Abstract
The metabolism of hydroxycinnamic acids by strictly heterofermentative lactic acid bacteria (19 strains) was investigated as a potential alternative energy route. Lactobacillus curvatus PE5 was the most tolerant to hydroxycinnamic acids, followed by strains of Weissella spp., Lactobacillus brevis, Lactobacillus fermentum, and Leuconostoc mesenteroides, for which the MIC values were the same. The highest sensitivity was found for Lactobacillus rossiae strains. During growth in MRS broth, lactic acid bacteria reduced caffeic, p-coumaric, and ferulic acids into dihydrocaffeic, phloretic, and dihydroferulic acids, respectively, or decarboxylated hydroxycinnamic acids into the corresponding vinyl derivatives and then reduced the latter compounds to ethyl compounds. Reductase activities mainly emerged, and the activities of selected strains were further investigated in chemically defined basal medium (CDM) under anaerobic conditions. The end products of carbon metabolism were quantified, as were the levels of intracellular ATP and the NAD+/NADH ratio. Electron and carbon balances and theoretical ATP/glucose yields were also estimated. When CDM was supplemented with hydroxycinnamic acids, the synthesis of ethanol decreased and the concentration of acetic acid increased. The levels of these metabolites reflected on the alcohol dehydrogenase and acetate kinase activities. Overall, some biochemical traits distinguished the common metabolism of strictly heterofermentative strains: main reductase activity toward hydroxycinnamic acids, a shift from alcohol dehydrogenase to acetate kinase activities, an increase in the NAD+/NADH ratio, and the accumulation of supplementary intracellular ATP. Taken together, the above-described metabolic responses suggest that strictly heterofermentative lactic acid bacteria mainly use hydroxycinnamic acids as external acceptors of electrons.
INTRODUCTION
Phenolic acids include aromatic secondary plant metabolites which possess a carboxylic acid functional group. They account for almost one-third of the dietary phenols. Hydroxycinnamic acids, such as caffeic, p-coumaric, and ferulic acids, are the most abundant class of phenolic acids in fruits, vegetables, and cereal grains. They either are covalently bonded to the plant cell wall or are present in soluble form within the cytoplasm (1, 2).
During plant fermentation, lactic acid bacteria have to interact with inherent phenolics. Depending on the chemical structure and concentration of phenolic acids of the food matrices, bacterial growth and viability are diversely affected. Low concentrations of gallic acid exert stimulatory effects on the growth and malolactic activity of lactic acid bacteria (3). Conversely, high concentrations of phenolic acids (>3 mM) negatively affect the integrity of the cell wall and membrane (3) and dissipate the pH gradient (4). Phenolic acids also delay the metabolism of carbohydrates by lactic acid bacteria (3).
To the best of our knowledge, the adaptation to and metabolism of phenolic acids have been investigated exclusively for Lactobacillus plantarum and rarely for other Lactobacillus spp. (e.g., Lactobacillus brevis and Lactobacillus fermentum) and lactic acid bacteria used for wine making (e.g., Oenococcus oeni) (3). On the contrary, the metabolism of phenolic acids by Weissella spp. and Leuconostoc mesenteroides, which are the main lactic acid bacterial species, together with L. plantarum, which is responsible for fruit and vegetable fermentation, has not been investigated or has been only poorly investigated. Strains of Weissella cibaria, Weissella confusa, and Leuc. mesenteroides were proposed to be used as starter cultures to guarantee high-quality standards during vegetable and fruit fermentations (5–8). Because of the volatile compounds that are released, the bacterial metabolism of hydroxycinnamic acids may positively reflect on the aroma and sensory properties of fermented foods (3, 9). Moreover, the human health benefits associated with the consumption of phenolics are, in part, dependent on microbial conversion (10). More in-depth knowledge of the bioconversion pathways of phenolic acids may facilitate the selection of microbial starters for improved competitiveness and optimal technology and functional features.
Although the metabolism of phenolics by lactic acid bacteria was demonstrated (3), its physiological significance has also been poorly investigated. Degradation of phenolics by lactic acid bacteria is certainly a mechanism to detoxify such compounds (4, 11). Reduction of phenolic acids may involve the reoxidation of the reduced cofactor NADH, providing an energy advantage through NAD+ regeneration (12). According to this hypothesis, when L. plantarum and Lactobacillus collinoides grew under anaerobic conditions or in the absence of additional electron acceptors, they reduced 4-vinylphenol to 4-ethylphenol for increasing the availability of NAD+ (9).
The exploitation of the real significance of the reduction of phenolic acids may be of particular interest mainly for strictly heterofermentative lactic acid bacteria, which have lower energy yields than bacteria with a homofermentative metabolism. During food fermentations, the competitiveness of strictly heterofermentative lactic acid bacteria depends on the use of external electron acceptors. Several examples of cosubstrates used to oxidize the cofactor NADH by heterofermentative species were previously shown (13). The reduction of aldehydes or oxidized glutathione was also coupled with the oxidation of NADH to NAD+, which allowed heterofermentative strains to synthesize additional ATP (14).
This study aimed at investigating the metabolism of hydroxycinnamic acids by strictly heterofermentative lactic acid bacteria as a potential alternative energy route.
The interpretation of strain behavior under the conditions used in this study should help highlight the effects of hydroxycinnamic acids on bacterial growth, physiology, and fermentation end products and should allow the selection of potential starters with high metabolic performance, especially to ferment food matrices with high levels of these compounds.
MATERIALS AND METHODS
Bacterial strains.
Nineteen strains of strictly heterofermentative lactic acid bacteria belonging to the Culture Collection of the Department of Soil, Plant and Food Science, University of Bari Aldo Moro, Bari, Italy, were used in this study (Table 1). All strains were previously isolated from fruits and vegetables. Strains were identified by partial sequencing of the 16S rRNA, recA, pheS, and rpoA genes. Cultures were maintained as stocks in 15% (vol/vol) glycerol at −80°C and routinely propagated at 30°C for 24 h in MRS broth (Oxoid, Basingstoke, Hampshire, United Kingdom). Strains of Weissella cibaria, Weissella confusa, and Leuconostoc mesenteroides were used for their leading role during fruit and vegetable fermentations (5–8, 18). Lactobacillus rossiae, Lactobacillus brevis, Lactobacillus fermentum, and Lactobacillus curvatus strains were used, as they are frequently involved during spontaneous fermentations of plant substrates (18).
TABLE 1.
Lactic acid bacteria used in this studya
| Strain(s) | Source | Reference or source |
|---|---|---|
| Weissella cibaria P9, P10 | Papaya | 15 |
| W. cibaria 3XMR3, 3XMR5 | Pineapple | 16 |
| W. cibaria/W. confusa POM12, POM15, POM16, POM17 | Tomato | 8 |
| Lactobacillus brevis POM2, POM4 | Tomato | 8 |
| Lactobacillus fermentum F1 | French beans | 7 |
| Lactobacillus curvatus PE5 | Peppers | 17 |
| Leuconostoc mesenteroides PES7, PES1 | Peach | R. Di Cagno (unpublished data) |
| Leuc. mesenteroides KI6 | Kiwi fruit | R. Di Cagno (unpublished data) |
| Lactobacillus rossiae 2MR6, 2MR8, 2MR10, 2MR15 | Pineapple | 16 |
Nineteen strains were tested.
Chemicals.
Caffeic acid, p-coumaric acid, ferulic acid, dihydrocaffeic acid, phloretic acid, dihydroferulic acid, ethylphenol, ethylcatechol, ethylguaiacol, and vinylguaiacol were obtained from Sigma-Aldrich (St. Louis, MO). p-Vinylphenol was obtained from Lancaster Synthesis (Windham, NH).
MIC.
The MICs of hydroxycinnamic acids (caffeic, p-coumaric, or ferulic acid) during the growth of lactic acid bacteria were determined by the critical dilution assay (11, 19). The phenolic compounds were dissolved in methanol and diluted in MRS broth to a final concentration of 30 mM. The pH was adjusted to 7.0 with 2 N NaOH, in order to exclude the effect of pH on the activity of phenolic acids. Serial 2-fold dilutions were made with MRS broth (pH 7.0) in sterile 96-well microtiter plates (Greiner Labortechnik). Lactic acid bacterial strains were subcultured in MRS broth under the above-described conditions. Logarithmic-phase cells (ca. 108 CFU/ml) were harvested by centrifugation (8,000 × g for 10 min), washed twice with 10 mM phosphate buffer, pH 7.0, and adjusted to ca. 105 CFU/ml. Each well was inoculated (10%, vol/vol). The final concentration of phenolic acids in sterile 96-well microtiter plates ranged from 27.3 mM to 1.7 mM. Microtiter plates were incubated for 24 h. After incubation, bacterial growth was determined by measuring the optical density at 620 nm (OD620). The MIC was defined as the lowest concentration of phenolic acids inhibiting bacterial growth. Control wells contained all the components except phenolic acids, which were replaced with distilled water (positive control) or with chloramphenicol (100 μg/ml; negative control).
Metabolism of phenolic acids during growth in MRS broth.
MRS broth was supplemented with caffeic, p-coumaric, or ferulic acid at a concentration of 1 mM (1, 11). Single cells from an overnight culture were inoculated (5%, vol/vol) into supplemented MRS medium and incubated for 24 h at 30°C. Sterile media containing the corresponding phenolic compounds, without a bacterial inoculum, were used as the control. Viable cells were enumerated by surface plating onto MRS agar. The pH was measured by use of a Foodtrode electrode (Hamilton, Bonaduz, Switzerland). After incubation, cells were removed by centrifugation (10,000 × g for 10 min), and the supernatant was acidified to pH 1.5 with hydrochloric acid. Ethyl acetate (3 ml) was used for liquid-liquid extraction. The extracts (20 μl) were analyzed by high-performance liquid chromatography (HPLC), using an Äkta purifier system (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) equipped with an XTerra MS C18 column (particle size, 5 μm; 4.6 by 250 mm; Waters, Brussels, Belgium) and a UV-900 detector (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) at 280 nm. Eluent A consisted of 0.1% (vol/vol) formic acid in HPLC-grade water, and eluent B consisted of 0.1% (vol/vol) formic acid in acetonitrile (30%, vol/vol) and HPLC-grade water (10%, vol/vol). Extracts were eluted with the following gradient: 0% eluent B (3 min), 0 to 40% eluent B (10 min), 40 to 60% eluent B (60 min), 60 to 100% eluent B (10 min), 100% eluent B (20 min), and 100 to 0% eluent B (15 min). The efficiency of conversion of hydroxycinnamic acids into their derivatives was calculated as the amount (mmol) of end products produced in a defined period divided by the amount (mmol) of hydroxycinnamic acid consumed in the same period times 100. Identification was confirmed by nano-liquid chromatography (LC)-electrospray ionization (ESI)-mass spectrometry on a Finningan LCQ Deca XP Max ion trap mass spectrometer (Thermo Electron, San Jose, CA) equipped with a nano-ESI interface. MS spectra were automatically collected by Xcalibur software (Thermo Electron) in the negative-ion mode. The total ion current (m/z range, 50 to 2,000) and selected ion-monitoring spectra were recorded and processed with BioWorks software (version 3.2; Thermo Electron). External standards analyzed under the same conditions were used for the identification and quantification of hydroxycinnamic acids and hydroxycinnamic acid derivatives. Vinylcatechol (for which a standard is not available) was identified by comparing its retention time and molecular ion with data reported in the literature.
Growth in CDM.
Lactic acid bacterial strains showing phenolic acid reductase activity were grown (with the initial cell number corresponding to ca. 108 CFU/ml) anaerobically at 30°C for 24 h in chemically defined basal medium (CDM), which was prepared as described by McFeeters and Chen (20), supplemented with caffeic, p-coumaric, or ferulic acid at a concentration of 2 mM. This concentration was well below the MIC. CDM not supplemented with phenolic acids was used as the control. Growth was determined by measuring the optical density at 620 nm. The kinetics of growth were determined and modeled according to the Gompertz equation, as modified by Zwietering et al. (21): y = k + A exp{−exp[(μmax e/A)(λ − t) + 1]}, where y is the OD620 at time t, k is the initial level of the dependent variable to be modeled (OD620 units), A is the difference in cell density between the inoculum and the stationary phase, μmax is the maximum growth rate (expressed as OD620 units h−1), λ is the length of the lag phase (expressed in hours), and t is time. The consumption of hydroxycinnamic acids was determined by HPLC, as described above. After 24 h of incubation, cells and supernatants recovered through centrifugation (10,000 × g, 10 min, 4°C) were used for further analyses.
Glucose consumption and main fermentation end products.
The supernatants recovered from cultures grown in CDM were filtered through a Millex-HA 0.22-μm-pore-size filter (Millipore Co.) and used to determine glucose, lactic acid, acetic acid, acetaldehyde, and ethanol by HPLC. An Äkta purifier system (GE Healthcare) was equipped with an Aminex HPX-87H column (ion exclusion; Bio-Rad), a UV detector operating at 210 nm, and a PerkinElmer 200a refractive index detector (PerkinElmer, Waltham, MA, USA) operating at 32°C. Elution was at 60°C with a flow rate of 0.6 ml min−1, and 10 mM H2SO4 was used as the mobile phase (22).
Acetic acid and ethanol were also determined by use of a headspace (HD)-gas chromatograph (GC)-mass spectrometer. A Clarus 680 gas chromatograph (PerkinElmer, Shalton, CT, USA) was equipped with a Clarus SQ 8 C mass spectrometer (PerkinElmer, Shalton, CT, USA) and a Combipal CTC autosampler (CTC Analytics AG, Switzerland) with a 2.5-ml HD syringe. The headspace program required vial equilibration at 60°C for 10 min, stirring at 250 rpm, and a syringe temperature of 90°C. The headspace vials (20 ml) were filled with 5 ml of supernatants recovered from cultures in CDM. The GC program used a start temperature of 50°C for 5 min and was increased by 10°C/min to 240°C and held for 5 min at 240°C. The capillary column was an ELITE 624SIL MS (length, 30 m; inside diameter, 0.25 mm; film thickness [DF], 1.4 μm). Helium was used as the carrier gas. Mass spectra were recorded in the positive mode from 0 to 29 min over an m/z range from 40 to 200. The scan time was 0.2 s, and interscan delay was 0.02 s. In order to understand the magnitude of hydroxycinnamic acid metabolism in cofactor NAD+ regeneration, the carbon and electron balances and the theoretical ATP/glucose yield were estimated. Carbon balances were calculated by assuming the production of 1 mmol CO2 per mmol glucose. Electron balances were calculated by considering the stoichiometry of the electron donors and electron acceptors involved in glucose metabolism.
Quantification of cofactors NAD+/NADH and intracellular ATP.
Cells grown in CDM at 30°C for 24 h under anaerobic conditions were harvested and washed in cold phosphate-buffered saline (Sigma, St. Louis, MO). Cells (ca. 109 CFU/ml) were suspended in 400 μl of NADH/NAD extraction buffer or ATP assay buffer (Abcam, Cambridge, MA, USA) and transferred into Lysing Matrix E tubes (MP Biomedicals, Santa Ana, CA, USA). Cells were broken through a FastPrep FP120 instrument (Bio 101, Savant Inc., Holbrook, NY, USA) for 40 s at a speed of 6.0. The levels of NAD+ and NADH were determined according to the guidelines provided by the manufacturer (Abcam). The results are expressed as the NAD+/NADH ratio. The level of intracellular ATP was determined by use of a colorimetric ATP assay kit according to the manufacturer's guidelines. The results are expressed as the number of nmol ATP/mg protein. The concentration of proteins was determined according to the Bradford method (23).
ADH and AK activities.
Cells grown in CDM at 30°C for 24 h were harvested and washed twice in 50 mmol/liter phosphate-buffered saline. Cells (ca. 109 CFU/ml) were suspended in phosphate-buffered saline. Cell extracts were prepared in Lysing Matrix E tubes by using the FastPrep FP120 instrument for 40 s at a speed of 6.0. After centrifugation (14,000 × g, 5 min, 4°C), the supernatant was collected. The alcohol dehydrogenase (ADH) activity of the cell extracts was determined by measuring the rate of oxidation of NADH at 25°C (24). A mixture without substrate was used as the control, and the rate of formation of NAD+ was subtracted from that obtained in the presence of the substrate. The acetate kinase (AK) activity of the cell extracts was determined as described by Fowler et al. (25). A control assay without substrate was carried out. Activities are expressed as the number of μmol of substrate depleted per minute per milligram of total cell protein.
Statistical analyses.
Data (at least three replicates) were subjected to one-way analysis of variance, and pairwise comparison of treatment means was achieved by Tukey's procedure at a P value of <0.05, using the statistical software Statistica for Windows (version 7.0).
RESULTS AND DISCUSSION
Antimicrobial activity of hydroxycinnamic acids.
The bactericidal effect of phenolic acids on Lactobacillus spp. was previously documented (3), but no studies have considered heterofermentative species such as Weissella cibaria, W. confusa, Leuconostoc mesenteroides, and Lactobacillus rossiae. After 24 h of cultivation on MRS broth containing caffeic, p-coumaric, or ferulic acid (27.3 to 1.7 mM), the MIC was determined (Table 2). The capacity to tolerate hydroxycinnamic acids (caffeic, p-coumaric, and ferulic acids) was species dependent. Under the experimental conditions of this study, Lactobacillus curvatus PE5 was the most tolerant, followed by strains of Weissella spp., Lactobacillus brevis, Lactobacillus fermentum, and Leuc. mesenteroides, which had the same MIC values. The highest sensitivity to the three hydroxycinnamic acids was found for strains of L. rossiae. This different behavior among the species may be somewhat related to their abundance on matrices that are particularly rich in phenolics (e.g., raw fruits and vegetables containing hydroxycinnamic acids at concentrations up to 2 g/kg [fresh weight]) (26–28). In fact, L. rossiae was mainly isolated from the sourdough microbiota (29–32) and from the gastrointestinal tract of humans (33) and animals (34). To the best of our knowledge, the only example of isolation from sources highly rich in phenolics was the pineapple (16). On the contrary, Weissella spp., Leuc. mesenteroides, and L. curvatus are some of the lactic acid bacterial species most frequently isolated from fruits and vegetables and used as starters for controlled fermentations to get optimal hygienic, sensory, and nutritional features (18). Although the degradation of phenolic acids is the main route used to detoxify these compounds (4, 11), the possibility of other mechanisms of adaptation may not be excluded. Leuc. mesenteroides strains and L. fermentum F1, which did not metabolize hydroxycinnamic acids, had higher MIC values than other species capable of using these compounds (e.g., L. rossiae).
TABLE 2.
Antimicrobial activities of caffeic, p-coumaric, and ferulic acids toward lactic acid bacterial strainsa
| Strain(s) | MIC (mM) |
||
|---|---|---|---|
| Caffeic acid | p-Coumaric acid | Ferulic acid | |
| Weissella cibaria P9, P10 | 13.6 | >27.3 | 27.3 |
| W. cibaria 3XMR3, 3XMR5 | 13.6 | >27.3 | >27.3 |
| W. cibaria/W. confusa POM12, POM15, POM16, POM17 | 13.6 | >27.3 | 27.3 |
| Lactobacillus brevis POM2, POM4 | 13.6 | 27.3 | 27.3 |
| Lactobacillus fermentum F1 | 13.6 | 27.3 | 27.3 |
| Lactobacillus curvatus PE5 | 27.3 | >27.3 | >27.3 |
| Leuconostoc mesenteroides PES7, PES1, KI6 | 13.6 | 27.3 | 27.3 |
| Lactobacillus rossiae 2MR6, 2MR8, 2MR10, 2MR15 | 6.8 | 13.6 | 13.6 |
The final concentration of the hydroxycinnamic acids in the sterile 96-well microtiter plates ranged from 27.3 mM to 1.7 mM. Further details are included in Material and Methods. The data are from three independent experiments.
Metabolism of hydroxycinnamic acids during growth in MRS broth.
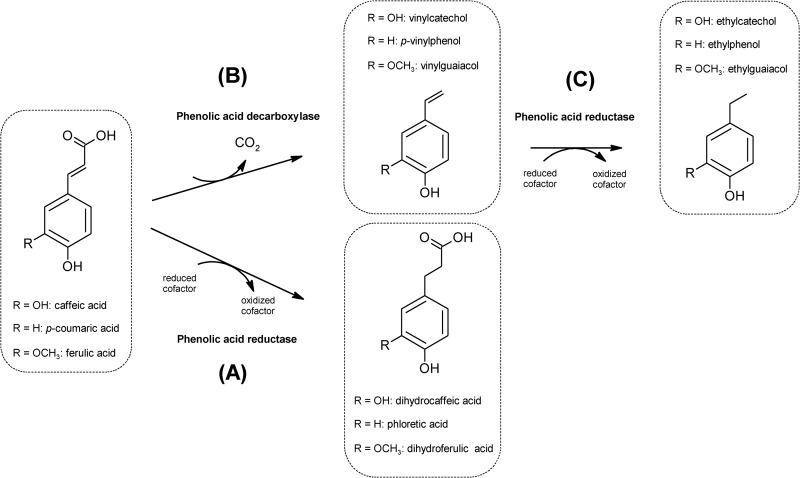
Viable cell counts and pH were determined during 24 h of fermentation in MRS broth supplemented with 1 mM caffeic, p-coumaric, or ferulic acid (data not shown). The bacterial inoculum was ca. 8.0 log CFU/ml. Compared to the growth of the control, supplementation with phenolic acids did not significantly (P > 0.05) affect growth. Regardless of the type of hydroxycinnamic acid used, the cell density increased from 1.4 to 1.7 log CFU/ml for almost all strains. After fermentation, the change in pH (ΔpH) agreed with that from cell growth (data not shown). The metabolism of hydroxycinnamic acids through the activities of phenolic acid decarboxylases and reductases (Fig. 1) was proven after 24 h of fermentation. Substrate consumption and synthesis of metabolites varied depending on the strains and the phenolic acids (Table 3). In particular, caffeic, p-coumaric, and ferulic acids may be reduced to dihydrocaffeic, phloretic, and dihydroferulic acids, respectively (Fig. 1, pathway A), or decarboxylated to the corresponding vinyl derivatives (vinylcatechol, p-vinylphenol, and vinylguaiacol, respectively) (Fig. 1, pathway B). Subsequently, vinyl derivatives may be reduced to their corresponding ethyl derivatives (ethylcatechol, ethylphenol, and ethylguaiacol, respectively) (Fig. 1, pathway C) (3). The percent conversion efficiency was strain dependent. L. fermentum F1 and Leuc. mesenteroides strains did not metabolize hydroxycinnamic acids. All the other strains almost completely consumed caffeic and p-coumaric acids. W. cibaria 3XMR5 showed the lowest percentages of degradation of caffeic and p-coumaric acids (72 and 65%, respectively). Except for W. cibaria 3XMR3 and 3XMR5 and L. brevis POM2 and POM4, which did not degrade ferulic acid, all the other strains completely consumed this compound. Metabolites identified through HPLC and nano-LC-ESI-MS analyses confirmed the degradation through the metabolic pathways of Fig. 1.
FIG 1.
Metabolic pathways (A, B, and C) of caffeic, p-coumaric, and ferulic acids by lactic acid bacteria (for a review, see reference 3).
TABLE 3.
Metabolism of caffeic, p-coumaric, and ferulic acids by lactic acid bacterial strains in MRS brotha
| Strain(s) | Caffeic acid |
p-Coumaric acid |
Ferulic acid |
|||
|---|---|---|---|---|---|---|
| % substrate consumed | Metabolite (% conversion efficiency)b | % substrate consumed | Metabolite(s) (% conversion efficiency) | % substrate consumed | Metabolite (% conversion efficiency) | |
| Weissella cibaria P9, P10 | 100 | Dihydrocaffeic acid (92.4–95.7) | 100 | Phloretic acid (80.2–81.3) | 100 | Dihydroferulic acid (78.2–80.2) |
| W. cibaria 3XMR3 | 100 | Ethylcatechol (84.3) | 100 | Ethylphenol (82.1) | 0 | |
| W. cibaria 3XMR5 | 72 | Vinylcatechol,c dihydrocaffeic acid (35.5) | 65 | p-Vinylphenol (53.2), phloretic acid (41.2) | 0 | |
| W. cibaria/W.confusa POM12, POM15, POM16, POM17 | 100 | Dihydrocaffeic acid (92.9–98.6) | 100 | Phloretic acid (78.2–80.7) | 100 | Dihydroferulic acid (80.2–85.6) |
| Lactobacillus brevis POM2 | 100 | Ethylcatechol (89.3) | 100 | p-Vinylphenol (85.8) | 0 | |
| L. brevis POM4 | 100 | Vinylcatecholc | 100 | p-Vinylphenol (82.9) | 0 | |
| Lactobacillus fermentum F1 | 0 | 0 | 0 | |||
| Lactobacillus curvatus PE5 | 100 | Dihydrocaffeic acid (95.1) | 100 | Phloretic acid (82.8) | 100 | Dihydroferulic acid (80.9) |
| Leuconostoc mesenteroides PES7, PES1, KI6 | 0 | 0 | 0 | |||
| Lactobacillus rossiae 2MR6, 2MR8, 2MR10, 2MR15 | 100 | Ethylcatechol (82.7–86.3) | 100 | Ethylphenol (76.2–82.9) | 100 | Dihydroferulic acid (76.1–82.4) |
The data are the means of three independent experiments. Strains in bold were selected to study phenolic hydroxycinnamic acid metabolism as external acceptors of electrons.
The efficiency of conversion of hydroxycinnamic acids into their derivatives was calculated as the amount (mmol) of end products produced in a defined period divided by the amount (mmol) of hydroxycinnamic acid consumed in the same period times 100.
A standard was not available.
This study first provides a comprehensive overview of the degradation products of hydroxycinnamic acids for a consistent number of lactic acid bacterial species. Phenolic volatile derivatives resulting from the bioconversion pathways may contribute to the aroma attributes of fermented foods. Strains of L. brevis, L. rossiae, and, in part, W. cibaria released these compounds from caffeic and p-coumaric acids. p-Vinylphenol is one of the permitted food additives and is approved for use as a flavoring agent. Ethylphenol is considered one of the most important flavor components of fermented soy sauce (3). Overall, phenol derivatives may exert biological activities. Dihydrocaffeic acid, the reduced derivative of caffeic acid, has well-known and higher antioxidant activity than its precursor (35, 36). The screening of strains based on enzymatic activities toward hydroxycinnamic acids may allow the selection of starter cultures with both optimal technology and functional features. Knowledge of the metabolism of phenolics in lactic acid bacteria is of great interest in food science; nevertheless, only tannase, p-coumaric acid decarboxylase, and benzyl alcohol dehydrogenase have been genetically characterized for L. plantarum (3, 37–39), while the activities of other enzymes on phenolics (e.g., reductase) remain biochemically and genetically uncharacterized.
Under the experimental conditions of this study, reductase activities emerged mainly from the analysis of the metabolism of hydroxycinnamic acids, thus suggesting a specific physiological significance for strictly heterofermentative species. Almost all strains of Weissella spp. and L. curvatus exhibited extensive reductase activity toward all three hydroxycinnamic acids. L. rossiae reduced only ferulic acid.
Heterofermentative lactic acid bacteria benefit energetically from the utilization of external electron acceptors to regenerate the reduced cofactor NADH, which allows the synthesis of acetic acid from acetyl phosphate with the concomitant gain of additional ATP. Fermented foods are ecosystems where such metabolic pathways may occur either inductively through the addition of alternative electron acceptors (e.g., fructose during sourdough fermentation) (13) or inherently due to the presence of alternative electron acceptors (e.g., aldehydes during fruit and vegetable juice fermentation) (40). Phenolics are classical examples of the above-described inherent compounds and are particularly abundant in raw fruits and some vegetables (26–28). Tolerance of high concentrations of phenolic acids is indispensable to get optimal microbial growth on these matrices. The metabolic pathways of phenolic acids have been highlighted for L. plantarum (3), but the physiological significance has not yet been fully elucidated. May heterofermentative lactic acid bacteria use phenolic acids as external electron acceptors and gain additional metabolic energy to counteract the stressful conditions generated by phenolics?
To study the use of phenolic hydroxycinnamic acids as external acceptors of electrons, further analyses considered only strains that showed reductase activities (Fig. 1, metabolic pathway A; Table 3). Representative strains for each species were considered.
Metabolism of hydroxycinnamic acids during growth in CDM.
The biochemistry and physiology of strictly heterofermentative lactic acid bacteria showing phenolic acid reductase activity in MRS broth were investigated in CDM (under anaerobic conditions) to simplify the system and to exclude the possibility of the presence of other potential external acceptors of electrons (e.g., oxygen and fructose). The end products of carbon metabolism were quantified, as was the NAD+/NADH ratio, electron and carbon balances, and theoretical ATP/glucose yield (Table 4). Hydroxycinnamic acids were supplemented in CDM at a final concentration of 2 mM, which was markedly lower than the values of the MICs. After 24 h of fermentation, all hydroxycinnamic acids were completely metabolized (data not shown). Compared to the growth of the control, the presence of phenolic acids did not significantly (P > 0.05) affect the growth of most of the strains (Table 4). The only exceptions found were W. cibaria P9 and L. rossiae 2MR8, which showed significantly (P < 0.05) higher increases in cell density and growth rate (μmax) when p-coumaric and ferulic acids were added, respectively. Compared to the controls, W. cibaria/W. confusa POM12 and L. curvatus PE5 showed a lower level of consumption of glucose when p-coumaric acid was added to CDM (25 and 20 mmol/liter, respectively, for the controls and 20 and 12 mmol/liter, respectively, with p-coumaric acid). The consumption of glucose did not significantly (P > 0.05) vary for the other strains under the other conditions of cultivation (Table 4). Overall, almost stoichiometric ratios between the amounts of fermentation end products and the levels of consumption of glucose were found (Table 4). Under most of the conditions, the concentration of lactic acid did not differ significantly (P > 0.05) between growth with and without hydroxycinnamic acids (Table 4). Compared to the controls, the only significant (P < 0.05) differences in lactic acid production were found when CDM supplemented with p-coumaric acid was fermented with W. cibaria/W. confusa POM12 and L. curvatus PE5 (27 ± 1 and 22 ± 2 mmol/liter, respectively for the controls and 22 ± 1 and 18 ± 2 mmol/liter, respectively, with p-coumaric acid). The lowest concentration of lactic acid production was shown by L. rossiae 2MR8. Compared to the controls, the concentrations of ethanol significantly (P < 0.05) decreased (14 to 27%) when W. cibaria P9, W. cibaria/W. confusa POM12, and L. curvatus PE5 were grown with all phenolic acids (Table 4). No significant (P > 0.05) variations were found for L. rossiae 2MR8. Both HPLC and HD-GC-MS analyses confirmed these results. Acetic acid was detected only through HD-GC-MS (Table 4). For W. cibaria P9 and L. curvatus PE5, compared to the acetic acid concentrations for the controls (921 ± 205 and 2,385 ± 234 μmol/liter, respectively), the concentrations of acetic acid significantly (P < 0.05) increased when W. cibaria P9 and L. curvatus PE5 fermented CDM supplemented with caffeic acid (1,948 ± 225 and 3,136 ± 263 μmol/liter, respectively) and p-coumaric acid (1,908 ± 106 and 3,332 ± 201 μmol/liter, respectively). W. cibaria/W. confusa POM12 showed significant (P < 0.05) increases (30 to 56%) of the concentration of acetic acid with all three hydroxycinnamic acids added compared to that for the control (1,522 ± 207 μmol/liter). Acetic acid production also significantly (P < 0.05) increased during the cultivation of L. rossiae 2MR8 with ferulic acid. The values of these end products affected electron balances, which showed a downward trend when hydroxycinnamic acids were added, suggesting the presence of others external acceptors of electrons. Compared to the ratio for the controls, the ratio between cofactors (NAD+/NADH) significantly (P < 0.05) increased when W. cibaria P9 fermented CDM with p-coumaric; W. cibaria/W. confusa POM12 and L. curvatus PE5 were grown with all three phenolic acids and with p-coumaric and ferulic acids, respectively; and L. rossiae 2MR8 fermented CDM with ferulic acid (Table 4). Overall, the energetic advantage derived from NAD+ regeneration through hydroxycinnamic acid metabolism was also confirmed by the theoretical ATP/glucose yield. Compared to the theoretical ATP/glucose yield for the controls, it significantly (P < 0.05) increased when W. cibaria P9 fermented CDM with caffeic acid and W. cibaria/W. confusa POM12 and L. curvatus PE5 were grown with p-coumaric acid (Table 4). The intracellular concentration of ATP significantly (P < 0.05) increased when the results for the control were compared to those for CDM to which hydroxycinnamic acids were added (see Table S1 in the supplemental material). The only exceptions were found for W. cibaria P9 and L. rossiae 2MR8, which showed significant (P < 0.05) decreases when fermenting CDM supplemented with p-coumaric acid (16.7 nmol ATP/mg protein versus 25.0 nmol ATP/mg protein for the control) and ferulic acid (15.1 nmol ATP/mg protein versus 31.0 nmol ATP/mg protein for the control), respectively. The gain of energy for these strains was probably spent mostly for growth. W. cibaria P9 and L. rossiae 2MR8 were the lactic acid bacteria which showed the highest increases of cell density and growth rate (μmax) during growth in CDM.
TABLE 4.
Increases of cell density, maximum growth rate, glucose consumption, and organic acid (lactic and acetic acid) and ethanol production in CDM supplemented with caffeic, p-coumaric, and ferulic acidsa
| Strain | Mediumb | ΔOD620 | μmax (OD620 unit h−1)c | Glucose consumption (mmol/liter) | Lactic acid production (mmol/liter) | Acetic acid production (μmol/liter) | Ethanol production (mmol/liter) | Electron balance (%)d | Carbon balance (%)e | NAD+/NADH ratio | Theoretical ATP/glucose yield |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W. cibaria P9 | Control | 0.244 ± 0.020C | 0.027 ± 0.006CD | 25 ± 2C | 25 ± 1A | 921 ± 205F | 30 ± 2A | 111 ± 8AB | 105 ± 8A | 37 ± 2E | 1.03 ± 0.04F |
| Caffeic acid | 0.279 ± 0.022C | 0.036 ± 0.003BC | 25 ± 1C | 26 ± 2A | 1,948 ± 225C | 24 ± 1C | 94 ± 7BCD | 102 ± 3A | 38 ± 3E | 1.15 ± 0.03DE | |
| p-Coumaric acid | 0.315 ± 0.018AB | 0.049 ± 0.004A | 24 ± 2C | 23 ± 3A | 1,908 ± 106C | 22 ± 2C | 91 ± 3D | 95 ± 13A | 46 ± 3C | 1.06 ± 0.07EF | |
| Ferulic acid | 0.259 ± 0.022C | 0.031 ± 0.006BCD | 25 ± 3C | 25 ± 2A | 1,122 ± 261EF | 25 ± 2B | 98 ± 5BCD | 101 ± 12A | 30 ± 5E | 1.05 ± 0.09EF | |
| W. cibaria/W. confusa POM12 | Control | 0.281 ± 0.023C | 0.030 ± 0.005BCD | 25 ± 3C | 27 ± 1A | 1,522 ± 207DE | 29 ± 2AB | 112 ± 7AB | 108 ± 13A | 15 ± 10F | 1.13 ± 0.04DE |
| Caffeic acid | 0.302 ± 0.021ABC | 0.039 ± 0.005B | 23 ± 1C | 25 ± 2A | 2,369 ± 234B | 23 ± 2C | 96 ± 4CD | 105 ± 10A | 36 ± 3E | 1.20 ± 0.04CD | |
| p-Coumaric acid | 0.271 ± 0.016C | 0.031 ± 0.002CD | 20 ± 1B | 22 ± 1B | 2,189 ± 123BC | 22 ± 1C | 101 ± 3AB | 108 ± 9A | 39 ± 3DE | 1.22 ± 0.03C | |
| Ferulic acid | 0.286 ± 0.022ABC | 0.029 ± 0.003D | 25 ± 2C | 28 ± 1A | 2,194 ± 132BC | 23 ± 2C | 85 ± 8D | 104 ± 11A | 45 ± 4CD | 1.21 ± 0.04CD | |
| L. curvatus PE5 | Control | 0.333 ± 0.020A | 0.028 ± 0.003D | 20 ± 3BC | 22 ± 2AB | 2,385 ± 234B | 28 ± 2AB | 124 ± 18A | 115 ± 16A | 48 ± 4C | 1.21 ± 0.08BCD |
| Caffeic acid | 0.337 ± 0.021A | 0.030 ± 0.004BCD | 17 ± 2B | 20 ± 3AB | 3,136 ± 263A | 24 ± 1C | 121 ± 25ABC | 118 ± 19A | 50 ± 4C | 1.37 ± 0.08B | |
| p-Coumaric acid | 0.346 ± 0.019A | 0.033 ± 0.002BCD | 12 ± 1A | 18 ± 2C | 3,332 ± 201A | 21 ± 3C | 141 ± 37A | 132 ± 31A | 74 ± 5B | 1.83 ± 0.12A | |
| Ferulic acid | 0.331 ± 0.025A | 0.032 ± 0.002BCD | 18 ± 3B | 20 ± 2B | 2,585 ± 261B | 23 ± 2C | 104 ± 19ABCD | 111 ± 18A | 65 ± 4B | 1.27 ± 0.07BC | |
| L. rossiae 2MR8 | Control | 0.154 ± 0.031D | 0.012 ± 0.002E | 10 ± 2A | 12 ± 1D | 1,277 ± 237DEF | 13 ± 1D | 124 ± 21AB | 118 ± 19A | 13 ± 2F | 1.32 ± 0.08BC |
| Ferulic acid | 0.251 ± 0.026C | 0.032 ± 0.002BCD | 13 ± 1A | 14 ± 2D | 2,050 ± 224BC | 12 ± 2D | 83 ± 17CD | 106 ± 8A | 175 ± 9A | 1.24 ± 0.09BCD |
Values were obtained after fermentation for 24 h at 30°C with lactic acid bacterial strains. Means within the column with different superscript letters are significantly different (P < 0.05). ΔOD620, increase in cell density; μmax, maximum growth rate.
CDM was prepared as described by McFeeters and Chen (20) and supplemented with caffeic, p-coumaric, or ferulic acid at a concentration of 2 mM. CDM not supplemented with hydroxycinnamic acids was used as a control. The data are the means of three independent experiments ± standard deviations (n = 3).
Growth data were modeled according to the Gompertz equation, as modified by Zwietering et al. (21).
Electron balances were calculated by considering the stoichiometry of the electron donors and electron acceptors involved in glucose metabolism.
Carbon balances were calculated by assuming the production of 1 mmol CO2 per mmol glucose.
Despite the inevitable interference, the complementary techniques used and the number of analyzed metabolites allowed highlight a clear trend. Under most of the culture conditions, the reductase activity toward hydroxycinnamic acids was correlated with the highest levels of ATP (intracellular ATP and theoretical ATP/glucose yield) and the highest NAD+/NADH ratios.
ADH and AK activities.
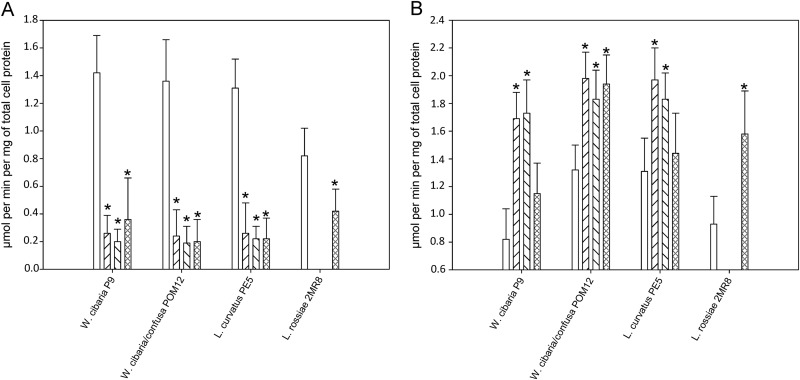
Under most of the culture conditions, the synthesis of ethanol decreased when CDM was supplemented with hydroxycinnamic acids. The concentration of acetic acid increased. The levels of these metabolites reflected on the ADH and AK activities. As shown in Fig. 2, the ADH activities of all the strains significantly (P < 0.05) decreased when cultivated with hydroxycinnamic acids. For W. cibaria P9 and L. curvatus PE5, compared to the AK activities of the controls (0.82 ± 0.22 and 1.31 ± 0.24 μmol per min per mg of protein, respectively), the AK activities of W. cibaria P9 and L. curvatus PE5 significantly (P < 0.05) increased when CDM was supplemented with caffeic acid (1.69 ± 0.19 and 1.97 ± 0.23 μmol per min per mg of protein, respectively) and p-coumaric acid (1.73 ± 0.24 and 1.83 ± 0.19 μmol per min per mg of protein, respectively). For W. cibaria/W. confusa POM12, compared to the AK activity of the control (1.32 ± 0.18 μmol per min per mg of protein), the AK activity significantly (P < 0.05) increased (30 to 50%) when all three hydroxycinnamic acids were used. The AK activity also significantly (P < 0.05) increased when L. rossiae 2MR8 fermented CDM with ferulic acid.
FIG 2.
Alcohol dehydrogenase (A) and acetate kinase (B) activities (μmol per min per mg of total cell protein) of lactic acid bacterial strains after growth (24 h at 30°C) in CDM which was supplemented with caffeic acid ( ), p-coumaric acid (
), p-coumaric acid ( ), and ferulic acid (
), and ferulic acid ( ) (2 mM). CDM not supplemented with hydroxycinnamic acids was used as the control (□). Data are shown as the means ± standard deviations of three independent experiments. *, significant difference (P < 0.05) from the control.
) (2 mM). CDM not supplemented with hydroxycinnamic acids was used as the control (□). Data are shown as the means ± standard deviations of three independent experiments. *, significant difference (P < 0.05) from the control.
Overall, some biochemical traits distinguished the common metabolism of strictly heterofermentative strains: main reductase activity toward hydroxycinnamic acids, a shift from alcohol dehydrogenase to acetate kinase activities, an increase of the NAD+/NADH ratio, and the accumulation of supplementary intracellular ATP. These findings, which were highlighted when the strains directly reduced hydroxycinnamic acids (Fig. 1, pathway A), might be also found during the reduction of vinyl derivatives (Fig. 1, pathways B and C).
Conclusion.
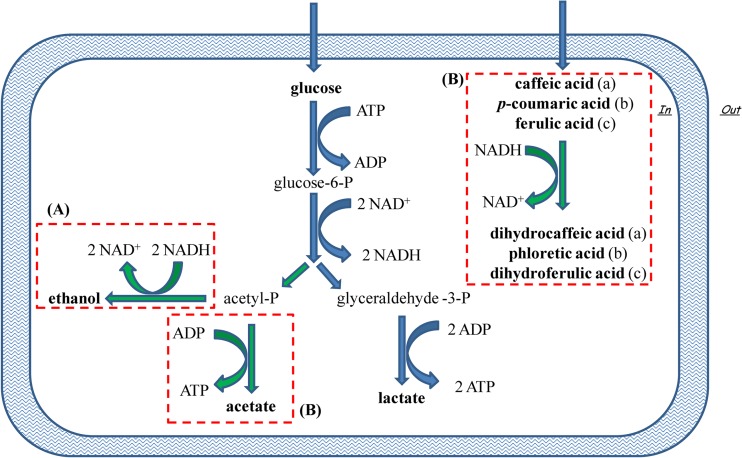
Taken together the metabolic responses observed in this study suggest that strictly heterofermentative lactic acid bacteria use the hydroxycinnamic acids as external acceptors of electrons (Fig. 3). This ability may be one of the metabolic strategies taken to tolerate the hostile environment generated by the presence of phenolic acids. At the same time, this strategy may provide a competitive advantage. A further raw plug was added to the complex framework and has not yet been fully elucidated: the metabolism of phenolic acids by lactic acid bacteria. The practical relevance of the use of external acceptors, as shown in this study, is represented by the substantial modification of the fermentation quotient (lactic and acetic acid ratio), which may have a strong impact on the fermented food flavor. Thus, the choice of starter strains with specific enzymatic activities involving hydroxycinnamic acids may be useful as a tool to influence the levels of products of hexose metabolism in fermented foods.
FIG 3.
Schematic representation of the presumptive NAD+/NADH recycling mechanism adopted by strictly heterofermentative lactobacilli. Heterofermentative metabolism via the pentose-phosphate pathway generates 1 mol of ATP when acetyl phosphate (acetyl-P) is used as the electron acceptor to synthesize ethanol (A) or 2 mol of ATP in the presence of other electron acceptors (B), which enables additional ATP synthesis through the conversion of acetyl phosphate to acetate. Alternative electron acceptors suggested by this study are caffeic, p-coumaric, and ferulic acids, which may be reduced to dihydrocaffeic, phloretic, and dihydroferulic acids, respectively. glucose-6-P, glucose-6-phosphate; glyceraldehyde-3-P, glyceraldehyde-3-phosphate.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the Ministero dell'Istruzione, dell'Università e della Ricerca, the Ministero dello Sviluppo Economico, and the Fondo Europeo di Sviluppo Regionale (PON02_00186_3417037, project PROINNO_BIT).
Footnotes
Published ahead of print 26 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02413-14.
REFERENCES
- 1.Rodrìguez H, Landete JM, Rivas BDL, Muñoz R. 2008. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 107:1393–1398. 10.1016/j.foodchem.2007.09.067. [DOI] [Google Scholar]
- 2.Naczk M, Shahidi F. 2006. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 41:1523–1542. 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Rodrìguez H, Curiel JA, Landete JM, de Las Rivas B, de Felipe FL, Gòmez-Cordovés C. 2009. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 132:79–90. 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Barthelmebs L, Divies C, Cavin JF. 2000. Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl. Environ. Microbiol. 66:3368–3375. 10.1128/AEM.66.8.3368-3375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung JY, Lee SH, Lee HJ, Seo HY, Park WS, Jeon CO. 2012. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 153:378–387. 10.1016/j.ijfoodmicro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Kostinek M, Specht I, Edward VA, Pinto C, Egounlety M, Sossa C, Mbugua S, Dortu C, Thonart P, Taljaard L, Mengu M, Franz CMAP, Holzapfel WH. 2007. Characterisation and biochemical properties of predominant lactic acid bacteria from fermenting cassava for selection as starter cultures. Int. J. Food Microbiol. 114:342–351. 10.1016/j.ijfoodmicro.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 7.Di Cagno R, Surico RF, Siragusa S, De Angelis M, Paradiso A, Minervini F, De Gara L, Gobbetti M. 2008. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, French beans or marrows. Int. J. Food Microbiol. 127:220–228. 10.1016/j.ijfoodmicro.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Di Cagno R, Surico RF, Paradiso A, De Angelis M, Salmon JC, Buchin S, De Gara L, Gobbetti M. 2009. Effect of autochthonous lactic acid bacteria starters on health-promoting and sensory properties of tomato juices. Int. J. Food Microbiol. 128:473–483. 10.1016/j.ijfoodmicro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Silva I, Campos FM, Hogg T, Couto JA. 2011. Factors influencing the production of volatile phenols by wine lactic acid bacteria. Int. J. Food Microbiol. 145:471–475. 10.1016/j.ijfoodmicro.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Selma MV, Espin JC, Tomas-Barberan FA. 2009. Interaction between phenolics and gut microbiota: role in human health. J. Agric. Food Chem. 57:6485–6501. 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Maldonado AF, Schieber A, Gänzle MG. 2011. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 111:1176–1184. 10.1111/j.1365-2672.2011.05141.x. [DOI] [PubMed] [Google Scholar]
- 12.Filannino P, Bai Y, Di Cagno R, Gobbetti M, Gänzle MG. 2014. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 46:272–279. 10.1016/j.fm.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Minervini F, De Angelis M, Di Cagno R, Gobbetti M. 2014. Ecological parameters influencing microbial diversity and stability of traditional sourdough. Int. J. Food Microbiol. 171:136–146. 10.1016/j.ijfoodmicro.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen N, Czerny M, Gänzle MG, Schieberle P, Vogel RF. 2007. Reduction of (E)-2-nonenal and (E,E)-2,4-decadienal during sourdough fermentation. J. Cereal Sci. 45:78–87. 10.1016/j.jcs.2006.07.002. [DOI] [Google Scholar]
- 15.Di Cagno R, Minervini G, Rizzello CG, De Angelis M, Gobbetti M. 2011. Effect of lactic acid fermentation on antioxidant, texture, color and sensory properties of red and green smoothies. Food Microbiol. 28:1062–1071. 10.1016/j.fm.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Di Cagno R, Cardinali G, Minervini G, Antonielli L, Rizzello CG, Ricciuti P, Gobbetti M. 2010. Taxonomic structure of the yeasts and lactic acid bacteria microbiota of pineapple (Ananas comosus L. Merr.) and use of autochthonous starters for minimally processing. Food Microbiol. 27:381–389. 10.1016/j.fm.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Di Cagno R, Surico RF, Minervini G, De Angelis M, Rizzello CG, Gobbetti M. 2009. Use of autochthonous starters to ferment red and yellow peppers (Capsicum annum L.) to be stored at room temperature. Int. J. Food Microbiol. 130:108–116. 10.1016/j.ijfoodmicro.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Di Cagno R, Coda R, De Angelis M, Gobbetti M. 2013. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 33:1–10. 10.1016/j.fm.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Rizzello CG, Losito I, Gobbetti M, Carbonara T, De Bari MD, Zambonin PG. 2005. Antibacterial activities of peptides from the water-soluble extracts of Italian cheese varieties. J. Dairy Sci. 88:2348–2360. 10.3168/jds.S0022-0302(05)72913-1. [DOI] [PubMed] [Google Scholar]
- 20.McFeeters RF, Chen KH. 1986. Utilization of electron acceptors for anaerobic mannitol metabolism by Lactobacillus plantarum. Compounds which serve as electron acceptors. Food Microbiol. 3:73–81. [Google Scholar]
- 21.Zwietering MH, Jongeberger I, Roumbouts FM, Van't Riet K. 1990. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 56:1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeppa G, Conterno L, Gerbi V. 2001. Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J. Agric. Food. Chem. 49:2722–2726. 10.1021/jf0009403. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Amárita F, Fernández-Esplá D, Requena T, Pelaez C. 2001. Conversion of methionine to methional by Lactococcus lactis. FEMS Microbiol. Lett. 204:189–195. 10.1111/j.1574-6968.2001.tb10884.x. [DOI] [PubMed] [Google Scholar]
- 25.Fowler ML, Ingram-Smith CJ, Smith KS. 2011. Direct detection of the acetate-forming activity of the enzyme acetate kinase. J. Vis. Exp. 2011:3474. 10.3791/3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manach C, Scalbert A, Morand C, Rémés C, Jiménez L. 2004. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 79:727–747. [DOI] [PubMed] [Google Scholar]
- 27.Clifford MN. 1999. Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J. Sci. Food Agric. 79:362–372. . [DOI] [Google Scholar]
- 28.Macheix JJ, Fleuriet A, Billot J. 1990. Fruit phenolics. CRC Press, Boca Raton, FL. [Google Scholar]
- 29.Corsetti A, Settanni L, van Sinderen D, Felis GE, Dellaglio F, Gobbetti M. 2005. Lactobacillus rossii sp. nov. isolated from wheat sourdough. Int. J. Syst. Evol. Microbiol. 55:35–40. 10.1099/ijs.0.63075-0. [DOI] [PubMed] [Google Scholar]
- 30.Siragusa S, Di Cagno R, Ercolini D, Minervini F, Gobbetti M, De Angelis M. 2009. Taxonomic structure and monitoring of the dominant population of lactic acid bacteria during wheat flour sourdough type I propagation using Lactobacillus sanfranciscensis starters. Appl. Environ. Microbiol. 75:1099–1109. 10.1128/AEM.01524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheirlinck I, Van der Meulen R, De Vuyst L, Vandamme P, Huys G. 2009. Molecular source tracking of predominant lactic acid bacteria in traditional Belgian sourdoughs and their production environments. J. Appl. Microbiol. 106:1081–1092. 10.1111/j.1365-2672.2008.04094.x. [DOI] [PubMed] [Google Scholar]
- 32.Minervini F, De Angelis M, Di Cagno R, Pinto D, Siragusa S, Rizzello CG, Gobbetti M. 2010. Robustness of Lactobacillus plantarum starters during daily propagation of wheat flour sourdough type I. Food Microbiol. 27:897–908. 10.1016/j.fm.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Di Cagno R, Rizzello CG, Gagliardi F, Ricciuti P, Ndagijimana M, Francavilla R, Guerzoni ME, Crecchio C, Gobbetti M, De Angelis M. 2009. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl. Environ. Microbiol. 75:3963–3971. 10.1128/AEM.02793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Angelis M, Siragusa S, Berloco M, Caputo L, Settanni L, Alfonsi G, Amerio M, Grandi A, Ragni A, Gobbetti M. 2006. Selection of potential probiotic lactobacilli from pig feces to be used as additives in pelleted feeding. Res. Microbiol. 157:792–801. 10.1016/j.resmic.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Silva FAM, Borges F, Guimarães C, Lima JLFC, Matos C, Reis S. 2000. Phenolic acids and derivatives: studies on the relationship among structure, radical scavenging activity, and physicochemical parameters. J. Agric. Food Chem. 48:2122–2126. 10.1021/jf9913110. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, de Paulis T, May JM. 2004. Antioxidant effects of dihydrocaffeic acid in human EA.hy926 endothelial cells. J. Nutr. Biochem. 15:722–729. 10.1016/j.jnutbio.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Cavin JF, Barthelmebs L, Diviès C. 1997. Molecular characterization of an inducible p-coumaric acid decarboxylase from Lactobacillus plantarum: gene cloning, transcriptional analysis, overexpression in Escherichia coli, purification, and characterization. Appl. Environ. Microbiol. 63:1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwamoto K, Tsuruta H, Nishitani Y, Osawa R. 2008. Identification and cloning of a gene encoding tannase (tannin acylhydrolase) from Lactobacillus plantarum ATCC 14917T. Syst. Appl. Microbiol. 31:269–277. 10.1016/j.syapm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Landete JM, Rodríguez H, de las Rivas B, Muñoz R. 2008. Characterization of a benzyl alcohol dehydrogenase from Lactobacillus plantarum WCFS1. J. Agric. Food Chem. 56:4497–4503. 10.1021/jf800500v. [DOI] [PubMed] [Google Scholar]
- 40.Filannino P, Cardinali G, Rizzello CG, Buchin B, De Angelis M, Gobbetti M, Di Cagno R. 2014. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 80:2206–2215. 10.1128/AEM.03885-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.