supernatants was added to TZM-bl reporter cells cultured in a 96-well format, and the infectivity of HIV-1 was determined 48 h after infection by analysis of firefly luciferase activity (67, 68).
Migration and activation assays.
Analyses of SDF-1α-mediated T lymphocyte chemotaxis and membrane ruffling as well as TCR CD3-induced actin rearrangements were described previously (34, 40, 62).
Coprecipitation assay.
For coimmunoprecipitation analysis, 293T cells (5 × 105 cells/well) transiently expressing wt Vpu or Nef.myc together with YFP-tagged TSPANs were harvested at 24 h posttransfection and lysed on ice for 30 min in a buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 50 mM MgCl2, 1% Brij-99 (Vpu) or 1% Brij-99 (Nef), and a protease inhibitor cocktail (Sigma) before clarification at 6,000 × g for 10 min at 4°C. Twenty percent of each lysate was preserved to control for protein expression (input). The remaining cell lysate was incubated for 2 h with GFP-Trap beads (Chromothek). Immunoprecipitates were then washed three times with lysis buffer, resuspended in nonreducing SDS lysis buffer, and boiled for 10 min in order to release the immunoprecipitated proteins from the beads. The eluates were then analyzed for the presence of YFP-tagged TSPANs, Vpu, and Nef.myc by Western blotting.
Western blotting.
Washed cell pellets (for the infectivity assays) or eluates from the GFP-Trap beads were lysed in SDS lysis buffer. Proteins were separated on 12.5% SDS-PAGE and blotted onto nitrocellulose membranes. Blocked membranes were probed with the following primary antibodies: sheep anti-HIV-1 p24CA antiserum (from Barbara Müller), rabbit polyclonal anti-Vpu (Biozol), sheep anti-Nef, rabbit anti-c-Myc (Santa Cruz), rat anti-GFP (Chromothek), and anti-p42/p44 MAPK (Erk1/2) (Cell Signaling Technology). Secondary antibodies were conjugated to horseradish peroxidase for enhanced chemiluminescence (ECL)-based detection.
Statistical analysis.
Statistical analysis of data sets was carried out by using Microsoft Excel and GraphPad Prism. The statistical significance of parametrically and not-normally distributed data sets was analyzed by the Student t test and Mann-Whitney U test, respectively.
RESULTS
Nef and Vpu share the ability to reduce cell surface exposure of a broad range of receptors.
The surface exposure of a number of host cell receptors is modulated by expression of the accessory proteins Nef and Vpu of HIV-1. In order to gain an overview of the full potential of both viral proteins for modulation of cell surface receptor exposure and to identify their target specificity, we screened the cell surface expression of a large panel of receptors for its sensitivity to Nef and Vpu expression. To this end, we transiently expressed GFP or YFP fusion proteins of HIV-1SF2 Nef, HIV-1NL4-3 Vpu, or SIVmac239 Nef in A3.01 CD4 T cells and determined relative cell surface levels (Fig. 1; see also Table S1 in the supplemental material). The mean fluorescence intensity (MFI) levels of individual receptor stainings for GFP/YFP-positive cells were compared to those of untransfected control cells in the same sample (Fig. 1A, gate R3 versus gate R2). This allowed us to determine the effect of ectopic viral gene expression on the relative steady-state surface exposure of each receptor analyzed (see Fig. 1A for primary data for CD4 and CD49a, which are efficiently downregulated and unaffected, respectively, by expression of HIV-1 Nef, HIV-1 Vpu, or SIV Nef). Nef and Vpu expression levels in these cells transiently expressing GFP fusion proteins were slightly lower than those of nonfusion proteins produced in the context of HIV-1 infection (Fig. 1B).
Since these initial results validated our experimental approach, we next screened all receptors detected by antibodies of the Lyoplate human screening panel (BD) by flow cytometry. A total of 105 receptors were analyzed, for which the antibodies of this panel resulted in a robust and specific signal on the surface of A3.01 CD4 T cells. This analysis revealed that significant upregulation of surface levels was rare (observed only for CD69 by Nef) and identified a number of surface molecules that remained unaffected by the expression of any of the three viral proteins (e.g., CD49d [Fig. 1A] or CD50 [ICAM-3]), emphasizing that receptor downregulation is selective. Expectedly (19), downregulation of cell surface CD3 was specific for SIV Nef (Fig. 1D and E). Considering the suggested direct connector model, a surprisingly high number of surface-exposed receptors was found to be downregulated in the presence of HIV-1 Nef: surface levels of 36 out of 105 receptors were reduced to <65% (Fig. 1D and E). This included previously described targets such as CXCR4 (CD184) and MHC-I receptors but also transferrin receptor (CD71), which was reported to be downregulated by Nef in an allele- and cell type-dependent manner (69 – 71). In addition, a number of new targets with potentially interesting links to HIV biology were identified, such as CD37, Lamp-1 (CD107a), Lamp-2 (CD107b), ICAM-1 (CD102), or gamma interferon receptor (CD119). HIV-1 Vpu also lowered the surface exposure of a large set of receptors, and the downregulation activity of Vpu across the panel of surface receptors analyzed correlated remarkably well with that of Nef (Fig. 1C). Vpu downregulated 32 out of the 105 receptors to at least 80%, and all of these receptors were also targeted by Nef (Fig. 1D and E). This included MHC-I, for which downregulation by Vpu is somewhat controversial in the literature (7, 72). The effects of Vpu on individual receptors were overall less pronounced than those induced by Nef, except for CD164, CD317 (data not shown), and CD71, which, in contrast to our experiments with T lymphocytes, is unaffected by Vpu in 293T and HeLa cells (54, 73). With the exception of CD3 (Fig. 1D and E) and CD317 (data not shown), which are potently downregulated by SIV Nef, the receptor downregulation pattern induced by SIV Nef closely matched that of HIV-1 Vpu. These results reveal a remarkable overlap in the specificity of HIV-1 Nef and Vpu for the downmodulation of a broad but nevertheless specific set of cell surface receptors upon overexpression in A3.01 T cells, with Nef generally exerting a stronger downregulation activity than Vpu. Whether and by which mechanism the downregulation of individual receptors by these viral proteins plays pathophysiological roles in the context of natural infection require specific in-depth analyses.
Nef and Vpu reduce the cell surface levels of TSPANs.
Since the above-described results revealed that, upon overexpression in A3.01 T cells, HIV-1 Nef and Vpu largely overlap in functions related to cell surface receptor downregulation, we analyzed the characteristics of the set of receptors thus far unknown to be targeted by both viral proteins in more detail. Among the group of affected surface receptors was a considerable number of members of the tetraspanin (TSPAN) protein family (Fig. 1D and E). Indeed, all six TSPANs included in the screen (CD37 [also referred to as TSPAN-26], CD53 [TSPAN-25], CD63 [TSPAN-30], CD81 [TSPAN-28], CD151 [TSPAN-24], and CD231 [TSPAN-7]) were significantly downregulated from the cell surface by HIV-1 Nef, with CD37 and CD81 being two of the most severely affected molecules in the total investigation (25% and 35% relative remaining surface expression, respectively) (Fig. 1D and E and 2A). TSPAN surface exposure was also modulated by Vpu and SIV Nef but with reduced efficiency compared to that of HIV-1 Nef. We therefore decided to focus this study on the TSPAN protein family.
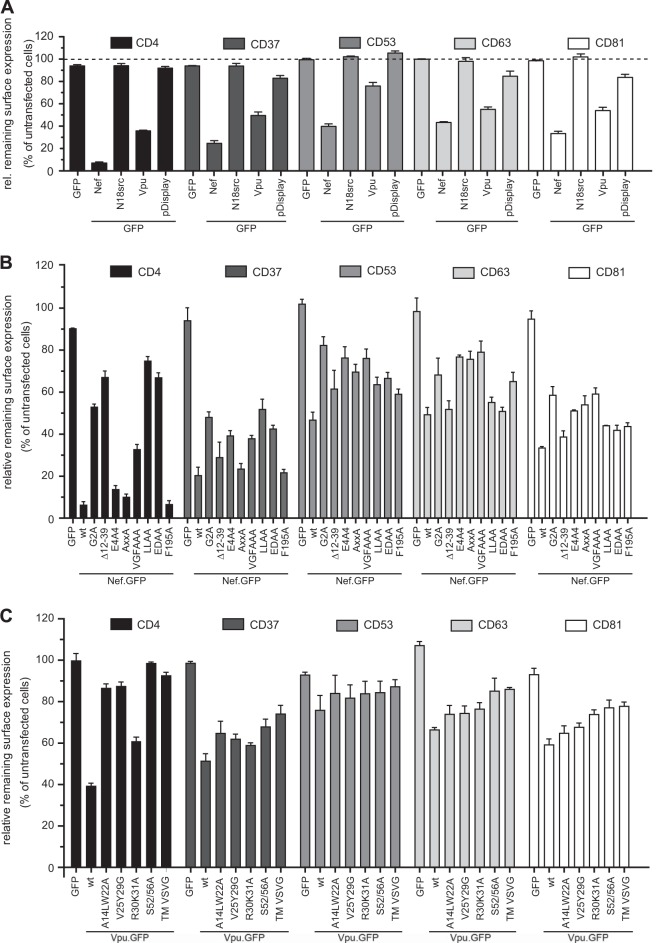
FIG 2.
Nef and Vpu affect the cell surface levels of TSPANs and show a distinct pattern of TSPAN downmodulation. A3.01 T lymphocytes were transiently transfected with the expression plasmids for GFP fusions of Nef or Vpu, unrelated proteins with properties similar to those of Nef or Vpu (N18.src and pDisplay, respectively) (pDisplay as YFP fusion) (A), the indicated Nef (B) and Vpu (C) mutant proteins, or the GFP control. Forty-eight hours later, cells were stained for flow cytometry analysis with selected antibodies for cell surface markers by indirect immunofluorescence. Receptor levels of GFP-positive cells were compared with those of untransfected cells within individual samples (Fig. 1). Shown are arithmetic mean values and standard errors of the means from three (two in the cases of Nef G2A, Δ12-39, and VGFAAA) experiments in triplicates.
Distinct molecular determinants in Nef and Vpu mediate cell surface downregulation of individual TSPANs.
The effects of Nef and Vpu on such a broad range of TSPAN family members could be the result of, e.g., an unspecific perturbation of membrane transport due to membrane association and insertion of these viral proteins, respectively. We therefore tested the effect of unrelated membrane-associated GFP fusion proteins on TSPAN cell surface exposure (Fig. 2A). N18src.GFP tethers GFP to the inner leaflet of the plasma membrane via a myristoylated SH4 domain in a manner analogous to that of Nef (65, 74), and pDisplay-YFP directs YFP to the transmembrane domain of CD4 to mimic Vpu (41). Both control proteins did not induce significant changes to TSPAN cell surface levels, indicating that TSPAN downregulation by Nef and Vpu is a specific process. We next asked whether the viral proteins employ conserved mechanisms to downregulate the individual TSPANs from the cell surface. We made use of a panel of characterized Nef and Vpu mutants to map the determinants involved in cell surface downmodulation of CD37, CD53, CD63, and CD81 in A3.01 cells transiently expressing Nef.GFP (Fig. 2B) or Vpu.GFP (Fig. 2C). For Nef, downregulation of CD4 was included as a positive control. Expectedly (18, 19, 52), mutations affecting the ability of Nef to interact with SH3 domain-containing host cell proteins (AxxA; VGFAAA), the phosphofurin acidic cluster sorting (PACS) adaptor (E4A4), or Pak2 kinase (F195A) had little to no effect on Nef-mediated CD4 downregulation. Mutations preventing Nef's N-terminal myristoylation, and thus efficient membrane associations (G2A) or interactions with the Nef-associated kinase complex (Δ12-39) or the endocytic machinery (adaptor complex for LLAA; catalytic subunit of the V-ATPase for EDAA), significantly impaired the activity of Nef on cell surface CD4. This mutant pattern is clearly distinct from that observed for receptors such as MHC-I or CXCR4, the downregulation of which depends on the motifs in Nef mediating interactions with SH3 domains (PxxP and VGF) but not endocytic adaptor complexes (31, 61, 75). Surprisingly, the molecular Nef determinants required for TSPAN cell surface downregulation differed for individual TSPANs, and no clear “CD4-like” or “MHC-I-like” mutant patterns were observed and no essential molecular determinant was determined for any of the TSPANs analyzed.
Similarly, the analysis of Vpu failed to identify an essential determinant of TSPAN downregulation (Fig. 2C). Surface exposure of both control receptors CD4 and CD317 (data not shown) was unaffected by Vpu mutants with a replaced diserine motif, and thus, coupling to beta-transducin repeat-containing protein (β-TrCP) (S52/56A) or its transmembrane domain was replaced by that of the vesicular stomatitis virus G protein (TM VSVG) (54). The A14L,W22A mutant, reported to be defective in transmembrane interactions of Vpu (76), was fully and partially defective in downregulating CD4 and CD317, respectively. A motif regulating Vpu's subcellular distribution (R30A,K31A) (77) was largely dispensable for the downregulation of both receptors, while a motif governing Vpu's lipid raft incorporation (V25G,Y29G) (68) was specifically required for the downregulation of cell surface CD4. With respect to TSPAN cell surface modulation, the diserine and TM substitution mutants of Vpu displayed a partial loss of function for all family members tested, while the additional mutants showed only slightly reduced activity. In contrast to Nef, the mutant pattern toward individual TSPANs was largely similar for Vpu.
Together, these results suggest that the effects of Nef and Vpu on TSPAN cell surface exposure may not be explained by the receptor modulation mechanisms already described for these viral proteins. In the case of Nef, even different molecular mechanisms may be employed toward individual TSPAN members.
Nef and Vpu counteract the upregulation of TSPAN cell surface expression levels in HIV-1-infected primary human CD4+ T lymphocytes.
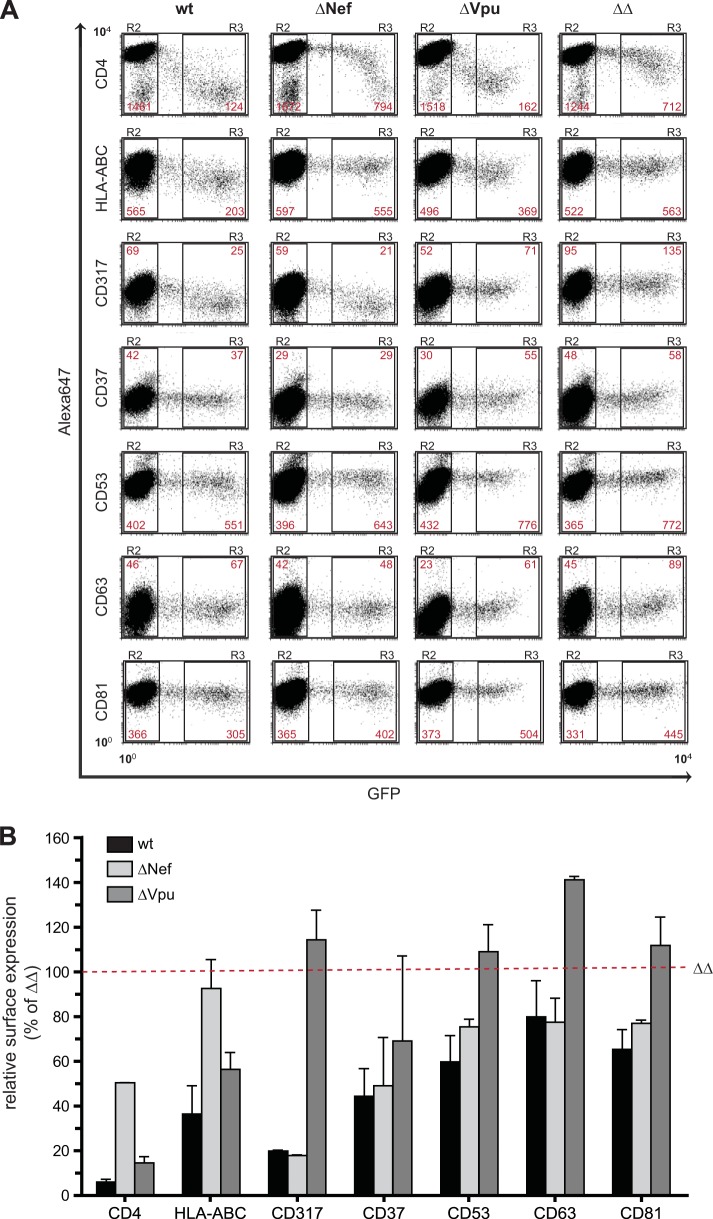
Since the above-described findings were obtained by using a human T cell line and transient overexpression of the accessory viral proteins, we next asked whether the effects of Nef and Vpu on TSPAN surface exposure are also observed upon HIV-1 infection of primary target cells. To this end, activated primary human CD4+ T lymphocytes were infected with either HIV-1NL4-3 (wt) or isogenic counterparts lacking the expression of Nef (ΔNef), Vpu (ΔVpu), or Nef and Vpu (ΔΔ). The proviruses used for the production of infectious progeny contain an IRES.GFP element that allows detection of productively infected cells by GFP expression.
At 2 days postinfection, CD4+ T cells were analyzed for surface expression of selected TSPANs (CD37, CD53, CD63, and CD81) or well-characterized reference receptors (CD4, HLA-ABC, and CD317) (Fig. 3A and B). The comparison demonstrated that surface levels of all receptors analyzed were lower in cells infected with wt HIV-1 than in cells infected with HIV-1 ΔΔ. For CD4, CD317, and MHC-I, this mirrored the net downregulation of cell surface receptors in wt HIV-1-infected (gate R3, GFP-positive) (Fig. 3A) relative to uninfected (gate R2, GFP-negative) (Fig. 3A) cells, which was abrogated in the absence of Nef and Vpu. For the TSPANs CD37, CD53, CD63, and CD83, surface levels were moderately upregulated in HIV-1 ΔΔ-infected cells by an unknown mechanism, and expression of Nef and Vpu reduced TSPAN exposure to the surface levels observed for uninfected cells (Fig. 3A and B). Expectedly, Nef was more critical than Vpu for the downregulation of CD4 and MHC-I in the context of primary CD4+ T cell infection, and downregulation of cell surface CD317 was exclusively dependent on Vpu (Fig. 3A and B). Given the results of the overexpression studies of A3.01 cells described above, it was surprising that Vpu affected TSPAN cell surface expression more efficiently than Nef in the infection context of primary CD4 T cells (Fig. 3B). Nef exerted only very mild effects for some of the TSPANs, and little synergy between Nef and Vpu was observed. Preliminary results from infection experiments in A3.01 T cells indicate that these differences in the relative activities of Nef and Vpu most likely reflect differences between this T cell line and primary cells (data not shown). Collectively, these results demonstrate that Nef and Vpu have the capacity to moderately modulate cell surface expression of individual TSPAN proteins in infected primary CD4+ T cells and that Vpu is the main contributor to the HIV-1-induced reduction of TSPAN cell surface levels in HIV-infected primary human CD4 T lymphocytes.
FIG 3.
Nef and Vpu affect the cell surface levels of tetraspanins in the context of infection. CD4-positive T cells were negatively isolated from fresh buffy coats, infected with wt HIV-1NL4-3-IRES-GFP (wt) or a Nef- and Vpu-defective counterpart (ΔΔ), and stained for surface expression of selected TSPANs 72 h later. Cells were fixed and analyzed by flow cytometry. (A) Representative flow cytometry dot plots of gated living cells. The MFI (YGeoMean) of uninfected (gate R2) and infected (GFP-positive) (gate R3) cells is shown in red for the indicated surface receptors. (B) Histogram of the relative surface expression of the depicted TSPANs and controls (CD4, HLA-ABC, and CD317) after CD4+ T cell infection with wt HIV-1NL4-3-IRES-GFP, ΔΔ, and the single Nef-defective (ΔNef) or Vpu-defective (ΔVpu) counterparts. For each marker, the MFI ratio of infected (R3) to uninfected (R2) cells was determined. Subsequently, the value for ΔΔ-infected cultures was set to 100%. Values are the arithmetic means of data from two independent experiments (two donors) and standard deviations.
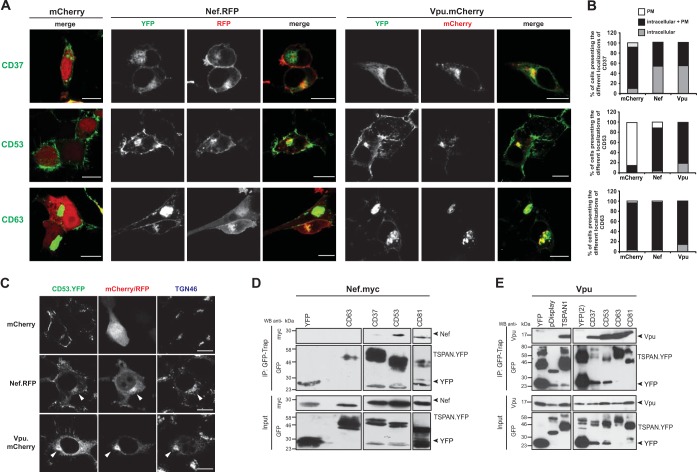
Nef and Vpu trigger accumulation of a variety of TSPANs in a perinuclear compartment.
We next wondered whether the modulation of TSPAN surface levels by Nef and Vpu is paralleled by a colocalization of these viral and cellular proteins or whether these proteins affect each other's subcellular localization. We therefore analyzed the subcellular localization of the YFP-tagged TSPANs CD37, CD53, and CD63 in the absence or presence of Nef and Vpu (expressed as mRFP and mCherry fusion proteins, respectively) by confocal microscopy (Fig. 4A and B). In control cells expressing only mCherry, CD37 and CD63 were detected at the PM as well as in intracellular compartments (referred to as intracellular + PM in Fig. 4B), with CD63 displaying pronounced intracellular accumulation. In contrast, CD53 localized predominately at the PM (Fig. 4B). For TSPANs with marked PM localization, and in accordance with the flow cytometric data, coexpression of Nef or Vpu resulted in a reduction of their PM localization that coincided with a marked accumulation of the TSPAN in a perinuclear area (Fig. 4A and B for CD37 and CD53; data not shown for CD9). Such a striking relocalization was not observed for TSPANs that displayed predominantly perinuclear localization in the absence of Nef or Vpu, such as CD63 (Fig. 4A and B), CD231, TSPAN1, TSPAN3, and TSPAN13 (data not shown). Vpu and Nef were always enriched in intracellular areas of TSPAN accumulation; however, this colocalization with perinuclear TSPANs was pronounced for Vpu, while Nef was detected in this area of the cell but colocalized less with distinct TSPAN-positive subcellular structures. Perinuclear TSPANs displayed a substantial overlap of the trans-Golgi marker TGN46 (Fig. 4C) and individual TSPANs; colocalization was also detected with markers for early endosomes and coatomer (data not shown). While the precise identity of the TSPAN-positive compartment in Vpu/Nef-expressing cells remains to be defined, the viral proteins induce the enrichment of TSPANs in anterograde transport and recycling compartments.
FIG 4.
Vpu and Nef colocalize with different TSPANs mainly in the trans-Golgi network and interact with a variety of TSPANs. (A) 293T cells, grown on cover glasses, were transfected with different YFP-tagged TSPAN-expressing plasmids together with vectors encoding either mCherry (left), Nef.RFP (middle), or Vpu.mCherry (right). Subsequently, cells were cultivated for 24 h and then fixed, mounted in Mowiol, and analyzed by confocal microscopy. Microphotographs shown are representative of three independent experiments. Bar = 10 μm. (B) For each YFP-tagged TSPAN analyzed, stainings were categorized into cells showing either solely plasma membrane staining (“PM”), exclusively intracellular staining (“intracellular”), or combined intracellular and plasma membrane stainings (“intracellular + PM”). Histogram bars depict the relative percentages of cells showing the different localization categories. At least 300 cells were analyzed out of three independent experiments. Bar = 10 μm. (C) 293T cells, grown on cover glasses, were transfected with different YFP-tagged TSPAN-expressing plasmids (green signal) together with vectors encoding either mCherry, Vpu.mCherry, or Nef.RFP (red signal). Subsequently, cells were cultivated for 24 h and then fixed, permeabilized, and stained with an anti-TGN46 secondary antibody (TGN marker) followed by an Alexa Fluor660-conjugated secondary antibody (blue staining) to visualize the different subcellular compartments. Cover glasses mounted in Mowiol were then analyzed by confocal microscopy. Bar = 10 μm. (D and E) 293T cells were cotransfected with Nef.Myc (D)- or Vpu (E)-encoding plasmids together with the indicated YFP-tagged TSPANs or the YFP expression constructs. Twenty-four hours later, the different YFP-tagged TSPAN variants were immunoprecipitated via their YFP tag from cell lysates by using GFP-Trap beads, and 20% of the lysates (“Input”) and 80% of the immunoprecipitates (“IP”) were subjected to SDS-PAGE and Western blotting. Black arrowheads in panels D and E indicate the running height for YFP, Nef, and Vpu. These blots are representative of two independent experiments.
Nef and Vpu associate with a variety of TSPANs.
Given the observed effects of Nef and Vpu on TSPAN surface exposure and subcellular localization, we next tested whether the viral proteins physically associate with this protein family. Vpu or a Myc-tagged version of Nef was expressed with YFP-tagged forms of various TSPANs in 293T cells, and TSPANs with associated proteins were immunoisolated by using GFP-Trap. YFP- or pDisplay.YFP-expressing cells served as negative controls. Nef weakly associated with some of the TSPANs analyzed (here CD53), but (nearly) no association was detected with other TSPANs (shown here for CD37 and CD63) (Fig. 4D). In contrast, robust coimmunoprecipitation of Vpu, but not the pDisplay.YFP control, was observed with all TSPANs analyzed (Fig. 4E and data not shown). In summary, Nef and Vpu broadly downmodulate cell surface levels of TSPANs and induce their perinuclear accumulation. The accessory viral proteins colocalize with many TSPAN family members in this perinuclear compartment and physically associate with them, but the physical link to TSPANs appears to be more pronounced for Vpu than for Nef. TSPANs thus emerge as an important cellular target of Nef and Vpu.
Nef and Vpu share the ability to affect the subcellular localization of the peripheral membrane protein Lck.
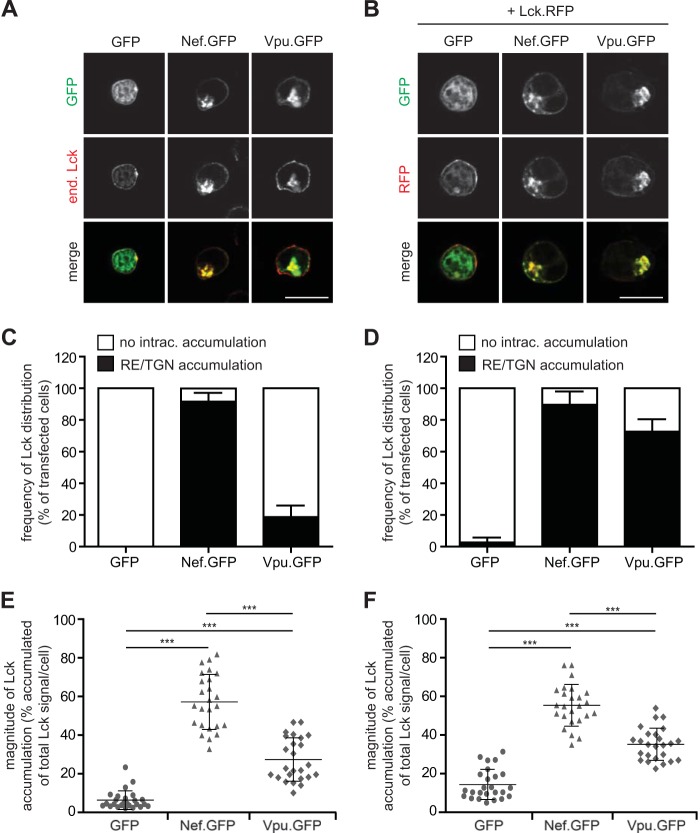
The identified broad similarities of Nef and Vpu in modulating the surface exposure of cell surface transmembrane receptors prompted us to investigate whether Vpu also exerts other activities ascribed to Nef. We first investigated effects on the subcellular localization of the Src family kinase and the peripheral membrane protein Lck. Rerouting of this key player in TCR signaling away from the PM to REs and the TGN is a well-characterized function of Nef in T lymphocytes. This effect does not require a physical interaction of Nef with Lck and is achieved by indirect inhibition of a yet-to-be-defined vesicular transport route for PM delivery of newly synthesized Lck and possibly other cargo molecules (35, 37, 38, 65). To elucidate whether Vpu has a similar effect on Lck, we analyzed the localization of endogenous Lck in A3.01 T cells transiently expressing either a GFP control or GFP fusion proteins of HIV-1 Nef or Vpu (Fig. 5A to F). Microscopic analyses revealed that Nef.GFP, but not GFP alone, induced a pronounced RE/TGN accumulation of Lck in the large majority of cells analyzed (92% versus 0% of cells with detectable Lck accumulation) (Fig. 5A and C). Expression of Vpu.GFP also induced Lck retargeting but with much-reduced frequency compared to Nef (19% of cells with Lck accumulation) (Fig. 5A and C). However, when the GFP versions of the viral proteins were coexpressed with an Lck.RFP fusion protein, Lck accumulation was observed with almost similar frequencies in Vpu- and Nef-expressing cells (73% and 90%, respectively) (Fig. 5B and D). This suggests that Vpu may affect the transport of newly synthesized Lck molecules but is less efficient than Nef in retargeting Lck pools that already reached their steady-state distribution following biosynthetic anterograde transport.
FIG 5.
Nef and Vpu relocalize the signaling molecule Lck. (A) A3.01 T lymphocytes were transiently transfected with plasmids expressing GFP, Nef.GFP, or Vpu.GFP. Twenty-four hours later, cells were fixed on poly-l-lysine-coated cover glasses and analyzed by immunofluorescence for endogenous (end.) Lck expression. Shown are representative confocal micrographs. (B) Confocal micrographs of A3.01 cells transiently coexpressing Lck.RFP together with GFP fusion proteins as described above for panel A. Bar = 10 μm. (C) Relative frequency of intracellularly accumulated (recycling endosomes/trans-Golgi network) Lck of cells, as described above for panel A, by categorizing transfected cells as those with or without intracellular Lck accumulation. Values are the means of data from 3 independent experiments and standard deviations in which 100 cells were counted per condition. (D) Quantification as described above for panel C for cotransfected cells as described above for panel B. (E and F) Quantification of Lck distribution in single cells. Depicted are the percentages of the total per-cell Lck signal detected in intracellular accumulations. Each symbol designates a value for an individual cell. Bars indicate the mean values of all cells analyzed (25 cells per condition). Statistical significance as assessed by the Mann-Whitney U test is indicated (***, P < 0.0001).
We next analyzed the magnitude of Lck retargeting in single cells in which this effect was observed (i.e., pixel quantification of the accumulated versus the total Lck signal per cell). In the presence of Nef, more than half of the total endogenous or ectopically expressed Lck signal per cell resided in intracellular accumulations (Fig. 5E). Vpu also induced more Lck accumulation than observed for control cells, but this effect was less pronounced than that of Nef (Fig. 5F). Overall, these results show that Vpu can affect the subcellular localization of peripheral membrane proteins such as Lck but with reduced efficacy compared to Nef.
Nef and Vpu enhance single rounds of virus replication via distinct mechanisms.
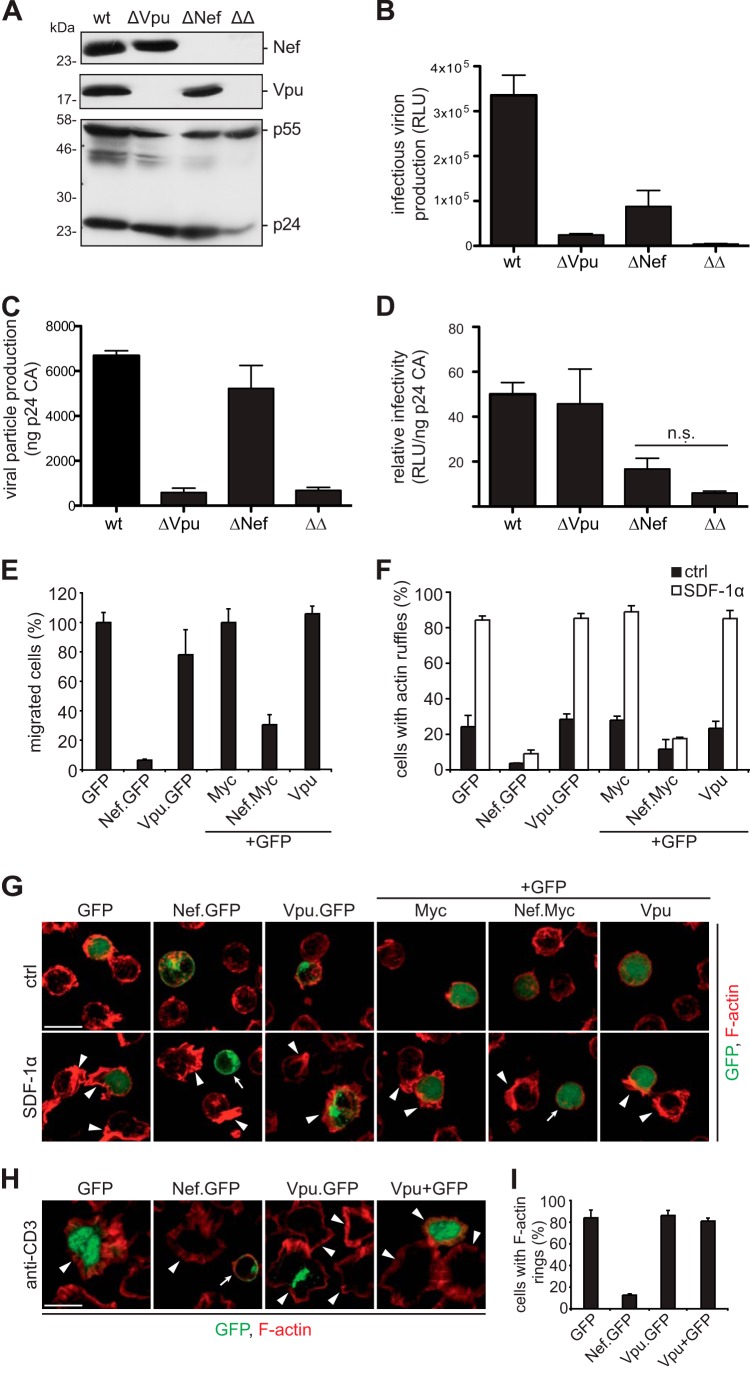
Nef enhances the infectivity of virus particles, while Vpu facilitates virus production from CD317-positive producer cells by antagonizing CD317 particle release restriction (46, 47, 78 – 80). To compare these activities side by side, virions were produced from CD317-expressing TZM-bl cells by transfection of proviral constructs encoding wt HIV-1 or derivatives thereof that lack expression of one or both accessory proteins (wt, ΔNef, ΔVpu, and ΔΔ) (Fig. 6A to I). Western blot analyses of lysates of these virus-producing cells confirmed the expected viral protein expression pattern (Fig. 6A). At 2 days posttransfection, the corresponding supernatants were used to test the production of infectious virions in a single-round infectivity assay on TZM-bl reporter cells (Fig. 6B). The lack of expression of either Nef or Vpu during virus production resulted in a significant drop in the production of infectious particles, and these effects were additive in the context of the ΔΔ double mutant. Quantification of the amount of p24CA released during virus production as a correlate for the release of physical virus particles (Fig. 6C) and of relative infectivity (infectious units per ng p24CA) (Fig. 6D) allowed us to differentiate between the effects of Nef and Vpu. Reflecting its role as a CD317 antagonist, Vpu exclusively enhanced virus production but not the infectivity of these particles. In contrast, Nef did not affect the production and release of physical particles but increased their infectivity. Consistent with previous reports analyzing the effects of Nef and Vpu separately, our direct comparison confirms that Nef and Vpu affect single rounds of HIV replication in CD317-positive cells via strictly distinct mechanisms and by acting at different stages of the viral life cycle.
FIG 6.
HIV-1 Nef and Vpu differ in interference with viral spread, cell migration, and actin rearrangement. (A) TZM-bl cells were transfected with 1 μg proviral constructs encoding wt HIV-1 or Nef- and Vpu-defective variants (ΔNef and ΔVpu, respectively) or a double mutant (ΔΔ). Shown are representative Western blot analyses of the corresponding cell lysates at 2 days posttransfection detecting viral Nef, Vpu, and capsid (p24CA) proteins. (B and C) Culture supernatants were used to analyze the amount of released viral particles by a p24CA ELISA (C) and the release of infectious HIV-1 virions in a single-round infectivity assay by infecting TZM-bl reporter cells (relative light units [RLU]) (B). (D) Relative infectious virus yield per ng p24CA (corresponding to data in panels B and C). Values are the arithmetic means and standard deviations of triplicates (B to D). Statistical significance is determined by the Student t test. n.s., nonsignificant. (E) Jurkat CCR7 T cells were transiently transfected to express the indicated GFP control, fusion proteins of Nef and Vpu, or nonfluorescent versions together with GFP and subjected to a transwell chemotaxis assay. Depicted is the percentage of GFP-positive cells that migrated toward 10 ng/ml SDF-1α over 2 h. Values are the means and standard deviations of data from 3 independent experiments. (F) Relative frequency of transiently transfected Jurkat CCR7 T cells (constructs as described above for panel E) that display membrane ruffling in response to treatment with 200 ng/ml SDF-1α or a solvent control (ctrl). Depicted are mean values from 3 independent experiments and standard deviations with at least 100 cells analyzed per condition. (G) Representative maximum projections of confocal z stacks of cells as described above for panel F. Shown are merged pictures of the GFP (green) and F-actin (phalloidin) (red) signals. Bar = 10 μm. Arrowheads indicate cells with F-actin-rich ruffles, and arrows indicate cells in which Nef prevented actin ruffling. (H) Jurkat CCR7 T cells were transiently transfected with the indicated expression plasmids and plated onto anti-CD3-coated cover glasses for 10 min to allow circumferential F-actin ring formation. After fixation, cells were stained with phalloidin to reveal F-actin. Shown are merged confocal mircrographs of the GFP (green) and F-actin (phalloidin) (red) signals. Bar = 10 μm. Arrowheads indicate cells with F-actin rings, and arrows indicate cells in which Nef prevented the formation of F-actin rings. (I) Frequency of cells shown in panel H that display F-actin-rich rings.
Vpu does not share Nef's ability to interfere with host cell actin remodeling.
We next asked whether Vpu also shares the ability of Nef to interfere with actin remodeling of T lymphocytes to affect their chemotaxis and migration. Jurkat T cells, which chemotax better than A3.01 cells (data not shown), showed the expected potent reduction in in vitro chemotaxis toward SDF-1α across transwell filters when transiently expressing GFP- or Myc-tagged versions of HIV-1 Nef (40, 81). In contrast, Vpu did not significantly influence the migration capacity of these cells (Fig. 6E). While Vpu reduces chemotaxis toward CCL19 by downregulating the corresponding receptor CCR7 from the cell surface (82), Vpu does not exert a generalized disruption of cell motility. Consistent results were obtained by investigating SDF-1α-induced F-actin-rich membrane ruffling, which was suppressed by Nef but not by Vpu (Fig. 6F and G). Furthermore, Vpu did not affect TCR-triggered actin polymerization into circumferential actin rings following surface-bound stimulation, a prominent activity of Nef (34, 62) (Fig. 6H and I). Thus, Nef and Vpu clearly differ in their abilities to interfere with host cell motility and actin rearrangements.
DISCUSSION
This study set out to advance our understanding of the breadth with which Nef and Vpu affect host cell receptor densities. Parallel analysis of 105 receptors detectable on the surface of A3.01 T lymphocytes revealed a previously unappreciated scope with which HIV-1 Nef remodels the cell surface of these HIV-1 target cells. Surprisingly, HIV-1 Vpu and SIV Nef largely shared this broad effect on host cell surface receptors, even though their activity against individual receptors was less pronounced than that of HIV-1 Nef. This redundancy in biological activity also extended to peripheral membrane proteins such as Lck, whose intracellular transport following biosynthesis was altered in a similar manner by Nef and Vpu. In contrast, other activities were not shared between both viral proteins: while Nef increases the relative infectivity of HIV-1 virions but does not antagonize the restriction of particle release imposed by CD317, Vpu, in our hands, does not affect the infectivity of HIV-1 particles but strongly facilitates their release from CD317-positive cells. Nef activities targeted at modulating host cell actin dynamics and motility were not shared by Vpu. Conversely, the ion channel activity of the transmembrane protein Vpu is likely not exerted by the peripheral membrane protein Nef. It thus emerges that (i) Nef and Vpu share an important set of biological activities, most notably remodeling of the surface receptor environment; (ii) both viral proteins promote single rounds of virus replication by divergent mechanisms; and (iii) additional modulatory activities are unique for either Nef or Vpu. This elaborate pattern of shared and individualized activities of Nef and Vpu likely represents the result of a detailed adaptation of HIV-1 to its human host.
Given that Nef and Vpu are completely distinct in their membrane topology, their domain organization, and the presence of signature motifs for interactions with host cell factors, we were surprised by the remarkable overlap in the receptor specificities of both viral proteins. As discussed in more detail below, this broad scope toward cell surface receptors does not appear compatible with the “direct connector model” but likely reflects the ability of Nef and Vpu to affect general vesicular transport pathways, of which the identified target molecules are cargo. Reduction of cell surface exposure of a given receptor can be achieved by limiting its delivery to the surface, its enhanced internalization from the cell surface, and/or impaired recycling. Notably, the effects of Nef on cell surface receptor levels were more pronounced than those of Vpu in most cases. This might reflect that Nef, depending on the cellular context, can affect internalization or recycling rates of receptors as well as their anterograde PM delivery (10, 19, 52, 83 – 87). In contrast, the action of Vpu appears to be limited mainly to anterograde transport and does not enhance receptor internalization (5, 7, 9, 48, 50, 53, 54, 88 – 92). A similar scenario may apply to peripheral membrane proteins such as Lck: Nef could efficiently reroute the entire cellular pool by affecting transport to as well as from the PM, while the activity of Vpu could be limited to the newly synthesized protein pool. This would explain why Nef affects endogenous as well as ectopically expressed Lck, while rerouting by Vpu is prominent only when ectopic Lck is coexpressed simultaneously with the viral protein. Depending on the trafficking and turnover rates of any given cargo molecule, Vpu thus fails to imprint the pronounced changes on the subcellular distribution of cargo observed in the presence of Nef. In this scenario, the different efficacies with which Nef and Vpu affected TSPAN cell surface levels in T cell lines and primary T lymphocytes may reflect that the contribution of individual transport pathways could be distinct in these two cell systems.
Another main aspect of this study is the identification of TSPANs as a major target for cell surface downregulation by Nef and Vpu. Even though the precise molecular mechanism by which these two viral proteins affect intracellular TSPAN transport remains to be defined, our results indicate that these mechanisms will be slightly different for Nef and Vpu. Vpu triggered the enrichment of TSPANs with natural PM exposure in a perinuclear compartment colocalized with all TSPANs analyzed in this TGN marker-positive compartment and physically associated with TSPANs. Similar results for surface downregulation, relocalization, and physical association, including in the context of HIV-1 infection, for Vpu and the TSPAN CD81 were independently obtained by the Thali laboratory, and the Schindler laboratory obtained evidence for the direct interaction of Vpu with CD81 in intact human cells in a FACS-based fluorescence resonance energy transfer (FRET) assay (M. Lambele, M. Schindler, and M. Thali, unpublished data). Taken together, these results strongly suggest that Vpu physically associates with TSPANs to alter their intracellular transport and reduce their cell surface exposure. This might reflect direct protein-protein interactions or coassembly into specialized membrane microdomains. While our results do not fully allow us to distinguish between these possibilities, we favor the direct interaction model since (i) TSPAN-Vpu interactions were also observed under buffer conditions known to disrupt TSPAN microdomains (data not shown), (ii) the lipid raft targeting motif of Vpu was dispensable for TSPAN downregulation, and (iii) FRET-based interactions between Vpu and CD81 were demonstrated. Since the Vpu transmembrane domain, which mediates its physical association with CD317 (59, 76, 93), was largely dispensable for Vpu's effect on TSPAN localization, the determinants for this interaction require further investigation.
In comparison to Vpu, the perinuclear colocalization of Nef with TSPANs was less pronounced, physical interactions were less frequent/stable, and a FACS-FRET interaction with CD81 was not observed by Lambele and colleagues (Lambele et al., unpublished). This is similar to the retargeting of Lck from the PM to RE/TGN compartments by Nef, for which association and colocalization are dispensable (38, 65, 94 – 96). Nef may thus act in an indirect manner on TSPAN trafficking. Therefore, Nef and Vpu likely affect the intracellular transport routes of a broad but nevertheless specific panel of cellular cargo molecules via distinct mechanisms, and it will be interesting to study how such specificity is achieved. At this point, we speculate that specificity is gained by the membrane microenvironment used for intracellular TSPAN transport. While Vpu may be positioned at a critical subcellular site of this transport route and can intercept transport by physical interaction, Nef may achieve similar results via yet-to-be-defined indirect mechanisms. In addition to TSPANs, Nef is also known to broadly affect the cell surface exposure and subcellular distribution of the chemokine receptor family (29 – 31), and Vpu was recently reported to reduce cell surface levels of the chemokine receptor CCL19 (82). Given that the presence of multiple membrane-spanning domains is a common feature of TSPANs (four) and chemokine receptors (seven), multipassing transmembrane proteins may be particularly prone to segregation into such Nef- and Vpu-sensitive transport platforms. Defining the nature of this transport pathway and its cargo specificity will be important steps toward unraveling the mechanisms by which Vpu and Nef interfere with it.
Another open question relates to the functional consequence of the observed alterations in TSPAN subcellular localization by Nef and Vpu. In line with the physiological role of TSPANs as molecular organizers for other transmembrane proteins that regulate cell adhesion, cell-cell fusion, and membrane morphology (97 – 102), TSPAN-containing microdomains are viewed as platforms for the release of infectious HIV-1 (103 – 107). The overexpression of individual TSPANs such as CD9 and CD63 or the addition of antibodies against individual TSPANs does not affect the efficiency of HIV particle release but reduces the infectivity of released virions or their ability to undergo cell-cell transmission, in particular by impairing virion fusion (104, 105, 108). TSPANs thus negatively regulate events during virus production that impact the quality but not the quantity of viral progeny and thus the efficiency of subsequent rounds of virus replication (104, 109, 110). In particular, given that Nef and Vpu have been demonstrated to promote cell-cell transmission under certain experimental conditions (111 – 113), it is tempting to speculate that these effects are achieved, at least in part, via the deregulation of TSPAN localization and function. Similar to such effects at the virological synapse affecting cell-to-cell spread, modified TSPAN surface exposure might affect cell-cell communication across the immunological synapse, a process potently disrupted by Nef (34 – 36, 38, 114).
Of note, silencing of the expression of individual TSPANs failed to produce phenotypes consistent with those observed upon TSPAN overexpression (104), an observation that might be explained by the broad effects of Nef and Vpu on the entire TSPAN family and a redundancy of function between individual TSPAN family members. In this scenario, the subtle effects that Vpu and Nef exert on surface levels of individual TSPAN family members in infected primary human T lymphocytes may add up to a biologically significant alteration of TSPAN surface exposure. If TSPAN rerouting is directly involved in the optimization of HIV-1 spread by Nef and Vpu, such an effect will thus not be due to the altered activity of one individual TSPAN. Dissecting the functional relevance of TSPAN deregulation will therefore depend on the prior identification of specific molecular determinants in Nef and Vpu that govern this activity.
From an evolutionary point of view, the emergence of HIV-1 nef and vpu genes from ancestral SIV nef resulted in the duplication of HIV's potential to remodel the cell surface composition of infected cells. Nef and Vpu are expressed to maximal levels early and late in the viral life cycle, respectively, which may allow the virus to control host cell vesicular transport at all steps of its replication cycle. Given that transport routes and kinetics of individual TSPANs likely vary in different HIV target cells, the existence of two independent mechanisms may also ensure the deregulation of vesicular transport in distinct cellular environments. Additionally, this functional duplication may provide a general backup strategy to prevent the loss of this activity altogether.
While these aspects clearly indicate the existence of high selection pressure on HIV's ability to alter host cell receptor exposure, the unexpected breadth of this activity suggests that the virus benefits from the global change rather than from the modulation of a few individual receptors. This is exemplified by the chemokine receptor and TSPAN families, where whole classes of proteins are affected by Nef and Vpu, with various degrees of effects on individual family members. Together with the remarkable ability of HIV accessory genes to rapidly (re)gain specific activities if subjected to specific selection pressures (115), encoding two versatile and multifunctional proteins such as Nef and Vpu might provide HIV-1 with the genetic and functional plasticity required to rapidly adapt to the ever-changing environment in its human host.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the Nikon Imaging Center for access to biosafety level 1 (BSL-1) and BSL-2 imaging facilities and service; Nadine Tibroni for expert technical help; Klaus Strebel, Juan Bonifacino, Mark Harris, Frank Kirchhoff, Barbara Müller, and Britta Brügger for the kind gifts of reagents; and Amanda Chase for proofreading of the manuscript. Markus Thali and his laboratory independently observed that HIV-1 Vpu and Nef reduce TSPAN cell surface levels, and we thank them for sharing their unpublished results.
This project is supported by the Deutsche Forschungsgemeinschaft (TRR83 project 15 to O.T.F., grant FA 378/11-1 to O.T.F., grant KE 741/4-1 to O.T.K., and a GRK1188 fellowship to M.L.). C.H. was supported by a fellowship from the postdoctoral program of the Medical Faculty Heidelberg.
O.T.F. is a member of cluster of excellence EXC81.
Footnotes
Published ahead of print 1 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02333-14.
REFERENCES
- 1.Malim MH, Emerman M. 2008. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe 3:388–398. 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Malim MH, Bieniasz PD. 2012. HIV restriction factors and mechanisms of evasion. Cold Spring Harb. Perspect. Med. 2:a006940. 10.1101/cshperspect.a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhoff F. 2010. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe 8:55–67. 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Garcia JV, Miller AD. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508–511. 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 5.Willey RL, Maldarelli F, Martin MA, Strebel K. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193–7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338–342. 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 7.Kerkau T, Bacik I, Bennink JR, Yewdell JW, Hunig T, Schimpl A, Schubert U. 1997. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J. Exp. Med. 185:1295–1305. 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, McCarthy C, Drakesmith H, Li D, Cerundolo V, McMichael AJ, Screaton GR, Xu XN. 2006. HIV-1 down-regulates the expression of CD1d via Nef. Eur. J. Immunol. 36:278–286. 10.1002/eji.200535487. [DOI] [PubMed] [Google Scholar]
- 9.Moll M, Andersson SK, Smed-Sorensen A, Sandberg JK. 2010. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood 116:1876–1884. 10.1182/blood-2009-09-243667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matusali G, Potesta M, Santoni A, Cerboni C, Doria M. 2012. The human immunodeficiency virus type 1 Nef and Vpu proteins downregulate the natural killer cell-activating ligand PVR. J. Virol. 86:4496–4504. 10.1128/JVI.05788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokarev A, Guatelli J. 2011. Misdirection of membrane trafficking by HIV-1 Vpu and Nef: keys to viral virulence and persistence. Cell. Logist. 1:90–102. 10.4161/cl.1.3.16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6:54–67. 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deacon NJ, Tsykin A, Solomon V, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, Lawson VA, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan JS, Cunningham A, Dwyer D, Dowton D, Mills J. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988–991. 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 15.Kestler HW, III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651–662. 10.1016/0092-8674(91)90097-I. [DOI] [PubMed] [Google Scholar]
- 16.Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, Jolicoeur P. 1998. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95:163–175. 10.1016/S0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 17.Rahim MM, Chrobak P, Hu C, Hanna Z, Jolicoeur P. 2009. Adult AIDS-like disease in a novel inducible human immunodeficiency virus type 1 Nef transgenic mouse model: CD4+ T-cell activation is Nef dependent and can occur in the absence of lymphophenia. J. Virol. 83:11830–11846. 10.1128/JVI.01466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geyer M, Fackler OT, Peterlin BM. 2001. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2:580–585. 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laguette N, Bregnard C, Benichou S, Basmaciogullari S. 2010. Human immunodeficiency virus (HIV) type-1, HIV-2 and simian immunodeficiency virus Nef proteins. Mol. Aspects Med. 31:418–433. 10.1016/j.mam.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Wildum S, Schindler M, Munch J, Kirchhoff F. 2006. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J. Virol. 80:8047–8059. 10.1128/JVI.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lama J, Mangasarian A, Trono D. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622–631. 10.1016/S0960-9822(99)80284-X. [DOI] [PubMed] [Google Scholar]
- 22.Collins KL, Chen BK, Kalams SA, Walker BD, Baltimore D. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397–401. 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 23.Swann SA, Williams M, Story CM, Bobbitt KR, Fleis R, Collins KL. 2001. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3-kinase-dependent pathway. Virology 282:267–277. 10.1006/viro.2000.0816. [DOI] [PubMed] [Google Scholar]
- 24.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671. 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 25.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. 2007. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J. Gen. Virol. 88:242–250. 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 26.Fausther-Bovendo H, Sol-Foulon N, Candotti D, Agut H, Schwartz O, Debre P, Vieillard V. 2009. HIV escape from natural killer cytotoxicity: nef inhibits NKp44L expression on CD4+ T cells. AIDS 23:1077–1087. 10.1097/QAD.0b013e32832cb26b. [DOI] [PubMed] [Google Scholar]
- 27.Stumptner-Cuvelette P, Morchoisne S, Dugast M, Le Gall S, Raposo G, Schwartz O, Benaroch P. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. U. S. A. 98:12144–12149. 10.1073/pnas.221256498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhry A, Das SR, Jameel S, George A, Bal V, Mayor S, Rath S. 2007. A two-pronged mechanism for HIV-1 Nef-mediated endocytosis of immune costimulatory molecules CD80 and CD86. Cell Host Microbe 1:37–49. 10.1016/j.chom.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Michel N, Ganter K, Venzke S, Bitzegeio J, Fackler OT, Keppler OT. 2006. The Nef protein of human immunodeficiency virus is a broad-spectrum modulator of chemokine receptor cell surface levels that acts independently of classical motifs for receptor endocytosis and Galphai signaling. Mol. Biol. Cell 17:3578–3590. 10.1091/mbc.E06-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel N, Allespach I, Venzke S, Fackler OT, Keppler OT. 2005. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 15:714–723. 10.1016/j.cub.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 31.Venzke S, Michel N, Allespach I, Fackler OT, Keppler OT. 2006. Expression of Nef downregulates CXCR4, the major coreceptor of human immunodeficiency virus, from the surfaces of target cells and thereby enhances resistance to superinfection. J. Virol. 80:11141–11152. 10.1128/JVI.01556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hrecka K, Swigut T, Schindler M, Kirchhoff F, Skowronski J. 2005. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to the chemokine stromal derived factor 1. J. Virol. 79:10650–10659. 10.1128/JVI.79.16.10650-10659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham L, Fackler OT. 2012. HIV-1 Nef: a multifaceted modulator of T cell receptor signaling. Cell Commun. Signal. 10:39. 10.1186/1478-811X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haller C, Rauch S, Michel N, Hannemann S, Lehmann MJ, Keppler OT, Fackler OT. 2006. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J. Biol. Chem. 281:19618–19630. 10.1074/jbc.M513802200. [DOI] [PubMed] [Google Scholar]
- 35.Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. 2006. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24:547–561. 10.1016/j.immuni.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Abraham L, Bankhead P, Pan X, Engel U, Fackler OT. 2012. HIV-1 Nef limits communication between linker of activated T cells and SLP-76 to reduce formation of SLP-76-signaling microclusters following TCR stimulation. J. Immunol. 189:1898–1910. 10.4049/jimmunol.1200652. [DOI] [PubMed] [Google Scholar]
- 37.Haller C, Rauch S, Fackler OT. 2007. HIV-1 Nef employs two distinct mechanisms to modulate Lck subcellular localization and TCR induced actin remodeling. PLoS One 2:e1212. 10.1371/journal.pone.0001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan X, Rudolph JM, Abraham L, Habermann A, Haller C, Krijnse-Locker J, Fackler OT. 2012. HIV-1 Nef compensates for disorganization of the immunological synapse by inducing trans-Golgi network-associated Lck signaling. Blood 119:786–797. 10.1182/blood-2011-08-373209. [DOI] [PubMed] [Google Scholar]
- 39.Stolp B, Abraham L, Rudolph JM, Fackler OT. 2010. Lentiviral Nef proteins utilize PAK2-mediated deregulation of cofilin as a general strategy to interfere with actin remodeling. J. Virol. 84:3935–3948. 10.1128/JVI.02467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stolp B, Reichman-Fried M, Abraham L, Pan X, Giese SI, Hannemann S, Goulimari P, Raz E, Grosse R, Fackler OT. 2009. HIV-1 Nef interferes with host cell motility by deregulation of cofilin. Cell Host Microbe 6:174–186. 10.1016/j.chom.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Stolp B, Imle A, Coelho FM, Hons M, Gorina R, Lyck R, Stein JV, Fackler OT. 2012. HIV-1 Nef interferes with T-lymphocyte circulation through confined environments in vivo. Proc. Natl. Acad. Sci. U. S. A. 109:18541–18546. 10.1073/pnas.1204322109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobile C, Rudnicka D, Hasan M, Aulner N, Porrot F, Machu C, Renaud O, Prevost MC, Hivroz C, Schwartz O, Sol-Foulon N. 2010. HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. J. Virol. 84:2282–2293. 10.1128/JVI.02230-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, Barker E. 2010. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe 8:397–409. 10.1016/j.chom.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schubert U, Ferrer-Montiel AV, Oblatt-Montal M, Henklein P, Strebel K, Montal M. 1996. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 398:12–18. 10.1016/S0014-5793(96)01146-5. [DOI] [PubMed] [Google Scholar]
- 45.Mehnert T, Lam YH, Judge PJ, Routh A, Fischer D, Watts A, Fischer WB. 2007. Towards a mechanism of function of the viral ion channel Vpu from HIV-1. J. Biomol. Struct. Dyn. 24:589–596. 10.1080/07391102.2007.10507148. [DOI] [PubMed] [Google Scholar]
- 46.Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 47.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt S, Fritz JV, Bitzegeio J, Fackler OT, Keppler OT. 2011. HIV-1 Vpu blocks recycling and biosynthetic transport of the intrinsic immunity factor CD317/tetherin to overcome the virion release restriction. mBio 2(3):e00036–11. 10.1128/mBio.00036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kueck T, Neil SJ. 2012. A cytoplasmic tail determinant in HIV-1 Vpu mediates targeting of tetherin for endosomal degradation and counteracts interferon-induced restriction. PLoS Pathog. 8:e1002609. 10.1371/journal.ppat.1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dube M, Paquay C, Roy BB, Bego MG, Mercier J, Cohen EA. 2011. HIV-1 Vpu antagonizes BST-2 by interfering mainly with the trafficking of newly synthesized BST-2 to the cell surface. Traffic 12:1714–1729. 10.1111/j.1600-0854.2011.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau D, Kwan W, Guatelli J. 2011. Role of the endocytic pathway in the counteraction of BST-2 by human lentiviral pathogens. J. Virol. 85:9834–9846. 10.1128/JVI.02633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roeth JF, Collins KL. 2006. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol. Mol. Biol. Rev. 70:548–563. 10.1128/MMBR.00042-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bolduan S, Hubel P, Reif T, Lodermeyer V, Hohne K, Fritz JV, Sauter D, Kirchhoff F, Fackler OT, Schindler M, Schubert U. 2013. HIV-1 Vpu affects the anterograde transport and the glycosylation pattern of NTB-A. Virology 440:190–203. 10.1016/j.virol.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magadan JG, Perez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. 2010. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog. 6:e1000869. 10.1371/journal.ppat.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grzesiek S, Stahl SJ, Wingfield PT, Bax A. 1996. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 35:10256–10261. [DOI] [PubMed] [Google Scholar]
- 56.Preusser A, Briese L, Baur AS, Willbold D. 2001. Direct in vitro binding of full-length human immunodeficiency virus type 1 Nef protein to CD4 cytoplasmic domain. J. Virol. 75:3960–3964. 10.1128/JVI.75.8.3960-3964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cluet D, Bertsch C, Beyer C, Gloeckler L, Erhardt M, Gut JP, Galzi JL, Aubertin AM. 2005. Detection of human immunodeficiency virus type 1 Nef and CD4 physical interaction in living human cells by using bioluminescence resonance energy transfer. J. Virol. 79:8629–8636. 10.1128/JVI.79.13.8629-8636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams M, Roeth JF, Kasper MR, Fleis RI, Przybycin CG, Collins KL. 2002. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. J. Virol. 76:12173–12184. 10.1128/JVI.76.23.12173-12184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dube M, Roy BB, Guiot-Guillain P, Binette J, Mercier J, Chiasson A, Cohen EA. 2010. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6:e1000856. 10.1371/journal.ppat.1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rauch S, Pulkkinen K, Saksela K, Fackler OT. 2008. Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vav1 via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity. J. Virol. 82:2918–2929. 10.1128/JVI.02185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meuwissen PJ, Stolp B, Iannucci V, Vermeire J, Naessens E, Saksela K, Geyer M, Vanham G, Arien KK, Fackler OT, Verhasselt B. 2012. Identification of a highly conserved valine-glycine-phenylalanine amino acid triplet required for HIV-1 Nef function. Retrovirology 9:34. 10.1186/1742-4690-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rudolph JM, Eickel N, Haller C, Schindler M, Fackler OT. 2009. Inhibition of T-cell receptor-induced actin remodeling and relocalization of Lck are evolutionarily conserved activities of lentiviral Nef proteins. J. Virol. 83:11528–11539. 10.1128/JVI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen KL, Ilano M, Akari H, Miyagi E, Poeschla EM, Strebel K, Bour S. 2004. Codon optimization of the HIV-1 vpu and vif genes stabilizes their mRNA and allows for highly efficient Rev-independent expression. Virology 319:163–175. 10.1016/j.virol.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 64.Tervo HM, Homann S, Ambiel I, Fritz JV, Fackler OT, Keppler OT. 2011. Beta-TrCP is dispensable for Vpu's ability to overcome the CD317/tetherin-imposed restriction to HIV-1 release. Retrovirology 8:9. 10.1186/1742-4690-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan X, Geist MM, Rudolph JM, Nickel W, Fackler OT. 2013. HIV-1 Nef disrupts membrane-microdomain-associated anterograde transport for plasma membrane delivery of selected Src family kinases. Cell. Microbiol. 15:1605–1621. 10.1111/cmi.12148. [DOI] [PubMed] [Google Scholar]
- 66.Coates K, Cooke SJ, Mann DA, Harris MP. 1997. Protein kinase C-mediated phosphorylation of HIV-I nef in human cell lines. J. Biol. Chem. 272:12289–12294. 10.1074/jbc.272.19.12289. [DOI] [PubMed] [Google Scholar]
- 67.Keppler OT, Allespach I, Schuller L, Fenard D, Greene WC, Fackler OT. 2005. Rodent cells support key functions of the human immunodeficiency virus type 1 pathogenicity factor Nef. J. Virol. 79:1655–1665. 10.1128/JVI.79.3.1655-1665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fritz JV, Tibroni N, Keppler OT, Fackler OT. 2012. HIV-1 Vpu's lipid raft association is dispensable for counteraction of the particle release restriction imposed by CD317/tetherin. Virology 424:33–44. 10.1016/j.virol.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Breuer S, Schievink SI, Schulte A, Blankenfeldt W, Fackler OT, Geyer M. 2011. Molecular design, functional characterization and structural basis of a protein inhibitor against the HIV-1 pathogenicity factor Nef. PLoS One 6:e20033. 10.1371/journal.pone.0020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koppensteiner H, Hohne K, Gondim MV, Gobert FX, Widder M, Gundlach S, Heigele A, Kirchhoff F, Winkler M, Benaroch P, Schindler M. 2014. Lentiviral Nef suppresses iron uptake in a strain specific manner through inhibition of transferrin endocytosis. Retrovirology 11:1. 10.1186/1742-4690-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madrid R, Janvier K, Hitchin D, Day J, Coleman S, Noviello C, Bouchet J, Benmerah A, Guatelli J, Benichou S. 2005. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 280:5032–5044. 10.1074/jbc.M401202200. [DOI] [PubMed] [Google Scholar]
- 72.Collins KL, Baltimore D. 1999. HIV's evasion of the cellular immune response. Immunol. Rev. 168:65–74. 10.1111/j.1600-065X.1999.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 73.Iwabu Y, Fujita H, Kinomoto M, Kaneko K, Ishizaka Y, Tanaka Y, Sata T, Tokunaga K. 2009. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 284:35060–35072. 10.1074/jbc.M109.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geist MM, Pan X, Bender S, Bartenschlager R, Nickel W, Fackler OF. 2014. Heterologous Src homology 4 domains support membrane anchoring and biological activity of HIV-1 Nef. J. Biol. Chem. 289:14030–14044. 10.1074/jbc.M114.563528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mangasarian A, Piguet V, Wang JK, Chen YL, Trono D. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 73:1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vigan R, Neil SJ. 2010. Determinants of tetherin antagonism in the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein. J. Virol. 84:12958–12970. 10.1128/JVI.01699-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dube M, Roy BB, Guiot-Guillain P, Mercier J, Binette J, Leung G, Cohen EA. 2009. Suppression of tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83:4574–4590. 10.1128/JVI.01800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aiken C, Trono D. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chowers MY, Spina CA, Kwoh TJ, Fitch NJ, Richman DD, Guatelli JC. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwartz O, Marechal V, Danos O, Heard JM. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 69:4053–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janardhan A, Swigut T, Hill B, Myers MP, Skowronski J. 2004. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2:E6. 10.1371/journal.pbio.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramirez PW, Famiglietti M, Sowrirajan B, DePaula-Silva AB, Rodesch C, Barker E, Bosque A, Planelles V. 2014. Downmodulation of CCR7 by HIV-1 Vpu results in impaired migration and chemotactic signaling within CD4(+) T cells. Cell Rep. 7:2019–2030. 10.1016/j.celrep.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laguette N, Bregnard C, Bouchet J, Benmerah A, Benichou S, Basmaciogullari S. 2009. Nef-induced CD4 endocytosis in human immunodeficiency virus type 1 host cells: role of p56lck kinase. J. Virol. 83:7117–7128. 10.1128/JVI.01648-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Giolo G, Neri F, Casartelli N, Potesta M, Belleudi F, Torrisi MR, Doria M. 2007. Internalization and intracellular retention of CD4 are two separate functions of the human immunodeficiency virus type 1 Nef protein. J. Gen. Virol. 88:3133–3138. 10.1099/vir.0.83164-0. [DOI] [PubMed] [Google Scholar]
- 85.Casartelli N, Giolo G, Neri F, Haller C, Potesta M, Rossi P, Fackler OT, Doria M. 2006. The Pro78 residue regulates the capacity of the human immunodeficiency virus type 1 Nef protein to inhibit recycling of major histocompatibility complex class I molecules in an SH3-independent manner. J. Gen. Virol. 87:2291–2296. 10.1099/vir.0.81775-0. [DOI] [PubMed] [Google Scholar]
- 86.Roeth JF, Williams M, Kasper MR, Filzen TM, Collins KL. 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J. Cell Biol. 167:903–913. 10.1083/jcb.200407031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wonderlich ER, Leonard JA, Collins KL. 2011. HIV immune evasion disruption of antigen presentation by the HIV Nef protein. Adv. Virus Res. 80:103–127. 10.1016/B978-0-12-385987-7.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. 1998. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1:565–574. 10.1016/S1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- 89.Binette J, Dube M, Mercier J, Halawani D, Latterich M, Cohen EA. 2007. Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum. Retrovirology 4:75. 10.1186/1742-4690-4-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. 2009. HIV-1 Vpu neutralizes the antiviral factor tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 5:e1000574. 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goffinet C, Allespach I, Homann S, Tervo HM, Habermann A, Rupp D, Oberbremer L, Kern C, Tibroni N, Welsch S, Krijnse-Locker J, Banting G, Krausslich HG, Fackler OT, Keppler OT. 2009. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5:285–297. 10.1016/j.chom.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 92.Lindwasser OW, Chaudhuri R, Bonifacino JS. 2007. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr. Mol. Med. 7:171–184. 10.2174/156652407780059177. [DOI] [PubMed] [Google Scholar]
- 93.McNatt MW, Zang T, Bieniasz PD. 2013. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS Pathog. 9:e1003299. 10.1371/journal.ppat.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baur AS, Sass G, Laffert B, Willbold D, Cheng-Mayer C, Peterlin BM. 1997. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity 6:283–291. 10.1016/S1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 95.Collette Y, Dutartre H, Benziane A, Ramos M, Benarous R, Harris M, Olive D. 1996. Physical and functional interaction of Nef with Lck. HIV-1 Nef-induced T-cell signaling defects. J. Biol. Chem. 271:6333–6341. [DOI] [PubMed] [Google Scholar]
- 96.Wolf D, Giese SI, Witte V, Krautkramer E, Trapp S, Sass G, Haller C, Blume K, Fackler OT, Baur AS. 2008. Novel (n)PKC kinases phosphorylate Nef for increased HIV transcription, replication and perinuclear targeting. Virology 370:45–54. 10.1016/j.virol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 97.Bassani S, Cingolani LA. 2012. Tetraspanins: interactions and interplay with integrins. Int. J. Biochem. Cell Biol. 44:703–708. 10.1016/j.biocel.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 98.Zhang XA, Huang C. 2012. Tetraspanins and cell membrane tubular structures. Cell. Mol. Life Sci. 69:2843–2852. 10.1007/s00018-012-0954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hemler ME. 2003. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 19:397–422. 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- 100.Spring FA, Griffiths RE, Mankelow TJ, Agnew C, Parsons SF, Chasis JA, Anstee DJ. 2013. Tetraspanins CD81 and CD82 facilitate alpha4beta1-mediated adhesion of human erythroblasts to vascular cell adhesion molecule-1. PLoS One 8:e62654. 10.1371/journal.pone.0062654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tejera E, Rocha-Perugini V, Lopez-Martin S, Perez-Hernandez D, Bachir AI, Horwitz AR, Vazquez J, Sanchez-Madrid F, Yanez-Mo M. 2013. CD81 regulates cell migration through its association with Rac GTPase. Mol. Biol. Cell 24:261–273. 10.1091/mbc.E12-09-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barreiro O, Zamai M, Yanez-Mo M, Tejera E, Lopez-Romero P, Monk PN, Gratton E, Caiolfa VR, Sanchez-Madrid F. 2008. Endothelial adhesion receptors are recruited to adherent leukocytes by inclusion in preformed tetraspanin nanoplatforms. J. Cell Biol. 183:527–542. 10.1083/jcb.200805076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nydegger S, Khurana S, Krementsov DN, Foti M, Thali M. 2006. Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1. J. Cell Biol. 173:795–807. 10.1083/jcb.200508165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krementsov DN, Weng J, Lambele M, Roy NH, Thali M. 2009. Tetraspanins regulate cell-to-cell transmission of HIV-1. Retrovirology 6:64. 10.1186/1742-4690-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jolly C, Sattentau QJ. 2007. Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J. Virol. 81:7873–7884. 10.1128/JVI.01845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thali M. 2009. The roles of tetraspanins in HIV-1 replication. Curr. Top. Microbiol. Immunol. 339:85–102. 10.1007/978-3-642-02175-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thali M. 2011. Tetraspanin functions during HIV-1 and influenza virus replication. Biochem. Soc. Trans. 39:529–531. 10.1042/BST0390529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weng J, Krementsov DN, Khurana S, Roy NH, Thali M. 2009. Formation of syncytia is repressed by tetraspanins in human immunodeficiency virus type 1-producing cells. J. Virol. 83:7467–7474. 10.1128/JVI.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ho SH, Martin F, Higginbottom A, Partridge LJ, Parthasarathy V, Moseley GW, Lopez P, Cheng-Mayer C, Monk PN. 2006. Recombinant extracellular domains of tetraspanin proteins are potent inhibitors of the infection of macrophages by human immunodeficiency virus type 1. J. Virol. 80:6487–6496. 10.1128/JVI.02539-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sato K, Aoki J, Misawa N, Daikoku E, Sano K, Tanaka Y, Koyanagi Y. 2008. Modulation of human immunodeficiency virus type 1 infectivity through incorporation of tetraspanin proteins. J. Virol. 82:1021–1033. 10.1128/JVI.01044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malbec M, Sourisseau M, Guivel-Benhassine F, Porrot F, Blanchet F, Schwartz O, Casartelli N. 2013. HIV-1 Nef promotes the localization of Gag to the cell membrane and facilitates viral cell-to-cell transfer. Retrovirology 10:80. 10.1186/1742-4690-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haller C, Tibroni N, Rudolph JM, Grosse R, Fackler OT. 2011. Nef does not inhibit F-actin remodelling and HIV-1 cell-cell transmission at the T lymphocyte virological synapse. Eur. J. Cell Biol. 90:913–921. 10.1016/j.ejcb.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 113.Casartelli N, Sourisseau M, Feldmann J, Guivel-Benhassine F, Mallet A, Marcelin AG, Guatelli J, Schwartz O. 2010. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 6:e1000955. 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rocha-Perugini V, Zamai M, Gonzalez-Granado JM, Barreiro O, Tejera E, Yanez-Mo M, Caiolfa VR, Sanchez-Madrid F. 2013. CD81 controls sustained T cell activation signaling and defines the maturation stages of cognate immunological synapses. Mol. Cell. Biol. 33:3644–3658. 10.1128/MCB.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gotz N, Sauter D, Usmani SM, Fritz JV, Goffinet C, Heigele A, Geyer M, Bibollet-Ruche F, Learn GH, Fackler OT, Hahn BH, Kirchhoff F. 2012. Reacquisition of Nef-mediated tetherin antagonism in a single in vivo passage of HIV-1 through its original chimpanzee host. Cell Host Microbe 12:373–380. 10.1016/j.chom.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.