Abstract
Most Staphylococcus aureus isolates produce either a serotype 5 (CP5) or 8 (CP8) capsular polysaccharide, and the CP antigens are targets for vaccine development. Since CP5 and CP8 have similar trisaccharide repeating units, it is important to identify an epitope shared by both CP5 and CP8. To characterize cross-reactivity between CP5 and CP8, the immunogenicity of CP5 and CP8 conjugate vaccines in mice and rabbits was evaluated by serological assays. Immune sera were also tested for functional activity by in vitro opsonophagocytic-killing assays and a murine bacteremia model. Antibodies to the CP5-cross-reactive material 197 (CRM197) conjugate vaccine bound only to purified CP5. In contrast, antibodies to the CP8-CRM conjugate vaccine reacted with CP8 and (to a lesser extent) CP5. De-O-acetylation of CP5 increased its reactivity with CP8 antibodies. Moreover, CP8 antibodies bound to Pseudomonas aeruginosa O11 lipopolysaccharide, which has a trisaccharide repeating unit similar to that of the S. aureus CPs. CP8-CRM antibodies mediated in vitro opsonophagocytic killing of S. aureus expressing CP5 or CP8, whereas CP5-CRM antibodies were serotype specific. Passive immunization with antiserum to CP5-CRM or CP8-CRM protected mice against bacteremia induced by a serotype 5 S. aureus isolate, suggesting that CP8-CRM elicits antibodies cross-reactive to CP5. The identification of epitopes shared by CP5 and CP8 may inform the rational design of a vaccine to protect against infections caused by CP5- or CP8-producing strains of S. aureus.
INTRODUCTION
Staphylococcus aureus is a major bacterial pathogen that causes skin and soft tissue infections and invasive diseases, such as bacteremia, pneumonia, osteomyelitis, endocarditis, and sepsis. Many virulence factors contribute to the pathogenesis of staphylococcal infections, including surface-associated adhesins, secreted exoproteins and toxins, and immune evasion factors (1, 2). Like many invasive bacterial pathogens, S. aureus produces a capsular polysaccharide (CP) that enhances its resistance to clearance by host innate immune defenses (3). Most strains of S. aureus are encapsulated, and those that produce a serotype 5 (CP5) or serotype 8 (CP8) capsule predominate among clinical isolates (4, 5). Encapsulated S. aureus strains are more virulent than isogenic acapsular mutants in rodent models of staphylococcal bacteremia (6, 7), surgical wound infection (8), septic arthritis (9), and subcutaneous (8) and renal (10) abscess formation.
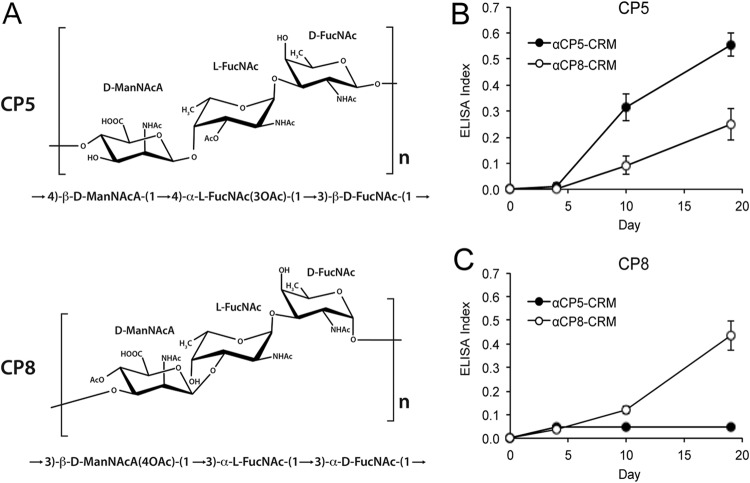
Staphylococcal CP5 and CP8 have similar trisaccharide repeating units (Fig. 1A) consisting of N-acetyl mannosaminuronic acid (ManNAc), N-acetyl-l-fucosamine (l-FucNAc), and N-acetyl-d-fucosamine (d-FucNAc) (11). CP5 and CP8 are reportedly serologically distinct (12, 13), and this has been attributed to differences in the linkages between the sugars and in the sites of O-acetylation. Staphylococcal CP conjugate vaccines elicit antibodies that promote opsonophagocytic killing (OPK) of S. aureus (6), and immunization has been shown to protect experimental animals against staphylococcal bacteremia, lethality, mastitis, osteomyelitis, and endocarditis (3, 14–18).
FIG 1.
S. aureus CP5 and CP8 conjugated to protein elicit capsular antibodies in mice. (A) CP5 and CP8 have similar trisaccharide repeating units comprised of ManNAc, l-FucNAc, and d-FucNAc. (B and C) Groups of ICR mice (n = 18) were immunized with 2.5 μg of CP5-CRM or CP8-CRM on days 0, 5, and 10. Each mouse serum was diluted 1:100 and tested by ELISA on plates coated with purified CP5 (B) or CP8 (C). The ELISA index was determined by dividing the absorbance reading of the test serum by the absorbance reading of a pool of high-titer immune mouse sera included on the same plate. The data shown are mean ELISA indices ± standard errors.
Bacterial CPs can elicit a long-lasting immune response in humans if they are coupled to a protein carrier that contains T-cell epitopes. However, production of these vaccine glycoconjugates is complex and expensive. Due to the nonspecific nature of the chemical conjugation, the vaccine is heterogeneous, variable from batch to batch, and produced with low yield. To improve CP conjugate vaccine production, some researchers have prepared synthetic carbohydrate-based vaccines based on the well-defined chemical structures of polysaccharides produced by Streptococcus pneumoniae, Haemophilus influenzae type b, and S. aureus (19–21). Other novel approaches include glycoengineering strategies, wherein bioconjugate vaccines are synthesized as recombinant glycoproteins by Escherichia coli (15, 22). For either approach, it is important to identify immunogenic epitopes that elicit opsonic and protective antibodies against the target microbe. Because S. aureus produces only two major capsular serotypes with similar structures, it would be of value to identify shared immunogenic epitopes. In this study, antibodies elicited by CP5 or CP8 conjugate vaccines were evaluated for their reactivity with purified S. aureus CP5 and CP8 in the native and de-O-acetylated forms. We identified a common epitope that elicits protective antibodies against both serotype 5 and 8 S. aureus.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. aureus Reynolds (CP5) and Reynolds (CP8) are isogenic strains that produce similar quantities of either CP5 or CP8; these strains and S. aureus Newman were described previously (7, 23). Reynolds Δcap5H produces wild-type levels of de-O-acetylated CP5 (24). NRS 382 (USA100) is a hospital-associated methicillin-resistant S. aureus isolate obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus program supported under NIAID NIH contract no. HHSN272200700055C. Bacteria were grown on tryptic soy agar or Columbia agar plus 2% NaCl plates at 37°C for 24 h.
Animal experiments.
Animal studies were approved by the Harvard Medical Area Institutional Animal Care and Use Committee. CP5 and CP8 conjugate vaccines, produced by conjugating CP5 or CP8 to cross-reactive material 197 (CRM197) (25), a nontoxic recombinant variant of diphtheria toxin, were provided by Wyeth Pharmaceuticals (Collegeville, PA). Rabbit antisera were obtained by immunization with 10-μg CP doses of the conjugate vaccines. CP5-exoprotein A (Epa) (from Pseudomonas aeruginosa) and CP8-Epa vaccines, purified IgG from rabbits immunized with the vaccines (15), and P. aeruginosa serotype O11 antiserum were provided by GlycoVaxyn AG (Schlieren, Switzerland). The conjugate vaccines were >90% O-acetylated.
Female ICR mice (3 to 4 weeks old), obtained from Harlan Sprague Dawley, Inc., were immunized by the subcutaneous route with 2.5 μg of CP5-CRM or CP8-CRM on days 0, 5, and 10. Mice immunized with CRM alone or with phosphate-buffered saline (PBS) served as controls, and all mice received aluminum hydroxide adjuvant. The mice were bled by tail vein puncture before each immunization and again 1 week after the last dose (day 17). Sera from individual mice were tested by enzyme-linked immunosorbent assay (ELISA), and sera from mice bled on day 17 were pooled for the OPK assay. For passive immunization, Swiss Webster mice (7 to 8 weeks old; Taconic Farms) were injected intraperitoneally with 0.5 ml sera from rabbits immunized with CP5-CRM or CP8-CRM. After 24 h, the mice were challenged by the intraperitoneal route with S. aureus Reynolds (CP5) (1 × 107 CFU/mouse). Heparinized blood was obtained by cardiac puncture 2 h after bacterial challenge for quantitative cultures.
Antibody binding assays.
CP5 and CP8 were purified and chemically de-O-acetylated as described previously (27). Sera were serially diluted and tested by ELISA in microtiter plates coated with purified CP5 or CP8 (4 μg/ml) coupled to poly-l-lysine (28). Immunoblots were performed on CP5-Epa and CP8-Epa vaccines separated on SDS-PAGE gels. The blots were probed with antibodies to Pseudomonas exotoxin A (Sigma) or sera from rabbits immunized with killed encapsulated CP5+ or CP8+ bacteria. The latter sera were rendered CP specific by absorption with strain Wood 46 (protein A negative) and isogenic acapsular mutant strains (7, 8) that were trypsinized to remove protein A.
OPK assays.
HL60 cells (ATCC) were cultured in RPMI 1640 containing l-glutamine (Mediatech) and supplemented with 10% heat-inactivated fetal bovine serum (HyClone), l-glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin (Mediatech) at 37°C in 5% CO2. The cells were differentiated to granulocytes by culturing in RPMI 1640 with 10% fetal bovine serum, 1% l-glutamine, and 0.8% N,N-dimethylformamide at a starting density of 4 × 105 cells/ml for 5 to 6 days.
The OPK assay was performed as described previously with modifications (29). Briefly, differentiated HL60 cells were washed in 1× Hanks' balanced salt solution (HBSS) and suspended in 1× HBSS containing Ca2+/Mg2+ and 0.1% gelatin (HBSS++). Trypan blue staining was used to distinguish dead from live leukocytes. The assay was performed in round-bottom polystyrene 96-well plates, and each well (80 μl) contained 4 × 105 viable HL60 cells, 1 × 103 CFU S. aureus, various dilutions of heat-inactivated rabbit or mouse serum, and 1% guinea pig serum (Cedarlane) as a complement source. Control samples included S. aureus incubated with complement and HL60 cells (no antibody), S. aureus incubated with antibodies and complement (no HL60 cells), and S. aureus and HL60 cells only. The 96-well plates were incubated at 37°C with shaking at 700 rpm. After 2 h, 20 μl 1% Triton X-100 was added to each well to lyse the HL60 cells. After a 3-min incubation at 37°C with shaking, 5-μl aliquots of the final reaction mixtures were plated in duplicate on tryptic soy agar plates. The percent change in the number of CFU/ml (i.e., killing) was defined as the reduction in the number of CFU/ml after 2 h compared with the number at time zero, and the opsonic titer was calculated as the highest serum dilution that yielded ≥50% bacterial killing.
Statistical analyses.
ELISA and OPK data were analyzed using the unpaired two-tailed Student t test (Prism 4 software). Quantitative culture data from mice were analyzed by the Mann-Whitney U test.
RESULTS
Serologic response of mice to CP5-CRM and CP8-CRM.
Since CP5 and CP8 alone do not elicit high-titer antibodies, CPs were conjugated to CRM197. Mice immunized with CP5-CRM or CP8-CRM produced serum antibodies to CP5 or CP8, respectively, that peaked a week after the last immunization (Fig. 1B and C). No detectable CP-specific antibodies were generated by vaccination with CRM alone or PBS (data not shown). Sera from mice vaccinated with CP5-CRM were nonreactive on microtiter plates coated with CP8. However, sera from mice immunized with CP8-CRM reacted with both CP8 (Fig. 1C) and CP5 (Fig. 1B), and we designated this one-way cross-reactivity.
Serologic response to CP5-Epa and CP8-Epa.
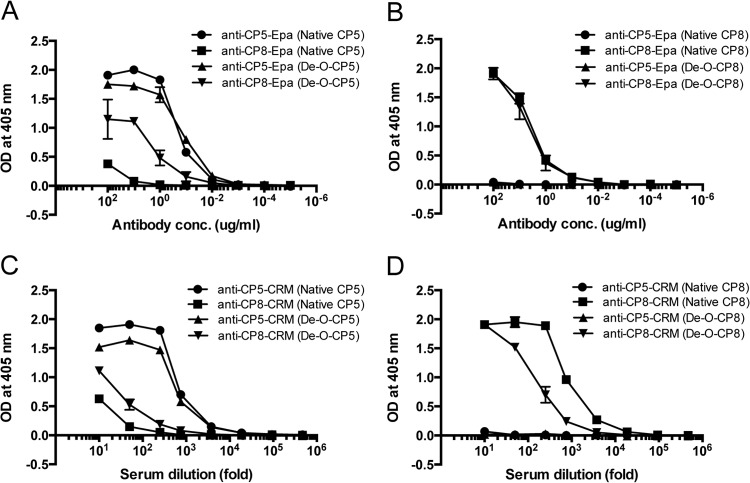
The one-way cross-reactivity that we observed between CP8-CRM antibodies and purified CP5 (but not CP5-CRM antibodies and purified CP8) was unexpected. We performed similar cross-reactivity experiments with rabbit antiserum raised to CP5- and CP8-Epa vaccines to ensure that the cross-reactivity that we observed was due to the polysaccharides and not due to some undetected property of the carrier protein.
Mice actively immunized with CP5-Epa or CP8-Epa showed a serotype-specific immune response (data not shown). Rabbits were immunized with CP5-Epa, and IgG prepared from these sera reacted by ELISA with purified CP5 (Fig. 2A), but not CP8 (Fig. 2B). In contrast, rabbits immunized with CP8-Epa produced antibodies reactive with CP8 (Fig. 2B) and, to a lesser extent, with CP5 (Fig. 2A). Recombinant Epa, CP8-Epa, and CP5-Epa vaccines were separated by SDS-PAGE and reacted by Western blotting with antibodies to Epa, CP5, or CP8. As shown in Fig. 3, CP5 antisera reacted only with the CP5 vaccine, whereas the CP8 antiserum bound to both CP5-Epa and CP8-Epa conjugates.
FIG 2.
Rabbit antibodies to CP8-Epa and CP8-CRM cross-react with CP5. Purified rabbit IgG to CP5-Epa or CP8-Epa (A and B) or rabbit immune serum to CP5-CRM or CP8-CRM (C and D) was serially diluted and tested by ELISA on plates coated with purified CP5, de-O-acetylated-CP5, CP8, or de-O-acetylated CP8. The data represent means (± standard errors) of three independent experiments. OD, optical density.
FIG 3.
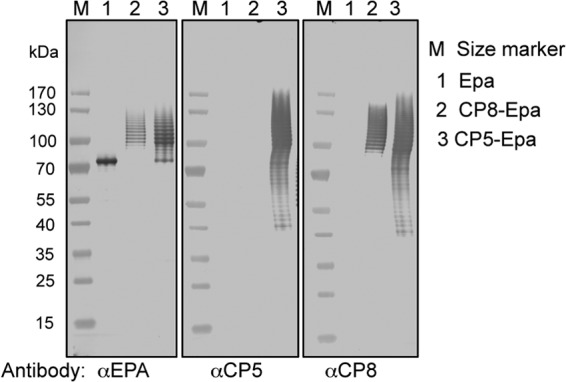
Serologic reactivity of CP5- and CP8-specific antiserum to Epa, CP8-Epa, and CP5-Epa assessed by immunoblotting. Epa, CP8-Epa, and CP5-Epa were separated by SDS-PAGE and detected by serum antibodies produced by immunization with rabbit serum raised to killed encapsulated bacteria (CP5+ or CP8+) and absorbed with acapsular strains to render the sera CP5 or CP8 specific.
Cross-reactive epitope.
Although CP5 and CP8 have similar trisaccharide repeating units (Fig. 1A), at least part of their serologic distinctiveness can be attributed to differences in O-acetylation of the sugar residues (11). O-Acetyl groups modify the third carbon of the CP5 l-FucNAc residue and the fourth carbon of the CP8 N-acetyl manosaminuronic acid residue (Fig. 1A). The substrate specificity of the O-acetyltransferase biosynthetic enzymes is consistent with the minimal homology (27% identity) that the cap5H and cap8J O-acetyltransferase gene products share with one another (30). The influence of the O-acetyl moieties on the cross-reactivity of the CPs was investigated by comparing the ELISA reactivities of rabbit sera to CP5-CRM or CP8-CRM on microtiter plates coated with either native or de-O-acetylated CPs. Rabbit IgG to CP5-Epa (Fig. 2A) and rabbit serum to CP5-CRM (Fig. 2C) were serotype specific and bound somewhat better to native CP5 than to de-O-acetylated CP5. Binding of CP5 antibodies to CP8 or de-O-acetylated CP8 was not detectable (Fig. 2B and D). CP8-CRM and CP8-Epa induced antibodies reactive with CP8 (Fig. 2B and D), and CP8-CRM rabbit serum showed better binding to native CP8 than to de-O-acetylated CP8 (Fig. 2D), confirming previous observations that O-acetyl groups are immunodominant CP epitopes (26). Of note, antibodies to CP8-Epa (Fig. 2A) and CP8-CRM (Fig. 2C) bound to CP5, and the level of cross-reactive antibody binding was enhanced after de-O-acetylation of CP5.
OPK of S. aureus with immune sera.
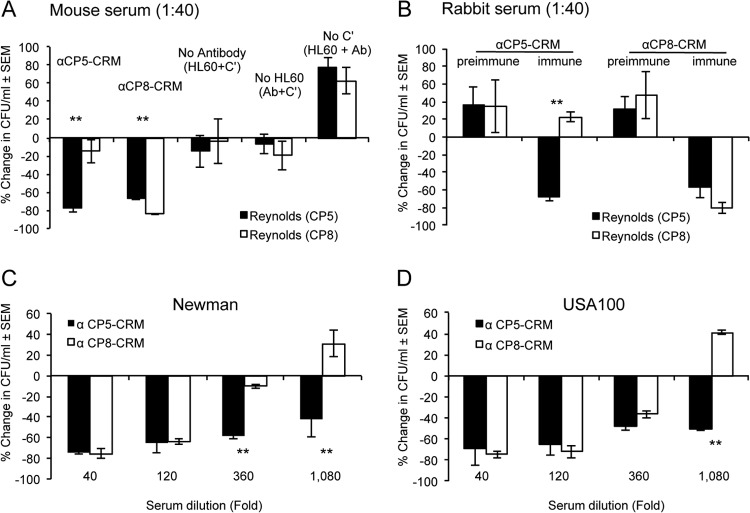
To determine whether the cross-reactive antibodies induced by CP8-CRM were functional, we evaluated the opsonic activity of CP5-CRM and CP8-CRM rabbit and mouse sera (diluted 1:40 [final]) against encapsulated S. aureus. Consistent with our ELISA results (Fig. 1), CP5-CRM mouse serum was opsonic only for Reynolds (CP5), whereas CP8-CRM mouse serum was opsonic for both S. aureus Reynolds (CP5) and Reynolds (CP8) (Fig. 4A). Samples lacking mouse serum, HL60 cells, or a complement source showed little to no bacterial killing. Likewise, rabbit serum raised to CP5-CRM showed serotype-specific opsonic activity, whereas rabbit serum to CP8-CRM promoted opsonophagocytic killing of both Reynolds (CP5) and Reynolds (CP8) (Fig. 4B). Preimmune rabbit serum was not opsonic. Although the levels of antibodies reacting with the CP5 antigen were low (as measured by ELISA), our results indicate that the cross-reactive antibodies induced by CP8-CRM were sufficient to opsonize a serotype 5 S. aureus strain for killing by phagocytes. Similarly, CP8-CRM antiserum showed opsonic activity when the serotype 5 strains Newman and USA100 were used as target strains in the OPK assay. The CP5-specific serum was opsonic at serum dilutions as high as 1:360 (strain Newman) or 1:1,080 (USA100), whereas CP8-CRM immune sera showed an opsonic titer of 120 for both S. aureus serotype 5 Newman (Fig. 4C) and USA100 (Fig. 4D).
FIG 4.
Opsonic activity of serum antibodies generated by immunization with CP5-CRM or CP8-CRM against S. aureus strains. The test mixture contained diluted sera, S. aureus, guinea pig serum as a complement source (concentration, 1%), and differentiated HL60 cells. (A and B) Pooled mouse serum (A) or rabbit serum (B) was tested at a 1:40 dilution. Controls lacking antibody, HL60 cells, and complement were included in each assay, as shown in panel A. (C and D) Serially diluted rabbit serum was analyzed against S. aureus serotype 5 strains Newman (C) and USA100 (D). The results are expressed as percent change in the number of CFU/ml at 2 h compared to the number of CFU/ml at time zero. The data shown are means (± standard errors) of two or more experiments. *, P < 0.05; **, P < 0.01.
OPK of S. aureus Δcap5H.
The de-O-acetylated CP5 structure contains the identical α-l-FucNAc-(1→3)-d-FucNAc (l-FucNAc-d-FucNAc) disaccharide present in CP8. We had previously constructed an isogenic mutant (Reynolds Δcap5H) of S. aureus that does not O-acetylate its CP5 (24). We used this mutant to determine whether the opsonic activity of CP8 antibodies would be enhanced against an S. aureus strain unable to O-acetylate its CP5. As shown in Fig. 5A, CP5-CRM antiserum had similar opsonic titers against Reynolds (CP5) and its Δcap5H mutant. In contrast, CP8-CRM antiserum showed greater opsonic activity against the de-O-acetylated mutant than the wild-type strain Reynolds (CP5) (Fig. 5B) at CP8-CRM antiserum dilutions of 1:360 (P < 0.05) and 1:1,080 (P < 0.01). Thus, the cross-reactive opsonic activity of CP8-CRM antiserum was enhanced against an S. aureus strain producing de-O-acetylated CP5, similar to the increased binding of CP8-CRM antiserum to de-O-acetylated CP5, with which ELISA plates were coated (Fig. 2C).
FIG 5.
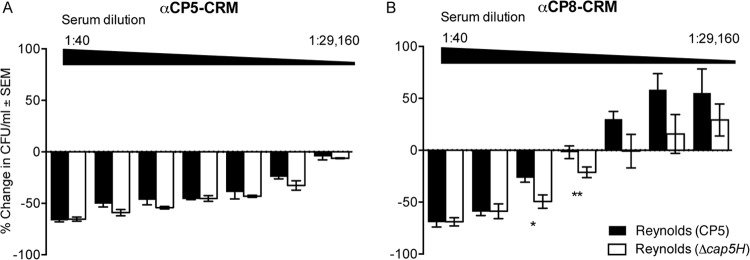
Opsonic activity of rabbit sera to CP5-CRM or CP8-CRM against S. aureus Reynolds (CP5) and Reynolds (Δcap5H). The rabbit sera were serially diluted 3-fold, starting at a 1:40 initial dilution. The results are expressed as percent change in the number of CFU/ml after 2 h. The data shown are means (± standard errors) of three experiments. Differences were analyzed by the Student t test. *, P < 0.05; **P < 0.001.
Cross-reactivity with P. aeruginosa serotype O11.
The increased serologic and functional activities of CP8 antibodies to de-O-acetylated CP5 suggest that the cross-reactive epitope lies in the l-FucNAc-d-FucNAc disaccharide common to the two CP types. To test this hypothesis, we measured the binding of CP5 and CP8 rabbit antisera to P. aeruginosa O11 lipopolysaccharide (LPS) (31), which also contains the l-FucNAc-d-FucNAc disaccharide in its repeating unit (Fig. 6). P. aeruginosa O11 antiserum showed high-titer reactivity against the homologous O11 antigen. CP8-CRM antibodies also bound to P. aeruginosa O11 LPS by ELISA, whereas control sera (CP5-CRM antiserum, O1-Epa antiserum, and preimmune serum) showed minimal reactivity. These findings lend support to the premise that the l-FucNAc-d-FucNAc is the cross-reactive CP5/8 epitope and that the O-acetylated CP5 disaccharide is immunodominant in CP5 conjugate vaccines.
FIG 6.
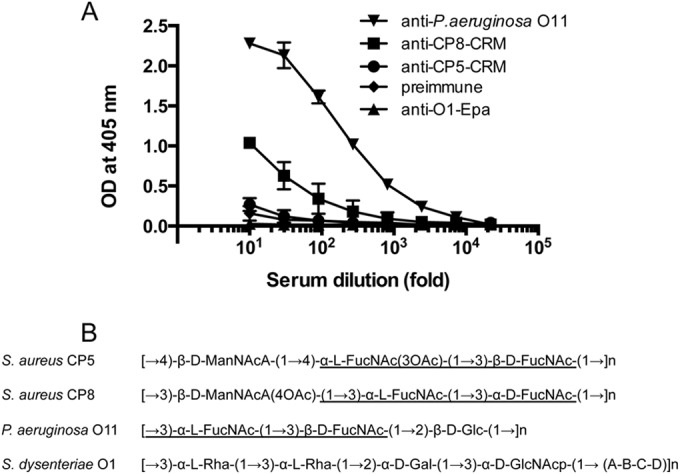
Binding of rabbit antibodies to P. aeruginosa O11 LPS by ELISA. (A) Ninety-six-well plates were coated with P. aeruginosa O11 LPS (2 μg/ml), and antibody samples were serially diluted 5-fold. Rabbit antiserum to O11-Epa was used as a positive control, and preimmune serum and Shigella O1-Epa IgG were used as negative-control sera. The data represent means (± standard errors) of two independent experiments. (B) Repeating units of polysaccharides from different bacterial genera. The shared structure of the repeating units is underlined.
In vivo cross protection of antibodies to CP8-CRM.
Having demonstrated that CP8-CRM immunization can induce cross-reactive antibodies against S. aureus CP5 in both ELISA and OPK in vitro assays, we assessed the protection afforded by passive immunization in a mouse bacteremia model. The results of a previous study had documented that antibodies to CP5 or CP8 significantly reduced bacteremia in mice when the animals were challenged with a serotype 5 or serotype 8 S. aureus strain, respectively (15). In this study, we passively immunized mice with 500 μl of either preimmune rabbit serum or rabbit antiserum to CP5-CRM or CP8-CRM. All animals were challenged 24 h after passive immunization with ∼107 CFU of S. aureus Reynolds (CP5). Treatment with either the homologous (CP5) or heterologous (CP8) antiserum resulted in significant (P ≤ 0.01) reductions in bacteremia compared to mice given preimmune rabbit serum (Fig. 7). As predicted by the results of the in vitro assays, the bacteremia levels were somewhat lower in mice that received the CP5-CRM antiserum than in mice that received CP8-CRM antiserum.
FIG 7.
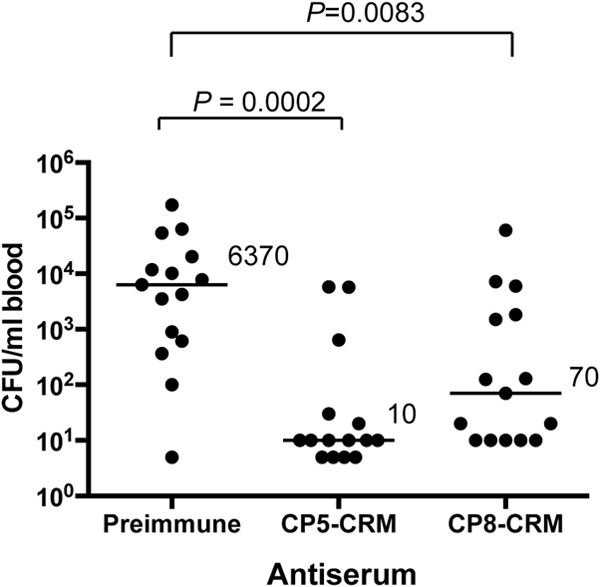
Rabbit antibodies to CP5-CRM or CP8-CRM reduce bacteremia induced by S. aureus Reynolds (CP5). Mice were passively immunized with 0.5 ml of preimmune serum or serum raised to CP5-CRM or CP8-CRM 24 h before challenge with 1 × 107 CFU S. aureus Reynolds (CP5). The data represent the results of quantitative blood cultures on individual animals, and the horizontal lines and numbers represent median numbers of CFU/ml blood for each group (n = 15). Bacteremia levels from mice given immune serum were compared by the Mann-Whitney test to those from mice given preimmune serum.
DISCUSSION
S. aureus CP5 and CP8 share the same trisaccharide repeating unit but differ structurally in the linkages between the sugars and in the sites of O-acetylation (Fig. 1A) (11). Despite the structural similarity between the two CP types, they are reported to be serologically distinct (12, 13). Consistent with previous findings, we demonstrate that immunization of mice or rabbits with CP5-CRM resulted in antibodies that were serotype specific and did not react with CP8. In contrast, immunization of rabbits with CP8-CRM or CP8-Epa elicited antibodies that reacted with CP8 and (to a lesser extent) with CP5. Moreover, serum antibodies induced by CP8-CRM showed in vitro opsonic killing against three different serotype 5 S. aureus strains. The one-way cross-reactivity (CP8 antibodies reactive with CP5) was increased when CP5 was de-O-acetylated, suggesting that the shared epitope was l-FucNAc-d-FucNAc, which is O-acetylated in the native CP5 state. The same disaccharide is found in the P. aeruginosa O11 LPS structure (31), and we showed that CP8-CRM rabbit serum bound well to O11 LPS.
Serologic cross-reactivity has also been observed in other species of Gram-positive encapsulated bacteria within their serogroups or sometimes across different serogroups, depending on the structural similarity of the CP types. A pneumococcal conjugate vaccine that includes S. pneumoniae serotype 6B induced cross-reactive antibodies to S. pneumoniae serotype 6A in both ELISA and OPK assays (32). A monoclonal antibody produced by vaccination with a pneumococcal 6B polysaccharide conjugate showed opsonic killing of a pneumococcal serotype 19A strain, resulting from a shared disaccharide [α-d-Glcp(1→3)α-l-Rhap] in their polysaccharide structures (33). To our knowledge, this is the first report of cross-reactivity between staphylococcal polysaccharides CP5 and CP8. Even though antibodies reactive with CP5 were less abundant in CP8-CRM antiserum than antibodies reactive with CP8, passive immunization with CP8-CRM antiserum protected against Reynolds (CP5) in a mouse bacteremia infection model.
De-O-acetylated CP5 has the same l-FucNAc-d-FucNAc disaccharide as CP8. Based on the structural similarity between de-O-acetylated CP5 and native CP8 (Fig. 1A), antibodies elicited by the CP8 l-FucNAc-d-FucNAc disaccharide likely bind to the de-O-acetylated counterpart of CP5, producing cross-reactivity. The reason that CP5 antibodies fail to react with CP8 is less clear. It is likely that the O-acetylated l-FucNAc-d-FucNAc disaccharide present in CP5 is immunodominant, although CP5-CRM and CP5-Epa antibodies still react with the de-O-acetylated CP5 antigen (Fig. 2). ManNAcA is common to both CP5 and CP8, but ManNAcA is O-acetylated in CP8. Moreover, the glycosidic linkage of ManNAcA to the FucNAc disaccharide differs for the two polysaccharides. It is likely that the three-dimensional structures of CP5 and CP8 differ markedly, and a complete structural analysis would be necessary for a full understanding of the one-way cross-reactivity that we report.
O-acetylation of CPs has been identified among many pathogenic bacteria, such as Neisseria meningitidis, Salmonella enterica serovar Typhi Vi, and S. pneumoniae, and O-acetyl groups are important immunogenic determinants (34–37). The S. aureus Δcap5H mutant was more susceptible to complement-mediated OPK and less virulent for mice than the isogenic parental strain (24). O-Acetyl groups are easily removed at alkaline pH, and as a result, they can be partially removed in the process of polysaccharide purification, activation, and chemical conjugation. We showed one-way cross-reactive opsonic killing by CP8-CRM immune sera against multiple S. aureus serotype 5 strains, including the Reynolds Δcap5H strain. Whether a CP8 conjugate vaccine will protect against multiple S. aureus strains in different infection models remains to be tested.
Vaccine candidates, such as CP5 and CP8 conjugates and IsdB, have been tested in clinical trials, but they failed to protect patients from staphylococcal infections (3, 38, 39). Correlates of protective immunity against S. aureus remain undefined. S. aureus also has a wide spectrum of virulence factors and disease types, and as such, a single vaccine may not protect against the broad range of staphylococcal infections. Efforts to create multicomponent vaccines and to induce both cellular and humoral immune responses are under way by several groups (40–42), and these multivalent vaccines often include CP antigens. Despite the failure in clinical trials of CP5 and CP8 conjugate vaccines, staphylococcal CPs are still important vaccine targets, since they are expressed in 70 to 80% of S. aureus clinical isolates and are essential for bacterial survival in blood (6). Moreover, capsular antibodies are opsonic and mediate clearance of S. aureus from the bloodstream (15). Based on our results of one-way cross-reactivity, the premise that a CP8 conjugate vaccine alone would protect against serotype 5 and 8 isolates of S. aureus merits further study.
ACKNOWLEDGMENTS
We thank Kelly Shields-Lapierre and Megan Dowd for technical assistance and Jeong-Mo Choi for useful discussions about carbohydrate structures.
Research reported in this publication was supported by GlycoVaxyn AG and by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01 AI088754.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sabina Gerber is an employee of GlycoVaxyn AG. Jean C. Lee is a paid consultant on vaccine development for Sanofi Pasteur and Pfizer and received research support from Centegen, Inc. Saeyoung Park reports no potential conflicts.
Footnotes
Published ahead of print 22 September 2014
REFERENCES
- 1.Foster TJ, Geoghegan JA, Ganesh VK, Höök M. 2014. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 12:49–62. 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loughman JA, Fritz SA, Storch GA, Hunstad DA. 2009. Virulence gene expression in human community-acquired Staphylococcus aureus infection. J. Infect. Dis. 199:294–301. 10.1086/595982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Riordan K, Lee JC. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17:218–234. 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochkeppel HK, Braun DG, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan EL, Boutonnier A, Fournier JM. 1987. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J. Clin. Microbiol. 25:526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbeit RD, Karakawa WW, Vann WF, Robbins JB. 1984. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2:85–91. 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 6.Thakker M, Park JS, Carey V, Lee JC. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66:5183–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. 2005. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect. Immun. 73:3502–3511. 10.1128/IAI.73.6.3502-3511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLoughlin RM, Solinga RM, Rich J, Zaleski KJ, Cocchiaro JL, Risley A, Tzianabos AO, Lee JC. 2006. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc. Natl. Acad. Sci. U. S. A. 103:10408–10413. 10.1073/pnas.0508961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson IM, Lee JC, Bremell T, Rydén C, Tarkowski A. 1997. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect. Immun. 65:4216–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portolés M, Kiser KB, Bhasin N, Chan KH, Lee JC. 2001. Staphylococcus aureus Cap5O has UDP-ManNAc dehydrogenase activity and is essential for capsule expression. Infect. Immun. 69:917–923. 10.1128/IAI.69.2.917-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones C. 2005. Revised structures for the capsular polysaccharides from Staphylococcus aureus types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr. Res. 340:1097–1106. 10.1016/j.carres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Melles DC, Taylor KL, Fattom AI, van Belkum A. 2008. Serotyping of Dutch Staphylococcus aureus strains from carriage and infection. FEMS Immunol. Med. Microbiol. 52:287–292. 10.1111/j.1574-695X.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 13.Karakawa WW, Fournier JM, Vann WF, Arbeit R, Schneerson RS, Robbins JB. 1985. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J. Clin. Microbiol. 22:445–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fattom A, Schneerson R, Watson DC, Karakawa WW, Fitzgerald D, Pastan I, Li X, Shiloach J, Bryla DA, Robbins JB. 1993. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect. Immun. 61:1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wacker M, Wang L, Kowarik M, Dowd M, Lipowsky G, Faridmoayer A, Shields K, Park S, Alaimo C, Kelley KA, Braun M, Quebatte J, Gambillara V, Carranza P, Steffen M, Lee JC. 2014. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J. Infect. Dis. 209:1551–1561. 10.1093/infdis/jit800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JC, Park JS, Shepherd SE, Carey V, Fattom A. 1997. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect. Immun. 65:4146–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuchscherr LP, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. 2008. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect. Immun. 76:5738–5744. 10.1128/IAI.00874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lattar SM, Noto Llana M, Denoël P, Germain S, Buzzola FR, Lee JC, Sordelli DO. 2014. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect. Immun. 82:83–91. 10.1128/IAI.01050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen WT, Snippe H. 2004. Short-chain oligosaccharide protein conjugates as experimental pneumococcal vaccines. Indian J. Med. Res. 119(Suppl):7–12. [PubMed] [Google Scholar]
- 20.Benaissa-Trouw B, Lefeber DJ, Kamerling JP, Vliegenthart JF, Kraaijeveld K, Snippe H. 2001. Synthetic polysaccharide type 3-related di-, tri-, and tetrasaccharide-CRM(197) conjugates induce protection against Streptococcus pneumoniae type 3 in mice. Infect. Immun. 69:4698–4701. 10.1128/IAI.69.7.4698-4701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danieli E, Proietti D, Brogioni G, Romano MR, Cappelletti E, Tontini M, Berti F, Lay L, Costantino P, Adamo R. 2012. Synthesis of Staphylococcus aureus type 5 capsular polysaccharide repeating unit using novel L-FucNAc and D-FucNAc synthons and immunochemical evaluation. Bioorg. Med. Chem. 20:6403–6415. 10.1016/j.bmc.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 22.Ihssen J, Kowarik M, Dilettoso S, Tanner C, Wacker M, Thöny-Meyer L. 2010. Production of glycoprotein vaccines in Escherichia coli. Microb. Cell. Fact. 9:61. 10.1186/1475-2859-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase; mode of action and antigenicity. J. Gen. Microbiol. 6:95–107. 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 24.Bhasin N, Albus A, Michon F, Livolsi PJ, Park JS, Lee JC. 1998. Identification of a gene essential for O-acetylation of the Staphylococcus aureus type 5 capsular polysaccharide. Mol. Microbiol. 27:9–21. 10.1046/j.1365-2958.1998.00646.x. [DOI] [PubMed] [Google Scholar]
- 25.Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, Scully IL, McNeil LK, Aste-Amézaga JM, Cooper D, Jansen KU, Anderson AS. 2013. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum. Vaccin. Immunother. 9:480–487. 10.4161/hv.23223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattom AI, Sarwar J, Basham L, Ennifar S, Naso R. 1998. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66:4588–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzianabos AO, Wang JY, Lee JC. 2001. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl. Acad. Sci. U. S. A. 98:9365–9370. 10.1073/pnas.161175598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray BM. 1979. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J. Immunol. Methods 28:187–192. 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- 29.Burton RL, Nahm MH. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 13:1004–1009. 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sau S, Bhasin N, Wann ER, Lee JC, Foster TJ, Lee CY. 1997. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology 143:2395–2405. 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 31.Dean CR, Franklund CV, Retief JD, Coyne MJ, Hatano K, Evans DJ, Pier GB, Goldberg JB. 1999. Characterization of the serogroup O11 O-antigen locus of Pseudomonas aeruginosa PA103. J. Bacteriol. 181:4275–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Väkeväinen M, Eklund C, Eskola J, Käyhty H. 2001. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J. Infect. Dis. 184:789–793. 10.1086/322984. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Parameswar AR, Demchenko AV, Nahm MH. 2009. Identification of a simple chemical structure associated with protective human antibodies against multiple pneumococcal serogroups. Infect. Immun. 77:3374–3379. 10.1128/IAI.00319-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szu SC, Li XR, Stone AL, Robbins JB. 1991. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 59:4555–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fusco PC, Farley EK, Huang CH, Moore S, Michon F. 2007. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin. Vaccine Immunol. 14:577–584. 10.1128/CVI.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michon F, Huang CH, Farley EK, Hronowski L, Di J, Fusco PC. 2000. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev. Biol. (Basel) 103:151–160. [PubMed] [Google Scholar]
- 37.Berry DS, Lynn F, Lee CH, Frasch CE, Bash MC. 2002. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 70:3707–3713. 10.1128/IAI.70.7.3707-3713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, Chan IS, McNeely TB, Kartsonis NA, Guris D, Onorato MT, Smugar SS, DiNubile MJ, Sobanjo-ter Meulen A. 2013. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309:1368–1378. 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 39.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, Muenz L, Fuller S, Johnson J, Fireman B, Alcorn H, Naso R. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491–496. 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 40.Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, Jansen KU. 2012. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum. Vaccin. Immunother. 8:1585–1594. 10.4161/hv.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. 2012. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS One 7:e46648. 10.1371/journal.pone.0046648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi A, Pancari G, Cope L, Bowman EP, Cua D, Proctor RA, McNeely T. 2012. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum. Vaccin. Immunother. 8:336–346. 10.4161/hv.18946. [DOI] [PMC free article] [PubMed] [Google Scholar]