Abstract
Understanding protective immunity to malaria is essential for the design of an effective vaccine to prevent the large number of infections and deaths caused by this parasitic disease. To date, whole-parasite immunization with attenuated parasites is the most effective method to confer sterile protection against malaria infection in clinical trials. Mouse model studies have highlighted the essential role that CD8+ T cells play in protection against preerythrocytic stages of malaria; however, there is mounting evidence that antibodies are also important in these stages. Here, we show that experimental immunization of mice with Plasmodium yoelii fabb/f− (Pyfabb/f−), a genetically attenuated rodent malaria parasite that arrests late in the liver stage, induced functional antibodies that inhibited hepatocyte invasion in vitro and reduced liver-stage burden in vivo. These antibodies were sufficient to induce sterile protection from challenge by P. yoelii sporozoites in the absence of T cells in 50% of mice when sporozoites were administered by mosquito bite but not when they were administered by intravenous injection. Moreover, among mice challenged by mosquito bite, a higher proportion of BALB/c mice than C57BL/6 mice developed sterile protection (62.5% and 37.5%, respectively). Analysis of the antibody isotypes induced by immunization with Pyfabb/f− showed that, overall, BALB/c mice developed an IgG1-biased response, whereas C57BL/6 mice developed an IgG2b/c-biased response. Our data demonstrate for the first time that antibodies induced by experimental immunization of mice with a genetically attenuated rodent parasite play a protective role during the preerythrocytic stages of malaria. Furthermore, they highlight the importance of considering both the route of challenge and the genetic background of the mouse strains used when interpreting vaccine efficacy studies in animal models of malaria infection.
INTRODUCTION
Malaria remains a daunting public health challenge that causes close to one million deaths per year (1). A vaccine capable of blocking malaria infection and transmission by preventing blood-stage infection would be a critical tool for malaria eradication. The preerythrocytic stages of infection (from inoculation in the skin to liver egress) are asymptomatic and constitute a bottleneck due to the small number of parasites that invade and develop in the liver—making it an ideal vaccine target. The most effective experimental malaria vaccines, with up to 100% efficacy in controlled human malaria infection trials, consist of live attenuated Plasmodium sporozoites (2). These parasites are able to invade hepatocytes but subsequently die in the liver or early in the blood stage, exposing the immune system to a variety of parasite antigens without subjecting the host to parasitemia-associated disease.
Protection against preerythrocytic stages of malaria has been shown to involve both T cells and antibodies (Abs) (reviewed in reference 3). For example, animal model studies using whole-parasite vaccines have shown that gamma interferon-positive (IFN-γ+) CD8+ T cells are essential for protection of mice against sporozoite challenge and that protection is likely mediated by direct killing of parasite-infected hepatocytes (4–10). These findings agree with human clinical trial data showing that the level of sporozoite-specific T cells elicited by immunization with whole-parasite malaria vaccines correlates with protection (11–14). Multiple lines of evidence suggest that antibodies are also involved in protection. For example, analyses of sera from human trials with both the most advanced preerythrocytic malaria subunit vaccine, RTS,S, and whole-parasite vaccines showed that efficacy is partially dependent on antibodies against the preerythrocytic circumsporozoite protein (CSP) and sporozoites (12, 14–16). In animal studies, anti-parasite antibodies reduce the number of viable sporozoites injected into the skin by the mosquito and block motility of sporozoites in the dermis, thus decreasing the likelihood of a parasite entering the circulation and invading hepatocytes (17–20). Monoclonal antibodies (MAbs) against CSP can also inhibit liver infection by binding to the sporozoite in the bloodstream and blocking sporozoite invasion of hepatocytes (21–23). Finally, antibodies against either sporozoites or CSP have been shown to induce opsonization and to promote the uptake and destruction of sporozoites by monocytes and macrophages in vitro (24, 25).
In spite of these data, the majority of rodent model studies employing whole-parasite vaccines have concluded that antibodies are not sufficient to confer protection when animals are given a sporozoite challenge. This conclusion arose from the observation that protection from sporozoite challenge is ablated in the absence of CD8+ T cells but not in the absence of antibodies or CD4+ T cells (8, 9, 26–28). However, in those studies, mice were challenged with sporozoites by intravenous (i.v.) injection—an unnatural route of infection that results in liver invasion by sporozoites within minutes and bypasses antibody-based immune mechanisms in the skin (18). It was recently shown that passive transfer of anti-CSP monoclonal antibodies induces sterile protection in mice challenged by mosquito bite, while intravenous challenge results in only partial protection (22, 29). While this experimental approach does not completely recapitulate natural infection, it shows that the route of sporozoite delivery should be considered when interpreting the role of antibodies in protection from malaria infection.
In addition, different strains of mice display different susceptibilities to Plasmodium and differ in their patterns of immune response and protection upon sporozoite challenge (30). For example, BALB/c mice are easier to protect against P. yoelii infection than C57BL/6 mice (6). This difference could be attributable to intrinsic genetic differences between the two mouse strains. For example, C57BL/6 mice differ from BALB/c mice in their tendency to mount a Th1-biased response versus a Th2-biased response, which could in turn shape the distribution of IgG isotypes generated by each strain in response to infection (31, 32). To our knowledge, the balance between different IgG isotypes and their roles in the differential levels of protection of mouse strains against preerythrocytic stages of malaria remain to be elucidated.
Here, we used experimental vaccination with a whole genetically attenuated late liver stage-arresting parasite, P. yoelii fabb/f− (Pyfabb/f−) (33), to show that antibodies are able to control preerythrocytic stages of malaria infection in our murine model of experimental infection in the absence of T cell immunity. Furthermore, we show that these responses depend on the challenge route and the mouse strain. Thus, our results help clarify previous conflicting data regarding protection against malaria in animal model studies. By demonstrating that antibody-based responses have an important role in inducing sterile protection, this work is relevant to the rational design of preerythrocytic malaria vaccines.
MATERIALS AND METHODS
Mice and parasites.
Female BALB/c and C57BL/6 mice (5 to 6 weeks old) were purchased from the Jackson Laboratory and maintained in a pathogen-free facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care at Seattle BioMed. Experiments were conducted in accordance with animal protocols approved by the Institutional Animal Care and Use Committee.
To begin the growth cycle of P. yoelii, 5-to-8-week-old female Swiss Webster mice were injected with blood from P. yoelii-green fluorescent protein-luciferase (PyGFP-luc) (34)- or Pyfabb/f− (33)-infected mice and were used to feed female Anopheles stephensi mosquitoes after gametocyte exflagellation. Salivary gland sporozoites were isolated 14 to 17 days after the infectious blood meal.
Immunization and challenge.
Mice were immunized intravenously (i.v.) via tail vein injection at 2-week intervals with two or three doses of 50,000 Pyfabb/f− sporozoites or, as a control, with salivary gland debris from uninfected mosquitos.
Immunized mice were challenged with 5,000 PyGFP-luc sporozoites by i.v. injection or by the bite of 15 to 20 A. stephensi PyGFP-luc-infected mosquitos, as previously described (22). Sterile protection was defined as the absence of detectable P. yoelii parasites in Giemsa-stained thin blood smear experiments performed daily from day 3 to day 17 after challenge.
In vivo bioluminescence imaging.
Luciferase activity was measured 42 to 44 h after mosquito bite or i.v. challenge of mice with PyGFP-luc as previously described (34).
Antibody inhibition of liver-stage-development assay (ILSDA).
HepG2 cells were cultured in 5% CO2 with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine. At 24 h before the assay, 30,000 cells were seeded in each well of a 96-well plate. Up to 30,000 PyGFP-luc sporozoites were added per well. Bioluminescence was quantified 42 to 44 h postinfection using a Centro XS3 LB 960 Microplate Luminometer (Berthold Technologies) under the Bright-Glo luciferase assay system protocol (Promega). For the comparative microscopic analysis, a range of 625 to 10,000 sporozoites was used. Infected hepatocytes were fixed with 2% paraformaldehyde (PFA), permeabilized with 0.1% Triton X-100 (Sigma), and stained using anti-CSP rabbit polyclonal antibody followed by fluorescein isothiocyanate (FITC)-conjugated anti-rabbit secondary antibody (eBioscience).
To measure the inhibition of sporozoite invasion, serially diluted (0 to 10 μg/ml in RPMI medium) anti-CSP monoclonal antibody 2F6 (22) or serum collected from Pyfabb/f− or mock-immunized BALB/c and C57BL/6 mice 9 days after the second immunization (diluted 1:10 in RPMI medium) was coincubated with 30,000 PyGFP-luc sporozoites for 20 min at room temperature (RT) before being transferred into wells containing HepG2 cells. The percentage of inhibition was calculated as the relative luminescence resulting from incubation of PyGFP-luc sporozoites with anti-CSP monoclonal antibody versus no antibody or with serum from Pyfabb/f−-immunized or mock-immunized mice versus serum from naive mice.
Passive transfer.
Serum samples from mice immunized with two doses of Pyfabb/f− sporozoites (or from mock-immunized mice) were collected on day 9 after the last immunization and pooled. Congenic naive mice were injected by i.v. administration with 300 μl of pooled serum and challenged by the bite of 15 to 20 PyGFP-luc-infected mosquitos 24 h postinjection, as described previously (22).
CD8+/CD4+ T cell depletion.
CD8+ and CD4+ T cells were depleted in mice as previously described (35). Briefly, 0.5 mg of anti-CD8 MAb 2.43 (TIB210; American Type Culture Collection) and 0.35 mg of anti-CD4 MAb 1.5 (BioXCell) or 0.85 mg of isotype control rat IgG2b (BioXCell) was injected by intraperitoneal (i.p.) administration into mice 24 h prior to parasite challenge. The day before the challenge, 50 to 100 μl of peripheral blood was collected via the retro-orbital plexus, and depletion of specific cell types was confirmed by surface staining of peripheral blood mononuclear cells (PBMC) with Pacific Blue-conjugated anti-CD3, peridinin chlorophyll protein (PerCP)/cy5.5-conjugated anti-CD8, and allophycocyanin (APC)-conjugated anti-CD4 antibodies (Biolegend) by flow cytometric analysis.
Protein production.
PyCSP (amino acids [aa] 21 to 362) was cloned into the pTT3 vector under the control of the cytomegalovirus (CMV) promoter (36). This plasmid was transfected into HEK293F cells as described previously (37) and purified by chromatography using a HisTrapp-FF nickel affinity column (GE Healthcare, Pittsburgh, PA) followed by a HiLoad 16/60 Superdex 200 size exclusion column (GE Healthcare, Pittsburgh, PA). Fractions containing monomeric PyCSP were collected, concentrated for final storage in phosphate-buffered saline (PBS) (pH 7.4), and analyzed by native PAGE and SDS-PAGE for size and purity.
Sporozoite lysate.
Sporozoites dissected from mosquito salivary glands were purified twice over 17% Accudenz density gradients as previously described (38). After pelleting by centrifugation at 13,500 rpm was performed, 1 million sporozoites were resuspended in 50 μl of lysis buffer (50 mM Tris-HCl [pH 7.5]; 140 nM NaCl; 1% Triton X-100; 1 mM MgCl; 1 mM EDTA) and incubated on ice overnight. Next, cellular debris was removed by centrifugation at 13,500 rpm, and the protein concentration of the supernatant was quantified using a Nanodrop 1000 spectrophotometer (Thermo Scientific).
ELISA.
Enzyme-linked immunosorbent assay (ELISA) plates (Corning, Inc.) were coated overnight at 4°C by adding 50 μl of 0.1 μg/ml of PyCSP protein or 10 μg/ml sporozoite lysate diluted in calcium bicarbonate-sodium carbonate coating buffer. Plates were washed three times with 200 μl PBS-T (1× PBS–0.05% Tween 20; Fisher) prior to blocking for 2 h in blocking buffer (5% milk–PBS–0.05% Tween 20; Fisher). Next, serum was diluted in blocking buffer at 1:20 for sporozoite lysate or 1:800 or 1:1,600 for PyCSP protein and 50 μl was added per well. Plates were incubated for 2 h at room temperature before being washed as described above. Next, 50 μl of a 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated anti-mouse IgG (Sigma-Aldrich) or IgG1, IgG2a, IgG2b, IgG2c, or IgG3 (SouthernBiotech) was added and incubated for an additional 2 h at room temperature. Finally, plates were washed again, and 50 μl of TMB (3,3′,5,5′-tetramethylbenzidine) liquid substrate system for ELISA (Sigma-Aldrich) substrate was added for 2 min. The reaction was stopped by addition of 50 μl of 1 N sulfuric acid prior to measurement of absorbance at 450 nm using a SpectraMax M2 microplate reader.
Statistical analysis.
Calculations were performed using GraphPad Prism. Mann-Whitney and unpaired t tests were used to evaluate the statistical significance of the results of comparisons between the groups. A P value of <0.05 was considered significant.
RESULTS
Immune sera from the late liver stage-arresting Pyfabb/f− GAP inhibit sporozoite invasion in vitro.
Immunization of mice with sporozoites from the late-arresting P. yoelii genetically attenuated parasite (PyGAP) Pyfabb/f− (33) results in a robust CD8+ T cell response capable of complete protection against challenge with wild-type P. yoelii sporozoites (39), but antibodies elicited by this vaccination strategy have received little investigation. Previous studies have shown that antibodies directed against preerythrocytic stages can block invasion of hepatocytes by sporozoites in vitro (40). In addition, studies in the rodent malaria model of experimental infection have shown that the type of immune response and the level of protection could depend on the mouse strain used (41). Thus, we first determined whether serum from BALB/c and C57BL/6 mice immunized with Pyfabb/f− sporozoites could also inhibit sporozoite invasion of hepatocytes in vitro. To do this, we modified our recently published method designed to measure hepatocyte killing by cytotoxic lymphocytes (42), which uses transgenic sporozoites that express a green fluorescent protein-luciferase fusion (PyGFP-luc) (34). Compared to the traditional method of quantification of malaria parasite-infected cells by microscopy, this high-throughput method uses bioluminescence to measure the levels of luciferase-expressing malaria parasites inside hepatocytes. PyGFP-luc sporozoites were incubated with HepG2:CD81 hepatoma cells (43, 44), and luciferase signal was measured 42 to 44 h later as an indication of liver-stage development. We first demonstrated a direct correlation between the level of infectivity determined by microscopy and that measured by bioluminescence for increasing numbers of sporozoites (from 625 to 10,000; Fig. 1A). Next, with the goal of optimizing this technique for the functional characterization of serum samples, we preincubated sporozoites with increasing concentrations of a monoclonal antibody against CSP (22), and we observed a concentration-dependent inhibition of sporozoite invasion of hepatocytes (Fig. 1B).
FIG 1.
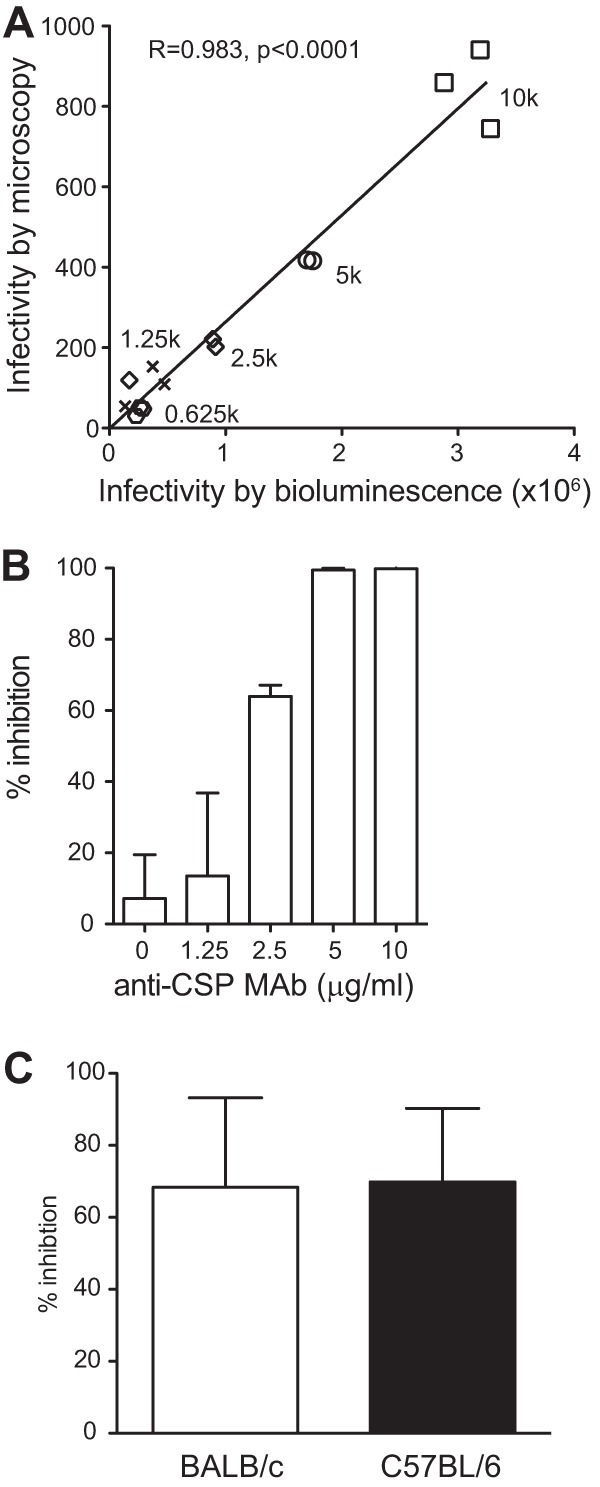
Immunization with Pyfabb/f− induces functional antibodies that inhibit hepatocyte invasion in vitro. (A and B) Optimization of the assay of antibody inhibition of liver-stage development. (A) Correlation between hepatocyte infection determined by bioluminescence (x axis) and by microscopy (y axis) for increasing amounts of sporozoites, as indicated. Pearson's correlation coefficient R = 0.983, P < 0.0001. (B) Inhibition of HepG2 cell invasion by P. yoelii PyGFP-luc sporozoites after incubation with increasing amounts of anti-CSP monoclonal antibody. The relative inhibition was calculated as a percentage of the average luminescence in the absence of antibody. Error bars correspond to standard deviations. (C) Inhibition of HepG2 cell invasion by PyGFP-luc sporozoites after incubation with a 1:10 dilution of serum obtained 9 days after the second dose of 50,000 Pyfabb/f− sporozoites from BALB/c (white bar, n = 5) or C57BL/6 (black bar, n = 5) mice. The relative inhibition was calculated as a percentage of the average luminescence resulting from incubation with serum from mock-immunized mice of the same strain (n = 5). Error bars correspond to standard deviations. These data are representative of the results of three independent experiments.
BALB/c and C57BL/6 mice were immunized with two doses of 50,000 Pyfabb/f− sporozoites (or were mock immunized) 2 weeks apart, and serum was collected 9 days after the second immunization, which fell within the peak of antibody response. We then used the technique described above to determine the ability of these sera to inhibit sporozoite invasion of hepatocytes in vitro. Sera were diluted 1:10 and preincubated with PyGFP-luc sporozoites for 20 min prior to infection of HepG2:CD81 cells and quantification as described above. For BALB/c and C57BL/6 mice immunized with Pyfabb/f− sporozoites, we observed similar levels of reduction of hepatocyte invasion (68% for BALB/c and 69% for C57BL/6 mice) compared to sera from mock-immunized mice of the same strain (Fig. 1C). These data show that immunization of mice with Pyfabb/f− induces functional antibodies that are capable of inhibiting the invasion of hepatocytes by P. yoelii sporozoites in vitro independently of the mouse strain used.
Immune sera from Pyfabb/f−-immunized mice reduce liver-stage burden in vivo.
We next determined if antibodies induced by Pyfabb/f− immunization could reduce liver-stage burden in vivo. Sera collected from both strains of mice immunized with Pyfabb/f− as described above (or mock immunized) were passively transferred to naive congenic mice 24 h prior to challenge with 15 to 20 bites of PyGFP-luc-infected mosquitos. After 42 to 44 h, liver-stage burden was measured by quantifying the bioluminescent signal in the mice, using an in vivo imaging system (34, 45, 46). Mice that received serum from either BALB/c or C57BL/6 mock-immunized mice showed similar levels of liver-stage burden as determined by in vivo bioluminescence (Fig. 2). In contrast, we observed a significant reduction in liver-stage parasite burden in mice that received serum from Pyfabb/f−-immunized BALB/c mice compared to the mock control versus little to no reduction in those that received serum from Pyfabb/f−-immunized C57BL/6 mice (Fig. 2). However, in spite of the reduced liver-stage burden observed in mice that received sera from Pyfabb/f−-immunized congenic BALB/c mice, all mice receiving sera from either mock- or Pyfabb/f−-immunized mice of both strains were positive for blood-stage parasitemia, although 5/9 of mice that received sera from Pyfabb/f−-immunized BALB/c mice showed a 2-day delay in the onset of parasitemia (data not shown).
FIG 2.
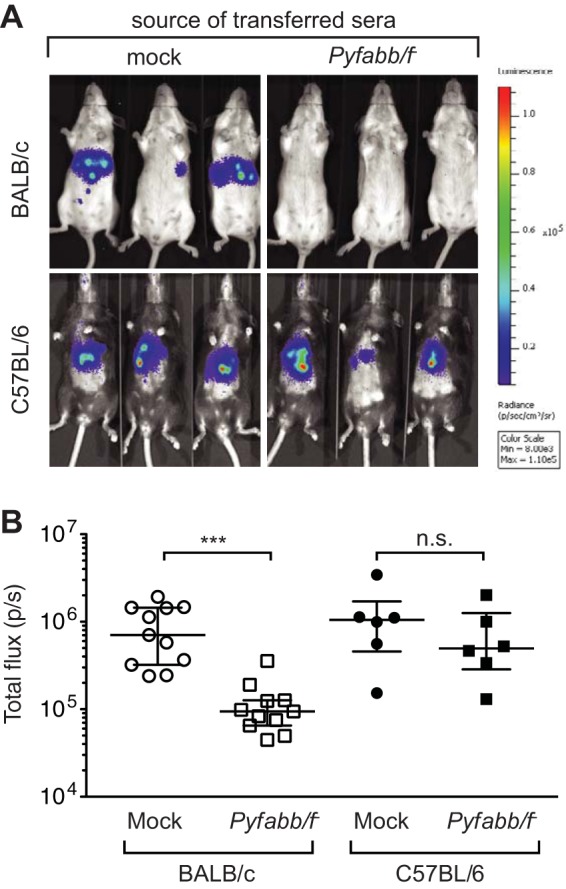
Transfer of immune sera from Pyfabb/f−-immunized mice induces functional antibodies that reduce liver-stage parasite burden. (A) In vivo luminescence imaging of BALB/c and C57BL/6 mice that received 300 μl serum from congenic mice immunized with Pyfabb/f− (or from mock-immunized control mice) 24 h prior to challenge by the bite of 15 to 20 mosquitoes infected with PyGFP-luc sporozoites. Liver-stage parasite burden was measured by bioluminescent imaging 42 to 44 h after challenge. The scale indicates radiance expressed as photons per second per centimeter squared per steradian (p/s/cm2/sr). Min, minimum; Max, maximum. (B) Quantification of the luciferase signal (measured as total flux per second). Data shown were obtained from 3 experiments with BALB/c (n = 9) and 2 experiments with C57BL/6 (n = 6) mice. The horizontal bars correspond to the median, and the error bars show the interquartile range. Significant differences between groups were calculated using the Mann-Whitney test; *** = P < 0.001; n.s. = nonsignificant.
Taken together, our data suggest that antibodies elicited by immunization of mice with Pyfabb/f− are capable of inhibiting hepatocyte invasion by sporozoites in vitro and significantly reducing liver-stage burden in vivo, although they did not result in sterile protection. Furthermore, while antibodies from the two strains of mice were able to inhibit in vitro hepatocyte infection to similar levels, BALB/c mice experienced a much more reduced liver burden than C57BL/6 mice.
Antibodies elicited by Pyfabb/f− immunization confer sterile immunity to challenge with P. yoelii sporozoites administered by mosquito bite.
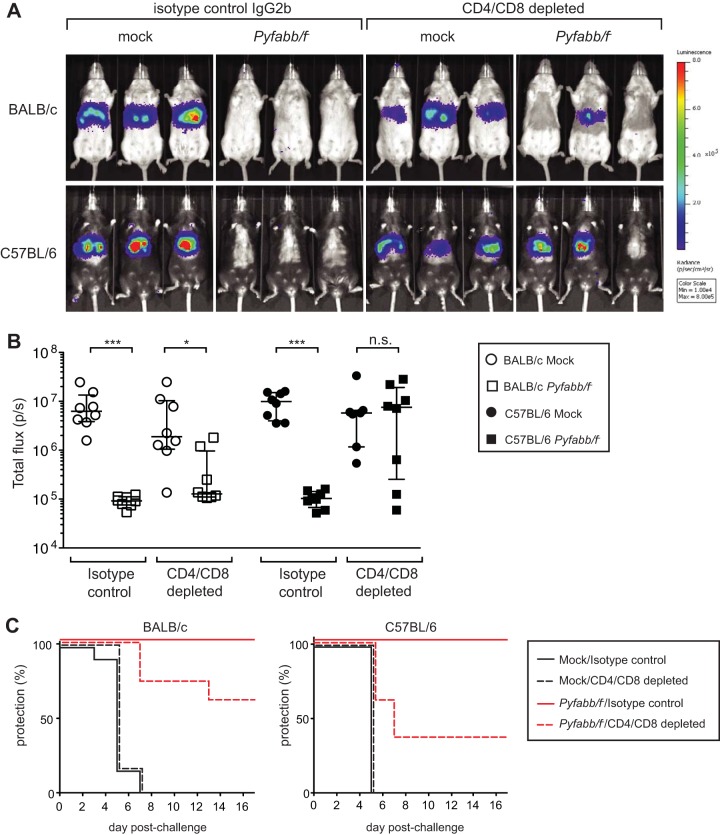
CD8+ T cells have been shown to have an essential role in protection against preerythrocytic stages of rodent malaria (8, 9, 26–28). Our data showing that antibody transfer can reduce liver-stage burden in BALB/c mice suggest that antibodies might also play an important role in this stage. However, the failure to observe sterile protection in these animals could have resulted from the fact that T cells are absolutely required for protection or, alternatively, could have been a consequence of the limitations of the experimental approach, since only a small amount of sera could be transferred from the immunized to the congenic naive mice. Therefore, we next examined the ability of these antibodies to yield sterile protection against sporozoite challenge at physiologically relevant levels and in the absence of T cell help. To optimize CD4+ and CD8+ depletion, we first treated mice immunized with two doses of 50,000 Pyfabb/f− sporozoites with anti-CD4 and anti-CD8 antibodies, and we identified conditions that depleted at least 98% of CD4+ and CD8+ T cells from liver and spleen, as determined by flow cytometry analysis, in comparison to the levels observed in isotype-treated, control mice (see Fig. S1A in the supplemental material). Then, to determine whether the antibodies were sufficient to protect mice against challenge with P. yoelii sporozoites, we depleted both CD4+ and CD8+ T cells from BALB/c and C57BL/6 mice 24 days after the last immunization, and we confirmed the efficiency of the depletion by analyzing the presence of CD4+ and CD8+ T cells in peripheral blood (see Fig. S1B and C). At 24 h after this treatment, mice were challenged via the bite of 15 PyGFP-luc-infected mosquitos and the liver-stage burden was determined by measuring in vivo luminescence 42 to 44 h later.
Mock-immunized BALB/c and C57BL/6 mice treated with the IgG2b isotype control antibody displayed very similar levels of liver-stage burden (Fig. 3A and B). Consistent with previous studies (39), immunization with Pyfabb/f− completely inhibited detectable liver-stage burden in both strains (Fig. 3A and B). For both strains, the difference in liver stage burden observed between the mock- and the Pyfabb/f−-immmunized mice treated with control IgG2b was statistically significant (P value = 0.0002 in both cases). In addition, all Pyfabb/f−-immunized mice treated with IgG2b isotype control antibody showed sterile protection, while disease became patent in all mock-immunized mice between day 3 and day 7 postchallenge (Fig. 3C). Strikingly, for Pyfabb/f−-immunized mice depleted of CD4+/CD8+ T cells, 6/8 (75%) of BALB/c mice and 3/8 (37.5%) of C57BL/6 mice had no detectable liver-stage burden. The difference observed between the mock- and the Pyfabb/f−-immunized mice depleted of CD4 and CD8 cells was significant for BALB/c mice (P = 0.001) but not for C57BL/6 mice (P = 0.78). More importantly, 5/8 (62.5%) of BALB/c mice and 3/8 (37.5%) of C57BL/6 mice immunized with Pyfabb/f− did not develop patent blood-stage infection despite their lack of CD8+ and CD4+ T cells. In addition, one BALB/c mouse showed a 6-day delay in patency, whereas two C57BL/6 mice showed a 3-day delay in patency. For both strains, the mice that did not develop blood-stage infection were those with the lowest levels of liver-stage burden (Fig. 3B and C). Although the differences between BALB/c and C57BL/6 mice immunized with Pyfabb/f− and depleted of CD4+/CD8+ T cells in liver-stage burden and disease patency were not statistically significant, these data demonstrate that immunization with a late liver stage-arresting GAP results in the acquisition of antibodies that are capable of inducing sterile protection in a subset of mice challenged with P. yoelii sporozoites and that the degree of protection might depend on the mouse strain tested. Moreover, they agree with recent studies in the rodent model of malaria showing that sporozoite challenge by mosquito bite engages antibody-mediated immune mechanisms in the skin that participate in controlling malaria infection (18, 19, 22).
FIG 3.
Immunization with Pyfabb/f− induces antibodies that are sufficient to protect mice against sporozoite challenge by mosquito bite. BALB/c or C57BL/6 mice (n = 7 to 8 mice/group) were immunized with 3 doses of 50,000 Pyfabb/f− sporozoites or mock immunized with uninfected salivary gland debris. At 24 days after the last dose, mice were treated with antibodies that deplete CD8 and CD4 T cells before challenge by the bite of 15 mosquitos infected with PyGFP-luc sporozoites. Control mice received equal amounts of isotype control IgG2b. Liver-stage burden was quantified by bioluminescent imaging 42 to 44 h after challenge. (A) Representative images of in vivo bioluminescence imaging. The scale indicates radiance expressed as p/s/cm2/sr. (B) Quantification of luciferase signal (measured as total flux per second). The horizontal bars correspond to the median, and the error bars show the interquartile range. Significant differences between groups were calculated using the Mann-Whitney test; * = P < 0.01; *** = P < 0.001; n.s. = nonsignificant. (C) Patency graphs of BALB/c (left panel) and C57BL/6 (right panel) mice. Parasitemia was determined by a Giemsa-stained thin blood smear procedure daily from day 3 to day 17 after challenge.
Antibodies elicited by Pyfabb/f− immunization are not sufficient to control challenge with P. yoelii sporozoites administered by i.v. injection.
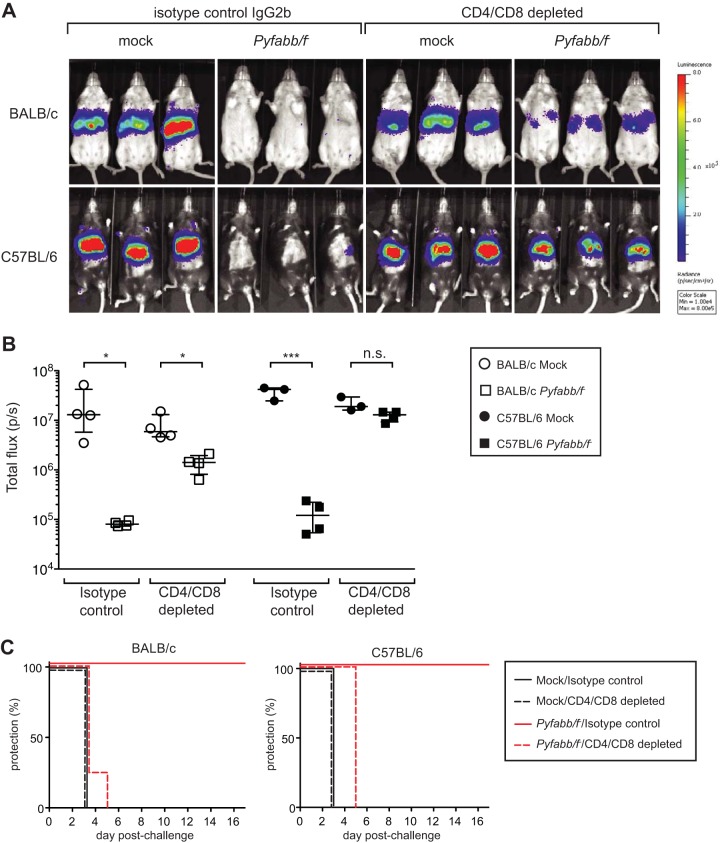
The data presented above contradict previously published studies in murine malaria that concluded that antibodies are dispensable for protection against preerythrocytic stages of malaria (8, 9, 26, 27). However, those studies utilized the method of challenge by i.v. administration, which bypasses antibody-based immune mechanisms in the skin (18, 19, 22). Thus, we hypothesized that, in comparison to challenge by mosquito bite, antibodies would not be sufficient to control malaria infection when sporozoites were delivered by i.v. challenge. To test this, we depleted both CD4+ and CD8+ T cells from Pyfabb/f−- and mock-immunized BALB/c and C57BL/6 mice as described above 24 h prior to i.v. challenge with 5,000 PyGFP-luc sporozoites. Similarly to what we observed with mosquito bite challenge, mice of both strains immunized with Pyfabb/f− displayed lower levels of liver-stage burden than mock-immunized mice (Fig. 4A and B). However, depletion of CD4+ and CD8+ T cells resulted in loss of inhibition of liver-stage burden in both mouse strains, although Pyfabb/f−-immunized BALB/c mice still displayed a significantly reduced level of liver infection compared to mock-immunized mice (Fig. 4A and B). Moreover, in contrast to the protection observed in mice challenged by mosquito bite (Fig. 3C), all Pyfabb/f−-immunized mice depleted of CD4+ and CD8+ T cells became patent between day 3 and day 5 post-sporozoite challenge by i.v. administration. These observations are in agreement with previously published data indicating that, although antibodies elicited by whole-parasite immunization are able to reduce liver infection, they are not sufficient to provide sterile immunity in the absence of T cells when mice are challenged with sporozoites through the i.v. route (8, 9, 26, 27).
FIG 4.
Antibodies from Pyfabb/f−-immunized mice are not sufficient to protect mice against sporozoite challenge by intravenous injection. BALB/c or C57BL/6 mice (n = 3 to 4 mice/group) were immunized with 3 doses of 50,000 Pyfabb/f− sporozoites or mock immunized with uninfected salivary gland debris. Mice were treated with CD8 and CD4 T cell-depleting antibodies 24 h prior to i.v. challenge with 5,000 PyGFP-luc sporozoites. Control mice received equal amounts of isotype control IgG2b. Liver-stage burden was quantified by bioluminescent imaging 42 to 44 h after challenge. (A) Representative images of in vivo bioluminescence imaging. The scale indicates radiance expressed as p/s/cm2/sr. (B) Quantification of luciferase signal (measured as total flux per second). The horizontal bars correspond to the median, and the error bars show the interquartile range. Significant differences between groups were calculated using the Mann-Whitney test; * = P < 0.01; *** = P < 0.001; n.s. = nonsignificant. (C) Patency curves of BALB/c (left panel) and C57BL/6 (right panel) mice. Parasitemia was determined by a Giemsa-stained thin blood smear procedure daily from day 3 to day 17 after challenge.
Immunization of BALB/c and C57BL/6 mice with Pyfabb/f− induces different patterns of anti-sporozoite antibody isotypes.
Different strains of mice have been shown to develop differing immune responses to and levels of protection against malaria infection (6, 30, 41). To investigate the possible contribution of antibodies to the differential levels of protection observed between BALB/c and C57BL/6 mice, we next compared the antibody responses induced by Pyfabb/f− immunization in these two strains.
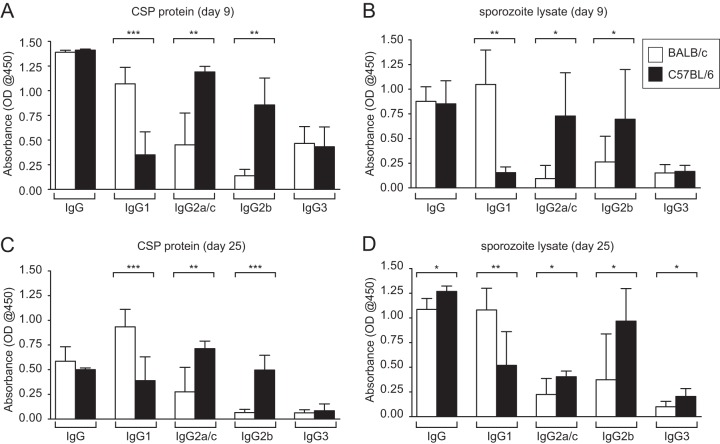
Mice were immunized with two doses of 50,000 Pyfabb/f− sporozoites administered 2 weeks apart or were mock immunized as a control. Serum was collected on day 9 and day 25 after the last immunization, and the levels of total IgG, IgG1, IgG2a and IgG2c (IgG2a/c), IgG2b, and IgG3 against the major sporozoite surface protein PyCSP and total sporozoite lysate were measured by ELISA (Fig. 5). Since C57BL/6 mice lack the allele for IgG2a and express IgG2c instead (47), we compared the levels of IgG2a in BALB/c mice to those of IgG2c in C57BL/6 mice. Pyfabb/f− immunization resulted in high titers of total IgG against PyCSP and sporozoite lysate that did not differ between BALB/c and C57BL/6 mice (Fig. 5), although we observed slightly higher significant reactivity to sporozoite lysate in sera collected from C57BL/6 mice on day 25 compared to those from BALB/c mice (Fig. 5D). The levels of IgG1 against both CSP protein and sporozoite lysate in sera from both day 9 and day 25 were consistently and significantly higher in BALB/c mice than in C57BL/6 mice (Fig. 5). In contrast, the levels of IgG2a/c and IgG2b against both CSP protein and sporozoite lysate were significantly higher in C57BL/6 mice than in BALB/c mice (Fig. 5). Overall, IgG3 displayed lower reactivity to both CSP protein and sporozoite lysate than the other isotypes (Fig. 5), and the differences between the strains in the levels of this isotype were not significant, with the exception of sera collected on day 25 from C57BL/6 mice, which showed slightly higher significant reactivity to sporozoite lysate than those from BALB/c mice (Fig. 5D). Our data show that immunization of mice with Pyfabb/f− induced high levels of antibodies against sporozoite proteins, including the main surface protein CSP. In addition, we observed different patterns of antibody isotypes between BALB/c and C57BL/6 mice, with the former displaying higher levels of IgG1 and the latter displaying higher levels of IgG2b and -c. It is possible that these differences in antibody isotypes could be related to the differential levels of protection observed between the two strains of mice, although it remains to be shown whether these differences in isotype levels directly contribute to the differential protection levels observed and whether other mechanisms are involved.
FIG 5.
Pyfabb/f− immunization of BALB/c mice and of C57BL/6 mice elicits different patterns of IgG isotypes. BALB/c (white bars, n = 5) or C57BL/6 (black bars, n = 5) mice were immunized with 2 doses of 50,000 Pyfabb/f− sporozoites or mock immunized. Sera were collected on days 9 (A and B) and 25 (C and D) after the last immunization and tested for reactivity to recombinant full-length P. yoelii CSP protein (A and C) and sporozoite lysate (B and D) in ELISAs measuring total IgG, IgG1, IgG2a/c, IgG2b, and IgG3. For each isotype, absorbance was normalized by subtracting from the signal observed in each immunized mouse the average signal observed in the mock-immunized mice of the corresponding strain. Error bars correspond to standard deviations. Significance was analyzed using unpaired t tests with Welch's correction; * = P < 0.05; ** = P < 0.01; *** = P < 0.001. OD@450, optical density at 450 nm.
DISCUSSION
There is an urgent need to develop a malaria vaccine capable of eliciting sterile protection that will both mitigate disease within individuals and hasten the eradication of malaria by stopping the human-mosquito transmission cycle. To date, the only human malaria vaccines that have yielded 100% sterilizing protection are those based on live attenuated parasites (12, 13, 48). The role of sporozoite-specific CD8+ T cells in this protection in both mice and humans is well established. While antibodies have also been implicated in protection, some rodent model studies have shown that they are dispensable for protection. This report presents the first demonstration that antibodies generated by immunization of mice with a genetically attenuated P. yoelii parasite are sufficient to confer sterile protection against the preerythrocytic stages of rodent malaria when sporozoites are delivered by mosquito bite.
Previous studies have shown that immunization of mice with live attenuated parasites elicits different immune responses and protection profiles depending on the murine strain tested (6, 30). This difference has been attributed to different susceptibilities to the malaria parasite as well as to the different cellular responses induced by immunization. To understand whether antibodies induced by whole-parasite immunization contribute to the different patterns of protection observed in different mouse strains, we compared the antibody responses induced by Pyfabb/f− immunization in BALB/c and C57BL/6 mice. To better understand the function of Pyfabb/f−-induced antibodies in preerythrocytic malaria infection, we measured the ability of mouse sera to inhibit hepatocyte invasion and/or liver-stage development using a modification of our recently published luminescence-based assay (42). Although we observed similarly high levels of in vitro inhibition using sera from BALB/c and C57BL/6 mice, passive transfer of sera from Pyfabb/f−-immunized mice into congenic mice followed by sporozoite challenge by mosquito bite showed a reduction of liver-stage burden only in BALB/c mice. However, in spite of this reduction, none of the mice displayed sterile protection as measured by blood parasitemia. This suggests that, although Pyfabb/f−-induced antibodies were able to reduce liver-stage burden in BALB/c mice, their presence was not sufficient to abrogate blood-stage infection.
Since this lack of protection could be due to the small volume of sera that can be passively transferred into mice, we next characterized the protective ability of Pyfabb/f−-induced antibodies at physiological levels in the absence of CD4+ and CD8+ T cells by depleting these cell types from immunized animals prior to challenge with sporozoites by mosquito bite. Using this method, we observed that 75% of Pyfabb/f−-immunized BALB/c mice and 37.5% of C57BL/6 mice depleted of T cells displayed liver-stage burdens similar to those of isotype-treated controls, suggesting that antibodies were sufficient to reduce liver-stage burden in these mice. Interestingly, this reduction in liver-stage burden resulted in the sterile protection of 50% of the mice immunized with Pyfabb/f− (i.e., in 62.5% of BALB/c mice and in 37.5% of C57BL/6 mice) that did not develop patent blood-stage infection despite their lack of CD8+ and CD4+ T cells. In addition, one of the BALB/c mice and two of the C57BL/6 mice showed a 6-day delay and a 2-day delay in the onset of parasitemia, respectively, compared to the corresponding controls. These data, taken together with previously published work, suggest that, although T cells have a clearly established role in protection against preerythrocytic stages of malaria infection, vaccination with whole parasites induces antibodies that can also significantly contribute to protection.
Although our data are apparently in conflict with previous reports showing that antibodies are dispensable for protection of mice against sporozoite challenge (8, 9, 26, 27), those studies used a nonnatural model of sporozoite challenge via the i.v. route, whereas we challenged the mice via experimental mosquito bite, which more closely reflects natural infection. It has been shown that sporozoites delivered by mosquito bite take longer to reach the liver than those administered by i.v. challenge, not only allowing antibodies time to block sporozoite invasion of hepatocytes in vivo but also blocking the number of sporozoites deposited in the skin by the mosquito (18, 19). In fact, when we repeated the experiment described above using i.v. challenge, we confirmed that this route of challenge essentially bypasses the antibody-based responses elicited by whole-parasite vaccination, since 100% of T cell-depleted mice of both strains challenged by i.v. administration developed blood-stage infection, although all BALB/c mice displayed significant reductions in liver-stage burden.
Taken together, our data show that the efficacy of Pyfabb/f−-induced antibodies in reducing liver-stage burden and conferring protection against sporozoite challenge depends not only on the route of sporozoite challenge but also on the strain of mouse used. In both passive-transfer and T cell depletion studies, antibodies from BALB/c mice consistently inhibited sporozoite infection of the liver more potently than those from C57BL/6 mice and resulted in a larger proportion of mice that acquired sterile protection against sporozoite challenge by mosquito bite. These differences cannot be explained only by strain-specific susceptibility to infection, since the two strains had similar levels of liver-stage burden and time to patency in control experiments, or by overall titers of total IgG, which were very similar for the two strains. However, isotyping of antibodies against both CSP and total sporozoite lysate revealed that the humoral immune response in BALB/c mice is biased toward the IgG1 isotype, whereas C57BL/6 mice make larger amounts of IgG2b and -c. Since different antibody isotypes differ in their Fc-mediated effector functions (32, 49), this bias could result in the activation of different mechanisms to eliminate parasites in vivo in the two mouse strains. For example, murine IgG1 is thought to act mainly through Fc-independent functions, such as pathogen neutralization, while IgG2b is more efficient at complement fixation and opsonization (50, 51). Thus, our observation that BALB/c and C57BL/6 mice display different patterns of parasite-specific IgG isotypes could partially explain the observed differences between the two strains in the levels of liver-stage burden and protection against sporozoite challenge. The differences in the IgG repertoire in BALB/c and C57BL/6 mice could also explain the discrepancy between the performance of sera from these strains between our in vitro and in vivo inhibition assays. In the in vitro sporozoite inhibition assays, which lack physiological levels of mouse complement and effector cells bearing Fc receptors, immune sera from the two strains performed equally well. However, in vivo experiments, in which Fc-dependent mechanisms were capable of acting, demonstrated that sera from BALB/c mice were significantly better at reducing liver burden following infectious mosquito bite. Together, these data provide a firm rationale for characterizing the roles of different IgG isotypes in protection against preerythrocytic malaria in the mouse model and for determining whether the results apply to human malaria.
Alternatively, it is possible that the observed differences in the abilities of the two strains of mice to mount an effective immune response against challenge with P. yoelii sporozoites could result from differences in the breadths of their antibody responses to parasite antigens or epitopes. Although we did not observe a difference between BALB/c and C57BL/6 mice in the total levels of IgG against the major sporozoite surface protein CSP or total sporozoite lysate, it is possible that these strains mount different responses to specific parasite antigens other than CSP that would not be discriminated by analyzing total sporozoite lysate. Finally, it is also possible that both the type and the specificity of antibodies elicited by the two strains of mice in response to malaria infection contribute to the differential levels of protection observed. Studies in human malaria have shown that acquisition of antibodies against non-CSP antigens such as TRAP and LSA1 as well as against other less extensively characterized antigens is associated with the level of protection against malaria (52–54). Thus, it will be important to identify which antigens are recognized by the antibodies that result from Pyfabb/f− immunization of the different mouse strains as well as to characterize whether differences in their functional activities correlated with protection.
Although our experimental challenge model is more biologically relevant than the use of i.v. challenge, one limitation is that it uses A. stephensi mosquitoes to deliver sporozoites instead of the natural vector of P. yoelii and therefore does not completely reflect natural rodent malaria infection. It is likely that our approach results in the delivery of lower numbers of sporozoites than natural infection, therefore resulting in higher levels of protection. However, we observed 100% infection in control mice upon challenge by the bite of at least 15 infected A. stephensi mosquitos, suggesting that the approach is a valid method to explore the role of antibodies in preerythrocytic stages of malaria.
In summary, we have shown for the first time that immunization of mice with a late liver stage-arresting GAP elicits antibodies that confer protection against the preerythrocytic stages of rodent malaria parasites in the absence of T cells. Multiple studies in the rodent malaria model have shown that sporozoite-specific CD8+ T cells are sufficient to eliminate liver stages (8, 9, 26–28). The hypothesis of the role of CD8+ T cells in malaria immunity is also supported by data from human clinical studies that showed that CD8+ T cells and IFN-γ are critical mediators of protection (11, 12). However, it was recently demonstrated that, to mediate effective immunity, T cells must exist in exceedingly large numbers that may be difficult to attain by vaccination of humans (6). Furthermore, some malaria vaccine clinical trials have suggested that T cell-directed subunit vaccines might have limited efficacy, while those trials have highlighted the importance of other immune mechanisms, including antibodies (15, 16, 55). Thus, future vaccine strategies designed to mediate effective antimalaria immunity should aim at eliciting both humoral and cellular immune responses. Mechanistic studies using the model used in this study can critically inform the development of the next generation of vaccines designed to elicit antibodies directed against pre-erythrocyte-stage antigens that contribute to protective immune responses in vivo.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant R01AI076498 to R.W. and by Bill and Melinda Gates Foundation grant OPP1016829 to R.W.
We are grateful to William W. Betz, Mark F. Kennedy, Heather Kain, and Jen C. C. Hume of the Seattle Biomedical Research Institute Insectary Facility for mosquito and sporozoite production, Tim Dawe and the Vivarium personnel for animal care, and Jessica Miller for critically reading the manuscript.
Footnotes
Published ahead of print 29 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02320-14.
REFERENCES
- 1.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. 2012. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431. 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, Richman A, Velmurugan S, Reyes S, Li M, Tucker K, Ahumada A, Ruben AJ, Li T, Stafford R, Eappen AG, Tamminga C, Bennett JW, Ockenhouse CF, Murphy JR, Komisar J, Thomas N, Loyevsky M, Birkett A, Plowe CV, Loucq C, Edelman R, Richie TL, Seder RA, Hoffman SL. 2011. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 334:475–480. 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 3.Doolan DL, Martinez-Alier N. 2006. Immune response to pre-erythrocytic stages of malaria parasites. Curr. Mol. Med. 6:169–185. 10.2174/156652406776055249. [DOI] [PubMed] [Google Scholar]
- 4.Cockburn IA, Amino R, Kelemen RK, Kuo SC, Tse SW, Radtke A, Mac-Daniel L, Ganusov VV, Zavala F, Menard R. 2013. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc. Natl. Acad. Sci. U. S. A. 110:9090–9095. 10.1073/pnas.1303858110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cockburn IA, Tse SW, Zavala F. 2014. CD8+ T cells eliminate liver stage Plasmodium parasites without detectable bystander effect. Infect. Immun. 82:1460–1464. 10.1128/IAI.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt NW, Butler NS, Badovinac VP, Harty JT. 2010. Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites. PLoS Pathog. 6:e1000998. 10.1371/journal.ppat.1000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330:664–666. 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 8.Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. U. S. A. 85:573-576. 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues M, Nussenzweig RS, Zavala F. 1993. The relative contribution of antibodies, CD4+ and CD8+ T cells to sporozoite-induced protection against malaria. Immunology 80:1–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Jobe O, Lumsden J, Mueller AK, Williams J, Silva-Rivera H, Kappe SH, Schwenk RJ, Matuschewski K, Krzych U. 2007. Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex class I-dependent interferon-gamma-producing CD8+ T cells. J. Infect. Dis. 196:599–607. 10.1086/519743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teirlinck AC, McCall MB, Roestenberg M, Scholzen A, Woestenenk R, de Mast Q, van der Ven AJ, Hermsen CC, Luty AJ, Sauerwein RW. 2011. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 7:e1002389. 10.1371/journal.ppat.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, Holman LA, James ER, Billingsley PF, Gunasekera A, Richman A, Chakravarty S, Manoj A, Velmurugan S, Li M, Ruben AJ, Li T, Eappen AG, Stafford RE, Plummer SH, Hendel CS, Novik L, Costner PJ, Mendoza FH, Saunders JG, Nason MC, Richardson JH, Murphy J, Davidson SA, Richie TL, Sedegah M, Sutamihardja A, Fahle GA, Lyke KE, Laurens MB, Roederer M, Tewari K, Epstein JE, Sim BK, Ledgerwood JE, Graham BS, Hoffman SL, VRC312 Study Team 2013. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341:1359–1365. 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 13.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, Spaarman L, de Mast Q, Roeffen W, Snounou G, Renia L, van der Ven A, Hermsen CC, Sauerwein R. 2009. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361:468–477. 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 14.Behet MC, Foquet L, van Gemert GJ, Bijker EM, Meuleman P, Leroux-Roels G, Hermsen CC, Scholzen A, Sauerwein RW. 2014. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum. Malar. J. 13:136. 10.1186/1475-2875-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, Mandomando I, Spiessens B, Guinovart C, Espasa M, Bassat Q, Aide P, Ofori-Anyinam O, Navia MM, Corachan S, Ceuppens M, Dubois MC, Demoitie MA, Dubovsky F, Menendez C, Tornieporth N, Ballou WR, Thompson R, Cohen J. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411–1420. 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 16.White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. 2013. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS One 8:e61395. 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart MJ, Nawrot RJ, Schulman S, Vanderberg JP. 1986. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect. Immun. 51:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderberg JP, Frevert U. 2004. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34:991–996. 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Kebaier C, Voza T, Vanderberg J. 2009. Kinetics of mosquito-injected Plasmodium sporozoites in mice: fewer sporozoites are injected into sporozoite-immunized mice. PLoS Pathog. 5:e1000399. 10.1371/journal.ppat.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollingdale MR, Zavala F, Nussenzweig RS, Nussenzweig V. 1982. Antibodies to the protective antigen of Plasmodium berghei sporozoites prevent entry into cultured cells. J. Immunol. 128:1929–1930. [PubMed] [Google Scholar]
- 21.Luo S, Liu D, Ye B, Shu H, Fu R. 1995. Effect of monoclonal antibodies on the entry and development of Plasmodium vivax sporozoite in cultured cells. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 13:284–289. [PubMed] [Google Scholar]
- 22.Sack BK, Miller JL, Vaughan AM, Douglass A, Kaushansky A, Mikolajczak S, Coppi A, Gonzalez-Aseguinolaza G, Tsuji M, Zavala F, Sinnis P, Kappe SH. 2014. Model for in vivo assessment of humoral protection against malaria sporozoite challenge by passive transfer of monoclonal antibodies and immune serum. Infect. Immun. 82:808–817. 10.1128/IAI.01249-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida N, Nussenzweig RS, Potocnjak P, Nussenzweig V, Aikawa M. 1980. Hybridoma produces protective antibodies directed against the sporozoite stage of malaria parasite. Science 207:71–73. 10.1126/science.6985745. [DOI] [PubMed] [Google Scholar]
- 24.Danforth HD, Aikawa M, Cochrane AH, Nussenzweig RS. 1980. Sporozoites of mammalian malaria: attachment to, interiorization and fate within macrophages. J. Protozool. 27:193–202. 10.1111/j.1550-7408.1980.tb04680.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwenk R, Asher LV, Chalom I, Lanar D, Sun P, White K, Keil D, Kester KE, Stoute J, Heppner DG, Krzych U. 2003. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 25:17–25. 10.1046/j.1365-3024.2003.00495.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen DH, Tigelaar RE, Weinbaum FI. 1977. Immunity to sporozoite-induced malaria infection in mice. I. The effect of immunization of T and B cell-deficient mice. J. Immunol. 118:1322–1327. [PubMed] [Google Scholar]
- 27.Egan JE, Weber JL, Ballou WR, Hollingdale MR, Majarian WR, Gordon DM, Maloy WL, Hoffman SL, Wirtz RA, Schneider I, Woollett GR, Young JF, Hockmeyer WT. 1987. Efficacy of murine malaria sporozoite vaccines: implications for human vaccine development. Science 236:453–456. 10.1126/science.3551073. [DOI] [PubMed] [Google Scholar]
- 28.Tarun AS, Dumpit RF, Camargo N, Labaied M, Liu P, Takagi A, Wang R, Kappe SH. 2007. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J. Infect. Dis. 196:608–616. 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- 29.Foquet L, Hermsen CC, van Gemert GJ, Van Braeckel E, Weening KE, Sauerwein R, Meuleman P, Leroux-Roels G. 2013. Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. J. Clin. Invest. 124:140–144. 10.1172/JCI70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doolan DL, Hoffman SL. 2000. The complexity of protective immunity against liver-stage malaria. J. Immunol. 165:1453–1462. 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 31.Schulte S, Sukhova GK, Libby P. 2008. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am. J. Pathol. 172:1500–1508. 10.2353/ajpath.2008.070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locksley RM, Heinzel FP, Sadick MD, Holaday BJ, Gardner KD., Jr 1987. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann. Inst. Pasteur Immunol. 138:744–749. 10.1016/S0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- 33.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH. 2009. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell. Microbiol. 11:506–520. 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller JL, Murray S, Vaughan AM, Harupa A, Sack B, Baldwin M, Crispe IN, Kappe SH. 2013. Quantitative bioluminescent imaging of pre-erythrocytic malaria parasite infection using luciferase-expressing Plasmodium yoelii. PLoS One 8:e60820. 10.1371/journal.pone.0060820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trimnell A, Takagi A, Gupta M, Richie TL, Kappe SH, Wang R. 2009. Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes. J. Immunol. 183:5870–5878. 10.4049/jimmunol.0900302. [DOI] [PubMed] [Google Scholar]
- 36.Sellhorn G, Caldwell Z, Mineart C, Stamatatos L. 2009. Improving the expression of recombinant soluble HIV Envelope glycoproteins using pseudo-stable transient transfection. Vaccine 28:430–436. 10.1016/j.vaccine.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Carbonetti S, Oliver BG, Glenn J, Stamatatos L, Sather DN. 2014. Soluble HIV-1 envelope immunogens derived from an elite neutralizer elicit cross-reactive V1V2 antibodies and low potency neutralizing antibodies. PLoS One 9:e86905. 10.1371/journal.pone.0086905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy M, Fishbaugher ME, Vaughan AM, Patrapuvich R, Boonhok R, Yimamnuaychok N, Rezakhani N, Metzger P, Ponpuak M, Sattabongkot J, Kappe SH, Hume JC, Lindner SE. 2012. A rapid and scalable density gradient purification method for Plasmodium sporozoites. Malar. J. 11:421. 10.1186/1475-2875-11-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. 2011. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9:451–462. 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zavala F, Hollingdale MR, Schwartz AL, Nussenzweig RS, Nussenzweig V. 1985. Immunoradiometric assay to measure the in vitro penetration of sporozoites of malaria parasites into hepatoma cells. J. Immunol. 134:1202–1205. [PubMed] [Google Scholar]
- 41.Nganou-Makamdop K, Sauerwein RW. 2013. Liver or blood-stage arrest during malaria sporozoite immunization: the later the better? Trends Parasitol. 29:304–310. 10.1016/j.pt.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Cooney LA, Gupta M, Thomas S, Mikolajczak S, Choi KY, Gibson C, Jang IK, Danziger S, Aitchison J, Gardner MJ, Kappe SH, Wang R. 2013. Short-lived effector CD8 T cells induced by genetically attenuated malaria parasite vaccination express CD11c. Infect. Immun. 81:4171–4181. 10.1128/IAI.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silvie O, Greco C, Franetich JF, Dubart-Kupperschmitt A, Hannoun L, van Gemert GJ, Sauerwein RW, Levy S, Boucheix C, Rubinstein E, Mazier D. 2006. Expression of human CD81 differently affects host cell susceptibility to malaria sporozoites depending on the Plasmodium species. Cell. Microbiol. 8:1134–1146. 10.1111/j.1462-5822.2006.00697.x. [DOI] [PubMed] [Google Scholar]
- 44.Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Renia L, Hannoun L, Eling W, Levy S, Boucheix C, Mazier D. 2003. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat. Med. 9:93–96. 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 45.Mwakingwe A, Ting LM, Hochman S, Chen J, Sinnis P, Kim K. 2009. Noninvasive real-time monitoring of liver-stage development of bioluminescent Plasmodium parasites. J. Infect. Dis. 200:1470–1478. 10.1086/606115. [DOI] [PubMed] [Google Scholar]
- 46.Ploemen IH, Prudencio M, Douradinha BG, Ramesar J, Fonager J, van Gemert GJ, Luty AJ, Hermsen CC, Sauerwein RW, Baptista FG, Mota MM, Waters AP, Que I, Lowik CW, Khan SM, Janse CJ, Franke-Fayard BM. 2009. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One 4:e7881. 10.1371/journal.pone.0007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin RM, Brady JL, Lew AM. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212:187–192. 10.1016/S0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 48.Clyde DF, Most H, McCarthy VC, Vanderberg JP. 1973. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 266:169–177. 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Leatherbarrow RJ, Dwek RA. 1984. Binding of complement subcomponent C1q to mouse IgG1, IgG2a and IgG2b: a novel C1q binding assay. Mol. Immunol. 21:321–327. 10.1016/0161-5890(84)90103-2. [DOI] [PubMed] [Google Scholar]
- 50.Kipps TJ, Parham P, Punt J, Herzenberg LA. 1985. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J. Exp. Med. 161:1–17. 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oi VT, Vuong TM, Hardy R, Reidler J, Dangle J, Herzenberg LA, Stryer L. 1984. Correlation between segmental flexibility and effector function of antibodies. Nature 307:136–140. 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- 52.Doolan DL, Mu Y, Unal B, Sundaresh S, Hirst S, Valdez C, Randall A, Molina D, Liang X, Freilich DA, Oloo JA, Blair PL, Aguiar JC, Baldi P, Davies DH, Felgner PL. 2008. Profiling humoral immune responses to P. falciparum infection with protein microarrays. Proteomics 8:4680–4694. 10.1002/pmic.200800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Felgner PL, Roestenberg M, Liang L, Hung C, Jain A, Pablo J, Nakajima-Sasaki R, Molina D, Teelen K, Hermsen CC, Sauerwein R. 2013. Pre-erythrocytic antibody profiles induced by controlled human malaria infections in healthy volunteers under chloroquine prophylaxis. Sci. Rep. 3:3549. 10.1038/srep03549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trieu A, Kayala MA, Burk C, Molina DM, Freilich DA, Richie TL, Baldi P, Felgner PL, Doolan DL. 2011. Sterile protective immunity to malaria is associated with a panel of novel P. falciparum antigens. Mol. Cell. Proteomics 10:M111.007948. 10.1074/mcp.M111.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ewer KJ, O'Hara GA, Duncan CJ, Collins KA, Sheehy SH, Reyes-Sandoval A, Goodman AL, Edwards NJ, Elias SC, Halstead FD, Longley RJ, Rowland R, Poulton ID, Draper SJ, Blagborough AM, Berrie E, Moyle S, Williams N, Siani L, Folgori A, Colloca S, Sinden RE, Lawrie AM, Cortese R, Gilbert SC, Nicosia A, Hill AV. 2013. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat. Commun. 4:2836. 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.