Abstract
The plant-pathogenic bacterium Xylella fastidiosa is restricted to the xylem vessel environment, where mineral nutrients are transported through the plant host; therefore, changes in the concentrations of these elements likely impact the growth and virulence of this bacterium. Twitching motility, dependent on type IV pili (TFP), is required for movement against the transpiration stream that results in basipetal colonization. We previously demonstrated that calcium (Ca) increases the motility of X. fastidiosa, although the mechanism was unknown. PilY1 is a TFP structural protein recently shown to bind Ca and to regulate twitching and adhesion in bacterial pathogens of humans. Sequence analysis identified three pilY1 homologs in X. fastidiosa (PD0023, PD0502, and PD1611), one of which (PD1611) contains a Ca-binding motif. Separate deletions of PD0023 and PD1611 resulted in mutants that still showed twitching motility and were not impaired in attachment or biofilm formation. However, the response of increased twitching at higher Ca concentrations was lost in the pilY1-1611 mutant. Ca does not modulate the expression of any of the X. fastidiosa PilY1 homologs, although it increases the expression of the retraction ATPase pilT during active movement. The evidence presented here suggests functional differences between the PilY1 homologs, which may provide X. fastidiosa with an adaptive advantage in environments with high Ca concentrations, such as xylem sap.
INTRODUCTION
Xylella fastidiosa is a Gram-negative, insect-vectored plant-pathogenic bacterium that is the causal agent of a number of economically important diseases, most typically causing leaf scorch symptoms that are associated with bacterial colonization of xylem vessels (1). The development of diseases caused by X. fastidiosa depends on the ability of the bacterium to spread and become systemic within the xylem vessels (2). The formation of bacterial biofilm during this process is thought to produce disease symptoms (3). X. fastidiosa produces two types of fimbrial adhesins, type I pili (chaperone-usher type) (4) and type IV pili (TFP), both located at one pole of the cell (5). Both types of pili are involved in biofilm formation, attachment of the cell to the substrate and other cells, and motility; however, the type I pilus is required primarily for cell anchoring, whereas the TFP functions mainly as a facilitator of twitching motility and cell autoaggregation (5–7). Twitching motility, mediated by TFP, is the sole means of motility in X. fastidiosa and has been demonstrated in microfluidic chambers and in planta, where it allows cell movement against the transpiration stream for basipetal migration and long-distance colonization (5).
Twitching motility is characterized by extension, attachment to surfaces, and retraction of the TFP, which pulls the cell forward (8). TFP and twitching motility have been observed in other Gram-negative bacteria, including mammalian pathogens, such as Pseudomonas aeruginosa (9), Neisseria meningitidis (10, 11), Neisseria gonorrhoeae (12, 13), and Kingella kingae (14), and plant pathogens, such as Ralstonia solanacearum (15), Acidovorax citrulli (16), Xanthomonas spp. (17–20), and Pseudomonas spp. (21–23). Twitching motility is a key virulence trait for many of these bacteria, and besides this function, TFP are important in cell attachment for epiphytic or endophytic colonization, aggregation, biofilm formation, and DNA transformation (24).
Several genes involved in TFP biogenesis, function, and regulation have been identified by mutagenesis of model organisms such as Pseudomonas aeruginosa (8, 24). The filamentous structure of TFP is composed of numerous units of a small protein called pilin, encoded by pilA, which requires the processing of the peptidase/N-methylase PilD to become mature. Two ATPases, PilB and PilT, are responsible for the assembly and disassembly of the polymeric structure for the extension and retraction of the pilus, respectively, while the porin PilQ is implicated in their secretion (8). It has been shown in Neisseria spp. that pilus extension and retraction are linked to PilC (referred to as PilY1 in other species), a protein predicted to be at the tip of the TFP. Although PilC is dispensable for pilus biogenesis, transcriptional regulation of pilC in N. meningitidis has been directly correlated with the level of cell piliation (11). The model based on these findings proposed that pilC upregulation counteracts the expression of pilT, keeping the pilus extended by antagonizing its retractile action, while pilC downregulation allows PilT function (11).
In X. fastidiosa, many of the genes involved in TFP function and structure have also been identified. Mutation of the type IV pilus genes pilQ, pilB, fimT, pilX, and pilO prevented TFP production and motility in vitro (5, 25), and mutation of one pilY1 homolog (PD0023) caused diminished motility (25, 26). Additionally, different regulatory systems have been implicated in twitching modulation, such as the quorum-sensing signaling molecule diffusible signal factor (DSF), which represses motility (27); pilR-pilS, a two-component regulatory system that activates pilA transcription (25); and the chemosensory Pil-Chp operon, which regulates TFP production and response (28). Furthermore, disruption of genes implicated in the production or function of TFP in X. fastidiosa resulted in delayed and less-severe symptoms in planta (28).
X. fastidiosa is restricted to the xylem vessel environment, where mineral nutrients are transported through the plant host; therefore, it is likely that changes in the concentrations of these elements impact the growth and virulence of this bacterium. Previously, our research group reported that calcium (Ca) increases biofilm formation and twitching motility speed (29), indicating a role for Ca in modulating these virulence traits. The present study was initiated in order to understand the molecular mechanism mediating the Ca-dependent increase in twitching motility in X. fastidiosa. In particular, it focuses on the roles of the three pilY1 homologs, PD0023, PD0502, and PD1611. Here the requirement for a single pilY1 homolog (PD1611), containing a C-terminal 9-amino-acid Ca-binding motif, for the regulation of Ca-dependent twitching motility is reported.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Xylella fastidiosa strain Temecula (30) was used as the wild-type (WT) strain. The Temecula mutants used in this study are shown in Table 1. All X. fastidiosa strains were grown on PW solid medium (31) or in PD2 liquid medium (32) at 28°C. Bacterial stocks were stored in liquid PD2 medium with 20% glycerol at −80°C.
TABLE 1.
Xylella fastidiosa strain Temecula mutants used in this study
| Mutated gene | Locus tag | Strain characteristic | Reference |
|---|---|---|---|
| rpfF | PD0407 | Defective in DSF production | 62 |
| rpfC | PD0406 | Defective in DSF sensing | 63 |
| fimA | PD0062 | Lacks type I pili | 5 |
| hxfA | PD1792 | Lacks hemagglutinin A | 59 |
| pilY1-0023 (mutant TM14) | PD0023 | Lacks pilY1 homolog PD0023 | 25 |
| pilY1-1611 | PD1611 | Lacks pilY1 homolog PD1611 | This study |
Quantification of twitching motility. (i) Agar plates.
Twitching motility was quantified by measuring the peripheral fringe widths of bacterial colonies grown on agar plates. Bacterial cells were spotted in quadruplicate onto three independent agar plates (n = 12) and were incubated for 5 days. The peripheral fringe width was quantified via microscopy with a 40× phase-contrast objective on an Eclipse Ti inverted microscope (Nikon, Melville, NY) using NIS-Elements software, version 3.01 (Nikon). Three measurements were taken for each colony, for a total of 36 measurements per treatment. The twitching motility of X. fastidiosa was assessed on unsupplemented PW agar plates and PW plates supplemented with either 0.25 mM CuSO4, 2.0 mM MgSO4, 1.5 mM FeSO4, 1.0 mM ZnSO4, 2 mM CaCl2, 1.5 mM EGTA (an extracellular Ca chelator), or 100 μM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, tetra(acetoxymethyl ester) (BAPTA/AM), an intracellular Ca chelator.
(ii) Microfluidic chambers.
Microfluidic chambers were used as described previously (33) to assess the twitching speeds of the X. fastidiosa pilY1-1611 and pilY1-0023 mutants (the latter was identified previously as mutant TM14 [25]) with different Ca concentrations. Briefly, microfluidic chambers consist of two parallel independent channels with separate inlets for bacteria and medium. Microfluidic chambers were mounted onto an Eclipse Ti inverted microscope (Nikon, Melville, NY) and were observed with a 40× phase-contrast objective to monitor the cell speed. Cells were introduced into the chambers and were incubated for 2 h before time lapse images were recorded with a Nikon (Melville, NY) DS-Q digital camera every 30 s for 1 h. For the experiments with Ca chelators (BAPTA/AM or EGTA), cells were initially incubated in unsupplemented medium. One day after inoculation, when twitching was observed, the medium was supplemented with the chelators until the end of the experiment, and the speed of the cells was calculated every 2 h for 14 h. The movement of X. fastidiosa cells was quantified by tracking the cells' positions using NIS-Elements (Nikon, Melville, NY), and cell speed was calculated by measuring the upstream displacement with respect to time (26). Experiments were repeated three times.
In silico prediction and analysis of PilY1 Ca-binding motif.
Conserved domains in PilY1 were identified using the NCBI Conserved Domain Search (CD-Search) tool against the Pfam database. Amino acid sequences of X. fastidiosa PilY1 were retrieved from NCBI and were analyzed with InterProScan to look for the presence of previously reported functional PilY1 Ca-binding motifs found in other species: DX(D/N)XDGXXD, found in P. aeruginosa and K. kingae (14, 34, 35); DXDXNXXXD, found in P. aeruginosa (35); and DX(D/N)XDXXXXXX(D/E), with homology to the canonical EF-hand human calmodulin, found in K. kingae (14). Secondary-structure prediction was carried out with PSIPRED, version 3.3 (36, 37). Protein secondary-structure alignments were done using T-Coffee (38).
Protein purification and assessment of Ca binding.
A truncated version of pilY1-PD1611 (encoding residues 943 to 1472) from X. fastidiosa strain Temecula was amplified for cloning into pHis-parallel 2 using primers For PilY1_SpeI and Rev PilY1_NotI (Table 2). Expression of the truncated protein in Escherichia coli BL21(DE3) was induced by 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) when room temperature bacterial cultures reached an optical density at 600 nm (OD600) of ∼0.6 to 0.8. Cells were grown for an additional 1.5 h at room temperature and were harvested by centrifugation at 4,000 rpm for 10 min at 4°C. Pellets were resuspended in molecular-grade water, centrifuged at 4,000 rpm for 10 min at 4°C, and stored at −80°C. Thawed pellets were suspended in lysis buffer (25 mM glycine [pH 8.2], 10 mM imidazole) and were sonicated on ice for 30 s three times. The cell lysate was centrifuged at 4°C for 30 min at 12,000 rpm. Supernatants were transferred to a Ni-nitrilotriacetic acid (NTA) column. The column was washed with 5 column volumes of lysis buffer before elution (25 mM glycine [pH 8.2], 200 mM imidazole). Purified protein was evaluated by SDS-PAGE, and the presence of Ca was determined using inductively coupled plasma optical emission spectrometry (ICP-OES) (PerkinElmer 7300 DV spectrometer).
TABLE 2.
PCR primers and qPCR primer–TaqMan probe sets used in this study
| Primer or probea name | Sequence (5′–3′) |
|---|---|
| Mutagenesis | |
| pilY1 D_F | ACGGGCGCGCCCACCGATGAACATGACGCAAA |
| pilY1 D_R | ACGGAATTCGTGAAGATTCGGCAGTGTTGAGC |
| PUp-For | ACGGAATTCGTTGTGTGCGGGTTTTGGT |
| PUp-Rev | ACGGGCGCGCCGTGGGAACGTGTGAGCAAG |
| Kan Asc1_F | TTAGGCGCGCCGTCTGCCTCGTGAAG |
| Kan Asc1_R | AAAGGCGCGCCAAGCCACGTTGTGT |
| Mutation confirmation | |
| PY_DR | GGAGTGGAGTCCTTGCAATTT |
| Kan_F | CACCTGATTGCCCGACATTA |
| PY_UF | CCTCGAACTCGTAGCCTTTC |
| Kan_R | ACTGGTCCACCTACAACAAAG |
| Gene expression | |
| pilY1 1611_F | GGTCGCATTGCAATATCCAGGCAA |
| pilY1 1611_R | ACAGTAATGATGGGACGCGCAGTA |
| pilY1 1611_P* | AGTTTGTAGGGCTGTTGTCCTGCACA |
| pilY1 0023_F | TGATTGATGGTGGTGTCCTG |
| pilY1 0023_R | CTACCCGTGTCCATTTGACC |
| pilY1 0023_P* | GACAACCCTGATCCTGAGGA |
| pilY1 0052_F | ATTCCAAGCGTGTCCGCTAT |
| pilY1 0052_R | ATAGTACGACCCACCCGATGAGTA |
| pilY1 0052_P* | CCCAATCCTGAGGATGCTAA |
| pilB_F | AAGCTGGGTATCGACAAACTCGGT |
| pilB_R | TCTTCGGCTGTGGAGATATTGCGT |
| pilB_P* | ACTGCGTTAGGCATCCTCAATGACGA |
| pilT_F | CCGCAAACTCATTGAAGAACCGCA |
| pilT_R | CGCAGCCATGATCGACCATATCAACA |
| pilT_P* | AGCACTTCTGTGAGGTGTGAACGA |
| pilA_F | AAACACCGGACTTGCCAACATCAC |
| pilA_R | AAACCATCGCTTGGAATCGTAGCGTCGA |
| pilA_P* | TGTTGCATGTCCACTGACCTCCAT |
| gyrB_F | TTGGCGTGATGGTTACCACTACCA |
| gyrB_R | ATGTAGTACCGCGTTTCGCTGACA |
| gyrB_P* | TGGGTGAGCCGCAATATCCTCTTAAGCA |
| nuoA_F | AGACGCACGGATGAAGTTCGATGT |
| nuoA_R | ATTCCAGCGCTCCCTTCTTCCATA |
| nuoA_P* | TTCATCGTGCCTTGGACTCAGGTGTT |
| Protein expression and mutagenesis | |
| ForPilY1_SpeI | GCGCGCGACTAGTTATGATTAATTCAA |
| RevPilY1_NotI | ATCATCGCGGCCGCTCATCGCAACTG |
| PilY1_M2_R | AGCGACCAACCCATCGCCATCCGTATCCAACACCGC |
| PilY1_M3_F | GATACGGATGGCGATGGGTTGGTCGCTATGGTGTAC |
| PilY1_MFV2 | GCTACGGCTGGCGCTGGGTTGGTCGCTATGGTGTAC |
| PilY1_MRV2 | AGCGACCAACCCAGCGCCAGCCGTAGCCAACACCGC |
| Protein confirmation | |
| PilY1_F | GTCGGGCTGTGCTCTTTAT |
| T7_F | TAATACGACTCACTATAGGG |
| PilY1_R | ACTTCCAAACCTCCCGTAATC |
| T7_R | CTAGTTATTGCTCAGCGGTG |
All probes (marked with asterisks) were labeled with FAM at the 5′ end and BHQ1 at the 3′ end.
Gene expression analysis.
The expression of the TFP genes pilT, pilB, and pilA and the three pilY1 homologs (PD0023, PD0502, and PD1611) was analyzed in three different settings (agar plates, glass test tubes, and microfluidic chambers) under Ca-replete and Ca-depleted conditions (2 mM CaCl2 or 1.5 mM EGTA, respectively). WT X. fastidiosa cells were grown on PW agar plates for 8 days and were passaged to new plates, and 8-day-old cultures were used as the inocula for all settings. Test tubes containing 5 ml of liquid PD2 medium were inoculated with a 10-μl cell suspension (OD600, 0.8) and were incubated with shaking (150 rpm) for 2 days. Liquid medium was removed, and biofilm cells were scraped from the air-liquid interface and were resuspended in 1 ml of molecular-grade water. For agar plates, cells were spotted onto the agar and were incubated for 2 days, and individual colonies were separately collected and were resuspended in 0.5 ml of molecular-grade water. In the microfluidic chambers, the two PD2 medium conditions (supplementation with CaCl2 or EGTA) were tested simultaneously; each of the two channels containing either treatment was inoculated with a cell suspension in liquid PD2 medium. After 2 days, when cell twitching was observed, cells from each treatment were independently collected by increasing the speed of medium flow until all cells were detached. Total RNA from each treatment was extracted by using the Quick-RNA MiniPrep kit (Zymo Research, Irvine, CA) according to the manufacturer's instructions. RNA was treated with DNase I (Thermo Scientific, Pittsburgh, PA) to remove contaminant DNA, and sample concentrations were determined with a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Scientific, Pittsburgh, PA). Two hundred nanograms of RNA from each sample was used to synthesize single-strand cDNA by reverse transcription using the Maxima First Strand cDNA synthesis kit for reverse transcription-quantitative PCR (RT-qPCR) (Thermo Scientific, Pittsburgh, PA). A 1.5-μl volume of cDNA was then used for qPCR. Primers for the TFP genes and the internal control genes, nuoA and gyrB (Table 2), were designed using PrimerQuest software (Integrated DNA Technologies). qPCR mixtures (total volume, 20 μl) consisted of 1× ABsolute Blue QPCR ROX mix (ABgene-Thermo Fisher Scientific, Surrey, United Kingdom), 0.4 μM 6-carboxyfluorescein (FAM)–black hole quencher 1 (BHQ1)-labeled TaqMan probe, 0.2 μM each primer, and 1.5 μl of 200-ng/μl cDNA. Samples were amplified on an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA) using the following cycling parameters: 95°C for 1 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The fold change in gene expression was calculated according to the 2−ΔΔCT method (39) using DataAssist software, version 3.01 (Life Technologies, Grand Island, NY). Data were normalized using nuoA (for microfluidic chambers) or gyrB (for plates and tubes) as an endogenous control. Changes in gene expression were assessed by t tests (fold change ≠ 1; P < 0.05) as implemented in DataAssist.
Mutagenesis and mutant characterization.
Homologous recombination was used to generate an X. fastidiosa pilY1 (PD1611)-null mutant, pilY1-1611. X. fastidiosa WT genomic DNA was extracted using a modified cetyltrimethylammonium bromide (CTAB) protocol (40). PCR was then performed to amplify the 800-bp upstream and downstream fragments flanking the pilY1 coding region. For this purpose, primers PUp-For, PUp-Rev, pilY1 D_F, and pilY1 D_R (Table 2) were designed using PrimerQuest software (Integrated DNA Technologies), and they contained the AscI restriction site sequences added to the 5′ ends of the forward and reverse primers of the upstream and downstream segments. PCR mixtures contained 1× buffer, 0.2 mM deoxynucleoside triphosphates (dNTPs), 0.2 μM (each) forward and reverse primers, 0.625 U Taq polymerase, and 50 ng X. fastidiosa DNA in 25 μl. PCR was performed using the following parameters: 94°C for 1 min, followed by 49 cycles of 94°C for 30 s and 70°C for 1 min, and a final extension step of 70°C for 10 min. The PCR product was visualized by gel electrophoresis, digested with AscI, ligated with T4 ligase (Fisher, Pittsburgh, PA), and cloned into a pJET1.2/blunt cloning vector (Thermo Fisher Scientific, Pittsburgh, PA). The resulting plasmid, pJET_pilY1, was transformed into Escherichia coli NEB 5-alpha (New England BioLabs, Ipswich, MA). A kanamycin cassette was amplified from pUC4K with primers containing the AscI restriction site sequence (Table 2). pJET_pilY1 and the kanamycin segment were digested with AscI and were ligated with T4 ligase. To avoid religation of the plasmid, pJET_pilY1 was dephosphorylated with shrimp alkaline phosphatase (Affymetrix, USB, Santa Clara, CA) after restriction with AscI. The final construct was electroporated into E. coli EAM1, a strain expressing an X. fastidiosa methyltransferase (PD_1607) (41), at 2.5 kV for 5.7 ms. Transformed EAM1 was incubated at 37°C with shaking at 200 rpm overnight in Luria-Bertani (LB) medium containing 1 mM IPTG and 50 mg ml−1 kanamycin to select for resistance. Electrocompetent X. fastidiosa Temecula cells were prepared as described previously (42). Fifty microliters of electrocompetent X. fastidiosa and 200 ng of methylated plasmid DNA were transferred to a 2-mm cuvette (Eppendorf, Hauppauge, NY) and were electroporated at 2.5 kV for 5.7 ms. Immediately, 1 ml of PD3 medium (43) was added to the transformed cells, which were incubated at 28°C with shaking at 150 rpm overnight. Two hundred microliters of incubated cells was then spread-plated onto solid PD3 medium (solidified with Gelrite instead of agar) containing 30 μg ml−1 kanamycin. Plates were incubated at 28°C until transformants were visible (∼10 days). DNA was extracted from the resulting transformants by using the CTAB protocol mentioned above, PCR amplified with primers designed from the regions flanking the recombination region (PY_DR and PY_UF) paired with primers designed from the kanamycin cassette (Kan_F and Kan_R) (Table 2), and sequenced (Eurofins MWG Operon, Huntsville, AL) to confirm the mutation. BLAST (GenBank) was used to confirm the correct insertion of the kanamycin cassette and the absence of sequence mismatches.
The confirmed pilY1-1611 mutant and the previously published pilY1-0023 mutant (26) were evaluated for biofilm formation, adhesion force, and cell-to-cell aggregation as described previously (29). Briefly, biofilm formation was quantified in 96-well plates with crystal violet as described previously (44). Bacterial adhesion force was evaluated in microfluidic chambers as described previously (33). The extent of cell-to-cell aggregation was visualized in the microfluidic chambers in the presence of 2 mM CaCl2 (29). Transmission electron microscopy was performed to confirm pilus biosynthesis. For this purpose, mutant pilY1-1611 cells obtained from the peripheral fringes of 3-day-old bacterial colonies were scraped off plates and were suspended in 100 μl of 1× phosphate-buffered saline. Ten microliters of this cell suspension was deposited on Formvar-coated grids and was allowed to settle for 1 min. The cells were subsequently stained with 50 μl of phosphotungstic acid; the excess liquid was removed; and the grids were air dried and were observed on a Zeiss EM10 transmission electron microscope (Carl Zeiss, Jena, Germany). Images were acquired using MaxIm DL software, version 5 (Diffraction Limited, Ottawa, Ontario, Canada).
Data analysis.
All analyses were conducted with the GLIMMIX procedure of SAS, version 9.2 (SAS Institute, Cary, NC), by following a completely randomized design (CRD). Comparison of the treatments was performed with least-squares means, with a level of significance (P value) of ≤0.05.
RESULTS
Ca specifically increases twitching motility.
The range of divalent cations affecting twitching motility was explored by growth on media supplemented with these elements. Colony fringe width measurements showed that of all metal elements tested, only Ca supplementation produced a significant increase (P = 0.001) in the fringe width of WT X. fastidiosa over that with unsupplemented medium (Fig. 1). In contrast, significant decreases in fringe width were observed for colonies grown on media supplemented with Zn or Cu (P ≤ 0.0001) from that with unsupplemented medium (Fig. 1). The fringes observed in plates supplemented with Mg (P = 0.12) or Fe (P = 0.80) were not different from those observed in unsupplemented plates (Fig. 1).
FIG 1.

Effects of medium supplementation with different metal elements on the twitching motility of Xylella fastidiosa. Shown are the colony fringe widths of WT X. fastidiosa spotted onto PW agar alone and onto PW agar supplemented with 2 mM CaCl2, 2 mM MgSO4, 1.5 mM FeSO4, 1 mM ZnSO4, or 0.25 mM CuSO4. Different letters above the bars indicate statistically significant differences according to the GLIMMIX procedure (P ≤ 0.05). Error bars represent the standard errors of the means (n = 36).
Ca chelation reduces twitching motility.
To assess the requirement for intracellular versus extracellular Ca in the Ca-mediated twitching response, growth was assessed on media containing different Ca chelators. A much smaller fringe was observed for colonies grown in medium supplemented with the extracellular Ca chelator EGTA than for colonies grown on unsupplemented PW plates, as reported previously (29). In contrast, colonies grown on plates containing the intracellular Ca chelator BAPTA/AM exhibited movement, as evidenced by their colony fringe, although it was significantly smaller than that observed with unsupplemented or Ca-supplemented PW medium (P ≤ 0.001) (Fig. 2A). Analysis of the twitching motility in microfluidic chambers under flow conditions after exposure to chelators indicated that the movement of cells exposed to EGTA decreased more rapidly over time than that of cells exposed to BAPTA/AM (Fig. 2B).
FIG 2.
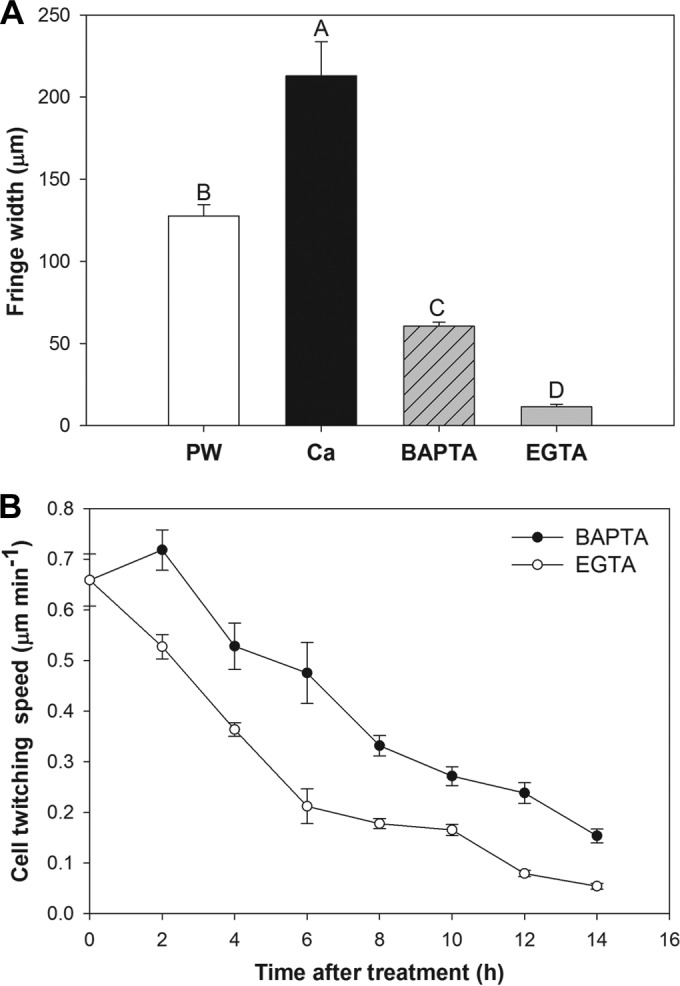
Effect of medium supplementation with Ca, the extracellular Ca chelator EGTA, or the intracellular Ca chelator BAPTA/AM on the twitching motility of WT X. fastidiosa. (A) Colony fringe widths of X. fastidiosa cultured on PW agar plates alone or supplemented with 2 mM CaCl2, 1.5 mM EGTA, or 100 μm BAPTA. Different letters above the bars indicate statistically significant differences according to the GLIMMIX procedure (P ≤ 0.05). Error bars represent standard errors of the means (n = 36). (B) Assessment of twitching motility in microfluidic chambers after the addition of EGTA or BAPTA. The movement of the cells was recorded 14 h after chelator addition. The speed of the cells was calculated for each 2-h time lapse. Error bars at each point indicate the standard error of the mean (n = 4).
Mutants with alterations in non-TFP genes known to influence twitching do not exhibit an impaired response to Ca.
To determine if biosynthetic genes implicated in twitching motility modulation are linked to Ca-increased twitching, X. fastidiosa fimA (encoding type I pili), hxfA (hemagglutinin), rpfF (DSF biosynthesis), and rpfC (DSF sensing) mutants were assessed for twitching motility on unsupplemented plates and plates supplemented with 2 mM CaCl2 or 1.5 mM EGTA. Twitching motility, evaluated by measuring the colony fringe width, was significantly increased with the addition of Ca to the medium for all mutants evaluated (Fig. 3), with P values of 0.04 for the fimA, hxfA, and rpfC mutants and ≤0.0001 for the rpfF mutant. Additionally, a decrease or complete stop in movement was observed in EGTA-supplemented medium relative to movement in unsupplemented medium for all these mutants (data not shown). All mutants had significantly greater fringe widths than the WT in both PW medium alone and PW medium supplemented with Ca, with P values of ≤0.0001 for the fimA and hxfA mutants, 0.002 for the rpfF mutant, and 0.02 for the rpfC mutant.
FIG 3.

Effect of Ca on the twitching motility of X. fastidiosa mutants defective in genes involved in the production of fimbriae (fimA, encoding type I pili) and afimbrial adhesins (hxfA, encoding hemagglutinin) and in DSF molecule biosynthesis (rpfF) and sensing (rpfC). Twitching motility was quantified on PW plates alone and PW plates supplemented with 2 mM CaCl2. The WT fringe length values are taken from reference 29 for comparative purposes. Different letters above paired bars (PW, PW+Ca) indicate significant differences according to the GLIMMIX procedure (P ≤ 0.05). Error bars represent the standard errors of the means (n = 36).
Only one of the three PilY1 homologs in X. fastidiosa has a conserved Ca-binding motif.
Conserved-domain analysis of the amino acid sequences of the three pilY1 homologs present in X. fastidiosa revealed the presence of the Neisseria PilC beta-propeller domain (pfam05567) in the C-terminal ends of the proteins, spanning approximately 500 amino acids. In addition, the PilY1 homologs in X. fastidiosa were examined for the presence of the Ca-binding signature pattern DX(D/N)XDGXXD as an indication of the EF-hand-like Ca-binding site that has been shown to be conserved in other pathogens with TFP (14, 34). This analysis indicated that of the three pilY1 homologs in X. fastidiosa, only PD1611 contains the Ca-binding motif, a stretch of 9 amino acids (DTDGDGLVD) located between two β-strands according to the secondary-structure prediction (data not shown). The structural context of this region agrees with what has been described previously for other PilY1 (PilC) proteins. None of the X. fastidiosa homologs contained either of the other PilY1 Ca-binding motifs: DXDXNXXXD, found in P. aeruginosa (35), or DX(D/N)XDXXXXXX(D/E), found in K. kingae (14).
Recombinant PilY1-1611 can bind Ca.
To evaluate the binding of Ca to PilY1, a truncated PilY1-1611 protein consisting of residues 943 to 1472 was purified after recombinant expression in E. coli. The final purified fraction was >90% pure, and Ca was associated with the final fractions (1.4 ± 0.8 mol Ca/mol protein [n = 5]). Dialysis of the purified PilY1-1611943–1472 protein resulted in slight depletion of bound Ca (90% ± 10% retained [n = 3]). The mineral element profile obtained by ICP-OES showed that Mg (25 to 50% of Ca) was also present in the purified protein, with negligible levels of all other elements included in the analysis (Co, Cu, Fe, Mn, K, Na, Ni, Zn). Site-directed mutants with alterations in the 9-amino-acid Ca-binding motif (D1177A or both D1169A and D1177A) were created, and although these mutant proteins were expressed in E. coli, they were not soluble under any condition tested. The solubility of PilY1-1611943–1472 was enhanced by the addition of Ca to the medium at the time of induction, but no concentration of added Ca promoted mutant solubility.
Ca regulates transcription of TFP ATPases.
Of the TFP genes analyzed (pilT, pilB, pilA, and the three pilY1 homologs PD0023, PD0502, and PD1611), pilB was differentially expressed in X. fastidiosa grown in Ca-replete conditions (2 mM CaCl2) compared to X. fastidiosa grown in Ca-depleted conditions (1.5 mM EGTA) in test tubes and agar plates (Fig. 4). pilB was significantly downregulated in tubes (fold change = −2.38, P = 0.0062) and plates (fold change = −2.63, P = 0.042) under the replete conditions. In contrast, in the microfluidic chambers under Ca-replete and Ca-depleted conditions, only pilT was differentially expressed (Fig. 4). pilT was significantly upregulated in X. fastidiosa grown in microfluidic chambers (fold change = 2.35, P = 0.0094) under Ca-replete conditions.
FIG 4.
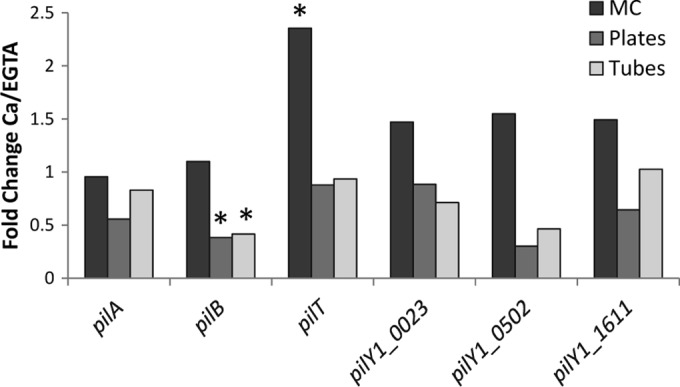
Analysis of expression of type IV pilus genes in three different settings: agar plates, glass tubes, and microfluidic chambers (MC). Results are expressed as the fold change in gene expression with the Ca treatment; EGTA chelation was considered the basal condition. Asterisks indicate significant differences in gene expression as determined by the t test (fold change ≠ 1; P < 0.05).
The pilY1-1611 mutant is impaired in the Ca-mediated twitching response.
Transmission electron micrographs revealed that the pilY1-1611 mutant forms TFP (Fig. 5A), as observed previously for the pilY1-0023 mutant (26). To confirm that the pilY1-1611 TFP were functional and to assess the abilities of both pilY1 mutants to respond to Ca, twitching motility on agar plates and in microfluidic chambers was assessed. Twitching motility was confirmed for the pilY1-1611 mutant in both systems. The colony fringe width in Ca-supplemented PW medium did not differ significantly from that in unsupplemented PW medium for the pilY1-1611 mutant (P = 0.17), but it was significantly increased (P = 0.02) on Ca-supplemented plates for the pilY1-0023 mutant (Fig. 5B). No colony fringe was observed for either mutant on EGTA-supplemented plates. Similarly, in microfluidic chambers, the cell twitching speeds of the pilY1-1611 mutant in unsupplemented and CaCl2-supplemented media did not differ significantly (P = 0.55), but the cell twitching speed of the pilY1-0023 mutant was significantly increased (P ≤ 0.001) in Ca-supplemented medium (Fig. 5C). The pilY1-0023 mutant moved significantly more slowly than the WT in PD2 medium (P = 0.0007), as demonstrated previously (26), while the speed of the pilY1-1611 mutant did not significantly differ from that of the WT (P = 0.53).
FIG 5.
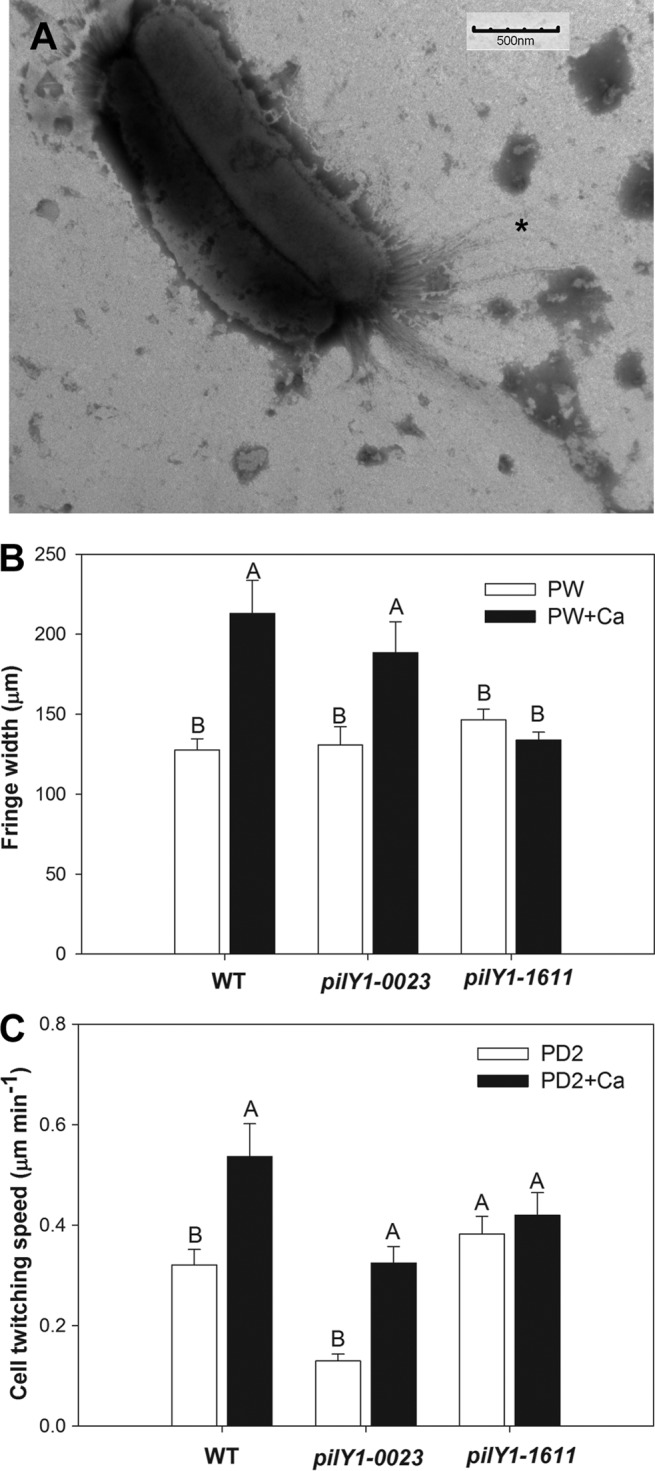
Twitching phenotypes of pilY1 mutant homologs. (A) Transmission electron micrograph of negatively stained pilY1-1611 mutant depicting both short type I pili and longer type IV pili (indicated by an asterisk). (B) Colony fringe widths of X. fastidiosa mutant strains cultured on unsupplemented PW agar or PW agar supplemented with 2 mM CaCl2. Different letters above paired bars indicate statistically significant differences according to the GLIMMIX procedure (P ≤ 0.05). Error bars represent standard errors of the means (n = 12). (C) Assessments of the twitching speeds of cells incubated in unsupplemented PD2 medium or PD2 medium supplemented with 2 mM CaCl2 in microfluidic chambers. Different letters above paired bars indicate significant differences according to the GLIMMIX procedure (P ≤ 0.05). Error bars represent standard errors of the means (n = 10). In panels B and C, the WT values are taken from reference 29 for comparative purposes.
The pilY1-1611 mutant is impaired in the Ca-mediated increase in single-cell attachment to surfaces but not in biofilm formation.
The pilY1-0023 and pilY1-1611 mutants were further characterized by assessing biofilm formation relative to that by the WT. A positive correlation between Ca concentration and biofilm formation was confirmed for the WT (as shown previously [29]) and the mutants for the range of Ca concentrations evaluated (Fig. 6A). No differences in the accumulation of biofilms were observed between the mutants and the WT. For the WT and mutants, the amount of biofilm formed in the medium with the highest Ca concentration added was ∼500% to 1,000% higher than the amount in the unsupplemented medium. Quantification of the force of adhesion of individual cells to the glass surface inside microfluidic chambers indicated that, in contrast to the findings for the WT (29), the addition of 2 mM Ca to the medium did not result in a significant increase in attachment for the pilY1-0023 (P = 0.4279) or pilY1-1611 (P = 0.7741) mutant (Fig. 6B). Comparison of attachment between the WT and the mutants indicated a significantly higher adhesion force for the pilY1-1611 mutant in unsupplemented medium (P = 0.0385). Visual assessment of cell-to-cell attachment, exemplified by aggregates formed in the microfluidic chambers, did not differ for the WT and mutants grown in PD2 medium. Aggregates appeared 2 days after inoculation and exhibited similar sizes and numbers for the WT and mutants during the time of the experiment (data not shown).
FIG 6.

Characterization of biofilm formation and surface attachment of X. fastidiosa pilY1-0023 and pilY1-1611 mutants compared to those of the wild type (WT). (A) Biofilm quantification in PD2 liquid medium supplemented with different concentrations of Ca in 96-well plates. Average values and standard errors of the means from one representative experiment are shown (n = 3). (B) Adhesion force in unsupplemented PD2 medium and in PD2 medium supplemented with 2 mM CaCl2 in microfluidic chambers. The WT adhesion force values are taken from reference 29 for comparative purposes. Different letters above paired bars indicate significant differences according to the GLIMMIX procedure (P ≤ 0.05). Error bars represent standard errors of the means (n = 5).
DISCUSSION
Calcium concentrations regulate the twitching motility of X. fastidiosa (29). In this study, we conclude that Ca-responsive twitching is mediated by PilY1-PD1611, one of three PilY1 homologs present in X. fastidiosa. This is the first report of a plant-associated bacterium using PilY1 containing a functional Ca-binding motif in twitching motility. Previously, Ca binding by the TFP PilY1/PilC1 proteins of P. aeruginosa (34), Neisseria spp. (45), and K. kingae (14) has been demonstrated to regulate the twitching and adhesion of these organisms.
Metal elements have been described as modulators of bacterial movement. Iron has been associated with the production of the quorum-sensing signal N-butanoyl homoserine lactone in P. aeruginosa (46) and with the upregulation of TFP genes in X. fastidiosa (47), features linked with twitching motility. In Vibrio fischeri, motility and flagellum production are dependent on Mg (48). Here, examination of elements, such as Ca, Mg, Fe, Zn, and Cu, previously shown to promote or suppress X. fastidiosa biofilm formation (29) indicated that only Ca increased twitching motility in vitro. Ca binding was confirmed for the recombinant PilY1-1611 protein, and while Mg was also detected in the purified protein, the failure of this element to modulate twitching suggests that this could be due to the fact that Ca-binding motifs can also bind other divalent cations (49, 50). Chelation of extracellular Ca by EGTA produced larger (on agar plates) and faster (in microfluidic chambers) decreases in twitching than intracellular chelation (BAPTA/AM), a finding consistent with the easy removal of Ca from exposed proteins or other molecules. In microfluidic chambers, where medium flow decreases the likely depletion of intracellular stores of Ca, the immediate effect of extracellular Ca chelation, in the absence of cell division during the experimental time frame (X. fastidiosa doubling time, ∼8 h [51]), was to decrease the motility of X. fastidiosa, indicating that Ca may interact with an extracellular structure (such as TFP) to elicit effects on motility. On agar plates, extracellular chelation also depletes intracellular Ca, since multiple generations are produced in the low-Ca environment. The enhanced reduction of motility by intracellular chelation may be partially explained by the disturbance of other essential roles of Ca in bacterial cell physiology.
PilY1-PD1611 is not essential for twitching but is needed for the Ca-mediated increase in twitching.
The experimental results confirm that X. fastidiosa PilY1 homologs have different functions, and they contribute to twitching motility to various degrees depending on environmental conditions. PilY1-PD1611 is involved in the Ca-mediated increase in twitching but does not affect movement when Ca concentrations are lower (∼0.02 mM Ca in PD2 medium [52]). However, PilY1-PD0023 is necessary for full twitching speed, regardless of the Ca concentration. The pilY1-PD0023 mutant showed ∼70% lower twitching speed than the WT under flow conditions (26), while the pilY1-PD1611 mutant was not significantly different from the WT under the same conditions. As with K. kingae and N. gonorrhoeae (14, 53), mutation of individual X. fastidiosa PilY1 homologs does not appear to affect piliation, indicating that neither pilY1-PD0023 nor pilY1-PD1611 is required for pilus biogenesis. However, when all homologs in K. kingae, N. gonorrhoeae, and P. aeruginosa are mutated, no pili are produced (14, 34, 45). To verify that all three X. fastidiosa PilY1 homologs are involved in pilus biogenesis, simultaneous mutation is required.
We speculate that the presence of the Ca-binding motif in bacterial PilY1 proteins could be directly related to the occurrence and concentration of Ca in the niches of these bacteria. X. fastidiosa lives in xylem sap, which is nutritionally poor, with a high concentration of Ca (about 4 mM) (54). Accordingly, with K. kingae, a human pathogen also containing the Ca-binding motif, disease is usually found in human joints and bones, where high Ca concentrations (4 mM or greater) are available (14). Therefore, the evolutionary adaptation of the regulation of twitching by Ca seems to be a feature of bacteria living under high Ca conditions.
Regulation of TFP gene expression by Ca.
PilB is required for TFP extension, but extension does not result in cell movement; only retraction mediated by PilT activity does (55). To facilitate equal extension and retraction, the expression of pilB and pilT is likely in equilibrium, resulting in wild-type twitching motility (34). It has been shown here that modification of Ca concentrations altered pilB and pilT expression under certain conditions. Significant upregulation of pilT was detected only under flow conditions (microfluidic chambers) and was not found in batch cultures (tubes and plates). Microfluidic chambers, where most cells are motile against the flow (5, 7), are a better representation of xylem vessels than batch cultures, because they reduce interference by nonmotile cells. The lack of detectable pilT upregulation under Ca-replete conditions in batch cultures may be due to higher numbers of nonmotile cells. In plates, entire colonies were used for expression analyses, but actively twitching cells are probably located only at the colony fringe. In tubes, biofilm cells were used for expression analyses, and it is likely that a low percentage of these cells were actively twitching. In the case of pilB, required for pilus assembly in X. fastidiosa (5) and other species (56, 57), downregulation in plates and tubes under Ca-replete conditions likely reflects the promotion of attachment and biofilm formation in X. fastidiosa under high-Ca conditions (29), a process that does not need TFP.
PilY1-PD1611 is not essential for biofilm formation but regulates the Ca-mediated increase in single-cell surface attachment.
The process of biofilm formation involves various steps, including cell attachment to surfaces, cell-to-cell attachment, and exopolysaccharide production (58). Deletion of either of the two pilY1 homologs studied here did not affect the biofilm formation process as a whole and did not modify the increases in biofilm production observed previously (29) when Ca concentrations were increased. These observations reflect the fact that other fimbrial (6) and afimbrial (59) adhesins, as well as exopolysaccharides (60), play a stronger role in the biofilm process than TFP. Besides its effect on PilY1, Ca likely has effects on these other adhesins. For example, a direct effect on exopolysaccharide formation is suggested by the fact that upregulation of exopolysaccharide biosynthetic genes and greater accumulations of exopolysaccharides were detected at higher Ca concentrations (L. F. Cruz, unpublished data).
When surface attachment (adhesion force) in Ca-supplemented medium was compared with that in unsupplemented medium, the pilY1-PD0023 mutant showed a tendency (though nonsignificant) to increased attachment in Ca-supplemented medium, while the pilY1-PD1611 mutant did not show differing levels of attachment in the different medium types. However, when overall adhesion force values for the mutants and WT were compared, the pilY1-PD1611 mutant had a significantly higher level of surface attachment than the WT, a phenotype different from that observed for the pilY1-PD0023 mutant. However, in X. fastidiosa, type I pili, formed at the same cell pole as TFP, have a stronger role in adhesion (33). Also, TFP may physically obstruct cell contact with the substrate (33). Although pilY1-1611 was able to produce TFP, it is possible that pili were produced at a lower level than in the WT or that the mutation affected TFP function, as suggested previously for N. gonorrhoeae mutants with alterations in different PilY1 homologs, where it is difficult to observe differences in the number of pili and other characteristics accurately (45). Fewer TFP could result in more access for type I pili and, therefore, in increased attachment. Additionally, it has been proposed that PilY1 in Pseudomonas spp. functions as a Ca-regulated switch that controls an alternation between pilus extension and retraction to generate twitching motility, where Ca-bound PilY1 inhibits PilT and Ca-free PilY1 facilitates PilT-mediated retraction (34, 61). Like P. aeruginosa PilY1 (34), a recombinantly expressed truncated PilY1-PD1611 protein was able to bind Ca. The loss of PilY1-PD1611 could simulate the Ca-free situation, increasing TFP retraction with lower rates of extension, allowing increased access for type I pili and thus resulting in increased single-cell adhesion. Further research is needed to understand the effect of this gene on adhesion.
A possible role for Ca in affecting other adhesins was tested by assessing the twitching of mutants lacking hemagglutinin (hxfA mutant [59]) and type I pili (fimA mutant [5]) in different Ca concentrations. The response to Ca in twitching was conserved in these mutants, suggesting that the effect of Ca in enhancing twitching is not an indirect effect on the adhesion functions of these proteins. Similarly, quantification of the twitching motilities of rpfF (biosynthesis) and rpfC (sensing) DSF mutants in different Ca concentrations indicates that the mechanism of Ca-responsive twitching motility is not associated with this quorum-sensing molecule. Interestingly, it is demonstrated here that X. fastidiosa DSF (as suggested previously [27]), hemagglutinin, and type I pilus (as shown previously [26]) mutants have increased twitching motility in both media tested, probably due to the weaker level of attachment of these mutant cells to surfaces.
Conclusions.
This study demonstrates that the PilY1 homolog PD1611 is necessary for the observed Ca-mediated increase in twitching motility by X. fastidiosa. As a modulator of twitching motility, Ca likely plays an important role in regulating the spread of X. fastidiosa through the plant host. Accordingly, the availability of Ca in the host plant, specifically in the xylem vessels, may determine features of disease progression that may have interesting implications for the development of disease management strategies. Further research is necessary to explore the host colonization capabilities of the X. fastidiosa pilY1-1611 mutant relative to those of the wild type with different Ca fertilization schemes in order to elucidate potential disease management programs.
ACKNOWLEDGMENTS
This project was supported by Agriculture and Food Research Initiative Competitive Grants Program grant 2010-65108-20633 from the USDA National Institute of Food and Agriculture and the Alabama Agricultural Experiment Station (AAES) Hatch Grant Program.
We thank Harvey C. Hoch and Thomas J. Burr (Cornell University) for X. fastidiosa mutant strains and Michele Igo (University of California, Davis) for constructs and strains for mutagenesis. We acknowledge the Auburn University Research Instrumentation Facilities for the use of their microscope and Michael E. Miller for assistance with its operation.
Footnotes
Published ahead of print 12 September 2014
REFERENCES
- 1.Purcell AH, Hopkins DL. 1996. Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 34:131–151. 10.1146/annurev.phyto.34.1.131. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins DL, Purcell AH. 2002. Xylella fastidiosa: cause of Pierce's disease of grapevine and other emergent diseases. Plant Dis. 86:1056–1066. 10.1094/PDIS.2002.86.10.1056. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins DL. 1989. Xylella fastidiosa: xylem-limited bacterial pathogen of plants. Annu. Rev. Phytopathol. 27:271–290. 10.1146/annurev.py.27.090189.001415. [DOI] [Google Scholar]
- 4.Zaini PA, Burdman S, Igo MM, Parker JK, De La Fuente L. Fimbrial and afimbrial adhesins involved in bacterial attachment to surfaces. In Graham JH. (ed), Virulence mechanisms of plant pathogenic bacteria, in press American Phytopathological Society Press, St. Paul, MN. [Google Scholar]
- 5.Meng YZ, Li YX, Galvani CD, Hao GX, Turner JN, Burr TJ, Hoch HC. 2005. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 187:5560–5567. 10.1128/JB.187.16.5560-5567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De La Fuente L, Burr TJ, Hoch HC. 2008. Autoaggregation of Xylella fastidiosa cells is influenced by type I and type IV pili. Appl. Environ. Microbiol. 74:5579–5582. 10.1128/AEM.00995-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De La Fuente L, Galvani CD, Cursino L, Burr TJ, Hoch HC. 2007. Xylella fastidiosa movement and biofilm formation studied in artificial xylem vessels. Phytopathology 97:S26. [Google Scholar]
- 8.Mattick JS. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289–314. 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 9.Burrows LL. 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 66:493–520. 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 10.Albiger B, Johansson L, Jonsson AB. 2003. Lipooligosaccharide-deficient Neisseria meningitidis shows altered pilus-associated characteristics. Infect. Immun. 71:155–162. 10.1128/IAI.71.1.155-162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morand PC, Bille E, Morelle S, Eugene E, Beretti JL, Wolfgang M, Meyer TF, Koomey M, Nassif X. 2004. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J. 23:2009–2017. 10.1038/sj.emboj.7600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfgang M, Park HS, Hayes SF, van Putten JPM, Koomey M. 1998. Suppression of an absolute defect in type IV pilus biogenesis by loss-of-function mutations in pilT, a twitching motility gene in Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 95:14973–14978. 10.1073/pnas.95.25.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Källström H, Islam MS, Berggren PO, Jonsson AB. 1998. Cell signaling by the type IV pili of pathogenic Neisseria. J. Biol. Chem. 273:21777–21782. 10.1074/jbc.273.34.21777. [DOI] [PubMed] [Google Scholar]
- 14.Porsch EA, Johnson MD, Broadnax AD, Garrett CK, Redinbo MR, St Geme JW., III 2013. Calcium binding properties of the Kingella kingae PilC1 and PilC2 proteins have differential effects on type IV pilus-mediated adherence and twitching motility. J. Bacteriol. 195:886–895. 10.1128/JB.02186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu HL, Kang YW, Genin S, Schell MA, Denny TP. 2001. Twitching motility of Ralstonia solanacearum requires a type IV pilus system. Microbiology 147(Part 12):3215–3229. [DOI] [PubMed] [Google Scholar]
- 16.Bahar O, Goffer T, Burdman S. 2009. Type IV pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp. citrulli. Mol. Plant Microbe Interact. 22:909–920. 10.1094/MPMI-22-8-0909. [DOI] [PubMed] [Google Scholar]
- 17.Ojanen-Reuhs T, Kalkkinen N, Westerlund-Wikstrom B, van Doorn J, Haahtela K, Nurmiaho-Lassila EL, Wengelnik K, Bonas U, Korhonen TK. 1997. Characterization of the fimA gene encoding bundle-forming fimbriae of the plant pathogen Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 179:1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su WC, Tung SY, Yang MK, Kuo TT. 1999. The pilA gene of Xanthomonas campestris pv. citri is required for infection by the filamentous phage Cf. Mol. Gen. Genet. 262:22–26. 10.1007/s004380051055. [DOI] [PubMed] [Google Scholar]
- 19.McCarthy Y, Ryan RP, O'Donovan K, He YQ, Jiang BL, Feng JX, Tang JL, Dow JM. 2008. The role of PilZ domain proteins in the virulence of Xanthomonas campestris pv. campestris. Mol. Plant Pathol. 9:819–824. 10.1111/j.1364-3703.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Makino S, Subedee A, Bogdanove AJ. 2007. Novel candidate virulence factors in rice pathogen Xanthomonas oryzae pv. oryzicola as revealed by mutational analysis. Appl. Environ. Microbiol. 73:8023–8027. 10.1128/AEM.01414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suoniemi A, Bjorklof K, Haahtela K, Romantschuk M. 1995. Pili of Pseudomonas syringae pathovar syringae enhance initiation of bacterial epiphytic colonization of bean. Microbiology 141(Part 2):497–503. 10.1099/13500872-141-2-497. [DOI] [Google Scholar]
- 22.Roine E, Raineri DM, Romantschuk M, Wilson M, Nunn DN. 1998. Characterization of type IV pilus genes in Pseudomonas syringae pv. tomato DC3000. Mol. Plant Microbe Interact. 11:1048–1056. 10.1094/MPMI.1998.11.11.1048. [DOI] [PubMed] [Google Scholar]
- 23.Taguchi F, Ichinose Y. 2011. Role of type IV pili in virulence of Pseudomonas syringae pv. tabaci 6605: correlation of motility, multidrug resistance, and HR-inducing activity on a nonhost plant. Mol. Plant Microbe Interact. 24:1001–1011. 10.1094/MPMI-02-11-0026. [DOI] [PubMed] [Google Scholar]
- 24.Burdman S, Bahar O, Parker JK, De La Fuente L. 2011. Involvement of type IV pili in pathogenicity of plant pathogenic bacteria. Genes 2:706–735. 10.3390/genes2040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Hao G, Galvani CD, Meng Y, De La Fuente L, Hoch HC, Burr TJ. 2007. Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell-cell aggregation. Microbiology 153(Part 3):719–726. 10.1099/mic.0.2006/002311-0. [DOI] [PubMed] [Google Scholar]
- 26.De La Fuente L, Burr TJ, Hoch HC. 2007. Mutations in type I and type IV pilus biosynthetic genes affect twitching motility rates in Xylella fastidiosa. J. Bacteriol. 189:7507–7510. 10.1128/JB.00934-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee S, Newman KL, Lindow SE. 2008. Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant Microbe Interact. 21:1309–1315. 10.1094/MPMI-21-10-1309. [DOI] [PubMed] [Google Scholar]
- 28.Cursino L, Galvani CD, Athinuwat D, Zaini PA, Li Y, De La Fuente L, Hoch HC, Burr TJ, Mowery P. 2011. Identification of an operon, Pil-Chp, that controls twitching motility and virulence in Xylella fastidiosa. Mol. Plant Microbe Interact. 24:1198–1206. 10.1094/MPMI-10-10-0252. [DOI] [PubMed] [Google Scholar]
- 29.Cruz LF, Cobine PA, De La Fuente L. 2012. Calcium increases Xylella fastidiosa surface attachment, biofilm formation, and twitching motility. Appl. Environ. Microbiol. 78:1321–1331. 10.1128/AEM.06501-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Sluys MA, de Oliveira MC, Monteiro-Vitorello CB, Miyaki CY, Furlan LR, Camargo LE, da Silva AC, Moon DH, Takita MA, Lemos EG, Machado MA, Ferro MI, da Silva FR, Goldman MH, Goldman GH, Lemos MV, El-Dorry H, Tsai SM, Carrer H, Carraro DM, de Oliveira RC, Nunes LR, Siqueira WJ, Coutinho LL, Kimura ET, Ferro ES, Harakava R, Kuramae EE, Marino CL, Giglioti E, Abreu IL, Alves LM, do Amaral AM, Baia GS, Blanco SR, Brito MS, Cannavan FS, Celestino AV, da Cunha AF, Fenille RC, Ferro JA, Formighieri EF, Kishi LT, Leoni SG, Oliveira AR, Rosa VE, Jr, Sassaki FT, Sena JA, de Souza AA, Truffi D, Tsukumo F, Yanai GM, Zaros LG, Civerolo EL, Simpson AJ, Almeida NF, Jr, Setubal JC, Kitajima JP. 2003. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 185:1018–1026. 10.1128/JB.185.3.1018-1026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis MJ, Purcell AH, Thomson SV. 1980. Isolation media for the Pierce's disease bacterium. Phytopathology 70:425–429. 10.1094/Phyto-70-425. [DOI] [Google Scholar]
- 32.Davis MJ, French WJ, Schaad NW. 1981. Axenic culture of the bacteria associated with phony disease of peach and plum leaf scald. Curr. Microbiol. 6:309–314. 10.1007/BF01566883. [DOI] [Google Scholar]
- 33.De La Fuente L, Montanes E, Meng YZ, Li YX, Burr TJ, Hoch HC, Wu MM. 2007. Assessing adhesion forces of type I and type IV pili of Xylella fastidiosa bacteria by use of a microfluidic flow chamber. Appl. Environ. Microbiol. 73:2690–2696. 10.1128/AEM.02649-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orans J, Johnson MDL, Coggan KA, Sperlazza JR, Heiniger RW, Wolfgang MC, Redinbo MR. 2010. Crystal structure analysis reveals Pseudomonas PilY1 as an essential calcium-dependent regulator of bacterial surface motility. Proc. Natl. Acad. Sci. U. S. A. 107:1065–1070. 10.1073/pnas.0911616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson MDL, Garrett CK, Bond JE, Coggan KA, Wolfgang MC, Redinbo MR. 2011. Pseudomonas aeruginosa PilY1 binds integrin in an RGD- and calcium-dependent manner. PLoS One 6:e29629. 10.1371/journal.pone.0029629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchan DWA, Ward SM, Lobley AE, Nugent TCO, Bryson K, Jones DT. 2010. Protein annotation and modelling servers at University College London. Nucleic Acids Res. 38:W563–W568. 10.1093/nar/gkq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones DT. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195–202. 10.1006/jmbi.1999.3091. [DOI] [PubMed] [Google Scholar]
- 38.Notredame C, Higgins DG, Heringa J. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302:205–217. 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Doyle J, Doyle JL. 1987. Genomic plant DNA preparation from fresh tissue—CTAB method. Phytochem. Bull. 19:11–15. [Google Scholar]
- 41.Matsumoto A, Igo MM. 2010. Species-specific type II restriction-modification system of Xylella fastidiosa Temecula1. Appl. Environ. Microbiol. 76:4092–4095. 10.1128/AEM.03034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto A, Young GM, Igo MM. 2009. Chromosome-based genetic complementation system for Xylella fastidiosa. Appl. Environ. Microbiol. 75:1679–1687. 10.1128/AEM.00024-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis MJ, Whitcomb RF, Gillaspie AGJ. 1981. Fastidious bacteria of plant vascular tissue and invertebrates (including so called rickettsia-like bacteria), p 2172–2188 In Starr MP, Stolp H, Truper HG, Balows A, Schlegel HG. (ed), The prokaryotes: a handbook on habitats, isolation, and identification of bacteria, vol 2 Springer-Verlag, Berlin, Germany. [Google Scholar]
- 44.Zaini PA, De La Fuente L, Hoch HC, Burr TJ. 2009. Grapevine xylem sap enhances biofilm development by Xylella fastidiosa. FEMS Microbiol. Lett. 295:129–134. 10.1111/j.1574-6968.2009.01597.x. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Y, Johnson MD, Burillo-Kirch C, Mocny JC, Anderson JE, Garrett CK, Redinbo MR, Thomas CE. 2013. Mutation of the conserved calcium-binding motif in Neisseria gonorrhoeae PilC1 impacts adhesion but not piliation. Infect. Immun. 81:4280–4289. 10.1128/IAI.00493-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patriquin GM, Banin E, Gilmour C, Tuchman R, Greenberg EP, Poole K. 2008. Influence of quorum sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J. Bacteriol. 190:662–671. 10.1128/JB.01473-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaini PA, Fogaca AC, Lupo FGN, Nakaya HI, Vencio RZN, da Silva AM. 2008. The iron stimulon of Xylella fastidiosa includes genes for type IV pilus and colicin V-like bacteriocins. J. Bacteriol. 190:2368–2378. 10.1128/JB.01495-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Shea TM, Klein AH, Geszvain K, Wolfe AJ, Visick KL. 2006. Diguanylate cyclases control magnesium-dependent motility of Vibrio fischeri. J. Bacteriol. 188:8196–8205. 10.1128/JB.00728-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawasaki H, Kurosu Y, Kasai H, Isobe T, Okuyama T. 1986. Limited digestion of calmodulin with trypsin in the presence or absence of various metal-ions. J. Biochem. (Tokyo) 99:1409–1416. [DOI] [PubMed] [Google Scholar]
- 50.Warren JT, Guo Q, Tang WJ. 2007. A 1.3-angstrom structure of zinc-bound N-terminal domain of calmodulin elucidates potential early ion-binding step. J. Mol. Biol. 374:517–527. 10.1016/j.jmb.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feil H, Purcell AH. 2001. Temperature-dependent growth and survival of Xylella fastidiosa in vitro and in potted grapevines. Plant Dis. 85:1230–1234. 10.1094/PDIS.2001.85.12.1230. [DOI] [PubMed] [Google Scholar]
- 52.Cobine PA, Cruz LF, Navarrete F, Duncan D, Tygart M, De La Fuente L. 2013. Xylella fastidiosa differentially accumulates mineral elements in biofilm and planktonic cells. PLoS One 8:e54936. 10.1371/journal.pone.0054936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morand PC, Drab M, Rajalingam K, Nassif X, Meyer TF. 2009. Neisseria meningitidis differentially controls host cell motility through PilC1 and PilC2 components of type IV pili. PLoS One 4:e6834. 10.1371/journal.pone.0006834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De La Fuente L, Parker JK, Oliver JE, Granger S, Brannen PM, van Santen E, Cobine PA. 2013. The bacterial pathogen Xylella fastidiosa affects the leaf ionome of plant hosts during infection. PLoS One 8:e62945. 10.1371/journal.pone.0062945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bulyha I, Lindow S, Lin L, Bolte K, Wuichet K, Kahnt J, van der Does C, Thanbichler M, Sogaard-Andersen L. 2013. Two small GTPases act in concert with the bactofilin cytoskeleton to regulate dynamic bacterial cell polarity. Dev. Cell 25:119–131. 10.1016/j.devcel.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 56.Jakovljevic V, Leonardy S, Hoppert M, Sogaard-Andersen L. 2008. PilB and PilT are ATPases acting antagonistically in type IV pilus function in Myxococcus xanthus. J. Bacteriol. 190:2411–2421. 10.1128/JB.01793-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Toole GA, Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295–304. 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 58.O'Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49–79. 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 59.Guilhabert MR, Kirkpatrick BC. 2005. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to Xylella fastidiosa biofilm maturation and colonization and attenuate virulence. Mol. Plant Microbe Interact. 18:856–868. 10.1094/MPMI-18-0856. [DOI] [PubMed] [Google Scholar]
- 60.Killiny N, Martinez RH, Dumenyo CK, Cooksey DA, Almeida RPP. 2013. The exopolysaccharide of Xylella fastidiosa is essential for biofilm formation, plant virulence, and vector transmission. Mol. Plant Microbe Interact. 26:1044–1053. 10.1094/MPMI-09-12-0211-R. [DOI] [PubMed] [Google Scholar]
- 61.Yasukawa K, Martin P, Tinsley CR, Nassif X. 2006. Pilus-mediated adhesion of Neisseria meningitidis is negatively controlled by the pilus-retraction machinery. Mol. Microbiol. 59:579–589. 10.1111/j.1365-2958.2005.04954.x. [DOI] [PubMed] [Google Scholar]
- 62.Newman KL, Almeida RP, Purcell AH, Lindow SE. 2004. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. U. S. A. 101:1737–1742. 10.1073/pnas.0308399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chatterjee S, Wistrom C, Lindow SE. 2008. A cell–cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci. U. S. A. 105:2670–2675. 10.1073/pnas.0712236105. [DOI] [PMC free article] [PubMed] [Google Scholar]


