Abstract
The use of bacteriophages in the treatment and prevention of infections by the fish pathogen Flavobacterium psychrophilum has attracted increased attention in recent years. It has been shown recently that phage delivery via the parenteral route resulted in immediate distribution of phages to the circulatory system and the different organs. However, little is known about phage dispersal and survival in vivo in rainbow trout after delivery via the oral route. Here we examined the dispersal and survival of F. psychrophilum phage FpV-9 in vivo in juvenile rainbow trout after administration by three different methods—bath, oral intubation into the stomach, and phage-coated feed—with special emphasis on the oral route of delivery. Phages could be detected in all the organs investigated (intestine, spleen, brain, and kidney) 0.5 h postadministration, reaching concentrations as high as ∼105 PFU mg intestine−1 and ∼103 PFU mg spleen−1 within the first 24 h following the bath and ∼107 PFU mg intestine−1 and ∼104 PFU mg spleen−1 within the first 24 h following oral intubation. The phages were most persistent in the organs for the first 24 h and then decreased exponentially; no phages were detected after 83 h in the organs investigated. Phage administration via feed resulted in the detection of phages in the intestine, spleen, and kidney 1 h after feeding. Average concentrations of ∼104 PFU mg intestine−1 and ∼101 PFU mg spleen−1 were found throughout the experimental period (200 h) following continuous delivery of phages with feed. These experiments clearly demonstrate the ability of the phages to survive passage through the fish stomach and to penetrate the intestinal barrier and enter the circulatory system after oral delivery, although the quantity of phages found in the spleen was 100- to 1,000-fold lower than that in the intestine. It was also shown that phages could tolerate long periods of desiccation on the feed pellets, with 60% survival after storage at −80°C, and 10% survival after storage at 5°C, for ∼8 months. Continuous delivery of phages via coated feed pellets constitutes a promising method of treatment and especially prevention of rainbow trout fry syndrome.
INTRODUCTION
Several studies have examined the potential of using bacteriophages (phages) for the control of infections with Flavobacterium psychrophilum (1–3), the Gram-negative fish pathogen responsible for rainbow trout fry syndrome (RTFS) in salmonid hatcheries worldwide (4–11). Today, RTFS is treated with antibiotics; however, the change in the antibiotic resistance patterns of F. psychrophilum (12, 13), combined with the fact that no commercial vaccine against RTFS is yet available (14–18), has led to increased attention to the use of phages for controlling F. psychrophilum infections in aquaculture.
Recently, application of F. psychrophilum phages isolated from Chilean salmon aquaculture was shown to reduce mortality in Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) (15 to 30 g) following intraperitoneal (i.p.) injection of the phages together with their host strain (109 PFU and 108 CFU per fish, respectively) (3). In a preliminary study, however, no significant reduction in fish mortality was detected following i.p. injection of F. psychrophilum phage FpV-9 (1) into rainbow trout fry that had been injected i.p. with F. psychrophilum (104 PFU and 104 CFU per fish, respectively) (unpublished results). It was suggested that the lack of therapeutic effect in this case was due to the low dose of phages administered.
Success in the application of phages against fish pathogens depends on the ability of the phages to reach the target organs in the fish at sufficiently high concentrations to control the pathogen (19–21), making phage delivery methods the key to successful therapeutic use of phages (22, 23). Different routes of phage administration have been used in studies with fish and shellfish; the most popular are the parenteral and oral routes, while immersion/bath or the addition of phages to the water has also been used (19, 23–25). The immediate distribution of phages to the circulatory system following parenteral delivery has proven it to be one of the most successful delivery methods in animal studies (23), and this has also been the method of choice in experiments with fish (3, 19, 26, 27). The disadvantages of this delivery method, however, are that the injection of phages is laborious when the number of animals is high, when the animals are very small, and when continuous treatment is required. Oral administration of phages, on the other hand, enables simultaneous treatment of a large number of fish and easy continuous delivery of phages (28).
One main issue with phage delivery via the oral route is phage stability in the highly acidic and proteolytically active environment of the stomach. It has been demonstrated that pH is very important for the infectivity of phages (2, 19, 23, 29) and that the infectivity of phage FpV-9 is immediately lost at pH <3 (19).
Another issue with oral delivery is the ability of orally applied phages to infiltrate from the intestinal tract into the circulatory system (30, 31). Studies in mice (32), rabbits (33), and goldfish (22) have shown that phages are capable of penetrating the intestinal wall, and administration of phage-coated feed has been used successfully to treat Pseudomonas plecoglossicida infections in ayu (24, 25, 34) and Flavobacterium columnare infections in catfish (35).
The lytic ability and survival of phages in the fish can be affected by numerous factors, e.g., the delivery mechanism, multiplicity of infection, fish immune response, physical and chemical environment, and development of phage-resistant bacteria (19). F. psychrophilum phage FpV-9 was rapidly spread to the internal organs following i.p. injection (19); however, the rate of phage decay in the target organs was high, a phenomenon also observed in other studies (2, 31, 32, 35, 36). Administration of a higher dose to compensate for the fast phage decay in vivo has been suggested (36). Alternatively, using phage-coated feed might overcome the problem, since it allows continuous delivery of phages.
Further development of the application of phages to control F. psychrophilum infections in aquaculture requires a better understanding of phage activity, survival, and dispersal in rainbow trout following oral administration. The aim of this study was to examine the dispersal and survival of F. psychrophilum phage FpV-9 in vivo in juvenile rainbow trout after administration by three different methods: bath, oral intubation into the stomach, and phage-coated feed.
MATERIALS AND METHODS
Bacterial strain.
In this study, a well-characterized Danish strain, Flavobacterium psychrophilum 950106-1/1 (serotype Fd, ribotype A, carrying a 3.3-kb plasmid, virulent) was used. The strain was originally isolated from a rainbow trout during a clinical outbreak of RTFS in a freshwater farm in 1995 and was identified both biochemically and serologically (37–39). The strain was stored at −80°C in tryptone-yeast extract-salts broth (TYES-B) medium (40) with 15 to 20% glycerol. The strain was inoculated straight from the −80°C stocks into 5 ml TYES-B and was incubated at 15°C with agitation for 48 h before further use in liquid cultures or on solid medium consisting of TYES-B with 1.1% agar (TYES-A).
Bacteriophage.
The bacteriophage used, FpV-9, was originally isolated from pond water from a Danish freshwater rainbow trout farm and has been characterized as belonging to the family Podoviridae, with a head size of ∼60 by 63 nm and a tail length of ∼172 nm. It has a genome of 48 kb, a latency time of 4 h, a burst size of ∼37 phages per infected cell, and an adsorption rate of ∼0.016 h−1 (1). FpV-9 has been described as lytic and infective to a broad range of strains of F. psychrophilum, including the host strain (1). High-titer concentrates of FpV-9 were prepared from plates with confluent lysis (1) and were kept in SM-buffer (50 mM Tris-Cl [pH 7.5], 99 mM NaCl, 8 mM MgSO4, 0.01% gelatin) at 5°C in the dark. The phage stocks used in the experiments were examined for bacterial contamination by plating 100 μl onto a TYES agar plate, and the plates were incubated for 5 to 7 days at 15°C before being checked for bacterial growth.
Fish used in the experiments.
Juvenile rainbow trout (Oncorhynchus mykiss) from the same batch of eggs originating from Fousing Trout Farm (Jutland, Denmark) were used in this study. Hatching and disinfection were carried out at AquaBaltic (Bornholm, Denmark). Upon arrival at the aquarium facilities, the fish were acclimatized for at least 1 month in 200-liter tanks containing 15°C recirculated tap water (nonchlorinated) with an air supply, while the ammonia, nitrite, and nitrate contents of the water were monitored with ammonia test strips (catalog no. 27553-25; Hach) and nitrate/nitrite test strips (catalog no. 27454-25; Hach) to ensure the functionality of biological filters. The fish were fed dry commercial feed (Inicio Plus; BioMar A/S, Denmark) at 1% of biomass per day. To ensure that the fish were free of bacterial infections upon arrival, bacteriological examination of 10 to 20 fish was conducted according to the procedure described by Henriksen et al. in 2013 (41). The fish showed no sign of disease at any time and were, to our knowledge, free of bacterial infections.
In both experiments (Table 1), the largest and the smallest fish were removed, in order to minimize size differences, before fish were randomly divided into the experimental groups. Water was changed twice a week, while the conditions of the fish and aquaria were monitored at least twice per day. Fish for sampling, moribund fish, and fish that were alive at the end of the experiment were killed by an overdose of 3-aminobenzoic acid ethyl ester (MS-222; Sigma) in water.
TABLE 1.
Fish and experimental parameters used in the studya
| Expt | Fish wt (g) | Fish length (cm) | Intestine wt (g) | Spleen wt (g) | Total no. of fish (no. per replicate) | Tank size (liters) |
|---|---|---|---|---|---|---|
| 1 | 5.6 (±2.0) | 7.8 (±1.2) | 0.028 (±0.013) | 0.0055 (±0.0054) | 144 (24) | 30 |
| 2 | 78.0 (±20.9) | 18.8 (±1.8) | 0.12 (±0.05) | 0.06 (±0.02) | 88b | 200 |
Values for fish weight, fish length, intestine weight, and spleen weight are means (SDs). The water temperature was 15°C in both experiments.
Experiment 2 was conducted without replicates. Thirty-eight fish in the control group received commercial feed; 12 fish were fed phage-coated feed once and then commercial feed for the rest of the study; and 38 fish were fed phage-coated feed throughout the experiment, except for the last sampling.
The fish experiments were carried out in accordance with the internationally accepted guidelines for the care and use of laboratory animals in research, and procedures were approved by the Committee for Animal Experimentation, Ministry of Justice, Copenhagen, Denmark (J.nr. 2012-15-2934-00629). The fish were inspected several times a day during the experiments, and precautions were taken to minimize stress by keeping the fish in opaque tanks, minimizing handling, and monitoring water chemistry.
Experiment 1. (i) Phage administration by bath or oral intubation into the stomach.
An experiment was carried out to examine the dispersal, distribution, and decay of phage FpV-9 in juvenile rainbow trout following phage administration by two different methods. Fish (total number, 144; mean weight, 5.6 g; standard deviation [SD], 2.0 [Table 1]) were randomly divided into six groups, each consisting of 24 fish, and were kept in 30-liter tanks. Fish in two tanks served as duplicates and were administered phages by bath treatment, whereas fish in one tank served as controls and received no treatment. In parallel duplicate tanks, fish were administered phages by oral intubation, with the final tank serving as a control where fish were administered sterile SM-buffer by oral intubation. Fish were bath challenged for 30 min in 2.5 liters of tap water containing bacteriophage at a concentration of 108 PFU ml−1 (phage concentrations were determined by the double-layer method described by Stenholm el al. in 2008 [1]) and were then transferred back to their original tanks (Table 2). Oral intubation of phages was carried out using a soft rubber tube with a diameter of ∼2 mm mounted on a 1-ml syringe. The fish were anesthetized with 3-aminobenzoic acid ethyl ester, and the rubber tube was gently guided through the oral cavity and into the stomach, where 0.1 ml of a phage stock containing 109 PFU/ml was inoculated (Table 2). Each fish was immediately transferred back to the original tank after phage injection. The same procedure was conducted for the control group, where 0.1 ml of sterile SM-buffer was inoculated. Throughout the experimental period, the fish were fed a minimum amount of feed.
TABLE 2.
Bacteriophage concentrations and volumes used in the studya
| Expt | Phage administration method | Vol or wt of phage solution or pellets | Phages (PFU) fish−1 |
|---|---|---|---|
| 1 | Bath | 2.5-liter bath (1 × 108 PFU ml−1) | |
| Oral intubation | 100 μl | 1 × 108 | |
| 2 | Phage-coated feed | 2% of fish body wt (1 g feed fish−1)b | 2.5 × 107 |
In all experiments, phage stock concentrations were 1 × 109 PFU ml−1.
Mean fish weight, measured prior to the start of experiment 2, was 50 g.
(ii) Sampling.
During the experiment, 1 ml water and 2 live fish were sampled from each tank (12 fish at each sampling), first at 0 h (before phage addition) and then at 0.5, 5, 11, 21, 27, 50, 70, 83, 123, 171, and 315 h after phage addition. The weight and length of each fish were measured, and organ samples from the intestine, spleen, brain, and kidney were transferred to Eppendorf tubes containing 300 μl SM-buffer and 5 μl of chloroform for qualitative detection of phages. Only samples from the intestine and spleen were weighed to allow a quantification of weight-specific phage abundance. Each organ was smashed with Eppendorf micropestles, sonicated for 15 s at 20 kHz (19) to homogenize the organs, and vortexed for 15 s. Fifteen microliters of chloroform was added to the water samples, and all the samples were stored at 5°C in the dark until further use for qualitative and quantitative examination of the presence of phages.
The fish from the final sampling (315 h) were further examined for the occurrence of F. psychrophilum. Samples from the spleen, brain, and kidney were streaked onto TYES plates and blood agar plates and were incubated for 7 days at 15°C for TYES plates and at 20°C for blood plates. No growth was seen on the agar plates.
Experiment 2. (i) Phage administration by phage-coated feed pellets.
A third method of phage administration was used in this experiment in order to determine whether phage-coated feed might be a suitable method for administering phages to the fish. Fish (mean weight, 78 g; SD, 20.9 [Table 1]) were randomly divided into three groups (three 200-liter tanks in all, and a total of 88 fish). One group of fish (12 fish in total) received phage-coated feed once and commercial fish feed for the rest of the experimental period. Another group of fish (38 fish in total) received phage-coated fish feed throughout the experiment except for the last feeding. Fish in the control group (38 fish in total) received commercial fish feed. The phage-coated feed used for the in vivo studies was stored at 5°C. Partly coated Inicio Plus pellets (2 mm; BioMar A/S, Denmark) suited for further coating with phages were coated with bacteriophage FpV-9. A 2.5-ml phage stock (containing 109 PFU ml−1) was applied per 100 g fish feed, using a spray bottle and a hand mixer, and the phage-coated feed was stored at 5°C (work done at BioMar A/S). All groups were fed at 2% of biomass per day (Table 2).
(ii) Sampling.
Sampling was conducted as described for experiment 1, with some minor changes. Two live fish were sampled from the control group and the group fed phage-coated feed through the whole experiment, first at 0 h (before phage addition) and then at 1, 7, 24, 25, 31, 48, 49, 55, 72, 73, 79, 144, 145, 151, 192, 193, 199, and 384 h after the first sampling, while two live fish were sampled from the group only fed once with phage-coated feed at 0 h (before phage addition) and at 1, 7, 24, 48, 72, 144, and 192 h after the first sampling. Water samples were taken at 0, 2, 5, 8, 11, and 14 h after the start of the experiment. Since the fish in experiment 2 were larger than those in experiment 1, organ samples were transferred to Eppendorf tubes containing 800 μl SM-buffer and 15 μl chloroform.
Qualitative detection of bacteriophages in fish organs.
Qualitative examination for the presence of phages in the intestine, spleen, kidney, and brain was carried out by the spot test method (1, 19). TYES plates with a bacterial lawn of the F. psychrophilum host strain were prepared by adding 300 μl of bacterial cells in the exponential-growth phase (optical density at 525 nm [OD525], 0.2 to 0.3; 48-h-old culture) to 4 ml of 43°C top agar (TYES broth with 0.4% agar), and the mixture was immediately poured onto a cold TYES agar plate and was incubated at 15°C to solidify (19). The diluted organ samples were briefly centrifuged at 5,000 × g for 2 min, and triplicate 5-μl samples from the supernatant of each sample were added to the TYES plates containing the bacterial lawn of the host strain and were incubated for 3 days at 15°C in the dark. Spot tests were considered positive if total lysis or single plaques were seen at the site of spots in one or more of the triplicate samples.
Quantification of bacteriophages in fish organs.
Quantitative examination of the abundance of infectious phages was carried out in the intestine and the spleen samples. The weight-specific phage abundance was quantified by the spot test method described above. Those of the spots obtained in the qualitative examination that contained a countable number of plaques (1 to 30 phages per 5-μl sample) were counted directly, while spots with total lysis or overlapping plaques were diluted in a 10-fold dilution series (5 μl of sample to 45 μl of SM-buffer), and triplicate 5-μl samples from each dilution were added to TYES agar plates as described in the preceding paragraph and were incubated for 3 days at 15°C in the dark. The number of plaques was then counted from the preferred dilution.
Bacteriophage infectivity on fish feed pellets.
In the experiment with fish feed pellets, the loss of phage infectivity over time at different temperatures was monitored after phage addition and desiccation of feed pellets. The feed pellets were coated with phage FpV-9 as described under “Experiment 2” above and were stored at 20°C, 5°C, or −80°C. Phage-coated fish feed samples were collected at days 1, 30, 60, 102, and 259. For each sampling, 0.1 g feed was added to triplicate 15-ml Falcon tubes containing 5 ml SM-buffer, vortexed for 20 s, and incubated at 5°C for 1 h, followed by homogenization by sonication at 47 kHz for 2 min in a water bath. The homogenized samples were diluted, and the abundance of infectious phages was determined by plaque assays using the host strain (1, 19). The exponential rate of infectivity loss (decay rate) was calculated for each treatment from the decrease in PFU over time, based on the linear regression of ln-transformed PFU data with time, using the SigmaPlot program, version 11.0. The decay rates thus represent the fraction of the phage population that was lost per unit of time of incubation.
RESULTS
Qualitative detection of bacteriophage FpV-9 in juvenile rainbow trout after phage administration by three different methods.
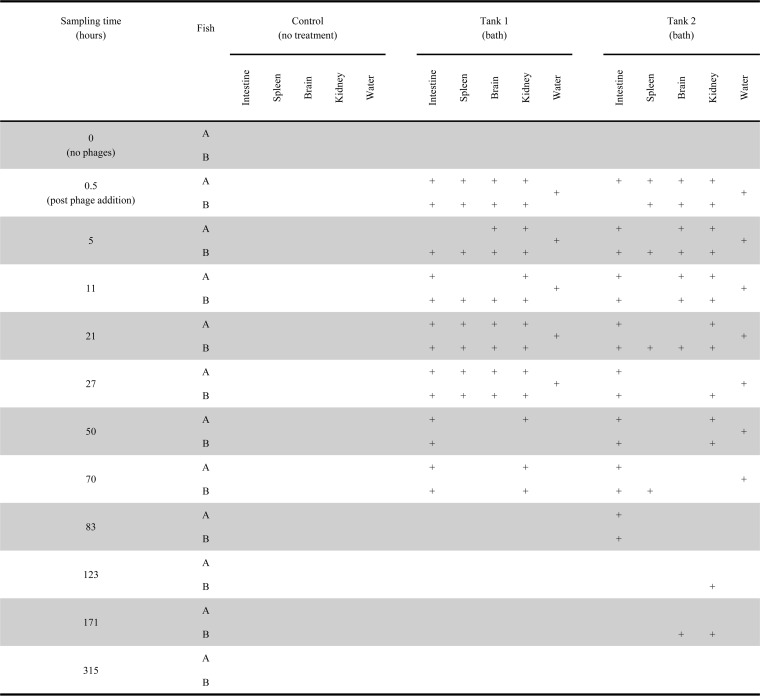
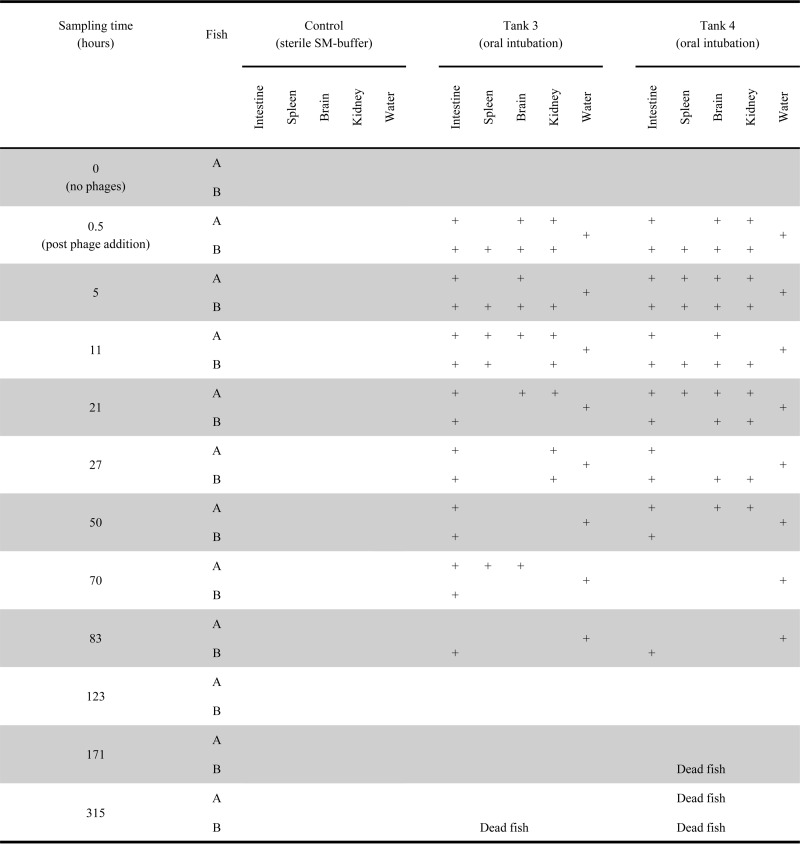
Phage administration by bath or oral intubation (experiment 1) resulted in a quick spread of phages to all the organs investigated for around 21 to 27 h, followed by a decrease in the frequency of phage detection (Fig. 1 and 2). The persistence of phages over time differed considerably for the different organs; infective phages were detected for ∼70 h in the intestine and kidney, whereas only half of the spleen and brain samples contained infective phages 21 to 27 h after addition. Phages were detected in brain and kidney samples from two fish after 123 and 171 h (Fig. 1).
FIG 1.
FpV-9 phages were administered to fish by bath challenge for 30 min in water containing a high concentration of phages. A plus sign indicates the presence of phages in the tissue, and a blank area indicates the absence of phages. Phages were detected by spotting samples onto the host strain F. psychrophilum 950106-1/1 in triplicate. Samples were considered positive if phages were detected in one or more of the triplicates.
FIG 2.
FpV-9 phages were administered to fish by oral intubation into the stomach. A plus sign indicates the presence of phages in the tissue, and a blank area indicates the absence of phages. Phages were detected by spotting samples onto the host strain F. psychrophilum 950106-1/1 in triplicate. Samples were considered positive if phages were detected in one or more of the triplicates.
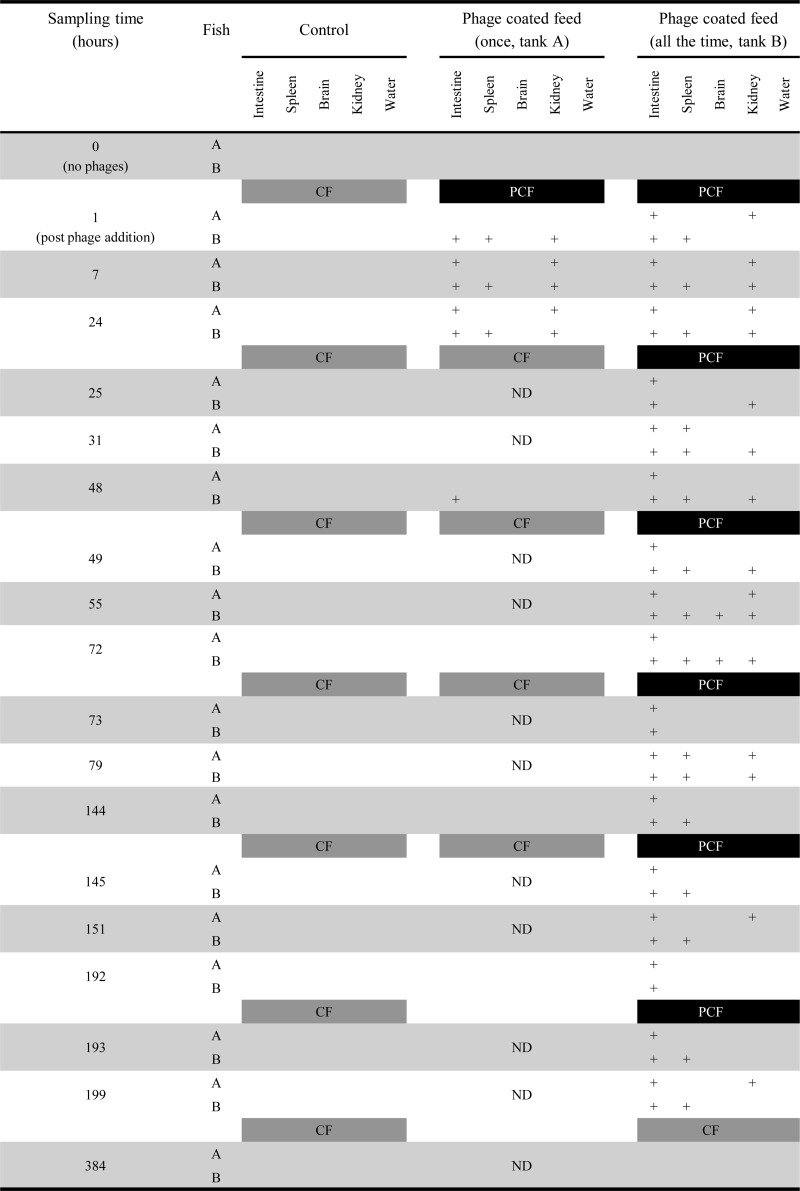
Phage administration by use of phage-coated feed pellets (experiment 2) resulted in the spreading of phage FpV-9 to the intestine, spleen, and kidney within the first hours after feeding, whereas phages were detected in only two of the brain samples examined (Fig. 3). In the tank where the fish were fed phage-coated feed only once before changing to commercial feed, all the intestine and kidney samples contained phages 7 h after phage addition, whereas phages were found in only ∼50% of the spleen samples examined within the first 24 h (Fig. 3). No phages were found in fish from this tank after 24 h, except for one intestine sampled at 48 h. Continuous feeding with phage-coated feed led to persistent detection of phages throughout the duration of the experiment. The detection of phages was most persistent in the intestine, while phages were in general found in only 40 to 50% of the spleen and kidney samples sampled. After the last feeding with phage-coated feed (at 192 h) in this tank (Fig. 3), the fish were fed commercial feed once every day for the rest of the experiment, resulting in complete loss of infective phages from all organs at the final sampling at 384 h.
FIG 3.
FpV-9 phages were administered to fish via phage-coated feed. A plus sign indicates the presence of phages in the tissue, and a blank area indicates the absence of phages. Phages were detected by spotting samples onto the host strain F. psychrophilum 950106-1/1 in triplicate. Samples were considered positive if phages were detected in one or more of the triplicates. CF, commercial feed; PCF, phage-coated feed; ND, not done (no samples taken). Bars giving the type of fish feed also indicate the time of feeding.
Quantification of bacteriophage FpV-9 in juvenile rainbow trout after phage administration by three different methods.
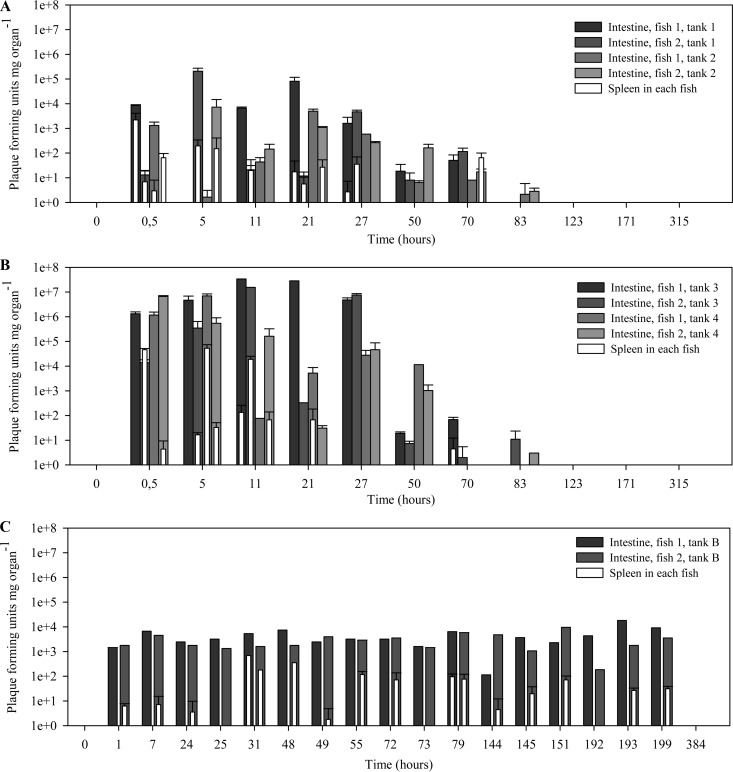
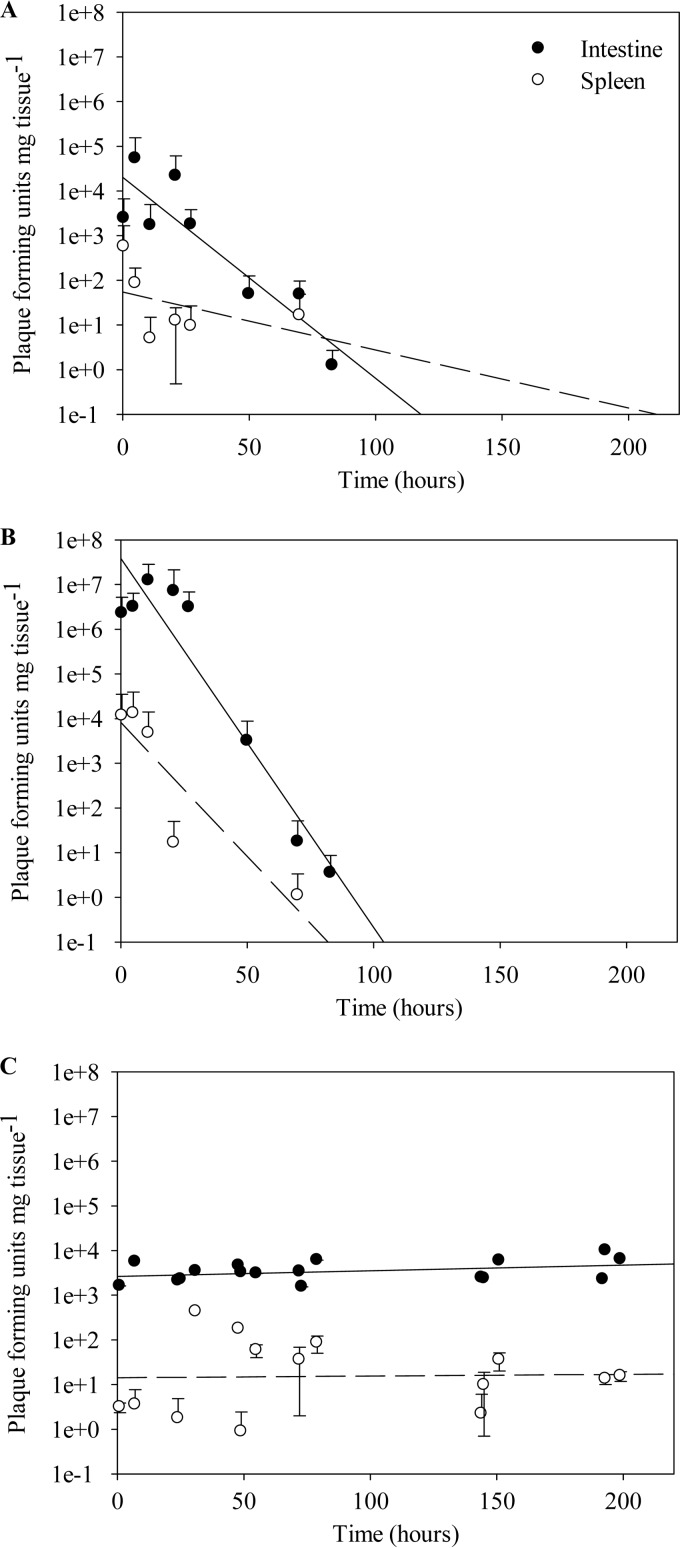
Quantitative analysis of the persistence of phage FpV-9 in the intestine and spleen supported the qualitative observations (Fig. 4 and 5; Table 3). After phage administration by bath (experiment 1), the abundance of phages could be quantified in the intestine and in the spleen after 0.5 h, reaching concentrations of ∼104 and ∼103 PFU mg organ−1, respectively, though with considerable variations between the fish (Fig. 4A). The phage concentration in the intestine reached a maximum of ∼105 PFU mg intestine−1 in one sample after 5 h (average concentration at 5 h, 5.3 × 104 PFU mg intestine−1), and then the detection frequency decreased to an average of 1.2 PFU mg intestine−1 at 83 h. No phages were found after this time point (Fig. 4A). In the spleen samples, phages were quantified during the first 27 h, reaching a maximum of ∼103 PFU mg spleen−1 in one sample after 30 min (average, 5.7 × 102 PFU mg spleen−1). No phages were detected in the spleen samples after 27 h except for one sample at 70 h. On average, phage density was ∼100-fold lower in the spleen than in the intestine between 0.5 h and 70 h post-phage administration. Plotting of the average phage density obtained in all samples from experiment 1 showed a significant exponential loss of infective phages from the intestine over time after the initial addition (Fig. 5A), at a rate of 0.10 ± 0.02 h−1 (P < 0.01) (Table 3). In the spleen, the loss of phage infectivity did not follow a significant exponential decay curve (decay rate, 0.03 ± 0.03 h−1; P > 0.05) (Fig. 5A; Table 3).
FIG 4.
FpV-9 phages were administered by three different methods. (A) Fish were immersed in a high-concentration bath of phages for 30 min immediately before sampling at 0.5 h. (B) Phages were administered by oral intubation into the stomach immediately before sampling at 0.5 h. (C) Phages were administered via phage-coated fish feed 1 h before sampling at 1 h, 25 h, 49 h, 73 h, 145 h, and 193 h. A total of four fish (A and B) or two fish (C) were sampled at each time point. Each shaded bar represents one sampled fish intestine, while each open bar represents the sampled spleen of the same fish.
FIG 5.
Mean quantification values for bacteriophage FpV-9 in rainbow trout intestine and spleen over time. Phages were administered by three different methods—bath (A), oral intubation into the stomach (B), and phage-coated fish feed (C)—1 h before sampling at 1 h, 25 h, 49 h, 73 h, 145 h, and 193 h. Mean values for each group are plotted along with regression lines from the log-transformed values and standard deviations. Statistics for regression are given in Table 3.
TABLE 3.
Phage decay/propagation rates in intestine and spleen samples calculated from linear regression
| Phage administration method | Tissue | Phage decaya |
Phage propagationa |
||
|---|---|---|---|---|---|
| Rate h−1 | r2 | Rate h−1 | r2 | ||
| Bath | Intestine | 0.10 (±0.02) | 0.81* | ||
| Spleen | 0.03 (±0.03) | 0.18 | |||
| Oral intubation | Intestine | 0.19 (±0.02) | 0.91* | ||
| Spleen | 0.14 (±0.04) | 0.81* | |||
| Phage-coated feed | Intestine | 0.003 (±0.002) | 0.14 | ||
| Spleen | 0.001 (±0.008) | 0.001 | |||
Phage decay/propagation rates were calculated from linear regression of ln-transformed data on PFU over time. Asterisks indicate significant rates of decay (P < 0.05).
Phage administration by oral intubation into the stomach resulted in ∼100-fold higher phage recovery in the intestine and 10-fold higher phage recovery in the spleen than those with the bath administration (Fig. 4A), reaching averages of ∼106 PFU mg intestine−1 and ∼104 PFU mg spleen−1 (Fig. 4B). The highest phage concentration in a sample was found at 11 h with ∼107 PFU mg intestine−1 and at 5 h with ∼104 PFU mg spleen−1. Phages were maintained at high densities in both intestine and spleen samples for the first 27 h and 21 h, respectively (Fig. 4B), followed by exponential decreases of 0.19 ± 0.02 h−1 and 0.14 ± 0.04 h−1, respectively (P < 0.05) (Fig. 5B; Table 3). Phages were not detected in the intestine after 83 h or in the spleen after 21 h, except for one spleen sample at 70 h (Fig. 4B). Around 1,000-fold fewer phages, on average, were found in the spleen than in the intestine from 0.5 h to 70 h post-phage administration.
Administration of bacteriophage via fish feed pellets (experiment 2) showed remarkably different results: the fish sustained a constant abundance of phages in the intestine samples throughout the experimental period, at an average of 4.5 × 103 PFU mg intestine−1 (1 to 199 h post-phage administration) (Fig. 4C). A similar result was seen for the spleen samples, although phages were found only in about 50% of the samples investigated; the abundance ranged from 2 PFU mg spleen−1 at 24 h to 6.9 × 102 PFU mg intestine−1 at 31 h, with an average of 5.2 × 101 PFU mg spleen−1 from 1 h to 199 h post-phage administration. On average, the abundance of phages in the spleen was 100- to 1,000-fold lower than that in the intestine (3.9 × 103 PFU mg intestine−1) throughout the experiment. Averaging the phage density over time showed a more or less constant recovery of phages over the duration of the experiment (200 h) with no significant changes (Fig. 5C; Table 3).
Quantification of bacteriophage FpV-9 on feed pellets.
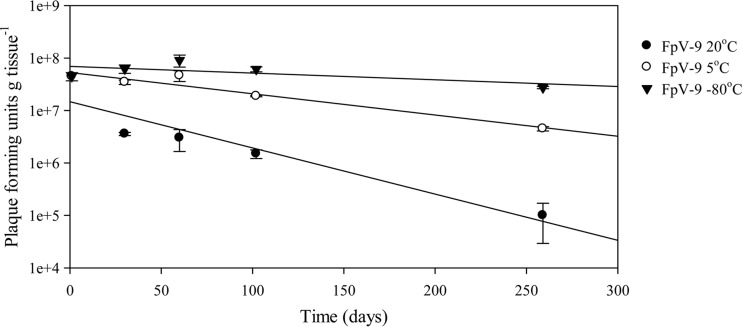
The rate of loss of the infectivity of phages used to coat fish feed pellets differed with storage temperature (Fig. 6; Table 4). The highest decrease in phage infectivity was seen for feed stored at 20°C, with a decrease from 4.7 × 107 PFU g feed−1 24 h postcoating to 1.0 × 105 PFU g feed−1 after 259 days, corresponding to a decay rate of 0.00084 ± 0.0018 h−1 (r2 = 0.89; P < 0.02) (0.2% survival after 259 days). Phage infectivity on feed stored at 5°C decreased from 4.7 × 107 PFU g feed−1 24 h postcoating to 4.5 × 106 PFU g feed−1 after 259 days, with a decay rate of 0.00039 ± 0.00006 h−1 (r2 = 0.94; P < 0.01), corresponding to 10% survival after 259 days. The best conservation was obtained at −80°C, with the phage infectivity decreasing from 4.7 × 107 PFU g feed−1 24 h postcoating to 2.7 × 107 PFU g feed−1 after 259 days. This corresponded to a decay rate (0.00012 ± 0.00008 h−1; r2 = 0.44; P > 0.05) 7-fold lower than that seen at room temperature and to 61% recovery after 259 days (Fig. 6; Table 4).
FIG 6.
Rate of decay of infective phages on fish feed pellets over time following storage at different temperatures. Statistics for regression are given in Table 4.
TABLE 4.
Survival of phage-coated feed
| Storage temp (°C) | Phage decaya |
|
|---|---|---|
| Rate h−1 | r2 | |
| 20 | 0.00084 (±0.00018) | 0.89* |
| 5 | 0.00039 (±0.00006) | 0.94* |
| −80 | 0.00012 (±0.00008) | 0.44 |
The phage decay rate was calculated from linear regression of ln-transformed data on PFU over time. Asterisks indicate significant rates of decay (P < 0.05).
DISCUSSION
The use of phages for the control of fish pathogens in infected fish depends on the ability of the phages to reach the target organs at a sufficient density and to maintain their lytic potential to control the pathogen. F. psychrophilum phage FpV-9 has been shown to spread rapidly to internal organs in trout after i.p. injection (107 PFU fish−1), but the fast phage decay in the organs resulted in almost no detection of phages after 72 h (19). The fast phage decay seen in fish as well as other animals (2, 31, 32, 35, 36) after phage delivery through the parenteral route makes the timing of phage injection crucial for effective control of the pathogen.
In the present experiment, we tested phage administration through the oral route as an alternative to the parenteral route. The dispersal and abundance of infectious phages in juvenile rainbow trout was investigated following the administration of phage FpV-9 by three different methods: bath, oral intubation into the stomach, and phage-coated fish feed pellets. The last administration method allowed continuous delivery of phages to the fish.
Dispersal and recovery of phages in fish organs.
In the groups where phages were administered by bath or oral intubation (experiment 1), the phages were rapidly and efficiently spread to all the organs investigated. Detection of phages in the spleen, brain, and kidney proves that orally administered phages can penetrate the intestinal barrier and can be absorbed into the circulatory system. This process has been described as similar to bacterial translocation, although the precise processes controlling the viral translocation are unknown (22, 23, 30). It has been suggested that phage passage is determined by a number of factors, including phage concentration and phage interaction with gut immune cells (30).
The same rapid and efficient spread of phages was seen in the groups to which phages were administered via phage-coated feed pellets (experiment 2), except for the brain samples investigated. The reduced phage recovery in the brain tissue is most likely an effect of the lower initial concentration of phage in fish treated by this method than in fish treated by the oral intubation or bath administration method used in experiment 1. Moreover, this finding is supported by other studies where the concentration of phages found in the brain was approximately 100 times lower than that in the blood following intravenous (i.v.) injection of phages into mice (32, 42). Together, these results indicate that a high phage concentration is important if the phages are to be spread to all the internal organs via the circulatory system, especially if they are to cross the blood-brain barrier.
It should also be noted that large differences in phage recovery were seen between fish in the same group at the same sampling point. This was possibly due to unequal consumption of phage-coated feed at each feeding, potentially resulting in unequal delivery of phages to individual fish.
Decay of phages following administration.
Even though the phages were rapidly spread to the internal organs following delivery by all three administration methods, fast decay of phages was observed in the intestine and spleen, reaching a rate of 0.03 or 0.14 h−1 in the spleen and 0.10 or 0.19 h−1 in the intestine after administration by bath or oral intubation, respectively. The decrease in the number of phages in the intestine is, according to our results, caused by the permeation of phages through the intestinal wall and into the bloodstream, although some might be lost in other ways, for example, in feces or by nonspecific adsorption. The 100- to 1,000-fold lower number of phages quantified in the spleen than in the intestine might indicate a loss of infective phages in the process of passing through the intestinal wall. These results correspond with the findings of Kawato and Nakai, where the number of phages found in the kidney was 104 to 105 times lower than that in the intestine following anal intubation of phages into goldfish (22). Here it was suggested that this inefficiency of transfer through the intestinal wall was caused by limited entry portals for phages in the intestine.
Many factors that could contribute to phage decay in the organs have been described. In a previous study, it was argued that irreversible adsorption to fish tissue might be one factor regulating phage infectivity (19). Although it was reported that no anti-phage antibodies were detected in yellowtail and ayu after oral treatment and intramuscular (i.m.) injections of bacteriophages (25), several other studies have reported humoral immunity in individuals subjected to phage therapy, leading to the production of phage-neutralizing antibodies that result in the inactivation of bacteriophages in vivo (21, 36, 43, 44). Phages are also known to interact with the innate immune system, which plays an important role in removing phages from higher organisms by filtration in the spleen, liver, and other filtrating organs of the reticuloendothelial system (31, 36, 43, 45). The concentration of phages found in the spleen in our study might be caused by this filtration. The impact that an immune response in fish might have on phage therapy is difficult to assess and needs further investigation, but reports from other animal studies clearly indicate that this is likely to be one factor contributing to phage decay.
An issue relating to phage delivery via the oral route is phage stability in the highly acidic and proteolytically active environment of the stomach (2, 19, 23, 29), and a loss of phages during passage through the stomach is expected. Previous studies have shown that pH is one important factor affecting the infectivity of phage FpV-9; there is an immediate loss of infectivity at pH ≤3 and a higher decay rate at pH 4.5 than at pH 6 and 7.5 (19). Sensitivity to pH might differ between phages, and each bacteriophage should be characterized independently (46). Furthermore, the pH in the stomach of the rainbow trout has been shown to increase from pH 2.7 to 4.9 within 1 h after the ingestion of a meal (29). The pH in the stomach of the fish in this study was most likely low (around pH 3), since feeding of the fish stopped 24 h prior to phage addition. Compared with phage administration by bath and oral intubation, administration with feed is thought to provide protection of the phages by increasing the pH in the stomach. Protection of the phages by an antacid that neutralizes the stomach acidity or by microencapsulation would likely result in a higher fraction of the phages passing the stomach and reaching the target organs (47).
Administration of phages via feed is the only method that allows easy continuous delivery of phages to the fish, while it also involves less stress and handling of the animals than the methods used in experiment 1 and the parenteral route (23). The constant density of phages maintained in the intestine and the spleen that was found in this study after feeding with phage-coated feed indicated that this delivery method compensated for the rate of phage decay in the organs investigated. Increasing the phage concentration on the feed should thus, in theory, result in an accumulation of phages in the organs, and thus, potentially, a more efficient treatment (36).
The results suggest continuous feeding with phage-coated feed as an ideal administration method for prophylactic treatment against F. psychrophilum infections, since the resulting constant, high abundances of phages in the fish could offer protection at the early stage of infection. Since the loss of appetite in fish is one of the clinical signs of RTFS (9), prophylactic treatment is the most promising method. The continuous delivery of phages into a system could, however, lead to inactivation of the phages by the adaptive immune defense mechanisms, which would require changing the phage or mixture of phages over time (31). Since outbreaks of RTFS often occur within the first months of feeding, when the immune system of the trout is not yet fully developed (9, 48), phage inactivation by this mechanism likely plays a minor role in rainbow trout fry, as also indicated in the current study.
Phage survival on feed.
Another promising prospect is the ability of the phage tested (FpV-9) to tolerate long periods of desiccation on the feed and still maintain high infectivity (19). To secure a low phage degradation rate on the feed, it is vital that the storage conditions be optimal, which was found to be the case at −80°C, where 60% of the phage remained infective after 8 months of storage, in contrast to the 10% survival rate found after storage at 5°C for the same period. These results are consistent with previous studies in which long-term storage in glycerol at −80°C proved to be an effective option for phage survival (19). The observed loss of infective phages on the feed over time could be explained by nonspecific adsorption to the surfaces of the feed particles (2, 19, 34). Phage degradation needs be tested at −20°C, since this is a more realistic storage temperature for the industry. These results are important if phage-coated feed is to have a commercial future.
Future perspectives.
In this study, we have shown that F. psychrophilum phage FpV-9 administered via the oral route could successfully reach the target organs investigated in juvenile rainbow trout. The results emphasize that phage administration via phage-coated feed successfully maintains a constant phage density in target organs. More research is needed, however, to optimize phage concentrations and protection on feed pellets, as well as to increase the site-specific delivery of phages, e.g., by development of phage formulations (23). The next step will be experimental challenge of rainbow trout with F. psychrophilum, where the effect of phages administered with the feed will be investigated. In addition to prophylactic treatment, it would be highly relevant to investigate different feeding regimens, as well as the administration dose, since this seems to be a critical factor in oral phage therapy.
ACKNOWLEDGMENTS
The study was supported by the Directorate for Food, Fisheries and Agribusiness and the Danish Council for Independent Research (FNU-09-072829).
We thank Jeanett Hansen and Lene Gertman for excellent technical support.
Footnotes
Published ahead of print 3 October 2014
REFERENCES
- 1.Stenholm AR, Dalsgaard I, Middelboe M. 2008. Isolation and characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 74:4070–4078. 10.1128/AEM.00428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Gomez DK, Nakai T, Park SC. 2010. Isolation and identification of bacteriophages infecting ayu Plecoglossus altivelis altivelis specific Flavobacterium psychrophilum. Vet. Microbiol. 140:109–115. 10.1016/j.vetmic.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Castillo D, Higuera G, Villa M, Middelboe M, Dalsgaard I, Madsen L, Espejo RT. 2012. Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. J. Fish Dis. 35:193–201. 10.1111/j.1365-2761.2011.01336.x. [DOI] [PubMed] [Google Scholar]
- 4.Bernardet JF, Baudin-Laurencin F, Tixerant G. 1988. First identification of Cytophaga psychrophila in France. Bull. Eur. Assoc. Fish Pathol. 8:104–105. [Google Scholar]
- 5.Lehmann J, Mock D, Stürenberg FJ, Bernardet JF. 1991. First isolation of Cytophaga psychrophila from a systemic disease in eel and cyprinids. Dis. Aquat. Organ. 10:217–220. 10.3354/dao010217. [DOI] [Google Scholar]
- 6.Lorenzen E, Dalsgaard I, From J, Hansen EM, Hørlyck V, Korsholm H, Mellergaard S, Olesen NJ. 1991. Preliminary investigations of fry mortality syndrome in rainbow trout. Bull. Eur. Assoc. Fish Pathol. 11:77–79. [Google Scholar]
- 7.Bruno DW. 1992. Cytophaga psychrophila (Flexibacter psychrophilus) histopathology associated with mortalities among farmed rainbow trout, Oncorhynchus mykiss Walbaum, in the UK. Bull. Eur. Assoc. Fish Pathol. 12:215–216. [Google Scholar]
- 8.Santos Y, Huntly PJ, Turnbull A, Hastings TS. 1992. Isolation of Cytophaga psychrophila (Flexibacter psychrophilus) in association with rainbow trout mortality in the United Kingdom. Bull. Eur. Assoc. Fish Pathol. 12:209–210. [Google Scholar]
- 9.Nematollahi A, Decostere A, Pasmans F, Haesebrouck F. 2003. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 26:563–574. 10.1046/j.1365-2761.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- 10.Jensen PA, Henriksen NH, Michelsen K, Madsen L, Dalsgaard I. 2003. Prevention of RTFS (rainbow trout fry syndrome) and reduction in the amount of used antibiotics in Danish rainbow trout hatcheries and fry farms. DFU report 124-03. Danish Institute for Fisheries Research, Copenhagen, Denmark: (In Danish.) [Google Scholar]
- 11.Nilsen H, Olsen AB, Vaagnes Ø, Hellberg H, Bottolfsen K, Skjelstad H, Colquhoun DJ. 2011. Systemic Flavobacterium psychrophilum infection in rainbow trout, Oncorhynchus mykiss (Walbaum), farmed in fresh and brackish water in Norway. J. Fish Dis. 34:403–408. 10.1111/j.1365-2761.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 12.Bruun MS, Schmidt AS, Madsen L, Dalsgaard I. 2000. Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture 187:201–212. 10.1016/S0044-8486(00)00310-0. [DOI] [Google Scholar]
- 13.Cabello FC. 2006. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8:1137–1144. 10.1111/j.1462-2920.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 14.Obach A, Laurencin FB. 1992. Vaccination of rainbow trout Oncorhynchus mykiss against the visceral form of coldwater disease. Dis. Aquat. Organ. 12:13–15. 10.3354/dao012013. [DOI] [Google Scholar]
- 15.Rahman MH, Ototake M, Iida Y, Yokomizo Y, Nakanishi T. 2000. Efficacy of oil-adjuvanted vaccine for coldwater disease in ayu Plecoglossus altivelis. Fish Pathol. 35:199–203. 10.3147/jsfp.35.199. [DOI] [Google Scholar]
- 16.LaFrentz BR, LaPatra SE, Jones GR, Cain KD. 2004. Protective immunity in rainbow trout Oncorhynchus mykiss following immunization with distinct molecular mass fractions isolated from Flavobacterium psychrophilum. Dis. Aquat. Organ. 59:17–26. 10.3354/dao059017. [DOI] [PubMed] [Google Scholar]
- 17.Sudheesh PS, LaFrentz BR, Call DR, Siems WF, LaPatra SE, Wiens GD, Cain KD. 2007. Identification of potential vaccine target antigens by immunoproteomic analysis of a virulent and a non-virulent strain of the fish pathogen Flavobacterium psychrophilum. Dis. Aquat. Organ. 74:37–47. 10.3354/dao074037. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzen E, Brudeseth BE, Wiklund T, Lorenzen N. 2010. Immersion exposure of rainbow trout (Oncorhynchus mykiss) fry to wildtype Flavobacterium psychrophilum induces no mortality, but protects against later intraperitoneal challenge. Fish Shellfish Immunol. 28:440–444. 10.1016/j.fsi.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Madsen L, Bertelsen SK, Dalsgaard I, Middelboe M. 2013. Dispersal and survival of Flavobacterium psychrophilum phages in vivo in rainbow trout and in vitro under laboratory conditions: implications for their use in phage therapy. Appl. Environ. Microbiol. 79:4853–4861. 10.1128/AEM.00509-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuzaki S, Rashel M, Uchiyama J, Sakurai S, Ujihara T, Kuroda M, Ikeuchi M, Tani T, Fujieda M, Wakiguchi H, Imai S. 2005. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J. Infect. Chemother. 11:211–219. 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- 21.Sulakvelidze A, Alavidze Z, Morris JG., Jr 2001. Bacteriophage therapy. Antimicrob. Agents Chemother. 45:649–659. 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawato Y, Nakai T. 2012. Infiltration of bacteriophages from intestinal tract to circulatory system in goldfish. Fish Pathol. 47:1–6. 10.3147/jsfp.47.1. [DOI] [Google Scholar]
- 23.Ryan EM, Gorman SP, Donnelly RF, Gilmore BF. 2011. Recent advances in bacteriophage therapy: how delivery routes, formulation, concentration and timing influence the success of phage therapy. J. Pharm. Pharmacol. 63:1253–1264. 10.1111/j.2042-7158.2011.01324.x. [DOI] [PubMed] [Google Scholar]
- 24.Park SC, Nakai T. 2003. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis. Aquat. Organ. 53:33–39. 10.3354/dao053033. [DOI] [PubMed] [Google Scholar]
- 25.Nakai T, Park SC. 2002. Bacteriophage therapy of infectious diseases in aquaculture. Res. Microbiol. 153:13–18. 10.1016/S0923-2508(01)01280-3. [DOI] [PubMed] [Google Scholar]
- 26.Nakai T, Sugimoto R, Park KH, Matsuoka S, Mori K, Nishioka T, Maruyama K. 1999. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis. Aquat. Organ. 37:33–41. 10.3354/dao037033. [DOI] [PubMed] [Google Scholar]
- 27.Verner-Jeffreys DW, Algoet M, Pond MJ, Virdee HK, Bagwell NJ, Roberts EG. 2007. Furunculosis in Atlantic salmon (Salmo salar L.) is not readily controllable by bacteriophage therapy. Aquaculture 270:475–484. 10.1016/j.aquaculture.2007.05.023. [DOI] [Google Scholar]
- 28.Oliveira J, Castilho F, Cunha A, Pereira MJ. 2012. Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquacult. Int. 20:879–910. 10.1007/s10499-012-9515-7. [DOI] [Google Scholar]
- 29.Bucking C, Wood CM. 2009. The effect of postprandial changes in pH along the gastrointestinal tract on the distribution of ions between the solid and fluid phases of chyme in rainbow trout. Aquacult. Nutr. 15:282–296. 10.1111/j.1365-2095.2008.00593.x. [DOI] [Google Scholar]
- 30.Górski A, Wazna E, Dabrowska B-W, Dabrowska K, Switała-Jeleń K, Miedzybrodzki R. 2006. Bacteriophage translocation. FEMS Immunol. Med. Microbiol. 46:313–319. 10.1111/j.1574-695X.2006.00044.x. [DOI] [PubMed] [Google Scholar]
- 31.Dabrowska K, Switała-Jeleń K, Opolski A, Weber-Dabrowska B, Gorski A. 2005. Bacteriophage penetration in vertebrates. J. Appl. Microbiol. 98:7–13. 10.1111/j.1365-2672.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 32.Keller R, Engley FB. 1958. Fate of bacteriophage particles introduced into mice by various routes. Proc. Soc. Exp. Biol. Med. 98:577–579. 10.3181/00379727-98-24112. [DOI] [PubMed] [Google Scholar]
- 33.Sechter I, Touitou E, Donbrow M. 1989. The influence of a non-ionic surfactant on rectal absorption of virus particles. Arch. Virol. 106:141–143. 10.1007/BF01311045. [DOI] [PubMed] [Google Scholar]
- 34.Park SC, Shimamura I, Fukunaga M, Mori KI, Nakai T. 2000. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microbiol. 66:1416–1422. 10.1128/AEM.66.4.1416-1422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prasad Y, Arpana , Kumar D, Sharma AK. 2011. Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn.) from columnaris disease. J. Environ. Biol. 32:161–168. [PubMed] [Google Scholar]
- 36.Carlton RM. 1999. Phage therapy: past history and future prospects. Arch. Immunol. Ther. Exp. (Warsz.) 47:267–274. [PubMed] [Google Scholar]
- 37.Dalsgaard I, Madsen L. 2000. Bacterial pathogens in rainbow trout, Oncorhynchus mykiss (Walbaum), reared at Danish freshwater farms. J. Fish Dis. 23:199–209. 10.1046/j.1365-2761.2000.00242.x. [DOI] [Google Scholar]
- 38.Madsen L, Dalsgaard I. 2000. Comparative studies of Danish Flavobacterium psychrophilum isolates: ribotypes, plasmid profiles, serotypes and virulence. J. Fish Dis. 23:211–218. 10.1046/j.1365-2761.2000.00240.x. [DOI] [Google Scholar]
- 39.Madsen L, Dalsgaard I. 1999. Reproducible methods for experimental infection with Flavobacterium psychrophilum in rainbow trout Oncorhynchus mykiss. Dis. Aquat. Organ. 36:169–176. 10.3354/dao036169. [DOI] [PubMed] [Google Scholar]
- 40.Holt RA, Rohovec JS, Fryer JL. 1993. Bacterial cold-water disease, p 3–23 In Inglis V, Roberts RJ, Bromage NR. (ed), Bacterial diseases of fish. Blackwell Scientific Publications, Oxford, United Kingdom. [Google Scholar]
- 41.Henriksen MM, Madsen L, Dalsgaard I. 2013. Effect of hydrogen peroxide on immersion challenge of rainbow trout fry with Flavobacterium psychrophilum. PLoS One 8:e62590. 10.1371/journal.pone.0062590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bogozova GG, Voroshilova NN, Bondarenko VM. 1991. The efficacy of Klebsiella pneumoniae bacteriophage in the therapy of experimental Klebsiella infection. Zh. Mikrobiol. Epidemiol. Immunobiol. 1991(4):5–8 (In Russian.) [PubMed] [Google Scholar]
- 43.Merril CR, Scholl D, Adhya SL. 2003. The prospect for bacteriophage therapy in Western medicine. Nat. Rev. Drug Discov. 2:489–497. 10.1038/nrd1111. [DOI] [PubMed] [Google Scholar]
- 44.Smith WH, Huggins MB, Shaw KM. 1987. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J. Gen. Microbiol. 133:1127–1135. [DOI] [PubMed] [Google Scholar]
- 45.Geier MR, Trigg ME, Merril CR. 1973. Fate of bacteriophage lambda in non-immune germ-free mice. Nature 246:221–222. 10.1038/246221a0. [DOI] [PubMed] [Google Scholar]
- 46.Brüssow H. 2005. Phage therapy: the Escherichia coli experience. Microbiology 151:2133–2140. 10.1099/mic.0.27849-0. [DOI] [PubMed] [Google Scholar]
- 47.Capparelli R, Parlato M, Borriello G, Salvatore P, Iannelli D. 2007. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob. Agents Chemother. 51:2765–2773. 10.1128/AAC.01513-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Decostere A, Haese ED, Lammens M, Nelis H, Haesebrouck F. 2001. In vivo study of phagocytosis, intracellular survival and multiplication of Flavobacterium psychrophilum in rainbow trout, Oncorhynchus mykiss (Walbaum), spleen phagocytes. J. Fish Dis. 24:481–487. 10.1046/j.1365-2761.2001.00322.x. [DOI] [Google Scholar]