ABSTRACT
The HIV-1 envelope protein (Env) is heavily glycosylated, with approximately 50% of the Env molecular mass being contributed by N-glycans. HIV-1 Env N-glycans shield the protein backbone and have been shown to play key roles in determining Env structure, surface exposure, and, consequently, antigenicity, infectivity, antibody neutralization, and carbohydrate and receptor binding. Studies of HIV-1 glycosylation have focused mainly on the position of glycosylation, rather than the types of glycans. Also, the role of Env glycan moieties on HIV-1 transmission has not been systematically defined. Using viruses with modified Env glycan content and heterogeneity, we examined the effects of Env glycan moieties on the major events of HIV-1 transmission. Compared to viruses with less oligomannose and more complex Env glycans, viruses with more oligomannose and less complex glycans more efficiently (i) transcytosed across an epithelial cell monolayer, (ii) attached to monocyte-derived macrophages (MDMs), (iii) bound monocyte-derived dendritic cells (MoDCs), and (iv) trans-infected primary lymphocytes via MoDCs. However, viruses with more oligomannose and less complex glycans displayed impaired infectivity in TZMbl cells, MDMs, primary lymphocytes, and fresh human intestinal tissue. Thus, N-linked Env glycans display discordant effects on the major events of HIV-1 transmission, with mature oligosaccharide structures on Env playing a crucial role in HIV-1 infection. Env glycosylation should be taken into consideration in the development of vaccine strategies to interdict HIV-1 transmission.
IMPORTANCE HIV-1 Env N-glycans shield the protein backbone and play key roles in determining Env structure and surface exposure, thereby impacting Env antigenicity, infectivity, antibody neutralization, and carbohydrate and receptor binding. Studies of HIV-1 glycosylation have focused mainly on the position of glycosylation, rather than the types of glycans. In the study described in this report, we investigated systematically the role of Env glycan moieties on HIV-1 transmission. We show that N-linked Env glycans display discordant effects on the major events of HIV-1 transmission. These data indicate that Env glycan moieties impact HIV-1 transmission and that modulation of Env glycan moieties offers a potential strategy for the development of therapeutic or prophylactic vaccines against HIV-1.
INTRODUCTION
An important feature of the HIV-1 envelope protein (Env) is that gp120 is heavily glycosylated. During synthesis and folding in the endoplasmic reticulum of the host cell, HIV-1 Env precursor gp160 is modified by N-linked glycosylation. After further folding of each monomer and glycan processing in the Golgi apparatus, the gp160 Env precursor is proteolytically cleaved into the surface subunit gp120 and the transmembrane subunit gp41 and then transported to the cell surface for incorporation into the virion as trimers of gp120/gp41 (1 – 3). The resultant Env gp120 is heavily glycosylated, with approximately 50% of the Env molecular mass being contributed by N-glycans and a small amount being contributed by O-glycans (4 – 6). N-Glycans include three basic structures, namely, high-mannose (i.e., oligomannose), hybrid, and complex glycans, all of which are present on HIV-1 Env (4 – 7). Importantly, N-glycans shield the Env backbone and have been shown to play key roles in determining the Env structure, epitope exposure, and, consequently, antigenicity, immunogenicity, antibody neutralization, infectivity, and carbohydrate and receptor binding (8 – 20).
The position of N-linked glycans on glycoproteins is genetically encoded, whereas the types of glycans at a given Asn in the consensus Asn-X-Ser/Thr-X sequence (where X is not Pro) are determined by the glycan branching processes in the cell endoplasmic reticulum and Golgi apparatus (21). However, some glycoproteins, including HIV-1 gp120, demonstrate protein-specific glycosylation (22 – 25). Consequently, the glycan profile of HIV-1 is divergent from the normal glycosylation of its host cell. Indeed, HIV-1 gp120 displays unusually high levels of oligomannose, the immature (i.e., incompletely processed) form of glycan chains (23, 26, 27). The N-linked glycans of native Env in primary HIV-1 isolates are predominantly oligomannose and independent of the production system or virus clade. In contrast, recombinant gp120 displays extensive glycan processing by cellular glycosylation enzymes, resulting in glycans composed of >70% complex glycans (26, 27). Studies of HIV-1 glycosylation have focused mainly on the Env structure and surface exposure and their effect on antigenicity, immunogenicity, and antibody neutralization (8 – 20) through mutation of the position of glycosylation, rather than modification of the type of glycans. Studies of the effect of glycosylation on HIV-1 infectivity and carbohydrate and receptor binding have also utilized deletion of the potential N-linked glycosylation sites (PNGSs) (15 – 18, 28).
The hypervariable loops of transmitted/founder (T/F) virus Env are generally shorter and contain fewer PNGSs than those of the Env characteristic of chronic viruses, potentially exposing key epitopes for receptor access and mucosal transmission. Specific PNGS signatures present in T/F viruses but not chronic viruses may promote the interaction between Env and the HIV-1 receptors CD4 and CCR5 on mononuclear target cells and the binding of Env to the lectin DC-SIGN on dendritic cells (DCs), promoting mucosal entry and infection. In this connection, N-glycans have been shown to impact HIV-1 infectivity and cytopathicity in cell lines (14 – 17, 28). In a recent study, HIV-1 replicated in cells from donors with a congenital disorder of glycosylation type IIb at significantly lower levels than it did in cells from healthy subjects, and the viruses produced were less infectious (29). However, the role of Env glycan moieties in HIV-1 transmission has not been systematically defined.
HIV-1 transmission in genital and gut mucosae involves three major events: (i) entry through/across the mucosal epithelium, (ii) infection and subsequent replication in subepithelial mononuclear target cells, and (iii) local dissemination and delivery to lymph nodes to initiate systemic infection (30 – 32). Here, we examined the impact of Env glycan moieties on these transmission events. We generated viruses with a different extent and complexity of Env glycans by producing viruses in 293T cells in the presence of different concentrations of the Golgi apparatus α-mannosidase inhibitor 1-deoxymannojirimycin (dMM). Using HT-29 model epithelial cells, peripheral blood lymphocytes (PBLs), monocyte-derived macrophages (MDMs), and monocyte-derived dendritic cells (MoDCs), we examined the impact of Env glycan moieties (high-mannose versus complex glycan) on the major events of HIV-1 transmission, including HIV-1 transcytosis across an epithelial cell monolayer, binding to and replication in PBLs and MDMs, and MoDC capture of HIV-1 and subsequent trans-infection of peripheral blood lymphocytes. We also examined the combined effect of Env glycan moieties on HIV-1 infection in human intestinal mucosa.
MATERIALS AND METHODS
Ethics statement.
All tissue and cell protocols were approved by the Institutional Review Board of the University of Alabama at Birmingham. Written informed consent was provided by the study participants.
HIV-1 molecular clone and viruses.
A replication-competent clone of R5 virus, YU2 (33), was transfected into 293T cells by use of the Fugene 6 reagent (Roche, Indianapolis, IN), according to the manufacturer's protocol and our recent publications (34, 35), in the presence of 0, 125, 250, and 500 μM the Golgi apparatus α-mannosidase inhibitor dMM. After 60 h, the supernatants were harvested, clarified by low-speed centrifugation, filtered through a 0.45-μm-pore-size filter, aliquoted, and stored at −80°C. Virus titers were determined by p24 enzyme-linked immunosorbent assay (ELISA; Perkin-Elmer, Boston, MA). YU2 viruses produced in the presence of 0, 125, 250, and 500 μM dMM were designated YU2, YU2.dMM125, YU2.dMM250, and YU2.dMM500, respectively.
Western blotting.
Western blotting was performed as described previously (36). Briefly, YU2 viruses were treated with endo-N-glycosidase H (endo H) from Streptomyces plicatus (Prozyme, St. Louis, MO) to remove high-mannose and hybrid N-glycans but not complex N-glycans and then subjected to SDS-PAGE, blotted onto a polyvinylidene difluoride membrane, and detected using anti-gp120 antibody.
Transcytosis assay in model columnar epithelium.
Epithelial cell monolayers were established in transwell chambers using HT-29 cells (ATCC, Manassas, VA) as described previously (35). Monolayers were used only when the transepithelial electrical resistance was 390 mΩ/cm2 or greater, consistent with nonpermeable, intact tight junctions. Transcytosis was assayed according to our previously described protocol (35). Briefly, HIV-1 containing 10 ng p24 was inoculated onto the apical surface of HT-29 cell monolayers, 2 h later the medium in the lower chamber was harvested, and virus that had entered the lower chamber was measured by p24 ELISA. Relative transcytosis efficiency was calculated by definition of the transcytosis efficiency of YU2 to be 100%.
Preparation of MDMs, PBLs, and MoDCs.
Peripheral blood monocytes and lymphocytes were purified by gradient sedimentation followed by magnetic anti-CD3 bead isolation or antiphycoerythrin (anti-PE) bead isolation of anti-CD14-PE-treated cells according to the manufacturer's manual (Miltenyi Biotec). To generate MDMs, purified monocytes were cultured in RPMI plus 10 ng/ml of macrophage colony-stimulating factor for 3 days (37) and then used for binding and infectivity experiments. To generate immature MoDCs, purified monocytes were cultured in RPMI plus interleukin-4 (IL-4; 200 U/ml) and granulocyte-macrophage colony-stimulating factor (800 U/ml) for 5 days. PBLs were cultured in RPMI with IL-2 (20 U/ml) and phytohemagglutinin (PHA; 5 μg/ml) for 3 days and then used in binding, infection, and trans-infection assays.
HIV-1 binding to MDMs and PBLs.
MDMs or PBLs (3 × 105 cells), prepared as described above, were inoculated with virus containing 10 ng p24, incubated for 2 h at 4°C, and analyzed by flow cytometry. Flow cytometry analysis was performed as described previously (34, 38), using CD13-allophycocyanin, CD3-PE, and KC57-fluorescein isothiocyanate (FITC) to identify MDMs, PBLs, and YU2 viruses, respectively.
HIV-1 infection of TZMbl cells, MDMs, and PBLs.
YU2 virus infectivity in TZMbl cells was determined as described previously (39). Briefly, TZMbl cell monolayers were inoculated with serially diluted viruses, incubated for 2 days, and stained with β-galactosidase. The infectious units were measured and normalized to the amount of p24. Virus infectivity in MDMs and PBLs was assessed as we have described previously (37). Briefly, MDMs or PBLs (2 × 105 cells), prepared as described above, were inoculated with viruses containing 10 ng p24 for 2 h at 37°C and then cultured for 16 days. Supernatant (100 μl) was harvested every 4 days and stored at −70°C until assayed for p24 by ELISA (Perkin-Elmer, Boston, MA).
MoDC capture of HIV-1 and trans-infection assay.
For MoDC capture of HIV-1, cultures of MoDCs (3 × 105 cells), prepared as described above, were inoculated with YU2 viruses containing 10 ng p24, incubated for 2 h at 37°C, and analyzed by flow cytometry for cells that contained HIV-1 using KC57-FITC antibody, as previously described (32, 38). For trans-infection, MoDC cultures (1 × 105 cells) were inoculated with viruses containing 10 ng p24, incubated for 2 h, washed 3 times with medium, cocultured with PHA-stimulated PBLs (4 × 105 cells) for 4 days, and analyzed by flow cytometry for HIV-1-positive lymphocytes using KC57 antibody.
HIV-1 infection in human intestinal mucosa.
Sections of human intestinal (jejunal) mucosa were obtained from otherwise healthy subjects undergoing gastric bypass for obesity. A leakproof intestinal explant system similar to our previously described vaginal, intestinal, and rectal explant system was constructed (30, 34, 35, 38, 40). YU2 viruses containing 10 ng p24 were inoculated onto the apical surface of intestinal tissue in 50-μl RPMI. After 2 h, the virus-containing medium was aspirated and the explants were treated with trypsin to remove residual virus still present on the epithelial surface. The explant systems were dissembled, and tissue specimens with equal wet weights were placed in medium and cultured for 3 days, after which the levels of p24 in the medium were determined by ELISA.
Statistical analysis.
Data are expressed as the mean ± standard deviation (SD) or the mean ± standard error of the mean (SEM), and statistical significance between groups was determined using a nonparametric Mann-Whitney test. P values of ≤0.05 were considered significant.
RESULTS
Generation of HIV-1 YU2 with different Env glycan moieties.
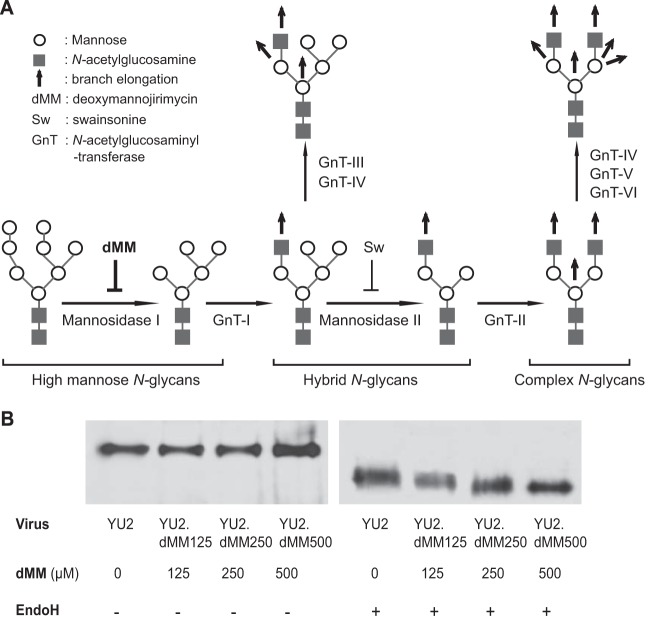
To generate viruses with different glycan moieties, i.e., Env glycans with different extents and complexities, we transfected 293T cells with an infectious clone of YU2 in the presence of the Golgi apparatus α-mannosidase inhibitor dMM. dMM inhibits Golgi apparatus α-mannosidase I, and treatment of cells with dMM blocks the branch elongation from high-mannose N-glycans to hybrid and complex N-glycans, resulting in immature oligosaccharide structures, specifically, N-linked carbohydrates that lack sialic acid and have a higher content of mannose residues and a reduced content of complex glycans (Fig. 1A). In the presence of various concentrations of dMM, the extent and complexity of Env glycans varied among the viruses that were produced. YU2 viruses produced in the presence of 0, 125, 250, and 500 μM dMM were named YU2, YU2.dMM125, YU2.dMM250, and YU2.dMM500, respectively (Fig. 1B). To confirm the different content and complexity of Env glycans, viruses were treated with endo-N-glycosidase H (endo H), which removes high-mannose and hybrid N-glycans but not complex N-glycans, and then were subjected to SDS-PAGE and Western blotting using anti-gp120 antibody for detection. Without endo H treatment, the gp120s of all four viruses showed similar gel mobility (Fig. 1B). After endo H treatment, the gel mobility of YU2, YU2.dMM125, YU2.dMM250, and YU2.dMM500 was gradually increased, indicating that the viruses produced in the presence of increasing concentrations of dMM contained more oligomannose and less complex glycans. Specifically, YU2 Env had the fewest oligomannose glycans and the most complex glycans, YU2.dMM500 had the most oligomannose and the fewest complex glycans, and YU2.dMM125 and YU2.dMM250 Env contained levels of oligomannose and complex glycans between those of YU2 and YU2.dMM500. We used these viruses in the following studies.
FIG 1.
HIV-1 YU2 with a variable extent and complexity of Env glycans. (A) Schematic diagram of cellular glycosylation pathways in the Golgi apparatus and the inhibition of α-mannosidase I by its inhibitor, 1-deoxymannojirimycin (dMM). (B) Viruses produced under the pressure of different concentrations of dMM displayed different extents and complexities of Env glycans. Viruses were treated with endo H to remove high-mannose and hybrid, but not complex, N-glycans and then subjected to SDS-PAGE and Western blotting using anti-gp120 antibody for detection.
HIV-1 with more oligomannose and less complex Env glycans is less infectious.
We first examined the effect of altering the glycan content of Env on HIV-1 infectivity in TZMbl cells. TZMbl cell monolayers were inoculated with serially diluted viruses, incubated for 2 days, and stained with β-galactosidase, and the infectious units were measured and normalized to the level of p24. YU2 was the most infectious virus, producing 8,125 infectious units/ng p24. Viruses produced in the presence of increasing concentrations of dMM (125, 250, and 500 μM) displayed a dose-dependent reduction in TZMbl cell infectivity, yielding 5,980, 4,910, and 4,740 infectious units/ng p24 for YU2.dMM125, YU2.dMM250, and YU2.dMM500, respectively (Fig. 2A and B). Thus, YU2 viruses with more oligomannose and less complex Env glycans are less infectious in TZMbl cells.
FIG 2.
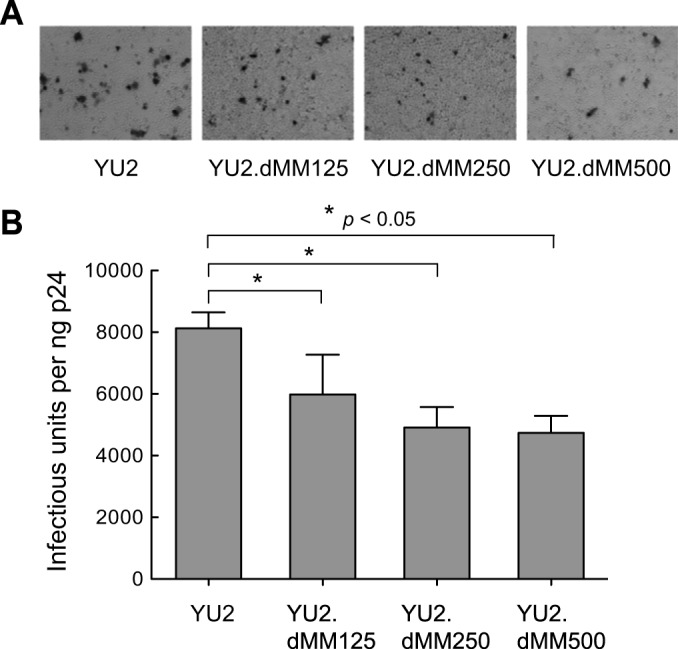
HIV-1 with more oligomannose and less complex Env glycans is less infectious. (A) Microscopy of TZMbl cells exposed to HIV-1 generated in the presence of increasing concentrations of dMM shows a dose-dependent reduction in the number of infected cells in a representative experiment (n = 4). (B) Mean number of infectious units ± SD for viruses that were generated in the presence of the indicated concentrations of dMM and that contained 1 ng p24 (n = 4). Statistical significance is indicated: *, P < 0.05.
HIV-1 with more oligomannose and less complex Env glycans transcytoses across HT-29 epithelial cell monolayers more efficiently.
A key event in HIV-1 mucosal transmission is the translocation of virus across the epithelium. Depending on the site of inoculation, the distinct structural features of mucosal epithelium permit the translocation of HIV-1 by different pathways, including transcytosis through the columnar epithelium in the intestinal, colonic, rectal, and endocervical mucosa and basal columnar cells in the vaginal epithelium (31, 35, 41, 42). To examine the effect of HIV-1 Env glycan moieties on transcytosis, equal amounts of viruses were inoculated onto tight HT-29 cell monolayers, and virus that transcytosed into the basolateral chamber was quantified 2 h later by p24 ELISA. The transcytosis efficiency of YU2.dMM125, YU2.dMM250, and YU2.dMM500 increased 2.4-, 2.7-, and 3.3-fold, respectively, compared with the transcytosis efficiency of YU2 (Fig. 3A). The increased transcytosis of YU2.dMM250 across the HT-29 cell monolayer was significantly diminished by d-mannose (0.25 M; P = 0.002) and IgG antibody against macrophage mannose receptor (anti-MMR; 10 μg/ml; P = 0.002) but only slightly by d-glucose (0.25 M; P = 0.30) and IgG isotype control antibody (10 μg/ml; P = 0.18) (Fig. 3B), indicating that the increased efficiency of transcytosis across the epithelial monolayers was mediated, at least in part, by mannose.
FIG 3.
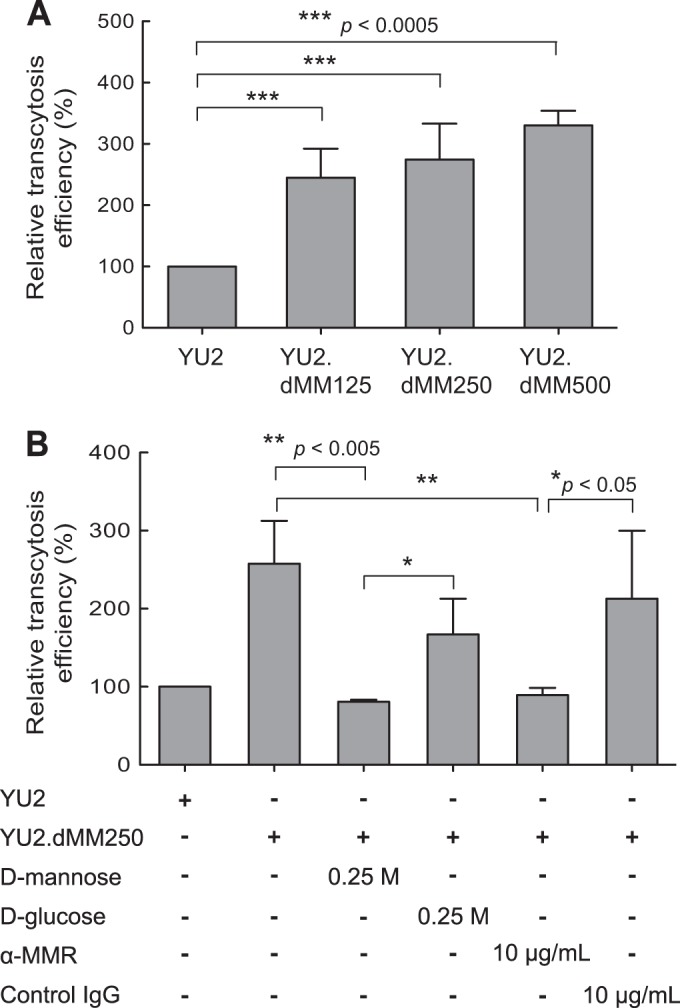
HIV-1 with more oligomannose and less complex Env glycans transcytoses more efficiently across an epithelial cell monolayer. (A) Equal amounts of viruses were inoculated onto tight HT-29 cell monolayers, and 2 h later, virus that transcytosed into the basolateral chamber was quantified by p24 ELISA, with the transcytosis efficiency of YU2 being defined as 100%. Error bars indicate SDs (n = 6). (B) An increased efficiency of transcytosis was mediated by mannose. HIV-1 generated in the presence of dMM (250 μM) was tested for its ability to transcytose across an epithelial monolayer, as described in the legend to panel A, in the presence of d-mannose, d-glucose, anti-macrophage mannose receptor (α-MMR) IgG antibody, or isotype control IgG. The transcytosis efficiency of YU2 was defined to be 100%. Error bars indicate SDs (n = 6). Statistical significance is indicated: *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
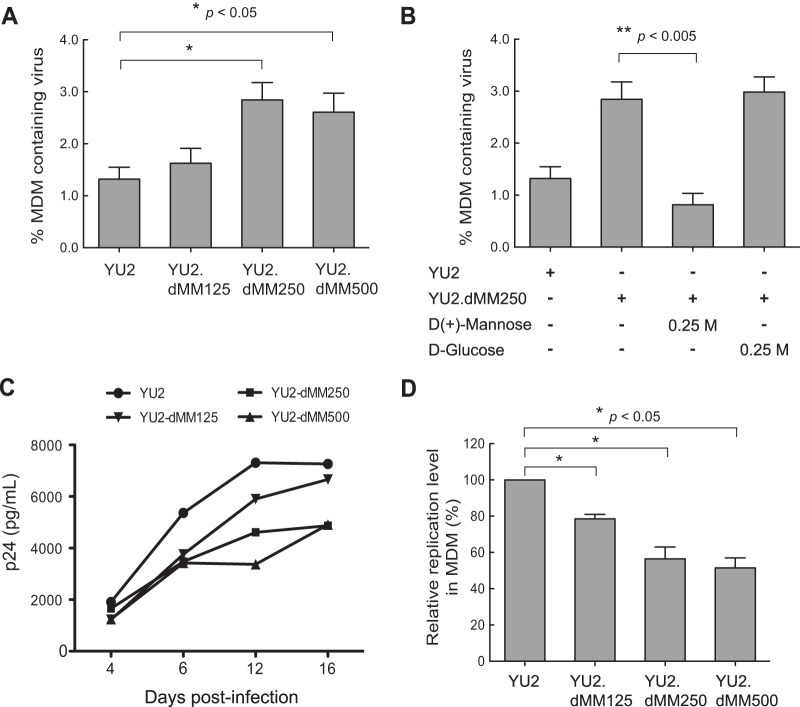
HIV-1 with more oligomannose and less complex Env glycans binds to MDMs more efficiently but displays lower MDM infectivity.
We next examined the effect of HIV-1 Env glycan moieties on HIV-1 binding to and infection of MDMs. Cultures of MDMs were inoculated with viruses produced in various concentrations of dMM and incubated for 2 h at 4°C, after which the MDMs were analyzed for HIV-1 using anti-KC57-FITC by flow cytometry. The proportion of MDMs containing bound HIV-1 were 1.25%, 1.63%, 2.84%, and 2.61% for YU2, YU2.dMM125, YU2.dMM250, and YU2.dMM500, respectively (Fig. 4A). The increased binding of YU2.dMM250 to MDMs was competitively blocked by d-mannose (0.25 M; P = 0.01), but not by d-glucose (0.25 M; P = 0.31) (Fig. 4B). Thus, HIV-1 with more oligomannose and less complex Env glycans binds to MDMs more efficiently, and the enhanced binding is mediated, at least partially, by mannose.
FIG 4.
HIV-1 with more oligomannose and less complex Env glycans binds to MDMs more efficiently but displays lower infectivity. (A) Cultures of MDMs were inoculated with YU2 viruses generated in the presence of different concentrations of dMM, and 2 h later, the cells were analyzed for HIV-1 by flow cytometry using KC57-FITC. Error bars indicate SDs (n = 4). (B) The increased efficiency of HIV-1 binding to MDMs is mediated by mannose. HIV-1 binding to MDMs was evaluated as described in the legend to panel A in the presence of d-mannose or d-glucose, with the binding efficiency of YU2 being defined as 100%. Error bars indicate SDs (n = 6). (C) Replication kinetics of viruses in MDMs. Cultures of MDMs were inoculated with viruses generated in the presence of different concentrations of dMM, and the p24 produced was assayed by ELISA at the indicated time points. Results from a representative experiment (n = 3) with MDMs from different donors are shown. (D) Relative replication levels of viruses in MDMs at 12 days postinoculation, with that of YU2 being defined as 100%. Error bars indicate SDs (n = 3). Statistical significance is indicated: *, P < 0.05; **, P < 0.005.
To assess the effect of variable Env glycans on HIV-1 infection of macrophages, cultures of MDMs were inoculated with viruses produced in increasing concentrations of dMM, and the levels of virus released into the culture supernatant were determined by p24 ELISA at 4-day intervals for 16 days (Fig. 4C). Surprisingly, YU2 displayed the highest level of infection at all time points, whereas YU2.dMM125, YU2.dMM250, and YU2.dMM500 produced progressively reduced levels of viral replication, as shown by the infection kinetics for each virus (Fig. 4C) and the summary of relative replication levels for each virus in MDMs at 12 days postinoculation (n = 3) (Fig. 4D). Thus, HIV-1 with more oligomannose and less complex Env glycans displayed reduced infectivity in MDMs.
HIV-1 with more oligomannose and less complex Env glycans displays impaired infectivity in lymphocytes.
In contrast to macrophages, lymphocytes do not express a mannose receptor; therefore, we anticipated that the Env glycan content would not affect HIV-1 binding to lymphocytes. Cultures of PHA-stimulated PBLs were inoculated with viruses produced in various concentrations of dMM, incubated for 2 h at 4°C, and analyzed for HIV-1-containing lymphocytes using KC57-FITC antibody by flow cytometry. Indeed, a high mannose content had no effect on the binding of HIV-1 to lymphocytes (data not shown). We also examined the effect of variable Env glycan contents on the infection of lymphocytes. PBLs were inoculated with viruses, and viral replication was assayed by p24 ELISA at 4-day intervals for 16 days (Fig. 5A). YU2 displayed the highest levels of infection at all time points, and YU2.dMM125, YU2.dMM250, and YU2.dMM500 produced progressively reduced levels of infection, as shown by a representative replication kinetics for each virus (Fig. 5A) and the relative replication levels in PBLs at 12 days postinoculation (n = 3) (Fig. 5B). Thus, increased amounts of oligomannose and reduced amounts of complex glycans on HIV-1 Env limit viral replication in both myeloid and lymphoid target cells in a dose-dependent manner.
FIG 5.
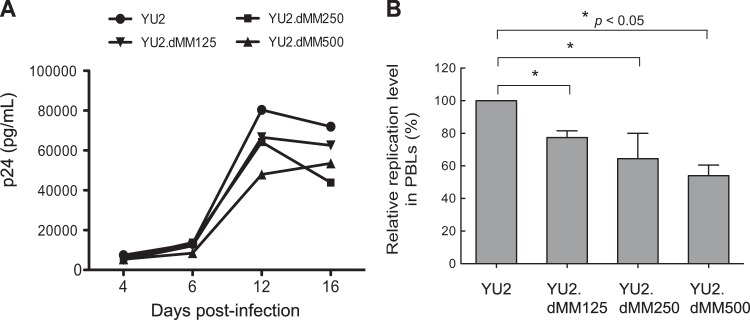
Viruses with more oligomannose and less complex Env glycans display impaired infectivity in lymphocytes. (A) Cultures of PHA-stimulated PBLs were inoculated with YU2 generated in the presence of different concentrations of dMM, and p24 production was determined at the indicated time points. Results are from a representative experiment (n = 3). (B) Relative mean ± SD replication levels of viruses in PBLs from three separate donors at 12 days postinoculation, with that of YU2 being defined as 100%. Error bars indicate SDs (n = 3). Statistical significance is indicated: *, P < 0.05.
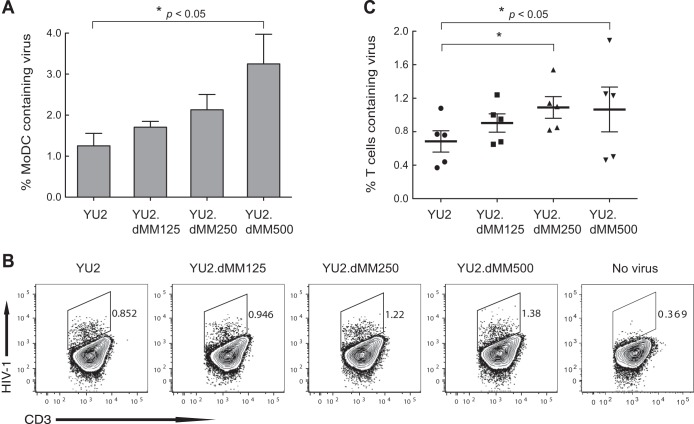
HIV-1 with more oligomannose and less complex Env glycans is captured by MoDCs more efficiently and displays enhanced trans-infection of PBLs.
To examine the effect of Env glycan moieties on HIV-1 capture by DCs, MoDC cultures were inoculated with viruses, incubated for 2 h at 37°C, and analyzed by flow cytometry for HIV-1 containing MoDCs using KC57-FITC antibody. The proportions of MoDCs that contained HIV-1 were 1.25%, 1.71%, 2.13%, and 3.24% for YU2, YU2.dMM125, YU2.dMM250, and YU2.dMM500, respectively (Fig. 6A). Again, the increased YU2.dMM250 binding to MoDCs was competitively blocked by d-mannose (0.25 M) (data not shown). These findings indicate that HIV-1 with more oligomannose and less complex Env glycans is captured by DCs more efficiently, and the enhanced capture is mediated by mannose.
FIG 6.
HIV-1 with more oligomannose and less complex Env glycans is captured by MoDCs more efficiently and displays enhanced trans-infection of primary lymphocytes. (A) Cultures of MoDCs were inoculated with viruses generated in the presence of different concentrations of dMM, incubated for 2 h, and analyzed by flow cytometry for HIV-1-containing MoDCs using KC57 antibody. Error bars indicate SEMs (n = 3). (B and C) trans-Infection of PBLs by virus-containing MoDCs. MoDC cultures were inoculated with viruses as described in the legend to panel A, cocultured with PHA-stimulated PBLs for 4 days, and analyzed by flow cytometry for HIV-1-positive CD3+ cells. Results in panel B are representative of those from 5 experiments with PBLs from separate donors. Values in panel C are means ± SEMs (n = 5). Statistical significance is indicated: *, P < 0.05.
To determine the effect of Env glycans on HIV-1 trans-infection of lymphocytes via DCs, cultures of MoDCs were inoculated with viruses, incubated for 2 h, washed 3 times with medium, cocultured with PHA-stimulated PBLs for 4 days, and then analyzed by flow cytometry for lymphocytes containing HIV-1 (Fig. 6B and C). As shown in representative experiments (n = 5, in which both MoDCs and PBLs were derived and isolated from different donors) (Fig. 6B), the percentages of lymphocytes infected with HIV-1 increased progressively for viruses generated in the presence of increasing concentrations of dMM. The mean proportions of lymphocytes infected by HIV-1 were 0.66%, 0.76%, 0.96%, and 0.99% for YU2, YU2.dMM125, YU2.dMM250, and YU2.dMM500, respectively (Fig. 6C), indicating that HIV-1 with more oligomannose and less complex Env glycans trans-infects lymphocytes via DCs more efficiently.
HIV-1 with more oligomannose and less complex Env glycans infects human intestinal tissue less efficiently.
Having shown that Env glycan moieties impact each major event in HIV-1 transmission in vitro, we next examined the effect of Env glycans on HIV-1 infection of human intestinal mucosa ex vivo to more closely mimic HIV-1 mucosal transmission. Viruses were inoculated onto the apical surface of explanted normal human intestinal mucosa for 2 h. After washing and trypsin treatment to remove viruses on the mucosal surface that had not entered the mucosa, tissue specimens with equal wet weights were cultured for 3 days and viral replication was assayed by p24 ELISA. In a representative experiment (n = 4) using tissue from different donors, p24 values at day 3 were 191, 91, 79, and 26 pg/ml for YU2, YU2.dMM125, YU2.dMM250, and YU2.dMM500, respectively (Fig. 7A). The p24 values at day 0 were similar for all viruses except YU2.dMM500, indicating that the wash and trypsin treatment had removed most the viruses on the tissue surface (Fig. 7A). Interestingly, the mean p24 values for YU2.dMM125, YU2.dMM250, and YU2.dMM500 were similar, being approximately 50% of that for YU2 (Fig. 7B). These results indicate that viruses with more oligomannose and less complex Env glycans are less infectious in human intestinal mucosa.
FIG 7.
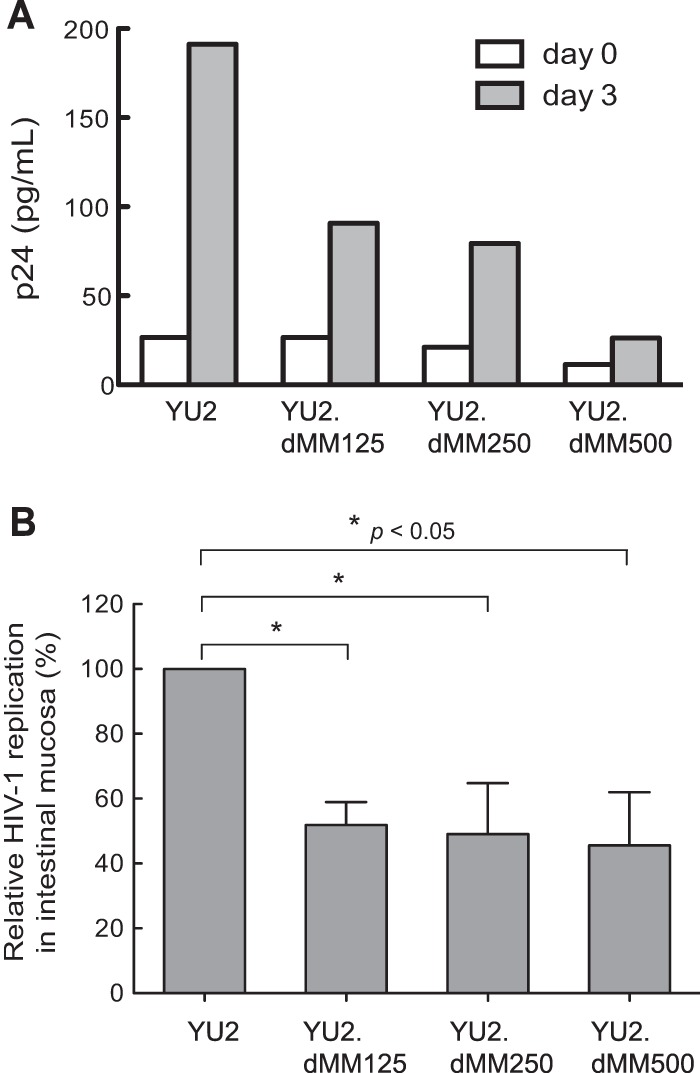
HIV-1 with more oligomannose and less complex Env glycans displays impaired infectivity in human intestinal mucosa. Viruses generated in the presence of different concentrations of dMM were inoculated onto the apical surface of explanted intestinal mucosa for 2 h, the explants were treated with trypsin and washed, and tissue specimens of equal wet weights were cultured for 3 days, after which p24 levels were determined by ELISA. (A) Level of p24 production in a representative intestinal tissue specimen (n = 4). (B) Replication levels of viruses from the assay whose results are presented in panel A in intestinal mucosa at 3 days postinoculation relative to the replication level of YU2, defined to be 100%. Error bars indicate SEMs (n = 4). Statistical significance is indicated: *, P < 0.05.
DISCUSSION
Our study focused on the role of the types of Env glycans, rather than the detailed glycan structure, on the major events of HIV-1 transmission. To produce viruses with progressively more oligomannose and less complex glycans on the Env glycan chains, HIV-1 YU2 was generated in the presence of increasing concentrations of the Golgi apparatus α-mannosidase inhibitor dMM, and the resultant viruses were progressively less infectious in TZMbl cells. These findings are consistent with the results of Montefiori et al. (14), who used an array of N-glycan processing inhibitors to generate HIV-1 isolates with attenuated infectivity in multiple T-cell lines. Similarly, Binley et al. (13) reported that HIV-1 pseudoviruses produced in 293S GnTI−/− cells, which lack the GlcNAc transferase I enzyme, the enzyme that initiates the conversion of oligonucleotide-mannose N-glycans into complex N-glycans, were markedly less infectious in CF2.Th.CD4.CCR5 cells. However, others have reported that the infectivity of HIV-1 produced in 293T cells in the presence of the glycan-processing inhibitor kifunensine or in 293S GnTI−/− cells in TZMbl cells was not affected (13, 43, 44).
We investigated the role of Env glycan moieties on HIV-1 entry events, beginning with the impact on transcytosis across epithelial cells. We used HT-29 model epithelial cells under a neutral pH condition. Other factors, such as acidic pH, primary human mucosal epithelium, and different virus isolates, will be taken into consideration in future studies. Viruses with more oligomannose and less complex glycans transcytosed across an epithelial cell monolayer more efficiently, and the enhanced transcytosis was mediated by mannose through engagement of the MMR, reflected in the ability of free d-mannose and anti-MMR antibody to block HIV-1 transcytosis. In this connection, N-glycan moieties on the rat neonatal Fc receptor (FcRn) have been shown to determine the directional transport of IgG mediated by FcRn, with transcytosis occurring mainly in an apical-to-basolateral direction (45), but our study shows the N-glycans impact on transcytosis of apically applied cargo, specifically, HIV-1, through model epithelium.
We also determined the effect of Env glycans on target cell binding and infectivity. Viruses with more oligomannose and less complex Env glycans bound MDMs more efficiently in a mannose-dependent manner. Despite enhanced binding, however, HIV-1 with more oligomannose and less complex Env glycans exhibited reduced infectivity in MDMs. Oligomannose glycans and complex glycans on the HIV-1 Env generally form two distinct regions: the former is present in the densely glycosylated gp120 outer domain, and the latter is present on the more exposed CD4 receptor-binding sites and the hypervariable loops (5, 23). Thus, our findings suggest that complex glycan regions are necessary for optimal infectivity in MDMs. In this regard, the mannose receptor on monocytes and macrophages binds to carbohydrates on a wide array of pathogens and mediates phagocytosis, endocytosis, and antigen presentation (46 – 48). The macrophage mannose receptor is involved in HIV-1 binding and entry (49, 50), likely through interaction with Env oligomannose glycan clusters, which are usually present distal to the CD4 binding site (5, 23). Approximately 60% of the initial association between HIV-1 and MDMs is mediated by the mannose receptor (49); however, mannose receptor-mediated binding does not lead to productive infection in MDMs, alveolar macrophages, or microglia (50). Thus, in the presence of more oligomannose and less complex Env glycans, HIV-1 binds to MDMs more efficiently but has lower infectivity.
In contrast to macrophages and DCs, lymphocytes do not express C-type lectin receptors and Env glycan moieties had no effect on HIV-1 binding to PBLs (data not shown). However, HIV-1 with more oligomannose and less complex Env glycans displayed reduced infectivity in PBLs. In agreement with this finding, the infectivity of HIV-1 produced in CD4+ T cells from subjects with a congenital disorder of glycosylation type IIb was reduced 50 to 80% compared with the infectivity of virus produced in cells from healthy subjects (29). This disorder is due to a defect in the gene encoding mannosyl-oligosaccharide glucosidase, the first enzyme in the pathway that processes the N-linked oligosaccharide precursor to its mature structure (51, 52). Together, our findings suggest that complex glycans and mature oligosaccharide structures on HIV-1 Env are crucial for optimal HIV-1 infection of CD4+ T cells.
Dendritic cells play a critical role in HIV-1 transmission (30, 32, 38, 40, 42, 53 – 56). In support of this function, we report that HIV-1 with more oligomannose and less complex Env glycans is more efficiently captured by MoDCs and displays enhanced trans-infection of T cells. Oligomannose glycan clusters are recognized by DC C-type lectin receptors, including DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN), mannose receptor, and DC immunoreceptor (DCIR), and enhance HIV-1 attachment to DCs and trans-infection of CD4+ T cells (57 – 62). N-Linked glycans also form the binding sites for the receptor langerin on Langerhans cells (63, 64). Notably, viruses that contain only oligomannose Env N-glycans also display more efficient capture by MoDCs and DC-SIGN-positive (DC-SIGN+) Raji cells (44). Thus, the enhanced trans-infection is likely due to the more efficient binding of HIV-1 with more oligomannose and less complex Env glycans to MoDCs. However, MoDC- and DC-SIGN+ Raji cell-captured viruses that contained only oligomannose Env N-glycans were substantially less able to trans-infect TZMbl cells (44), suggesting the possibility that the altered glycan phenotype that promoted virus binding to DC-SIGN restricted transmission of the virus to target cells (44), possibly due to stronger binding to DC-SIGN. The difference between this finding and the finding reported here could be due to the different target cells used, the presence of only oligomannose N-glycans on Env versus the mixture of oligomannose and complex Env glycans, and/or the different viral strains used.
To more closely mimic HIV-1 mucosal transmission, we examined the impact of Env glycan moieties on HIV-1 infection in human intestinal mucosa ex vivo. Use of this model allowed us to assess the composite effects of glycan diversity on HIV-1 infection at the site of virus inoculation. HIV-1 with progressively more oligomannose and less complex Env glycans displayed a progressive reduction in mucosal tissue infection, consistent with the reduced infectivity in macrophages and lymphocytes by viruses whose Env contains more oligomannose and less complex glycans. These findings indicate that, in addition to affecting the exposure of antigenic sites on Env and, consequently, immunogenicity, host-generated carbohydrates, specifically, N-linked oligosaccharide processing into mature oligosaccharide structures on Env, impact HIV-1 infection. However, viruses whose Env contained more oligomannose and less complex glycans displayed enhanced epithelial transcytosis and macrophage and DC binding, indicating the importance of infectivity in assessing the biological impact of Env carbohydrate diversity. Here we utilized in vitro model systems to dissect the effect of the type of glycan on HIV-1 transmission/infectivity. HIV-1 transmission/infectivity in vivo is highly complex; consequently, studies that utilize primary ex vivo human mucosal tissues and nonhuman primate models will further elucidate the impact of glycosylation on HIV-1 transmission/infectivity. Also, the underlying mechanisms of the discordant effects of N-linked Env glycans on the major steps in HIV-1 transmission warrant further investigation. Although our study will not lead immediately to new vaccine strategies, our findings underscore the role of Env glycans in HIV-1 infection and transmission and suggest that increasing the oligomannose content in the Env antigen in a prophylactic vaccine and using oligomannose as an adjuvant in a therapeutic vaccine might improve vaccine efficacy. Thus, modulation of Env glycan moieties should be taken into consideration in devising therapeutic or prophylactic vaccine strategies to protect against HIV-1 infection.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI093151 and AI106395 (to R.S.) and grants AI083027, RR-20136, and DK064400 (to P.D.S.); the UAB Center for AIDS Research (CFAR, NIH P30 AI027767) and Comprehensive Cancer Center (NIH P30 CA013148) Pilot Grant Program (to R.S.); the Ministry of School, Youth, and Sport (CZ.1.07/2.3.00/20.0164), Czech Republic (to M.R.); a UAB School of Medicine pilot grant (to J.N.); a grant from Immunology, Autoimmunity and Transplantation Strategic Planning (IAT) and amfAR, the Foundation for AIDS Research (108015-49-RGRL; to P.D.S.); and a grant from the Research Service of the U.S. Department of Veterans Affairs (to P.D.S.).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 1 October 2014
REFERENCES
- 1.Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk HD, Garten W. 1992. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 360:358–361. 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- 2.Fennie C, Lasky LA. 1989. Model for intracellular folding of the human immunodeficiency virus type 1 gp120. J. Virol. 63:639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moulard M, Decroly E. 2000. Maturation of HIV envelope glycoprotein precursors by cellular endoproteases. Biochim. Biophys. Acta 1469:121–132. 10.1016/S0304-4157(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 4.Go EP, Irungu J, Zhang Y, Dalpathado DS, Liao HX, Sutherland LL, Alam SM, Haynes BF, Desaire H. 2008. Glycosylation site-specific analysis of HIV envelope proteins (JR-FL and CON-S) reveals major differences in glycosylation site occupancy, glycoform profiles, and antigenic epitopes' accessibility. J. Proteome Res. 7:1660–1674. 10.1021/pr7006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Borchers C, Bienstock RJ, Tomer KB. 2000. Mass spectrometric characterization of the glycosylation pattern of HIV-gp120 expressed in CHO cells. Biochemistry 39:11194–11204. 10.1021/bi000432m. [DOI] [PubMed] [Google Scholar]
- 6.Geyer H, Holschbach C, Hunsmann G, Schneider J. 1988. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J. Biol. Chem. 263:11760–11767. [PubMed] [Google Scholar]
- 7.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019–2022. 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 9.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 10.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557. 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar-Gonzalez JF, Bailes E, Pham KT, Salazar MG, Guffey MB, Keele BF, Derdeyn CA, Farmer P, Hunter E, Allen S, Manigart O, Mulenga J, Anderson JA, Swanstrom R, Haynes BF, Athreya GS, Korber BT, Sharp PM, Shaw GM, Hahn BH. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952–3970. 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinones-Kochs MI, Buonocore L, Rose JK. 2002. Role of N-linked glycans in a human immunodeficiency virus envelope glycoprotein: effects on protein function and the neutralizing antibody response. J. Virol. 76:4199–4211. 10.1128/JVI.76.9.4199-4211.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binley JM, Ban YE, Crooks ET, Eggink D, Osawa K, Schief WR, Sanders RW. 2010. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J. Virol. 84:5637–5655. 10.1128/JVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montefiori DC, Robinson WE, Jr, Mitchell WM. 1988. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U. S. A. 85:9248–9252. 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolk T, Schreiber M. 2006. N-Glycans in the gp120 V1/V2 domain of the HIV-1 strain NL4-3 are indispensable for viral infectivity and resistance against antibody neutralization. Med. Microbiol. Immunol. 195:165–172. 10.1007/s00430-006-0016-z. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Jin W, Hu K, Luo S, Du T, Griffin GE, Shattock RJ, Hu Q. 2012. Highly conserved HIV-1 gp120 glycans proximal to CD4-binding region affect viral infectivity and neutralizing antibody induction. Virology 423:97–106. 10.1016/j.virol.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Nie J, Prochnow C, Truong C, Jia Z, Wang S, Chen XS, Wang Y. 2013. A systematic study of the N-glycosylation sites of HIV-1 envelope protein on infectivity and antibody-mediated neutralization. Retrovirology 10:14. 10.1186/1742-4690-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francois KO, Balzarini J. 2011. The highly conserved glycan at asparagine 260 of HIV-1 gp120 is indispensable for viral entry. J. Biol. Chem. 286:42900–42910. 10.1074/jbc.M111.274456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malenbaum SE, Yang D, Cavacini L, Posner M, Robinson J, Cheng-Mayer C. 2000. The N-terminal V3 loop glycan modulates the interaction of clade A and B human immunodeficiency virus type 1 envelopes with CD4 and chemokine receptors. J. Virol. 74:11008–11016. 10.1128/JVI.74.23.11008-11016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen PJ, Herschhorn A, Haim H, Salas I, Gu C, Sodroski J, Gabuzda D. 2014. Loss of a conserved N-linked glycosylation site in the simian immunodeficiency virus envelope glycoprotein V2 region enhances macrophage tropism by increasing CD4-independent cell-to-cell transmission. J. Virol. 88:5014–5028. 10.1128/JVI.02785-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kornfeld R, Kornfeld S. 1985. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54:631–664. 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 22.Crispin MD, Ritchie GE, Critchley AJ, Morgan BP, Wilson IA, Dwek RA, Sim RB, Rudd PM. 2004. Monoglucosylated glycans in the secreted human complement component C3: implications for protein biosynthesis and structure. FEBS Lett. 566:270–274. 10.1016/j.febslet.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 23.Scanlan CN, Offer J, Zitzmann N, Dwek RA. 2007. Exploiting the defensive sugars of HIV-1 for drug and vaccine design. Nature 446:1038–1045. 10.1038/nature05818. [DOI] [PubMed] [Google Scholar]
- 24.Iacob RE, Perdivara I, Przybylski M, Tomer KB. 2008. Mass spectrometric characterization of glycosylation of hepatitis C virus E2 envelope glycoprotein reveals extended microheterogeneity of N-glycans. J. Am. Soc. Mass. Spectrom. 19:428–444. 10.1016/j.jasms.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanisch FG, Breloy I. 2009. Protein-specific glycosylation: signal patches and cis-controlling peptidic elements. Biol. Chem. 390:619–626. 10.1515/BC.2009.043. [DOI] [PubMed] [Google Scholar]
- 26.Doores KJ, Bonomelli C, Harvey DJ, Vasiljevic S, Dwek RA, Burton DR, Crispin M, Scanlan CN. 2010. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc. Natl. Acad. Sci. U. S. A. 107:13800–13805. 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonomelli C, Doores KJ, Dunlop DC, Thaney V, Dwek RA, Burton DR, Crispin M, Scanlan CN. 2011. The glycan shield of HIV is predominantly oligomannose independently of production system or viral clade. PLoS One 6:e23521. 10.1371/journal.pone.0023521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auwerx J, Francois KO, Covens K, Van Laethem K, Balzarini J. 2008. Glycan deletions in the HIV-1 gp120 V1/V2 domain compromise viral infectivity, sensitize the mutant virus strains to carbohydrate-binding agents and represent a specific target for therapeutic intervention. Virology 382:10–19. 10.1016/j.virol.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Sadat MA, Moir S, Chun TW, Lusso P, Kaplan G, Wolfe L, Memoli MJ, He M, Vega H, Kim LJ, Huang Y, Hussein N, Nievas E, Mitchell R, Garofalo M, Louie A, Ireland DC, Grunes C, Cimbro R, Patel V, Holzapfel G, Salahuddin D, Bristol T, Adams D, Marciano BE, Hegde M, Li Y, Calvo KR, Stoddard J, Justement JS, Jacques J, Long Priel DA, Murray D, Sun P, Kuhns DB, Boerkoel CF, Chiorini JA, Di Pasquale G, Verthelyi D, Rosenzweig SD. 2014. Glycosylation, hypogammaglobulinemia, and resistance to viral infections. N. Engl. J. Med. 370:1615–1625. 10.1056/NEJMoa1302846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen R, Kappes JC, Smythies LE, Richter HE, Novak L, Smith PD. 2014. Vaginal myeloid dendritic cells transmit founder HIV-1. J. Virol. 88:7683–7688. 10.1128/JVI.00766-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hladik F, McElrath MJ. 2008. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8:447–457. 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen R, Richter HE, Smith PD. 2011. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am. J. Reprod. Immunol. 65:261–267. 10.1111/j.1600-0897.2010.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH. 1991. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J. Virol. 65:3973–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, Sankaran-Walters S, Dandekar S, Clapham PR, Smythies LE, Smith PD. 2009. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J. Virol. 83:3258–3267. 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen R, Drelichman ER, Bimczok D, Ochsenbauer C, Kappes JC, Cannon JA, Tudor D, Bomsel M, Smythies LE, Smith PD. 2010. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J. Immunol. 184:3648–3655. 10.4049/jimmunol.0903346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raska M, Takahashi K, Czernekova L, Zachova K, Hall S, Moldoveanu Z, Elliott MC, Wilson L, Brown R, Jancova D, Barnes S, Vrbkova J, Tomana M, Smith PD, Mestecky J, Renfrow MB, Novak J. 2010. Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J. Biol. Chem. 285:20860–20869. 10.1074/jbc.M109.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen R, Meng G, Ochsenbauer C, Clapham PR, Grams J, Novak L, Kappes JC, Smythies LE, Smith PD. 2011. Stromal down-regulation of macrophage CD4/CCR5 expression and NF-kappaB activation mediates HIV-1 non-permissiveness in intestinal macrophages. PLoS Pathog. 7:e1002060. 10.1371/journal.ppat.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen R, Smythies LE, Clements RH, Novak L, Smith PD. 2010. Dendritic cells transmit HIV-1 through human small intestinal mucosa. J. Leukoc. Biol. 87:663–670. 10.1189/jlb.0909605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896–1905. 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen R, Smith PD. 2014. Mucosal correlates of protection in HIV-1-exposed sero-negative persons. Am. J. Reprod. Immunol. 72:219–227. 10.1111/aji.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bomsel M, Alfsen A. 2003. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell Biol. 4:57–68. 10.1038/nrm1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen R, Richter HE, Smith PD. 2014. Interactions between HIV-1 and mucosal cells in the female reproductive tract. Am. J. Reprod. Immunol. 71:608–617. 10.1111/aji.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Montfort T, Eggink D, Boot M, Tuen M, Hioe CE, Berkhout B, Sanders RW. 2011. HIV-1 N-glycan composition governs a balance between dendritic cell-mediated viral transmission and antigen presentation. J. Immunol. 187:4676–4685. 10.4049/jimmunol.1101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eggink D, Melchers M, Wuhrer M, van Montfort T, Dey AK, Naaijkens BA, David KB, Le Douce V, Deelder AM, Kang K, Olson WC, Berkhout B, Hokke CH, Moore JP, Sanders RW. 2010. Lack of complex N-glycans on HIV-1 envelope glycoproteins preserves protein conformation and entry function. Virology 401:236–247. 10.1016/j.virol.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo TT, de Muinck EJ, Claypool SM, Yoshida M, Nagaishi T, Aveson VG, Lencer WI, Blumberg RS. 2009. N-Glycan moieties in neonatal Fc receptor determine steady-state membrane distribution and directional transport of IgG. J. Biol. Chem. 284:8292–8300. 10.1074/jbc.M805877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fearon DT, Locksley RM. 1996. The instructive role of innate immunity in the acquired immune response. Science 272:50–53. 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 47.Stahl PD, Ezekowitz RA. 1998. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10:50–55. 10.1016/S0952-7915(98)80031-9. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi K, Donovan MJ, Rogers RA, Ezekowitz RA. 1998. Distribution of murine mannose receptor expression from early embryogenesis through to adulthood. Cell Tissue Res. 292:311–323. 10.1007/s004410051062. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen DG, Hildreth JE. 2003. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur. J. Immunol. 33:483–493. 10.1002/immu.200310024. [DOI] [PubMed] [Google Scholar]
- 50.Trujillo JR, Rogers R, Molina RM, Dangond F, McLane MF, Essex M, Brain JD. 2007. Noninfectious entry of HIV-1 into peripheral and brain macrophages mediated by the mannose receptor. Proc. Natl. Acad. Sci. U. S. A. 104:5097–5102. 10.1073/pnas.0611263104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volker C, De Praeter CM, Hardt B, Breuer W, Kalz-Fuller B, Van Coster RN, Bause E. 2002. Processing of N-linked carbohydrate chains in a patient with glucosidase I deficiency (CDG type IIb). Glycobiology 12:473–483. 10.1093/glycob/cwf050. [DOI] [PubMed] [Google Scholar]
- 52.De Praeter CM, Gerwig GJ, Bause E, Nuytinck LK, Vliegenthart JF, Breuer W, Kamerling JP, Espeel MF, Martin JJ, De Paepe AM, Chan NW, Dacremont GA, Van Coster RN. 2000. A novel disorder caused by defective biosynthesis of N-linked oligosaccharides due to glucosidase I deficiency. Am. J. Hum. Genet. 66:1744–1756. 10.1086/302948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piguet V, Steinman RM. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28:503–510. 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, KewalRamani VN. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859–868. 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257–270. 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, Anton PA, Lee B. 2005. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J. Virol. 79:5762–5773. 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feinberg H, Mitchell DA, Drickamer K, Weis WI. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163–2166. 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 58.Lin G, Simmons G, Pohlmann S, Baribaud F, Ni H, Leslie GJ, Haggarty BS, Bates P, Weissman D, Hoxie JA, Doms RW. 2003. Differential N-linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC-SIGN and DC-SIGNR. J. Virol. 77:1337–1346. 10.1128/JVI.77.2.1337-1346.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turville S, Wilkinson J, Cameron P, Dable J, Cunningham AL. 2003. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J. Leukoc. Biol. 74:710–718. 10.1189/jlb.0503208. [DOI] [PubMed] [Google Scholar]
- 60.Geijtenbeek TBH, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GCF, Middel J, Cornelissen ILMHA, Nottet HSLM, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597. 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 61.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135–144. 10.1016/S1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 62.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295–1297. 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 63.de Witte L, Nabatov A, Geijtenbeek TB. 2008. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol. Med. 14:12–19. 10.1016/j.molmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 64.de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MA, de Gruijl T, Piguet V, van Kooyk Y, Geijtenbeek TB. 2007. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 13:367–371. 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]