ABSTRACT
Rabies virus (RABV) spread is widely accepted to occur only by retrograde axonal transport. However, examples of anterograde RABV spread in peripheral neurons such as dorsal root ganglion (DRG) neurons indicated a possible bidirectional transport by an uncharacterized mechanism. Here, we analyzed the axonal transport of fluorescence-labeled RABV in DRG neurons by live-cell microscopy. Both entry-related retrograde transport of RABV after infection at axon endings and postreplicative transport of newly formed virus were visualized in compartmentalized DRG neuron cultures. Whereas entry-related transport at 1.5 μm/s occurred only retrogradely, after 2 days of infection, multiple particles were observed in axons moving in both the anterograde and retrograde directions. The dynamics of postreplicative retrograde transport (1.6 μm/s) were similar to those of entry-related retrograde transport. In contrast, anterograde particle transport at 3.4 μm/s was faster, indicating active particle transport. Interestingly, RABV missing the glycoproteins did not move anterogradely within the axon. Thus, anterograde RABV particle transport depended on the RABV glycoprotein. Moreover, colocalization of green fluorescent protein (GFP)-labeled ribonucleoproteins (RNPs) and glycoprotein in distal axonal regions as well as cotransport of labeled RNPs with membrane-anchored mCherry reporter confirmed that either complete enveloped virus particles or vesicle associated RNPs were transported. Our data show that anterograde RABV movement in peripheral DRG neurons occurs by active motor protein-dependent transport. We propose two models for postreplicative long-distance transport in peripheral neurons: either transport of complete virus particles or cotransport of RNPs and G-containing vesicles through axons to release virus at distal sites of infected DRG neurons.
IMPORTANCE Rabies virus retrograde axonal transport by dynein motors supports virus spread over long distances and lethal infection of the central nervous system. Though active rabies virus transport has been widely accepted to be unidirectional, evidence for anterograde spread in peripheral neurons supports the hypothesis that in some neurons RABV also enters the anterograde pathway by so-far unknown mechanisms. By live microscopy we visualized fast anterograde axonal transport of rabies virus. The velocities exceeded those of retrograde movements, suggesting that active, most likely kinesin-dependent transport machineries are involved. Dependency of anterograde transport on the expression of virus glycoprotein G and cotransport with vesicles further suggest that complete enveloped virus particles or cotransport of virus ribonucleoprotein and G-containing vesicles occurred. These data provide the first insight in the mechanism of anterograde rabies virus transport and substantially contribute to the understanding of RABV replication and spread of newly formed virus in peripheral neurons.
INTRODUCTION
Although many viruses are able to enter the nervous system, only a limited set of viruses have evolved specific mechanisms for directed axonal transport to ensure neuroinvasion and virus replication in the peripheral nervous system and central nervous system (CNS) (1). Among those viruses, rabies virus (RABV) (Rhabdoviridae family) is a classic example of a pathogen that enters the nervous system by retrograde axonal transport. Though retrograde and anterograde transport of any cargo within the axon of a neuron depend on microtubules and associated motor complexes (2), detailed knowledge about checkpoints regulating the directionality of virus transport processes in neurons remains limited.
RABV is a nonsegmented negative-strand RNA virus that is transmitted from rabid animals by bites. The virus is transported through retrograde microtubule-dependent axonal transport (3) from the site of inoculation to the CNS, where virus replication is accompanied by progressive neuronal dysfunctions and lethal outcome of the disease. The genome of rabies virus encodes five virus proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and large polymerase (L). Rabies virus replication requires a ribonucleoprotein (RNP) complex consisting of the genomic virus RNA tightly packaged by nucleoprotein. Phosphoprotein P is associated with the RNP in chaperoning N to ensure specific RNA packaging and as a polymerase cofactor within the P/L-polymerase complex. Identification of a dynein light chain 1 (DLC1) binding motif in the rabies virus phosphoprotein (4, 5) supported the hypothesis that RABV RNPs directly bind to dynein motor complexes and thus enter the retrograde transport pathway after release from endosomes. Subsequent studies revealed that destruction of the DLC1 binding motif in P did not abrogate the ability of RABV to invade the CNS (6) but had a supportive effect on early virus transcription (7).
Fluorescence protein fusion to P has been used to label RNPs of RABV (8, 9) and other rhabdoviruses (10) to visualize intracellular RNP transport. By the combination of GFP-tagged rabies virus RNPs and incorporation of a membrane-anchored red fluorescent protein into virus particles, double-labeled rabies virus particles were further used to show retrograde axonal transport of complete, membrane-enveloped virions (11), suggesting that in neurons, receptor usage by the viral glycoprotein and subsequent receptor-dependent endocytosis are crucial for entering the long-distance transport to the cell soma. In neurons from mouse dorsal root ganglions (DRGs), most of the retrogradely transported RABV was cotransported with low-affinity nerve growth factor receptor (p75NTR) and colocalized with acidified transport vesicles (12), confirming RABV transport in vesicles. This provided direct evidence that one of the three proposed neuronal RABV receptors (13) is indeed a receptor that directs incoming virus particles in the retrograde transport pathway. Notably, RABV not only was cotransported with p75NTR but also accelerated the p75NTR retrograde axonal transport machinery, indicating that RABV modulates axonal transport in the course of retrograde infection (12).
In accordance with the model of receptor-dependent axonal transport, closely related vesicular stomatitis virus and unrelated retrovirus vectors have been directed to retrograde infection of neurons by incorporation of rabies virus glycoprotein (14–16), and cotransport with the potential RABV receptors with pseudotyped lentiviruses was shown (17).
RABV axonal transport has been considered unidirectional, and this led to the use of RABV as a retrograde transneuronal marker for tracing neuronal connections within the CNS (reviewed in references 18 and 19). Monosynaptically restricted transsynaptic tracing by green fluorescent protein (GFP) expressing G gene-deleted rabies viruses (20, 21) represented a pioneering step in neurobiology, as the system allowed reliable tracing of retrograde transsynaptic neuron connections. Similarly to the case for RABV, which was also modified to allow nonretrograde infection by introduction of vesicular stomatitis virus (VSV) G sequences (22, 23), the system was also adapted to VSV vectors for use as retro- and anterograde neuronal tracers in a G-dependent manner (16, 24).
Recent evidence for anterograde transsynaptic transfer of rabies virus in sensory neurons emphasized that rabies virus G protein also supports anterograde transsynaptic spread, at least in peripheral neurons (25). Accordingly, although unidirectional transsynaptic RABV spread in the CNS is commonly accepted from a large body of evidence, intraneuronal spread of rabies in peripheral neurons appears to be special, and the proposed exclusive retrograde RABV transmission to the CNS through motor neurons (18) may represent only one part of the involved transport processes. In agreement with anterograde RABV transport in sensory neurons (25), in vitro and in vivo virus tracking in DRG neurons showed that anterograde virus transport through axons of peripheral DRG neurons and other peripheral neurons is possible and that anterograde axonal transport of infectious RABV occurred at remarkable velocities of 100 to 400 mm/day (26, 27). However, mechanisms that explain the transport of RNPs or complete intracellular virus particles from the cell soma into axons have not been described. In addition to the infection of the CNS by anterograde pathways (25), a role of anterograde RABV transport in centrifugal spread from infected DRGs to peripheral sites such as skin and hair follicles has also been proposed. However, inefficient transport or passive diffusion in the anterograde direction was concluded from the late appearance of virus in these organs (28). Thus, a central unsolved question is whether anterograde transport of RABV in peripheral neurons indeed is mediated by passive diffusion or by directed anterograde transport processes of virus particles or subviral complexes. In the latter case, unknown mechanisms that allow anterograde RABV transport have to be unraveled. In particular, differences in these axonal transport processes in peripheral DRG and CNS neurons may be decisive for infections routes and pathogenesis of RABV and other neuroinvasive viruses.
In a compartmentalized cultivation system that allowed reliable directed outgrowth of axons that were more than 1.7 cm long, we characterized the axonal transport of GFP-labeled RABV directly after infection of peripheral DRG neurons at distal axon endings to follow entry-related retrograde axonal transport. Moreover, after virus replication in the neuron soma (postreplicative), axonal virus transport was further investigate (i) to see whether virus is indeed transported in the anterograde direction, (ii) to see whether anterograde transport velocities differ from early retrograde transport processes, and (iii) to determine whether virus transport depends on the presence of viral glycoprotein. Finally, we propose two possible models of glycoprotein-dependent anterograde axonal transport of newly formed RABV in peripheral neurons: RABV is transported either as a complete particle after intracellular budding in axonal transport vesicles or as a prebudding complex with cytoplasmic RNPs connected to glycoprotein-containing transport vesicles.
MATERIALS AND METHODS
Cells and viruses.
BSR T7/5 cells (29) were used for virus rescue from recombinant cDNA as described previously (30). Conditional expression of RABV matrix and glycoproteins in MGon cells (31) was used for amplification of G gene-deleted RABV. Virus stocks of autonomously replicating RABV were prepared on NA neuroblastoma cells provided by the Collection of Cell Lines in Veterinary Medicine (CCLV), FLI Riems.
All recombinant virus cDNAs were constructed by standard techniques on the basis of recombinant cDNA derived from attenuated RABV vaccine strain SAD B19 (32). The enhanced GFP (EGFP)-P-expressing recombinant rabies viruses (rRABV) encoded a fusion protein of N-terminal EGFP and downstream phosphoprotein P as previously described (8). In the mutant EGFP-P DLC1mut, two amino acid exchanges within a dynein light chain 1 (DLC1) binding motif were introduced (142-EDKSTQTT-149 → 142-EDASTATT-149) to eliminate binding to cellular DLC1 (9). In rRABV EGFP-P Gcvs and rRABV EGFP-P DLC1mut Gcvs, the authentic G protein of the attenuated RABV was replaced by the G-coding sequence (EU126641.1) of the neuroinvasive RABV strain CVS-11. In addition to the authentic RABV G, rRABV tmCherry encoded membrane-anchored mCherry protein with RABV G signal, transmembrane, and cytoplasmic domain sequences from an additional cistron. Recombinant Newcastle disease virus (rNDVF1) EGFP-P, used as a control, is based on NDV clone 30 modified by a polybasic amino acid sequence at the fusion protein cleavage site (33, 34) and the insertion of an extra gene coding for an EGFP-NDV P fusion protein introduced between the P and M genes.
DRG neuron preparation and cultivation.
Dorsal root ganglia were prepared from embryonic day 15.5 (E15.5) embryos of pregnant Sprague-Dawley rats that were obtained from the Department of Experimental Animal Facilities and Biorisk Management (Friedrich-Loeffler-Institut). Preparation of DRGs was approved by the competent authority of the Federal State of Mecklenburg-Western Pomerania (reference numbers LALLF 7221.3-2.1-011/13 and 7221.3-2.3-005/08), Germany, on the basis of actual national and European legislation for the protection of animals used for experiments. After euthanization with CO2, embryos were removed from the uterus and transferred to a tissue culture dish. The embryos were rinsed with Hanks balanced salt solution (HBSS) (Fisher Scientific), decapitated, and laminectomized, and the spinal cord was removed. The DRGs were collected in HBSS at 4°C. After dissolution of the DRG neurons by trypsin treatment for 20 min at 37°C, neurons were cultivated in Neurobasal medium with B27 supplement and GlutaMax (Invitrogen), penicillin-streptomycin (Sigma-Aldrich), and nerve growth factor 2.5S (50 ng/ml; Invitrogen). The neuron cultures were incubated at 37°C, and neuronal medium was replaced every 3 days.
Compartmentalized neuron culture.
After coating of μ-Slide 60.4 cultivation chambers (Ibidi) with poly-dl-ornithine (500 μg/ml; Sigma-Aldrich) and natural mouse laminin (10 μg/ml; Invitrogen), the channels of the μ-Slide connecting the two opposite chambers were filled with 30 μl of 0.5% agarose in Neurobasal medium. After agarose polymerization, the two chambers were filled with Neurobasal medium, and 30 μl of a DRG neuron suspension containing cells from 2.5 dorsal root ganglia was seeded into the “proximal” chamber. After cultivation overnight at 37°C, 40 μl medium was removed from the chambers and replaced by the same volume of Neurobasal medium with 10 μM arabinofuranosyl cytidine (AraC). After 3 days of AraC treatment, the medium was replaced with AraC-free medium and the neurons were further cultivated for 2 to 3 weeks.
Indirect immunofluorescence and live-virus imaging.
Indirect immunofluorescence with mouse monoclonal antibody (MAb) E559 (35), recognizing RABV G protein, and Alexa Fluor 568-conjugated secondary antibodies (Molecular Probes) was performed by standard techniques after fixation with 3.7% formaldehyde in phosphate-buffered saline (PBS) and permeabilization with 0.5% Triton X-100 in PBS. Images were acquired with a Leica SP5 confocal laser scanning microscope (63× objective; numerical aperture, 1.4) with sequential acquisition of the fluorophores in double-fluorescent specimen. Images were processed with the ImageJ software version 1.48b (36).
Live imaging of neuronal cultures was performed at 37°C on a Leica SP5 confocal laser scanning microscope equipped with a resonant scanner for fast image acquisition. Images were acquired at frame rates indicated in Results. To achieve better particle detection, in some experiments the pinhole was widened as indicated. Detection of mCherry and EGFP fluorescence occurred simultaneously without any time loss between acquisition of red and green channels. Quantitation of image stacks was performed by using ImageJ and the Manual Tracking plug-in developed by F.P. Cordelières (http://imagej.nih.gov/ij/plugins/track/Manual%20Tracking%20plugin.pdf). Transport velocities (μm/s) of virus particles were calculated for each time frame by the particle displacement divided by time required for image or z-stack acquisition (0.276 s to 0.503 s). Velocities were categorized by 0.5-μm/s intervals.
RESULTS
RABV infection and live imaging of DRG neurons.
To study RABV axonal transport, we established a compartmental platform as described in Materials and Methods. DRG neurons were cultured from 15.5-day-old rat embryos, and the cells were seeded into the proximal chambers of the compartmentalized channel slides (Fig. 1A). To prevent movement of cell bodies toward the opposite culture chamber, the connecting channels were filled with 0.5% agarose as a diffusion barrier. Neurons were cultivated for 2 weeks, and axon outgrowth through the 1.7-cm-long channels toward the opposite distal chamber was regularly controlled by microscopy. In addition, the absence of cell bodies in channels and distal medium reservoirs was verified.
FIG 1.
Chambered dorsal root ganglion (DRG) neuron cultivation, directed infection, and EGFP-P detection in infected neurons. (A) DRG neurons were prepared from rat embryos and seeded in cell culture devices in which the two chambers were connected by an agarose-filled channel. After 2 weeks, axonal growth cones reached the distal chamber. Directed infections at growth cones (distal chamber) or at the cell bodies (proximal chamber) allowed imaging of virus infections and intra-axonal transport within axons. Virus infections were monitored directly after infection (0 days postinfection [dpi]; retrograde virus entry) or postreplicative after 1 to 5 days of infection. (B) DRG neuron cultures were infected at the growth cones (right side) with rRABV EGFP-P DLC1mut Gcvs (genome organization is shown at the top). After 5 days of infection, GFP autofluorescence was detected in the cell bodies (left side), in bundled axons (middle), and in distal chambers (right side). Upper two rows, bright-field and fluorescence images of cultivation devices. The images were assembled from 48 fields of view. Lower two rows, magnifications from proximal and distal chambers (right and left sides, respectively) and from the middle of the channel (middle).
When axon growth cones reached the distal chamber after 2 weeks, neurons were infected at the axon endings with 104 focus-forming units (FFU) of recombinant RABV expressing EGFP-tagged phosphoprotein P. In addition to the EGFP, the recombinant virus rRABV EGFP-P DLC1mut Gcvs (Fig. 1B) contained a mutation in the dynein light chain 1 (DLC1) binding motif of P. Moreover, the glycoprotein G of the attenuated RABV backbone was replaced by a G sequence of the pathogenic CVS-11 strain. Whereas the glycoprotein replacement was intended to increase neuroinvasive properties to the attenuated rRABV backbone (9), the DLC1 binding mutation was inserted to exclude any effect of dynein motor complex binding of incoming RNPs during entry and the intracellular transport of newly formed RNPs after virus replication in the infected neuron.
Infection of DRG neurons was monitored by GFP fluorescence. After 1 day of infection, green fluorescence in proximal cell bodies and in axons (not shown) was detectable. After 2 to 5 days of infection, the neurons were completely filled with EGFP-tagged P protein, and axonal transport of EGFP-P particles in both directions was observed. Bundles of parallel fluorescent axons were detected in the channel, whereas infected cell bodies remained exclusively in the proximal chamber (Fig. 1B). Detection of fluorescent neurons even after 5 days of infection revealed that the compartmentalized neuron cultures not only allowed retrograde infection of the neurons but also allowed us to follow nonlytic RABV infections over a long time period. Successful infection at the axon terminals with rRABV EGFP-P DLC1mut Gcvs confirmed that DLC-1 binding by P is not required for retrograde transport. Similar results were obtained with rRABV EGFP-P Gcvs, in which the DLC-1 binding motif in P was not affected (not shown).
Retrograde transport of virus particles.
To visualize entry-related retrograde transport of virus particles after infection at the axon endings, 104 focus forming units (FFU) of rRABV EGFP-P DLC1mut Gcvs were added to the distal chambers, and axons were monitored by live confocal laser scanning microscopy. After 170 min, individual fluorescent particles were observed in the middle of the channel. The fluorescent particles were tracked by image acquisition at 0.276 s/frame. Trajectories of individual particles (Fig. 2A and B; see Movie S1 in the supplemental material) were captured, and velocities of transport of virus particles were calculated (Fig. 2C). A mean transport velocity of 1.49 μm/s was calculated from instantaneous velocities (n = 817) from 8 individual retrograde trajectories.
FIG 2.
RABV retrograde axonal transport. DRG neurons were infected with rRABV EGFP-P DLC1mut Gcvs at the distal growth cones. After 170 min, virus particle transport was observed by image acquisition in the middle part of the channels (0.276 s/frame; optical slice = 1.5 μm). (A) Transport of a single virus particle into the retrograde direction. From a total of 181 time frames, every 20th image is shown. The transported virus particle is marked by circles. (B) Time projection of 842 time frames. (C) Particle transport velocities were determined for each time frame and were categorized as indicated in the diagram. The frequencies of different transport velocities are shown. Data have been generated from eight trajectories, comprising a total of 817 individual transport events.
Anterograde transport of EGFP particles.
Strong GFP fluorescence in axons of rRABV EGFP-P DLC1mut Gcvs-infected neurons (Fig. 1B) indicated anterograde transport of soluble EGFP-tagged phosphoprotein and of newly formed EGFP-P-containing protein complexes in axons after virus replication in the cell soma. To clarify whether EGFP-P particles were indeed transported in axons, DRG neurons were infected with 104 FFU of rRABV EGFP-P DLC1mut Gcvs at the cell soma, and 2 days later, transport in the middle part of the channel was analyzed. Multiple particulate EGFP-P structures were detected within the channels (Fig. 3A; see Movie S2 in the supplemental material), indicating that newly formed EGFP-P particles had entered the axon after virus replication in the cell soma. Moreover, fast and directed transport of EGFP-P particles in both the antero- and retrograde directions was observed, further indicating that the EGFP-P particles indeed hijacked active cellular transport machineries within the axons. Trajectories of individual particles confirmed transport of particles in either the antero- or retrograde direction (Fig. 3A, left and right panels; see Movie S3 in the supplemental material).
FIG 3.
Postreplicative axonal transport. DRG neurons were infected in the proximal chamber, and EGFP-P particle transport in axons was monitored after 48 h of infection by z-stack acquisition (5 optical slices per stack; acquisition rate, 0.503 s/stack; optical slice = 1.5 μm; size depth = 6 μm). (A) Directed transport in the anterograde (left) and retrograde (right) directions was visualized by trajectories for individual particles. The left and right images represent identical time points and areas. (B) Particle transport velocities were determined for each time frame and were categorized as indicated in the diagram. The frequencies of different transport velocities are shown for retro- and anterograde transport processes (black and gray bars, respectively). Anterograde transport velocities were calculated from 15 trajectories consisting of 636 individual transport events. Retrograde transport velocities were calculated from seven trajectories consisting of 512 transport events.
To assess whether different transport mechanisms were involved in the observed postreplicative retro- and anterograde transport processes, the mean transport velocities of particles from anterograde and retrograde trajectories were determined (Fig. 3B). Notably, anterograde particle transport, at 3.37 μm/s (standard deviation [SD] = 0.18), was about twice as fast as retrograde transport processes. At 1.6 μm/s (SD = 0.39), the mean velocity of retrogradely transported particles was comparable to the velocity of entry-related retrograde transport after infection in the distal chamber, although the distribution of retrograde transport velocities differed slightly for the entry-related and postreplicative retrograde transport processes (Fig. 2C). The different transport kinetics for antero- and retrograde transport processes strongly suggested that different cellular transport mechanisms were involved, most likely by the use of different cellular motor complexes. Similar results were obtained with rRABV EGFP-P Gcvs (not shown), indicating that both entry-related retrograde transport and, after virus replication, axonal transport in both directions were independent of DLC-1 binding by P.
Colocalization of EGFP-P particles with nucleoprotein N.
Although EGFP-P has been previously used as a marker for extracellular virions (8) and retrograde axonal transport during virus entry (9, 11, 12), here we checked colocalization with nucleoprotein N to see whether EGFP-P particles transported in the axons at later phases of DRG infection indeed could represent viral RNPs. Therefore, DRG neurons were infected at the cell soma, and 3 days later the cells were fixed and immunostained with an N-specific antibody. Confocal laser scanning analysis in distal areas of the axons revealed that most of the EGFP-P particles also contained nucleoprotein N. This was evident from both maximum z-projections demonstrating complete axon bundles (Fig. 4A) and single confocal slices (Fig. 4B). These data indicated that the transported particles indeed represent viral RNPs.
FIG 4.
Colocalization of EGFP-P and nucleoprotein N in axons. Immunostaining against nucleoprotein N after 3 days of infection in distal parts of DRG axons is shown. (A) Maximum z-projection of 34 optical slices (0.772 μm each; size depth = 11.1 μm). (B) Detail from panel A, showing a single optical slice (z = 0.772 μm). Scale bar, 3 μm.
Exclusion of NDV-derived EGFP-P from anterograde transport in axons.
To assess whether the cultured DRG neurons still had mechanisms that regulate transport of specific protein complexes in axons, we investigated whether EGFP-labeled phosphoprotein of Newcastle disease virus (NDV) is also transported into axons after NDV replication and EGFP-P particle formation in the neurons cytoplasm.
In contrast to RABV, NDV only occasionally infects the CNS (37), and we considered the existence of specific motifs in NDV mediating axonal transport of NDV particles to be unlikely. After 4 days of infection at the cell soma with RABV EGFP-P Gcvs or rNDVF1 EGFP-P, GFP fluorescence was monitored in the cell soma, in a proximal area within the channel close to the cell soma (∼1 mm relative to the proximal chamber), and in a distal part (∼12 mm relative to the proximal chamber) of the channel (Fig. 5). Both viruses led to the accumulation of strong GFP-fluorescent cytoplasmic inclusions, indicating that both viruses successfully infected the DRG neurons at the cell soma. In contrast to RABV EGFP-P, where small EGFP-P particles were detected in outgrowths of the neurons, in rNDVF1 EGFP-P-infected cultures, particulate EGFP-P fluorescence was restricted to the cell soma and only a faint, unequally distributed GFP fluorescence was detectable in neuritic outgrowths (Fig. 5, proximal chamber, bright area). In the channels, NDV-infected neurons led to only a faint axoplasmic GFP fluorescence that decreased from the more proximal to the more distal part of the channel (Fig. 5, proximal and distal areas). Particulate structures were absent in the axons, showing that rNDVF1 EGFP-P particles do not enter the axonal transport machinery. In contrast to infection with rNDVF1 EGFP-P, infection with RABV EGFP-P resulted in the appearance of particulate structures in proximal and distal parts of the axons. These data support the idea that in the DRG neuron cultures used, specific axonal sorting occurs. Accordingly, we assume that GFP-labeled RABV particles are not randomly transported through axons, for instance as a result of abundant protein levels, but that specific mechanisms exist that allow intra-axonal transport of RABV particles in the anterograde direction.
FIG 5.
Exclusion of NDV phosphoprotein from anterograde transport in axons. DRG neurons were infected at the cell soma with rRABV EGFP-P Gcvs and recombinant NDV expressing EGFP-tagged NDV P protein (rNDVF1 EGFP-P). EGFP-P fluorescence was monitored at 4 dpi within the cell soma (proximal chamber) and in the channel at proximal areas close to the cell soma side (middle row) and in more distant distal areas (bottom row). Note that brightness/contrast was increased (to 100) for rNDVF1 EGFP-P fluorescence within the image section (cell soma) and in images from channel areas.
Anterograde transport of EGFP-P particles depends on glycoprotein G.
Axonal transport of newly formed EGFP-P particles could be due either to the transport of enveloped virus particles after budding in cellular membrane compartments or to the transport of cytoplasmic subviral protein complexes. To assess whether the transported EGFP-P particles represented enveloped and glycoprotein G-containing virus particles, postreplicative EGFP-P transport in rRABV EGFP-P Gcvs-infected DRG neurons was compared to that in DRG neurons that were infected with G gene-deleted RABV. Four days after infection of DRG neurons at the cell soma with 104 FFU of rRABV EGFP-P Gcvs or G gene-deleted virus rRABV EGFP-P ΔG, the GFP fluorescence was monitored in the cell soma (proximal chamber) and in the middle part of the channels. Both viruses efficiently infected the DRG neurons, as multiple cytoplasmic inclusions became visible in the cell soma (Fig. 6). Immunostaining against glycoprotein G confirmed the absence of G protein in the postreplicative phase of rRABV EGFP-P ΔG infection.
FIG 6.
Postreplicative axonal transport of EGFP-P particles is G protein dependent. DRG neurons were infected with rRABV EGFP-P Gcvs and G gene-deleted rRABV EGFP-P ΔG at the cell soma. Upper row, cells were fixed after 2 days of infection and were immunostained with G-specific monoclonal antibody E559. Merged images of red G signals and green EGFP-P autofluorescence are shown. Detail, G-specific fluorescence only. Lower row, after 4 days of infection, axonal EGFP-P fluorescence of living neurons was monitored within channels. The images represent maximum z-projections of seven optical slices (0.772 μm each; size depth = 4.5 μm).
Whereas some EGFP-P particles were detected in close proximity to the soma, images from middle parts of the channels revealed that in the absence of G, only diffuse GFP fluorescence was detectable in the axons, indicating that no EGFP-P-particles entered the anterograde transport pathway (Fig. 6). In contrast, RABV EGFP-P Gcvs led to intra-axonal transport of EGFP-P particles.
These data strongly suggested the axonal transport of glycoprotein-containing virus particles or at least cotransport of EGFP-P particles and vesicles of the cellular transport machinery containing RABV-G. Moreover, dependence of the anterograde axonal transport on newly synthesized G also excluded the possibility that the observed EGFP-P particles in the axons were due to an accumulation of input virus.
Colocalization of P particles with glycoprotein.
To assess whether G-dependent axonal transport of P particles correlated with colocalization of G and P, DRG neuron cultures were infected with rRABV EGFP-P Gcvs at the cell soma. After 2 days of infection, the DRGs were fixed and immunostained with G-specific MAb E559. Detection of EGFP-P particles and G vesicles (Fig. 7, green and red, respectively) in distal axon parts confirmed anterograde transport of both proteins. Colocalization of EGFP-P and G (arrowheads) further indicated cotransport of both virus proteins in particulate structures. In addition, noncolocalized EGFP-P and G particles were also observed in the distal chamber (arrows). Due to the deletion of G, neither EGFP-P particles nor G signals were detected in distal axon parts of rRABV EGFP-P ΔG-infected DRGs (Fig. 7, g to i), whereas diffusely distributed axoplasmic GFP fluorescence still indicated the presence of soluble EGFP-P protein even at distal parts of the axons. These data indicated that G and EGFP-P particles indeed accumulated in distal axon regions as a result either of cotransport through neurons or of particle assembly at distal membrane sites. As transport of EGFP-P particles depended on the presence of G, we hypothesized that cotransport of both viral protein components was causative for the observed colocalization. Some noncolocalizing particles may be due to the additional transport of G-containing secretory vesicles in the case of red particles and to partial dissociation of G and EGFP-P from cotransported particles at distal sites.
FIG 7.
Colocalization of RABV G and EGFP-P in distal chambers. DRG neuron cultures were infected with rRABV EGFP-P Gcvs or rRABV EGFP-P ΔG at the cell soma. At 2 dpi, neurons were fixed and immunostained with G-specific MAb E559. Optical slice = 0.772 μm. (a to c) Axons of rRABV EGFP-P Gcvs-infected neurons in the distal chamber. (d to f) Magnification of distal axons, with EGFP-P and G colocalization indicated by arrowheads. Particles exhibiting only green or red fluorescence are indicated by arrows. Scale bar, 1 μm. (g to i) Axons of rRABV EGFP-P ΔG-infected neurons in the distal chamber. Only diffusely distributed GFP fluorescence in axons and bouton-like axon swellings was observed, indicating the presence of soluble EGFP-P in the distal axon parts.
Cotransport of newly formed EGFP-P particles and tmCherry-labeled vesicles.
Since anterograde transport of EGFP-P particles depended on the expression of G in the infected cell, transport of newly formed virus particles or subviral structures through neurons was most likely. To show that EGFP-P particles were indeed cotransported with G-containing membranes, neurons were coinfected at the cell soma with rRABV EGFP-P Gcvs and rRABV tmCherry at the proximal chamber. In addition to its authentic G, rRABV tmCherry expressed a membrane-anchored mCherry fusion protein in which the ectodomain of RABV G was replaced by the red-fluorescent mCherry protein (Fig. 8B). As colocalization of EGFP-P and membrane-anchored tmCherry depended on coinfection and subsequent formation of new virus particles, anterograde cotransport of both labels was expected in the case of vesicle-associated axonal RNP transport.
FIG 8.
Cotransport of EGFP-P particles and tmCherry-labeled vesicles. DRG neuron cultures were coinfected with rRABV EGFP-P Gcvs and rRABV tmCherry in the cell soma. After 3 days of infection, axonal transport of EGFP-P (green) and tmCherry (red) was monitored within the channels (0.336 s/frame; optical slice = 0.772 μm). (A) Colocalization of EGFP and mCherry fluorescence in transported particles. Upper three rows, t-projections with red, green, and merged images. Arrows, coinfected neurons. The dashed lines indicate the time period and area used for single-particle tracking as shown below. The image sequence was derived from 25 time frames, and every third image is shown. Scale bar, 1 μm. (B) Genome organization of rRABV EGFP-P Gcvs and rRABV tmCherry. The tmCherry protein consists of RABV G-derived signal peptide and transmembrane and cytoplasmic sequences (SP, TM, and Cyt, respectively). (C) Single-particle analysis with time projection of an anterograde particle transport process. Shown are merged images (1-μm2 details of an individual transported particle) from 60 consecutive frames. The frame order is indicated at the left. (D) t-projections of neurons infected exclusively with rRABV EGFP-P Gcvs or rRABV tmCherry. Scale bar, 1 μm.
Indeed, at 3 days postinfection, anterograde axonal transport of EGFP-P particles and mCherry fluorescent vesicles was detectable in the axons. Double-infected neurons exhibited double-stained particles in axons and cotransport of EGFP-P and mCherry (Fig. 8A, arrows), as demonstrated by time projections and by image sequences of individual transported particles. These data clearly showed that newly formed EGFP-P particles were cotransported with membrane-anchored mCherry protein. Analysis of individually tracked particles further revealed that the ratio of red and green fluorescence was not constant over time (Fig. 8C; see Movie S4 in the supplemental material), suggesting that the mCherry-labeled membrane was not as tightly bound to the EGFP-P particle as may be expected in the case of complete bullet-shaped RABV particles. Nevertheless, these data strongly supported the hypothesis that anterograde axonal transport of EGFP-P particles occurs either in or at RABV glycoprotein-containing membrane vesicles.
DISCUSSION
By the use of EGFP-P-labeled viruses, we showed here that newly formed RABV particles are anterogradely transported into axons of peripheral rat DRG neurons. Though early and more recent work provided evidence for anterograde spread of RABV in peripheral neurons (25, 26), the involved mechanisms of anterograde RABV transport remained unclear. Here, by demonstrating the dependency of the anterograde transport on the expression of viral glycoprotein in the infected DRGs, we now provide a deeper insight in the mechanism of anterograde RABV spread. Glycoprotein-dependent transport of RABV particles (Fig. 6) and cotransport of membrane-anchored mCherry comprising the cytoplasmic and transmembrane domains of RABV G (Fig. 8; see Movie S4 in the supplemental material) strongly support a model in which either complete virus particles are transported within cellular transport vesicles or cytoplasmic RNPs are cotransported with glycoprotein-containing vesicles by sticking to the cytoplasmic side of the vesicles (Fig. 9). Moreover, direct observation of fast and directed anterograde RABV transport in peripheral sensory neurons strongly supports a model in which active anterograde transport machineries are involved. In view of current concepts of exclusive retrograde axonal transport of RABV in neurons (reviewed in reference 18), our data and recent evidence for anterograde transsynaptic RABV spread in sensory neurons (25) strongly support a more differential model of axonal RABV transport in which peripheral neurons could substantially differ in permitting RABV transport in the anterograde direction.
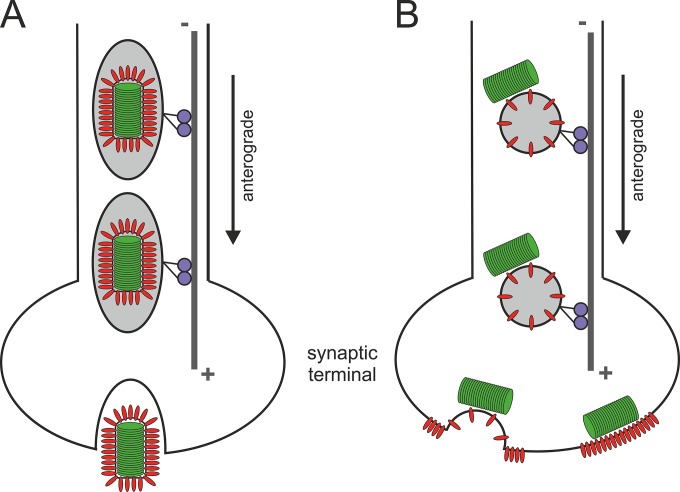
FIG 9.
Model of intraneuronal postreplicative RABV transport. Either complete, enveloped virus particles are transported within exocytotic vesicles (A) or cotransport of cytoplasmic RNPs and G-containing transport vesicles may occur (B). Whereas the former mode may allow release of complete virus particles at axon termini or presynaptic membranes, the cotransport model would allow local concentration of both viral glycoprotein and RNPs at distal sites of virus assembly. After fusion of the transport vesicle with presynaptic membranes, the RNP is positioned directly beneath the G-enriched presynaptic membrane, and virus particles may be assembled by a subsequent budding event.
Earlier work already supported anterograde axonal RABV transport in DRG and other peripheral neurons (26, 27, 38). Tsiang and colleagues (26) were able to demonstrate virus release after anterograde transport through DRG neurons, strongly supporting the hypothesis that anterograde axonal transport indeed led to the release of infectious virus at presynaptic membranes of DRG neurons. Although anterograde spread of RABV in peripheral neurons has also been recognized in vivo in the course of viral spread to peripheral sites, only inefficient transport or passive diffusion in the anterograde direction was concluded (28), and mechanistic insight into the involved processes remained poor. Fast and directed anterograde transport at velocities clearly exceeding those determined for retrograde RABV transport during entry (Fig. 2) and after virus replication in the cell (Fig. 3) strongly supports the idea that active, energy-dependent transport by kinesins is involved in anterograde RABV transport. This is also consistent with current concepts on directed intraneuronal transport (reviewed in reference 39). Physical barriers that prevent uncontrolled transport of large complexes into axons (40) may be one reason for the requirement of specific mechanisms to allow RABV particle transport. Since passive diffusion of virions, subviral particles, or even large viral protein complexes into axons is unlikely, the dependency of axonal transport on the expression of glycoprotein G suggests that the viral glycoprotein represents a sorting signal that either directs budding of virions into transport vesicles or allows recruitment of virus RNPs to vesicles that are transported into axons.
Different transport velocities for retro- and anterograde transport of viruses have been reported. For instance, bidirectional fast axonal transport of pseudorabies virus in chicken DRG neurons was characterized by average velocities of 1.97 and 1.2 μm/s for anterograde and retrograde transport processes, respectively (41), confirming that anterograde axonal virus transport occurs about twice as fast as retrograde virus transport. Higher velocities as determined for RABV transport in rat DRGs may be due to the use of different cellular motor proteins or to species-specific differences in the velocity of axonal transport in DRGs derived from rat or chicken embryos. The same reasons may also contribute to minor differences in retrograde RABV transport velocities in murine DRG neurons. In the murine DRG neurons, the average velocity of retrograde RABV transport, 0.93 μm/s (12), remained below the velocities of 1.5 μm/s and 1.6 μm/s as observed here for retrograde axonal RABV transport in the rat DRG neurons (Fig. 2 and 3). Notably, also in the murine system, subpopulations of fast RABV transport were identified, with velocities of 1.2 to 1.4 μm/s (12), indicating that also in the murine system RABV can achieve transport kinetics similar to those observed here in the rat DRG system.
Importantly, with average anterograde velocities of 3.4 μm/s (Fig. 3), we were clearly able to distinguish fast anterograde RABV transport processes from retrograde transport. The most reasonable explanation for the differences in the kinetics of retro- and anterograde transport processes is the use of different cellular motor complexes. The possibility to differentiate between slower retrograde and faster anterograde transport processes also revealed that the observed particle transport in opposite directions (Fig. 6) indeed depended on different transport mechanisms and was not just an effect of back-folding and growing of axons terminals toward the proximal chamber.
Although we have not yet directly shown a role of kinesin motors in the anterograde axonal rabies virus transport and no kinesin binding motifs in any RABV protein have been described, we hypothesize that kinesin-dependent axonal transport was involved in postreplicative anterograde transport processes. In view of the suggested models, vesicle-associated RABV transport indeed may not require specific kinesin binding sites in virus proteins, since cellular components of the transport vesicles could be used. Similar retrograde transport velocities in virus entry and after virus replication further indicated dynein-mediated back-transport into the retrograde direction, either by virus release and reinfection at axonal termini or by exchange of motor proteins and a subsequent switch in transport directionality. Indeed, back-transport of excess vesicles back to the soma has been described in Drosophila (42) and could easily explain the observed bidirectional transport of RABV particles after virus replication.
To show restricted transport of cytoplasmically expressed RNP proteins, we included recombinant NDV EGFP-P-expressing Newcastle disease virus. Exclusion of NDV EGFP-P particles from transport into axons of infected DRG neurons (Fig. 5) strongly supports the hypothesis that the particle transport for RABV occurs in an active and regulated way, Indeed, in hippocampal neurons a physical barrier for both lateral diffusion of membrane proteins and lipids (43, 44) and transport through the cytoplasm (40) in the axon initial segment (AIS) serves as filter for cargoes that are excluded from axons. In an actin-dependent manner, large 70-kDa dextrans were excluded from diffusion into axons by the cytoplasmic filter, whereas smaller GFP molecules or 10-kDa dextrans were not excluded (40). Remarkably, the molecular mass of monomeric 74-kDa NDV EGFP-P fusion protein is similar to that of 70-kDa dextran, and exclusion of even monomeric rNDVF1 EGFP-P is conceivable. At least partial exclusion of soluble rNDVF1 EGFP-P is supported by the finding that diffuse rNDVF1 EGFP-P fluorescence was detectable mainly in proximal axon areas, whereas fluorescence was hardly detectable in more distal parts (Fig. 5). More intense signals for the 62-kDa soluble RABV EGFP-P within axons may rely on a less restricted transport into axons because of the lower molecular mass or on the presence of sorting signals in P that may allow transport of soluble P through a cytoplasmic barrier within the proximal axon areas. In the case of transported RNPs, which represent large complexes of RNA and N, L, and P proteins (see the colocalization of EGFP-P particles with N in Fig. 4), a physical barrier in the axon initial segment is most likely to preclude passive diffusion in axons.
Glycoprotein-dependent axonal transport of EGFP-P particles (Fig. 6) revealed that transport of EGFP-P particles into axons requires envelope-dependent sorting or transport signals. This conclusion was supported (i) by the G-dependent appearance of P particles in axons, (ii) by colocalization of EGFP-P particles and G protein in distal axon areas (Fig. 7), and (iii) by intra-axonal cotransport of EGFP-P and tmCherry (Fig. 8). Cotransport of EGFP-P and membrane-anchored tmCherry further revealed that postreplicative anterograde axonal transport of EGFP-P is connected to vesicle transport, leading to the hypothesis that either complete virions were transported in cellular vesicles (Fig. 9A) or cytoplasmic P-containing RNPs are associated with RABV G-containing vesicles of the secretory pathway (Fig. 9B). Whereas the first model may result in release of preformed infectious virus at presynaptic membranes by exocytosis, the second would lead to local enrichment of G in the presynaptic membrane with the RNP already bound at the cytoplasmic side of the membrane. This would allow local concentration of virus components needed for budding at presynaptic membranes after long-distance transport through axons.
Secretory transport of complete virions within cellular vesicles (Fig. 9A) would imply intracellular budding of RABV particles. Indeed, intracellular virus particle formation is known to occur within RABV-infected cells (45, 46), and more recent data provided evidence for matrix protein-dependent accumulation of virus-like particles within the rough endoplasmic reticulum (rER) (30, 47). Transport of virus particles within secretory Golgi vesicles, however, has not been observed so far. Since most investigations have been performed with nonneuronal cell cultures, it is not yet clear whether ER virions indeed are not mobilized in infected neurons and whether the observed cotransport of EGFP-P and tmCherry could be due to mobilization of ER-associated RABV virions. However, the dynamics of the ER in neurons and motor-assisted vesicular ER transport within axons (48) could mobilize postreplicative virion accumulation within the ER of neurons without a requirement for Golgi vesicles.
EGFP-P and G colocalization in distal axon areas was not complete, as indicated by particles with EGFP fluorescence only (Fig. 7), and the red and green fluorescence intensities of transported particles were not perfectly overlaying (Fig. 8B and C), suggesting that no complete virions with a rigid bullet-shaped morphology were transported. Cotransport of G-containing vesicles and cytoplasmic RNPs on the cytoplasmic side of the vesicles would explain imperfect colocalization of EGFP-P particles and tmCherry and would support a model in which coenrichment of virus envelope proteins and RNPs allows coordinated budding at distal presynaptic membranes (Fig. 9B).
Our data confirm early results on RABV infection of peripheral neurons that claimed anterograde axonal transport in cultivated DRG neurons (26) and in vivo after intranasal and intramuscular inoculation (27, 38), the latter indicating that anterograde virus transport observed in primary peripheral DRG neuron cultures may not be a result of cultivation artifacts. Infection of mice by the olfactory route is an excellent example of anterograde spread of RABV toward the CNS after a first round of replication in olfactory receptor neurons, where infection of second-order neuron mitral cells as well as transsynaptic transmission to higher-order neurons must have included anterograde axonal spread (27). Recent evidence for anterograde RABV spread in vivo through sensory neurons (25) also supported the conclusion of different axonal transport modes in RABV-infected peripheral sensory and CNS neurons. Whereas previous work relied mainly on virus spread by the detection of infected neurons, we present here for the first time a dynamic insight into the postreplicative transport of newly formed RABV. We provide evidence that envelope-dependent transport of either complete anterograde virus particles or vesicle-RNP cotransport occurs in peripheral neurons, both providing the possibility of virus release and transmission to higher-order neurons at distal presynaptic membranes (Fig. 9).
As our data strongly indicate fast anterograde axonal transport after replication in DRG neurons, we conclude that anterograde axonal transport through sensory neurons, either toward the CNS or centrifugally to peripheral sites, occurs in an active manner, in contrast to previously suggested passive diffusion (28). Obviously, in addition to receptor-dependent retrograde entry into neurons (11), the G protein also determines long-distance transport in DRG neurons after virus replication in the cell soma (16). Similar to the case for RABV, intracellular transport of complete VSV within vesicles of the secretory pathway has not been demonstrated, and cotransport of cytoplasmic RNPs with G-containing vesicles, as discussed here for RABV, cannot be excluded.
We have shown that anterograde transport of newly formed RABV virions or subviral particles in axons of peripheral DRG neurons occurs in a glycoprotein-dependent manner. In view of the large body of evidence for exclusive unidirectional spread of RABV in motor neurons and within the CNS, our findings and those of others suggest that RABV transport depends on neuron type-specific mechanisms and that peripheral DRG neurons, and maybe also other peripheral neurons, are exceptional in supporting active anterograde RABV transport. Future identification of distinct mechanisms in peripheral and central axonal virus transport and their impact on virus spread and pathogenesis may be crucial for a detailed understanding of nervous system infections by RABV and other neurotropic viruses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Angela Hillner and Dietlind Kretzschmar for technical assistance, and we are grateful to Thomas C. Mettenleiter for helpful discussions. We thank Karl-Klaus Conzelmann for critical comments on the manuscript. We further thank the Department of Experimental Animal Facilities and Biorisk Management of the Friedrich-Loeffler-Institut for providing embryonated rats for DRG preparations.
This study was supported by the German-Israeli Foundation for Scientific Research and Development (grant no. 1107-73.1/2010) and the German Federal Ministry of Education and Research (grant no. 01KI1016A).
Footnotes
Published ahead of print 1 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02254-14.
REFERENCES
- 1.Koyuncu OO, Hogue IB, Enquist LW. 2013. Virus infections in the nervous system. Cell Host Microbe 13:379–393. 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salinas S, Schiavo G, Kremer EJ. 2010. A hitchhiker's guide to the nervous system: the complex journey of viruses and toxins. Nat. Rev. Microbiol. 8:645–655. 10.1038/nrmicro2395. [DOI] [PubMed] [Google Scholar]
- 3.Lycke E, Tsiang H. 1987. Rabies virus infection of cultured rat sensory neurons. J. Virol. 61:2733–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacob Y, Badrane H, Ceccaldi PE, Tordo N. 2000. Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J. Virol. 74:10217–10222. 10.1128/JVI.74.21.10217-10222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raux H, Flamand A, Blondel D. 2000. Interaction of the rabies virus P protein with the LC8 dynein light chain. J. Virol. 74:10212–10216. 10.1128/JVI.74.21.10212-10216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mebatsion T. 2001. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J. Virol. 75:11496–11502. 10.1128/JVI.75.23.11496-11502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan GS, Preuss MA, Williams JC, Schnell MJ. 2007. The dynein light chain 8 binding motif of rabies virus phosphoprotein promotes efficient viral transcription. Proc. Natl. Acad. Sci. U. S. A. 104:7229–7234. 10.1073/pnas.0701397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finke S, Brzozka K, Conzelmann KK. 2004. Tracking fluorescence-labeled rabies virus: enhanced green fluorescent protein-tagged phosphoprotein P supports virus gene expression and formation of infectious particles. J. Virol. 78:12333–12343. 10.1128/JVI.78.22.12333-12343.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finke S, Conzelmann KK. 2005. Replication strategies of rabies virus. Virus Res. 111:120–131. 10.1016/j.virusres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Das SC, Nayak D, Zhou Y, Pattnaik AK. 2006. Visualization of intracellular transport of vesicular stomatitis virus nucleocapsids in living cells. J. Virol. 80:6368–6377. 10.1128/JVI.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klingen Y, Conzelmann KK, Finke S. 2008. Double-labeled rabies virus: live tracking of enveloped virus transport. J. Virol. 82:237–245. 10.1128/JVI.01342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gluska S, Zahavi EE, Chein M, Gradus T, Bauer A, Finke S, Perlson E. 2014. Rabies virus hijacks and accelerates the p75NTR retrograde axonal transport machinery. PLoS Pathog. 10:e1004348. 10.1371/journal.ppat.1004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafon M. 2005. Rabies virus receptors. J. Neurovirol. 11:82–87. 10.1080/13550280590900427. [DOI] [PubMed] [Google Scholar]
- 14.Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, Carter EE, Barber RD, Baban DF, Kingsman SM, Kingsman AJ, O'Malley K, Mitrophanous KA. 2001. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum. Mol. Genet. 10:2109–2121. 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 15.Mentis GZ, Gravell M, Hamilton R, Shneider NA, O'Donovan MJ, Schubert M. 2006. Transduction of motor neurons and muscle fibers by intramuscular injection of HIV-1-based vectors pseudotyped with select rabies virus glycoproteins. J. Neurosci. Methods 157:208–217. 10.1016/j.jneumeth.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Beier KT, Saunders A, Oldenburg IA, Miyamichi K, Akhtar N, Luo L, Whelan SP, Sabatini B, Cepko CL. 2011. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc. Natl. Acad. Sci. U. S. A. 108:15414–15419. 10.1073/pnas.1110854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hislop JN, Islam TA, Eleftheriadou I, Carpentier DC, Trabalza A, Parkinson M, Schiavo G, Mazarakis ND. 2014. Rabies virus envelope glycoprotein targets lentiviral vectors to the axonal retrograde pathway in motor neurons. J. Biol. Chem. 289:16148–16163. 10.1074/jbc.M114.549980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ugolini G. 2011. Rabies virus as a transneuronal tracer of neuronal connections. Adv. Virus Res. 79:165–202. 10.1016/B978-0-12-387040-7.00010-X. [DOI] [PubMed] [Google Scholar]
- 19.Ugolini G. 2010. Advances in viral transneuronal tracing. J. Neurosci. Methods 194:2–20. 10.1016/j.jneumeth.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, Callaway EM. 2007. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53:639–647. 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wickersham IR, Finke S, Conzelmann KK, Callaway EM. 2007. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat. Methods 4:47–49. 10.1038/nmeth999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickersham IR, Sullivan HA, Seung HS. 2013. Axonal and subcellular labelling using modified rabies viral vectors. Nat. Commun. 4:2332. 10.1038/ncomms3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haberl MG, Viana da Silva S, Guest JM, Ginger M, Ghanem A, Mulle C, Oberlaender M, Conzelmann KK, Frick A. 2014. An anterograde rabies virus vector for high-resolution large-scale reconstruction of 3D neuron morphology. Brain Struct. Funct. 10.1007/s00429-014-0730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beier KT, Saunders AB, Oldenburg IA, Sabatini BL, Cepko CL. 2013. Vesicular stomatitis virus with the rabies virus glycoprotein directs retrograde transsynaptic transport among neurons in vivo. Front. Neural Circuits 7:11. 10.3389/fncir.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zampieri N, Jessell TM, Murray AJ. 2014. Mapping sensory circuits by anterograde transsynaptic transfer of recombinant rabies virus. Neuron 81:766–778. 10.1016/j.neuron.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsiang H, Lycke E, Ceccaldi PE, Ermine A, Hirardot X. 1989. The anterograde transport of rabies virus in rat sensory dorsal root ganglia neurons. J. Gen. Virol. 70:2075–2085. 10.1099/0022-1317-70-8-2075. [DOI] [PubMed] [Google Scholar]
- 27.Astic L, Saucier D, Coulon P, Lafay F, Flamand A. 1993. The CVS strain of rabies virus as transneuronal tracer in the olfactory system of mice. Brain Res. 619:146–156. 10.1016/0006-8993(93)91606-S. [DOI] [PubMed] [Google Scholar]
- 28.Ugolini G. 2008. Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis. Dev. Biol. 131:493–506. [PubMed] [Google Scholar]
- 29.Buchholz UJ, Finke S, Conzelmann KK. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finke S, Granzow H, Hurst J, Pollin R, Mettenleiter TC. 2010. Intergenotypic replacement of lyssavirus matrix proteins demonstrates the role of lyssavirus M proteins in intracellular virus accumulation. J. Virol. 84:1816–1827. 10.1128/JVI.01665-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finke S, Mueller-Waldeck R, Conzelmann KK. 2003. Rabies virus matrix protein regulates the balance of virus transcription and replication. J. Gen. Virol. 84:1613–1621. 10.1099/vir.0.19128-0. [DOI] [PubMed] [Google Scholar]
- 32.Schnell MJ, Mebatsion T, Conzelmann KK. 1994. Infectious rabies viruses from cloned cDNA. EMBO J. 13:4195–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramp K, Topfstedt E, Wackerlin R, Hoper D, Ziller M, Mettenleiter TC, Grund C, Romer-Oberdorfer A. 2012. Pathogenicity and immunogenicity of different recombinant Newcastle disease virus clone 30 variants after in ovo vaccination. Avian Dis. 56:208–217. 10.1637/9870-080311-Reg.1. [DOI] [PubMed] [Google Scholar]
- 34.Mebatsion T, Verstegen S, De Vaan LT, Romer-Oberdorfer A, Schrier CC. 2001. A recombinant newcastle disease virus with low-level V protein expression is immunogenic and lacks pathogenicity for chicken embryos. J. Virol. 75:420–428. 10.1128/JVI.75.1.420-428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider LG, Barnard BJH, Schneider HP, Odegaard OA, Müller J, et al. 1985. Mechanisms of rabies virus neutralization by glycoprotein-specific monoclonal antibodies. Springer, Berlin, Germany. [Google Scholar]
- 36.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown C, King DJ, Seal BS. 1999. Pathogenesis of Newcastle disease in chickens experimentally infected with viruses of different virulence. Vet. Pathol. 36:125–132. 10.1354/vp.36-2-125. [DOI] [PubMed] [Google Scholar]
- 38.Coulon P, Derbin C, Kucera P, Lafay F, Prehaud C, Flamand A. 1989. Invasion of the peripheral nervous systems of adult mice by the CVS strain of rabies virus and its avirulent derivative AvO1. J. Virol. 63:3550–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapitein LC, Hoogenraad CC. 2011. Which way to go? Cytoskeletal organization and polarized transport in neurons. Mol. Cell. Neurosci. 46:9–20. 10.1016/j.mcn.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. 2009. A selective filter for cytoplasmic transport at the axon initial segment. Cell 136:1148–1160. 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Smith GA, Gross SP, Enquist LW. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 98:3466–3470. 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong MY, Zhou C, Shakiryanova D, Lloyd TE, Deitcher DL, Levitan ES. 2012. Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell 148:1029–1038. 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winckler B, Forscher P, Mellman I. 1999. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397:698–701. 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- 44.Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K, Fujiwara T, Kusumi A. 2003. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 5:626–632. 10.1038/ncb1009. [DOI] [PubMed] [Google Scholar]
- 45.Murphy FA, Harrison AK, Winn WC, Bauer SP. 1973. Comparative pathogenesis of rabies and rabies-like viruses: infection of the central nervous system and centrifugal spread of virus to peripheral tissues. Lab. Invest. 29:1–16. [PubMed] [Google Scholar]
- 46.Murphy FA, Bauer SP, Harrison AK, Winn WC., Jr 1973. Comparative pathogenesis of rabies and rabies-like viruses. Viral infection and transit from inoculation site to the central nervous system. Lab. Invest. 28:361–376. [PubMed] [Google Scholar]
- 47.Pollin R, Granzow H, Kollner B, Conzelmann KK, Finke S. 2013. Membrane and inclusion body targeting of lyssavirus matrix proteins. Cell. Microbiol. 15:200–212. 10.1111/cmi.12037. [DOI] [PubMed] [Google Scholar]
- 48.Valenzuela JI, Jaureguiberry-Bravo M, Couve A. 2011. Neuronal protein trafficking: emerging consequences of endoplasmic reticulum dynamics. Mol. Cell. Neurosci. 48:269–277. 10.1016/j.mcn.2011.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.