Abstract
Vibriosis is a leading cause of seafood-associated morbidity and mortality in the United States. Typically associated with consumption of raw or undercooked oysters, vibriosis associated with clam consumption is increasingly being reported. However, little is known about the prevalence of Vibrio spp. in clams. The objective of this study was to compare the levels of Vibrio cholerae, Vibrio vulnificus, and Vibrio parahaemolyticus in oysters and clams harvested concurrently from Long Island Sound (LIS). Most probable number (MPN)–real-time PCR methods were used for enumeration of total V. cholerae, V. vulnificus, V. parahaemolyticus, and pathogenic (tdh+ and/or trh+) V. parahaemolyticus. V. cholerae was detected in 8.8% and 3.3% of oyster (n = 68) and clam (n = 30) samples, with levels up to 1.48 and 0.48 log MPN/g in oysters and clams, respectively. V. vulnificus was detected in 97% and 90% of oyster and clam samples, with median levels of 0.97 and −0.08 log MPN/g, respectively. V. parahaemolyticus was detected in all samples, with median levels of 1.88 and 1.07 log MPN/g for oysters and clams, respectively. The differences between V. vulnificus and total and pathogenic V. parahaemolyticus levels in the two shellfish species were statistically significant (P < 0.001). These data indicate that V. vulnificus and total and pathogenic V. parahaemolyticus are more prevalent and are present at higher levels in oysters than in hard clams. Additionally, the data suggest differences in vibrio populations between shellfish harvested from different growing area waters within LIS. These results can be used to evaluate and refine illness mitigation strategies employed by risk managers and shellfish control authorities.
INTRODUCTION
The incidence of vibriosis in the United States has increased over the past decade (1), and it continues to be a leading cause of seafood-borne illnesses in this country (2). Among the most common causes of seafood-associated vibriosis are Vibrio cholerae (nontoxigenic), Vibrio vulnificus, and Vibrio parahaemolyticus (1, 2). Nontoxigenic (non-O1/non-O139) V. cholerae lacks the major virulence factor, cholera toxin, associated with the disease cholera (3). Infection by these strains typically results in a relatively mild form of gastroenteritis (4), but certain serotypes can cause a cholera-like illness (5, 6). Similarly, infections by the leading cause of vibriosis, V. parahaemolyticus, typically manifest as mild to moderate gastrointestinal illness (2, 7). While there is still much uncertainty surrounding V. parahaemolyticus virulence, the presence of the thermostable direct hemolysin (tdh) and tdh-related (trh) genes is commonly recognized as an indicator of pathogenicity (8, 9). Although it is a less frequent cause of vibriosis, V. vulnificus can cause more severe illness, including septicemia and death, particularly in individuals with predisposing conditions (2, 10).
In addition to the apparent effects of an expanding geographical range of vibrios (11), there is evidence that hard-shelled clams (Mercenaria mercenaria), as well as oysters (Crassostrea spp.), can be a vehicle for illness (12–14) and that they are contributing to increasing incidence. The prevalence of V. parahaemolyticus and V. vulnificus in freshly harvested oysters and stored shellstock is well documented (15–21). To a lesser extent, the occurrence and distribution of nontoxigenic V. cholerae in oysters and the environment has also been studied (22, 23). However, few data exist regarding the levels of these human pathogens in clams (24–27).
During the summer of 2012, V. parahaemolyticus illnesses associated with shellfish (oysters and clams) harvested from New York and Connecticut waters in Long Island Sound were reported (28). In response, both states closed the implicated shellfish growing areas. While these areas were closed, shellfish were collected for laboratory analysis. While the intent of the sample collection effort was V. parahaemolyticus testing as part of growing area reopening plans, the availability of samples provided a unique opportunity to compare vibrio levels in clams and oysters. Therefore, the objective of this study was to examine the levels of V. cholerae, V. vulnificus, and V. parahaemolyticus in oyster and clam samples concurrently harvested from Long Island Sound growing waters.
MATERIALS AND METHODS
Sample collection.
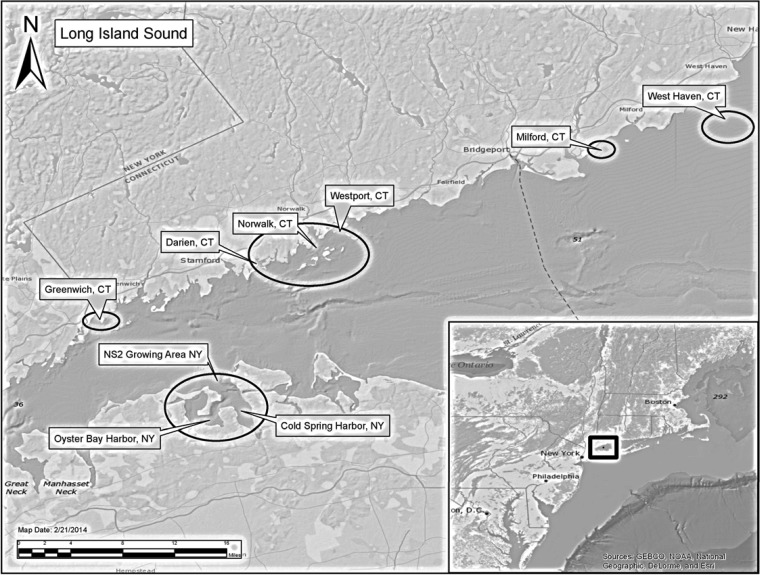
Shellfish samples consisting of oysters (Crassostrea virginica) and clams (M. mercenaria) from East and West Oyster Bay Harbor and outer Cold Spring Harbor, NY (Fig. 1), were collected by commercial harvesters under the direction of New York Department of Environmental Conservation personnel between 16 July and 18 September 2012. Department of Environmental Conservation personnel placed each bagged sample in an insulated cooler with a bubble wrap layer between the samples and wet ice. Collection times, as well as water temperatures and salinities, were recorded. Temperature and salinity were measured using a YSI Model 30 (YSI, Inc., Yellow Springs, OH).
FIG 1.
Study sites. The map shows New York and Connecticut sampling locations in Long Island Sound.
Shellfish samples were collected by Connecticut Department of Agriculture, Bureau of Aquaculture (DA-BA), staff from shellfish growing areas in Greenwich, Darien, Norwalk, Westport, Milford, and West Haven (Fig. 1) between 23 July and 24 September 2012. The majority of the shellfish samples were harvested by commercial harvesters under the direct supervision of DA-BA staff, with the exception of a few samples collected from dealer facilities. Cooler samples were harvested and held at a dealer facility no longer than 24 h under temperature control at ≤45°F until DA-BA collection. Collection time, water temperature, and salinity were recorded by DA-BA staff in the field at the time of collection. Temperature and salinity were measured using a YSI Model 30 or Pro30 (YSI, Inc.).
All samples were shipped via overnight delivery on insulated blue ice to FDA's Gulf Coast Seafood Laboratory (GCSL). A data logger was included with each shipment to ensure the samples were maintained at 50°F or less during transport. Any samples with an internal meat temperature of >50°F upon receipt were not included in the study report.
Sample analysis.
Analysis was initiated within 2 h of sample receipt and within 28 h of sample collection. Shellfish samples were analyzed for Vibrio spp. using most probable number (MPN)–real-time (RT) PCR as previously described (29). For each sample, the entire shell contents of 10 to 12 animals were aseptically removed and homogenized. The homogenate was used to prepare a three-tube, multiple-dilution MPN series in alkaline peptone water (APW) and incubated overnight at 35°C. The Bax Vibrio kit (DuPont Qualicon, Wilmington, DE) was used for simultaneous RT-PCR detection of V. parahaemolyticus, V. vulnificus, and V. cholerae from growth in APW following the manufacturer's recommended procedure.
A second multiplex RT-PCR method targeting the tdh and trh genes, with an internal amplification control (IAC), was used for identification of pathogenic V. parahaemolyticus (30). The tdh-trh RT-PCR was conducted in 25-μl reaction mixtures with 1× PCR buffer (Life Technologies, Foster City, CA), 5 mM MgCl2, 300 μM deoxynucleoside triphosphates (dNTPs) (mixed; equal concentrations), 200 μM each trh primer, 150 μM each tdh primer, 25 μM each IAC primer, 75 μM each trh and tdh nuclease style probe, 150 μM IAC probe, 2.25 U Platinum Taq DNA polymerase (Life Technologies), 2 μl IAC DNA template, and 2 μl target template (boiled lysates from APW growth). All primers and nuclease style probes were purchased from Integrated DNA Technologies (IDT) (Coralville, IA) or Life Technologies. Cycling was conducted on a SmartCyclerII system (Cepheid, Sunnyvale, CA) with an initial denaturation/polymerase activation at 95°C for 60 s, followed by 45 cycles of 95°C for 5 s and 59°C for 45 s with instrument optics turned on. Default instrument analysis parameters were used, except that the threshold was set at 15.
Statistical analysis.
Median vibrio levels are reported based on log-transformed values from all sample outcomes, with half the limit of detection (LOD) substituted for outcomes with nondetectable levels. Differences between distributions of abundances were evaluated by Mann-Whitney rank sum tests. These nonparametric tests were selected as generally applicable for all group level comparisons, given a high proportion of observations below the LOD for some gene targets (V. cholerae and pathogenic V. parahaemolyticus). Spearman correlation was used to assess the association between vibrio levels and environmental parameters. The statistical significances of observed differences and associations were determined using an alpha level of 0.05. All analyses were conducted using SigmaPlot 12.0 (Systat Software, Inc., San Jose, CA).
RESULTS
Vibrio levels by shellfish species.
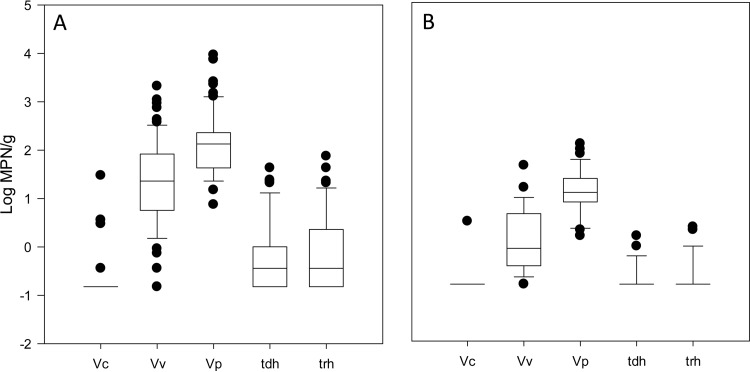
Figure 2 presents the levels of all Vibrio spp. tested in oysters and clams harvested from Long Island Sound. V. cholerae was detected in 6 of 68 oyster samples (8.8%) and in 1 of 30 clam samples (3.3%). In oysters, V. cholerae levels in samples above the LOD (−0.52 log MPN/g) ranged from −0.44 to 1.48 log MPN/g. The clam sample contained 0.48 log MPN/g of V. cholerae. V. vulnificus was detected in 66 of 68 oyster (97%) and 27 of 30 clam samples (90%). Levels ranged from below the LOD to 3.3 log MPN/g, with a median of 0.97 log MPN/g in oysters. In clams, V. vulnificus levels ranged from below the LOD to 1.6 log MPN/g, with a median of −0.08 log MPN/g. Total V. parahaemolyticus was detected in all 68 oyster and 30 clam samples, with median levels of 1.88 (range, 0.88 to 4.0) and 1.07 (range, 0.18 to 2.1) log MPN/g, respectively. Pathogenic (tdh+) V. parahaemolyticus was detected in 39 of 68 oyster (57%) and 5 of 30 clam (17%) samples tested. The levels of tdh+ V. parahaemolyticus ranged from below the LOD to 1.63 log MPN/g in oysters and 1.00 log MPN/g in clams. Similarly, trh+ V. parahaemolyticus was detected in 47 of 68 oyster (69%) and 7 of 30 clam (23%) samples, with ranges from below the LOD to 1.88 and 0.36 log MPN/g in oysters and clams, respectively.
FIG 2.
Vibrio species levels in shellfish harvested from Long Island Sound. Shown are box plots of V. cholerae (Vc), V. vulnificus (Vv), total V. parahaemolyticus (Vp), and potentially pathogenic (tdh+ and trh+) V. parahaemolyticus in oysters (A) and clams (B). For observations below the LOD (−0.52 log MPN/g), the value of 1/2 the LOD was substituted. The band inside each box indicates the median value. Lower and upper lines of the box represent the 25th and 75th percentiles, respectively. Lower and upper limits of the whiskers represent the 10th and 90th percentiles, respectively.
No significant difference in levels of V. cholerae between the shellfish species was observed (P = 0.342). However, the differences in distribution of V. vulnificus, as well as total and pathogenic V. parahaemolyticus, levels in oysters and clams were statistically significant (P < 0.001).
Vibrio levels by harvest state.
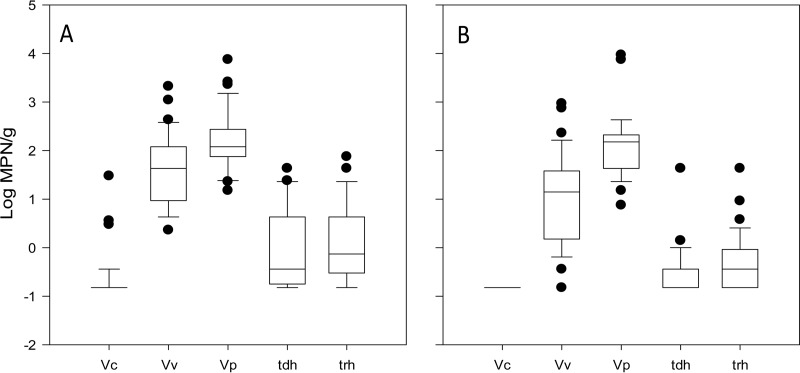
Sixty shellfish (35 oyster and 25 clam) samples from New York and 38 (33 oyster and 5 clam) samples from Connecticut were analyzed. As a significant difference in vibrio levels between shellfish species was identified and only five clam samples were collected in Connecticut, the distribution of vibrios across state growing areas was only examined using oysters (Fig. 3). All six oyster samples with detectable V. cholerae levels were harvested from New York waters on four separate sampling occasions. Median V. vulnificus levels were 1.63 (range, 0.36 to 3.32) log MPN/g and 1.15 (range, below the LOD to 2.97) log MPN/g from New York and Connecticut oysters, respectively. The median V. parahaemolyticus levels were 2.08 (range, 1.18 to 3.88) log MPN/g for New York oysters and 2.18 (range, 0.88 to 3.97) log MPN/g for Connecticut oysters. The median levels of tdh+ V. parahaemolyticus were −0.44 (range, below the LOD to 1.63) log MPN/g from New York oysters and −0.52 (range, below the LOD to 1.63) log MPN/g from Connecticut shellfish. The median trh+ V. parahaemolyticus levels were −0.13 (range, below the LOD to 1.88) log MPN/g and −0.44 (range, below the LOD to 1.63) log MPN/g in New York and Connecticut oysters, respectively.
FIG 3.
Distribution of vibrios between state waters. Shown are box plots of V. cholerae (Vc), V. vulnificus (Vv), total V. parahaemolyticus (Vp), and pathogenic (tdh+ and trh+) V. parahaemolyticus in oysters harvested from New York (A) and Connecticut (B) growing areas. For observations below the LOD (−0.52 log MPN/g), the value of 1/2 the LOD was substituted. The band inside each box indicates the median value. Lower and upper lines of the box represent the 25th and 75th percentiles, respectively. Lower and upper limits of the whiskers represent the 10th and 90th percentiles, respectively.
The differences in V. cholerae (P = 0.014), V. vulnificus (P = 0.010), and tdh+ V. parahaemolyticus (P = 0.002) levels in New York versus Connecticut oysters were statistically significant. The differences in trh+ V. parahaemolyticus levels were found to be marginally nonsignificant (P = 0.052). However, the differences in total V. parahaemolyticus levels in New York versus Connecticut oysters were not significant (P = 0.605).
Association of vibrios with environmental parameters.
Water temperature ranged from 20.2 to 26.0°C (mean, 23.3°C), and salinity ranged from 22.8 to 27.7 ppt (mean 26.2 ppt) during the sampling period (Table 1). No significant correlations (P > 0.05) between levels of V. cholerae or V. vulnificus in shellfish and temperature or salinity were determined. In addition, no significant correlation (P > 0.05) between temperature and total V. parahaemolyticus levels was identified; however, significant positive correlations were observed between temperature and pathogenic (tdh, Spearman's correlation coefficient [rs] = 0.317, P = 0.003; trh, rs = 0.348, P = 0.001) V. parahaemolyticus. A significant negative correlation was identified between salinity and levels of total (rs = −0.330, P = 0.002) and pathogenic (tdh, rs = −0.334, P = 0.002; trh, rs = −0.415, P < 0.001) V. parahaemolyticus.
TABLE 1.
Median temperature, salinity, and vibrio levels in shellfish from Long Island Sound over the study perioda
| Sampling date (2012) | nb | Temp (°C) | Salinity (ppt) | Level (log MPN/g) |
||||
|---|---|---|---|---|---|---|---|---|
| V. cholerae | V. vulnificus | Total V. parahaemolyticus | tdh+ V. parahaemolyticus | trh+ V. parahaemolyticus | ||||
| 16 July | 11 | 23.8 (22.9–24.3) | 25.5 (25.4–25.7) | −0.82 (−0.82 to −0.44) | 0.36 (−0.82–2.6) | 1.88 (0.97–3.18) | 0.18 (−0.82–1.63) | 0.30 (−0.82–1.88) |
| 23 July | 5 | NDc | ND | <LODd | −0.04 (−0.82–1.15) | 1.88 (0.88–2.18) | −0.82 (−0.82 to −0.44) | −0.44 (−0.82–0.36) |
| 30 July | 10 | 23.1 (22.0–23.5) | 26.2 (26.0–26.5) | <LOD | 0.63 (−0.82–1.36) | 1.36 (0.18–2.36) | −0.24 (−0.82–0.63) | −0.24 (−0.82–0.63) |
| 6 August | 19 | 25.1 (22.6–26.0) | 25.8 (25.2–26.0) | −0.82 (−0.82 to −0.44) | 0.88 (−0.82–3.32) | 2.18 (0.36–3.97) | −0.82 (−0.82–1.63) | −0.44 (−0.82–1.36) |
| 13 August | 8 | 23.1 (22.5–24.5) | 25.9 (25.6–26.1) | <LOD | 0.72 (−0.44–1.58) | 2.02 (1.18–2.63) | −0.82 (−0.82 to −0.04) | −0.63 (−0.82–0.58) |
| 4 September | 10 | 24.5 (23.8–24.7) | 26.8 (26.2–27.3) | <LOD | 0.97 (−0.44–2.58) | 1.97 (0.63–2.88) | −0.44 (−0.82–0.63) | −0.44 (−0.82–0.63) |
| 10 September | 8 | 23.7 (23.5–23.8) | 26.1 (26.9–26.1) | <LOD | 1.63 (1.36–2.97) | 2.32 (1.63–2.46) | −0.82 (−0.82 to −0.04) | −0.44 (−0.82–0.96) |
| 11 September | 10 | 23.1 (22.4–23.2) | 27.0 (22.8–27.6) | −0.82 (−0.82–0.56) | 1.14 (−0.14–2.36) | 1.47 (0.30–3.36) | −0.82 (−0.82 to −0.52) | −0.82 (−0.82 to −0.52) |
| 18 September | 10 | 22.5 (22.5–22.7) | 27.4 (27.4–27.7) | <LOD | 1.63 (−0.13–2.18) | 1.51 (0.88–2.32) | −0.82 (−0.82 to −0.44) | −0.82 (−0.82–0.36) |
| 24 September | 7 | 20.40 (20.2–21.2) | 26.0 (25.8–26.1) | <LOD | 15.8 (0.36–2.36) | 2.08 (1.36–2.63) | −0.82 (−0.82–0.15) | −0.82 (−0.82–0.15) |
Values are reported as median levels (range) from all independent shellfish samples collected on that date.
n, number of independent shellfish samples collected.
No temperature or salinity data were available for the 23 July sampling.
<LOD indicates all shellfish samples were below the limit of detection. For observations of Vibrio sp. levels of <LOD, the value of 1/2 the LOD was substituted.
DISCUSSION
This study compared the levels of Vibrio spp. of greatest human health concern in oysters (C. virginica) and clams (M. mercenaria) harvested concurrently from similar harvest areas. In this study, V. cholerae was detected sporadically and at low levels, which indicates a persistent but small population of total V. cholerae in Long Island Sound shellfish. This is consistent with previous findings concerning indigenous populations of nontoxigenic V. cholerae in the northeast (31, 32).
Oysters contained significantly higher levels of V. vulnificus and V. parahaemolyticus (total and pathogenic) than clams. As a result of the V. parahaemolyticus data generated during this study, New York and Connecticut growing area waters that were closed to shellfish harvest due to illness reports were reopened for clam harvesting earlier than for oyster harvesting. Interestingly, the difference between pathogenic V. parahaemolyticus levels in the two shellfish species may be mostly attributable to the significantly higher frequency of detection in oysters. This means that pathogenic V. parahaemolyticus is detected less frequently in clams than in oysters; however, the levels of pathogenic strains in clams are similar to those in oysters on the occasions when they are detected. This could help explain why illnesses are associated with clams, albeit at a much lower frequency than oyster-associated illnesses.
Similar to our results, Hood et al. (25) examined the microbiological levels in oysters (C. virginica) and clams (Mercenaria campechiensis) from a common harvest area and found the microbial loads to be significantly lower in the clam samples. While V. parahaemolyticus, V. cholerae, and “L+ vibrio” (V. vulnificus) were part of the total microbial load, the data presented in the Hood et al. study did not specifically compare the levels of these organisms between freshly harvested oysters and clams. They did, however, provide evidence for the growth of all three organisms in oysters and clams (25), contrary to a later study that suggested minimal changes of V. vulnificus levels in stored clams (M. mercenaria) (27). In the National Shellfish Sanitation Program, the internal temperature of shellstock must be ≤50°F before the product can be repacked or shipped (33). In our study, two clam samples were received at the analytical laboratory at temperatures of >50°F. These samples were not included in the data presented but had ∼0.5-log-unit higher V. parahaemolyticus and ∼2-log-unit higher V. vulnificus levels than any of the samples that were maintained at ≤50°F, suggesting growth of these Vibrio spp. in clams. More conclusive studies on the growth and survival of the Vibrio spp. in clams are needed to fully understand the associated public health risk and proper postharvest handling strategies.
In addition to examining the differences in vibrio loads between shellfish species, we looked for differences in vibrio levels between shellfish harvested from New York and shellfish harvested from Connecticut. This comparison was limited to oysters, as a similar number of samples were harvested from the two states and our data demonstrated that clams have lower vibrio levels than oysters, so inclusion of both species could potentially skew the data. Higher median levels of all vibrios tested were found in New York oysters, with the exception of total V. parahaemolyticus. It is interesting that, although there was no significant difference between total V. parahaemolyticus levels, tdh+ V. parahaemolyticus levels were significantly higher in New York oysters.
No correlation with temperature and salinity was observed with V. vulnificus levels in shellfish, most likely due to the narrow range of temperatures and salinities observed being equally permissive for V. vulnificus. An inverse correlation between water salinity and V. parahaemolyticus levels in oysters was observed. Previous reports have provided conflicting conclusions on the correlation of Vibrio spp. with water temperature and salinity (21, 34–36). Overall, it appears these associations are dependent on the geographical location, as well as the range of temperature and salinity occurring during the study period. The apparent linear relationship between salinity and V. parahaemolyticus levels observed in the present study is likely due to salinities being on the high end of optimal (15 to 25 ppt) for the species (17). These observations are not inconsistent with a nonlinear relationship observed over a wider salinity range, such as that identified by Johnson et al. (20). These results highlight the need for a better understanding of the suite of environmental variables that affect Vibrio sp. prevalence and abundance in the environment.
In summary, the current study examined the abundance of Vibrio spp. in oyster and clam samples harvested from New York and Connecticut waters in Long Island Sound from July to September 2012. The results indicate that V. cholerae, V. vulnificus, and total and pathogenic V. parahaemolyticus are more prevalent in oysters than in hard clams. Additionally, the data suggest differences in the prevalence and abundance of Vibrio spp. between New York and Connecticut shellfish, even though the growing area waters are all within Long Island Sound. This information can be used to evaluate and refine management strategies used by shellfish regulatory authorities.
ACKNOWLEDGMENTS
We thank Joe Orlando for sample collection in New York and Jennifer Yeadon and Alissa Dragan for sample collection in Connecticut. We are grateful for the critical review of the manuscript by members of the New York Department of Environmental Conservation laboratory staff and Captain W. Burkhardt III.
This project was supported, in part, by an appointment to the Research Fellowship Program for the Center for Food Safety and Applied Nutrition administered by the Oak Ridge Associated Universities through a contract with the FDA.
Footnotes
Published ahead of print 3 October 2014
REFERENCES
- 1.Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. 2012. Increasing rates of vibriosis in the United States, 1996-2010: review of surveillance data from 2 systems. Clin. Infect. Dis. 54(Suppl 5):S391–S395. 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwamoto M, Ayers T, Mahon BE, Swerdlow DL. 2010. Epidemiology of seafood-associated infections in the United States. Clin. Microbiol. Rev. 23:399–411. 10.1128/CMR.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandal S, Mandal MD, Pal NK. 2011. Cholera: a great global concern. Asian Pac. J. Trop. Med. 4:573–580. 10.1016/S1995-7645(11)60149-1. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. 2012. Bad bug book: foodborne pathogenic microorganisms and natural toxins, 2nd ed, p 42–45 Food and Drug Administration, Washington, DC. [Google Scholar]
- 5.Crump JA, Bopp CA, Greene KD, Kubota KA, Middendorf RL, Wells JG, Mintz ED. 2003. Toxigenic Vibrio cholerae serogroup O141-associated cholera-like diarrhea and bloodstream infection in the United States. J. Infect. Dis. 187:866–868. 10.1086/368330. [DOI] [PubMed] [Google Scholar]
- 6.Tobin-D'Angelo M, Smith AR, Bulens SN, Thomas S, Hodel M, Izumiya H, Arakawa E, Morita M, Watanabe H, Marin C, Parsons MB, Greene K, Cooper K, Haydel D, Bopp C, Yu P, Mintz E. 2008. Severe diarrhea caused by cholera toxin-producing Vibrio cholerae serogroup O75 infections acquired in the southeastern United States. Clin. Infect. Dis. 47:1035–1040. 10.1086/591973. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. 2012. Bad bug book: foodborne pathogenic microorganisms and natural toxins, 2nd ed, p 27–29 Food and Drug Administration, Washington, DC. [Google Scholar]
- 8.Shimohata T, Takahashi A. 2010. Diarrhea induced by infection of Vibrio parahaemolyticus. J. Med. Invest. 57:179–182. 10.2152/jmi.57.179. [DOI] [PubMed] [Google Scholar]
- 9.Su YC, Liu C. 2007. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 24:549–558. 10.1016/j.fm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. 2012. Bad bug book: foodborne pathogenic microorganisms and natural toxins, 2nd ed, p 46–49 Food and Drug Administration, Washington, DC. [Google Scholar]
- 11.McLaughlin JB, DePaola A, Bopp CA, Martinek KA, Napolilli NP, Allison CG, Murray SL, Thompson EC, Bird MM, Middaugh JP. 2005. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N. Engl. J. Med. 353:1463–1470. 10.1056/NEJMoa051594. [DOI] [PubMed] [Google Scholar]
- 12.Slayton RB, Newton AE, DePaola A, Jones JL, Mahon BE. 2014. Clam-associated vibriosis, USA, 1988-2010. Epidemiol. Infect. 142:1083–1088. 10.1017/S0950268813001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace BJ, Guzewich JJ, Cambridge M, Altekruse S, Morse DL. 1999. Seafood-associated disease outbreaks in New York, 1980-1994. Am. J. Prev. Med. 17:48–54. 10.1016/S0749-3797(99)00037-9. [DOI] [PubMed] [Google Scholar]
- 14.Wechsler E, D'Aleo C, Hill VA, Hopper J, Myers-Wiley D, O'Keeffe E, Jacobs J, Guido F, Huang A, Dodt SN, Rowan B, Sherman M, Greenberg A, Schneider D, Noone B, Fanella L, Williamson BR, Dinda E, Mayer M, Backer M, Agasan A, Kornstein L, Stavinsky F, Neal B, Edwards D, Haroon M, Hureley D, Colbert L, Miller J, Mojica B, Carloni E, Devine B, Cambridge M, Root T, Schoonmaker D, Shayegani M, Hastback E, Wallace B, Kondracki S, Smith P, Matiuck S, Pilot K, Acharya M, Wolf G, Manley W, Genese C, Brooks J, Hadler J. 1999. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York, 1998. MMWR Morb. Mortal. Wkly. Rep. 48:48–51. [PubMed] [Google Scholar]
- 15.Cook DW. 1994. Effect of time and temperature on multiplication of Vibrio vulnificus in postharvest Gulf Coast shellstock oysters. Appl. Environ. Microbiol. 60:3483–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook DW, Bowers JC, DePaola A. 2002. Density of total and pathogenic (tdh+) Vibrio parahaemolyticus in Atlantic and Gulf coast molluscan shellfish at harvest. J. Food Prot. 65:1873–1880. [DOI] [PubMed] [Google Scholar]
- 17.DePaola A, Nordstrom JL, Bowers JC, Wells JG, Cook DW. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 69:1521–1526. 10.1128/AEM.69.3.1521-1526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DePaola A, Jones JL, Woods J, Burkhardt W, III, Calci KR, Krantz JA, Bowers JC, Kasturi K, Byars RH, Jacobs E, Williams-Hill D, Nabe K. 2010. Bacterial and viral pathogens in live oysters: 2007 United States market survey. Appl. Environ. Microbiol. 76:2754–2768. 10.1128/AEM.02590-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellison RK, Malnati E, DePaola A, Bowers J, Rodrick GE. 2001. Populations of Vibrio parahaemolyticus in retail oysters from Florida using two methods. J. Food Prot. 64:682–686. [DOI] [PubMed] [Google Scholar]
- 20.Johnson CN, Flowers AR, Noriea NF, III, Zimmerman AM, Bowers JC, DePaola A, Grimes DJ. 2010. Relationships between environmental factors and pathogenic vibrios in the Northern Gulf of Mexico. Appl. Environ. Microbiol. 76:7076–7084. 10.1128/AEM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright AC, Hill RT, Johnson JA, Roghman MC, Colwell RR, Morris JG., Jr 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl. Environ. Microbiol. 62:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DePaola A, Presnell MW, Jr, Motes ML, McPhearson MR, Twedt RM, Becker RE, Zywno S. 1983. Non-O1 Vibrio cholerae in shellfish, sediment and waters of the U.S. Gulf coast. J. Food Prot. 46:802–806. [DOI] [PubMed] [Google Scholar]
- 23.Cantet F, Hervio-Heath D, Caro A, Le MC, Monteil C, Quemere C, Jolivet-Gougeon A, Colwell RR, Monfort P. 2013. Quantification of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae in French Mediterranean coastal lagoons. Res. Microbiol. 164:867–874. 10.1016/j.resmic.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu WT, Jong KJ, Lin YR, Tsai SE, Tey YH, Wong HC. 2013. Prevalence of Vibrio parahaemolyticus in oyster and clam culturing environments in Taiwan. Int. J. Food Microbiol. 160:185–192. 10.1016/j.ijfoodmicro.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Hood MA, Ness GE, Rodrick GE, Blake NJ. 1983. Effects of storage on microbial loads of two commercially important shellfish species, Crassostrea virginica and Mercenaria campechiensis. Appl. Environ. Microbiol. 45:1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utsalo SJ, Mboto CI, Gemade EI, Nwangwa MA. 1988. Halophilic Vibrio spp. associated with hard clams (Mercenaria spp.) from the Calabar river estuary. Trans. R. Soc. Trop. Med. Hyg. 82:327–329. 10.1016/0035-9203(88)90464-6. [DOI] [PubMed] [Google Scholar]
- 27.Brenton CE, Flick GJ, Jr, Pierson MD, Croonenberghs RE, Peirson M. 2001. Microbiological quality and safety of quahog clams, Mercenaria mercenaria, during refrigeration and at elevated storage temperatures. J. Food Prot. 64:343–347. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Urtaza J, Baker-Austin C, Newton AE, Jones JL, Gonzalez-Aviles GD, DePaola A. 2013. Transoceanic spread of Pacific Northwest Vibrio parahaemolyticus strains. N. Engl. J. Med. 369:1573–1574. 10.1056/NEJMc1305535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones JL, Hara-Kudo Y, Krantz JA, Benner RA, Jr, Smith AB, Dambaugh TR, Bowers JC, DePaola A. 2012. Comparison of molecular detection methods for Vibrio parahaemolyticus and Vibrio vulnificus. Food Microbiol. 30:105–111. 10.1016/j.fm.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Jones JL, Lüdeke CHM. 2012. Improved detection of pathogenic Vibrio parahaemolyticus from oyster, water, and sediment using real-time PCR, abstr. 2055 Abstr. 112th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC. [Google Scholar]
- 31.Heidelberg JF, Heidelberg KB, Colwell RR. 2002. Seasonality of Chesapeake Bay bacterioplankton species. Appl. Environ. Microbiol. 68:5488–5497. 10.1128/AEM.68.11.5488-5497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaper J, Lockman H, Colwell RR, Joseph SW. 1979. Ecology, serology, and enterotoxin production of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 37:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anonymous. 2011. National shellfish sanitation program (NSSP). Guide for the control of molluscan shellfish, 2011 revision. Food and Drug Administration, Interstate Shellfish Sanitation Conference. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/Food/GuidanceRegulation/FederalStateFoodPrograms/ucm2006754.htm. [Google Scholar]
- 34.Johnson CN, Bowers JC, Griffitt KJ, Molina V, Clostio RW, Pei S, Laws E, Paranjpye RN, Strom MS, Chen A, Hasan NA, Huq A, Noriea NF, III, Grimes DJ, Colwell RR. 2012. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Appl. Environ. Microbiol. 78:7249–7257. 10.1128/AEM.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louis VR, Russek-Cohen E, Choopun N, Rivera IN, Gangle B, Jiang SC, Rubin A, Patz JA, Huq A, Colwell RR. 2003. Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 69:2773–2785. 10.1128/AEM.69.5.2773-2785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobrinho PS, Destro MT, Franco BD, Landgraf M. 2010. Correlation between environmental factors and prevalence of Vibrio parahaemolyticus in oysters harvested in the southern coastal area of Sao Paulo State, Brazil. Appl. Environ. Microbiol. 76:1290–1293. 10.1128/AEM.00861-09. [DOI] [PMC free article] [PubMed] [Google Scholar]