Abstract
The liver fluke Fasciola hepatica is a highly evolved parasite that uses sophisticated mechanisms to evade the host immune response. The immunosuppressive capabilities of the parasite have been associated with antigens secreted through the parasite's tegument, called excretory-secretory products (ESPs). Proteomic studies have identified the fatty acid binding protein (FABP) as one of molecules present in the parasite ESPs. Although FABP has been investigated for potential use in the development of vaccines against fascioliasis, its direct interaction with cells of immune system has not been studied. In this study, FABP was purified in native form from soluble extracts of F. hepatica adult flukes using a combination of molecular sieving chromatography and preparative isoelectric focusing. The immunological effect of the purified protein, termed Fh12, was assayed in vitro using monocyte-derived macrophages (MDM) obtained from healthy human donors. Results from the assay indicate that Fh12 produced a significantly increased arginase expression and activity and induced the expression of chitinase-3-like protein (CHI3L1). The assay also showed that Fh12 downregulated the production of nitric oxide (NO) and the expression of nitric oxide synthase (NOS2). This indicates that Fh12 induced the production of alternatively activated macrophages (AAMϕ). The results also demonstrated the ability of Fh12 to downregulate the secretion of the proinflammatory and inflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-12 (IL-12), and IL-1βB, even after stimulation with lipopolysaccharide (LPS), as well as its ability to stimulate the overexpression of IL-10. These results suggest a potent anti-inflammatory role for Fh12, which could occur via targeting of Toll-like receptor 4 (TLR4).
INTRODUCTION
Fascioliasis, caused by the liver fluke Fasciola hepatica, is a chronic infection that affects more than 600 million animals in the world and produces economic loses estimated at more than 2 billion dollars per year. Fascioliasis is known to reduce the production of animal milk and meat, specifically in cattle and sheep (1). Moreover, fascioliasis is currently considered an emerging human disease, with an increased number of reported cases, observed particularly in the Andean rural regions (1, 2). During its life cycle, F. hepatica migrates across body cavities, penetrates host tissues, and settles in bile ducts, where flukes can then live for many years (1). During its migration, the parasite excretes and secretes a large number of antigens, which induce an immunosuppressive effect to prevent the innate immune response in the mammalian host. This results in the suppression of the effective priming of an adaptive immune response. This immunosuppressive status is what allows the long-term survival of F. hepatica and therefore the development of chronic infections. Proteins secreted by this parasite are termed excretory-secretory products (ESPs) and are known to contain a mixture of proteolytic enzymes (such as cathepsin-L [CatL]) and antioxidants (such as glutathione S-transferase [GST], thioredoxin peroxidase [TxP], and fatty acid binding proteins [FABPs]) (3, 4). Proteolytic enzymes are important for digestion of the host tissue and are used in the parasite's defense mechanism. The proteolytic enzymes and antioxidants have been proposed as vaccine candidates against fascioliasis (5–13).
Previous studies performed with recombinant forms of F. hepatica proteins, such as CatL1, GST, and TxP, have shown that these proteins interact with cells of immune system in animal model of fascioliasis (14–16). CatL1 and GST have been shown to activate dendritic cells via interaction with the pattern recognition receptor (PRR) Toll-like receptor 4 (TLR4) (15). F. hepatica and other helminth-derived peptides have also shown to suppress the activation of macrophages by microbial stimuli and to alter the response of B cells to cytokine stimulation (17, 18). Total ESPs and recombinant versions of thioredoxin peroxidase have been shown to induce the alternative activation of macrophages in murine (14, 19) and ruminant (16, 20) models of fascioliasis. The alternatively activated macrophages (AAMϕ) are associated with the Th2-type immune responses that are typically induced by F. hepatica, as well as many other helminth parasites, and that are involved in controlling Th1-type immune responses (21). In contrast, Th1-type immune responses with classically activated macrophages (CAMϕ) are usually developed in bacterial, viral, or protozoan infections (21, 22). It has been proposed that F. hepatica induces these AAMϕ as a manipulative survival strategy which allows this parasite to survive in the mammalian host for long periods of time (14, 19).
Studies on F. hepatica fatty acid binding proteins (FABPs) have been focused mainly on their potential application as vaccine candidates against fascioliasis (6, 10). However, no information is currently available on how FABPs interact with cells of the innate immune system to modulate host immunity. F. hepatica FABPs are immunogenic proteins with molecular masses of 12 to 15 kDa that play important roles in nutrient acquisition and parasite survival within the mammalian host. Because liver flukes express a highly reduced lipid metabolism, flukes are unable to synthesize long-chain fatty acids and steroids de novo and are unable to process lipids by β-oxidation. Proteomic studies have identified FABPs in the ESPs and in the tegumental coat antigen of F. hepatica (3, 4). Therefore, by virtue of its location, FABP is likely a molecule that facilitates the parasite's survival in the host. The objective of this study was to understand the effect of native forms of F. hepatica fatty acid binding protein on human monocyte-derived macrophages (MDM) in vitro.
MATERIALS AND METHODS
Parasites.
F. hepatica adult flukes were obtained from bile ducts of naturally infected cattle sacrificed at a local slaughterhouse. Parasites were washed with 0.05 M phosphate-buffered saline, pH 7.2 (PBS), to eliminate all traces of bile and blood and were transported to the laboratory in clean prewarmed RPMI 1640 medium.
Purification of native 12-kDa FABP.
Native F. hepatica fatty acid binding protein (Fh12) was purified from F. hepatica adult fluke extract using a combination of gel filtration and preparative isoelectric focusing (IEF) separations, as previously described (23). Briefly, F. hepatica adult flukes were homogenized in a Teflon homogenizer using PBS in the presence of a protease inhibitor cocktail. The solution was then centrifuged at 30, 000 × g for 30 min at 4°C. One hundred milligrams of the soluble extract protein was loaded onto a Sephadex G-50 (XK 26/100) column equilibrated with PBS and chromatographed at a flow rate of 3 cm/h. The column was calibrated using proteins of known molecular mass (cytochrome c [12.0 kDa] and carbonic anhydrase [29.0 kDa]). Proteins that eluted within a first high, broad peak were discarded. Proteins that eluted within a second small peak, with an elution volume equal to or greater than that for cytochrome c but lower than that for carbonic anhydrase, were collected, pooled, and dialyzed overnight against 1% glycine in the presence of 2% ampholytes (pH 3 to 10 and pH 5 to 7 at a ratio of 1:4) (Bio-Rad Laboratories, Hercules, CA, USA). The sample was loaded into a liquid isoelectric focusing (IEF) system (Rotofor; Bio-Rad) and maintained at 4°C. Two consecutive IEF runs were carried out following the manufacturer's instructions. Separation was achieved by applying constant power (12 W) until the voltage stabilized at approximately 1,400 V. Focusing was continued for an additional 30 min and then terminated (an IEF run lasts approximately 6 h). Individual IEF fractions were harvested, and their pH and absorbance at 280 nm were measured. Each aliquot was subjected to 12.5% SDS-PAGE, and the proteins were visualized by silver staining. Fractions from the second IEF run with a pH between 4.61 and 5.9 that exhibited a polypeptide band around 12 to 15 kDa were manually excised from the gel, washed twice with double-distilled water, digested with sequencing-grade trypsin (Promega, Madison, WI), and analyzed by matrix-assisted laser desorption ionization (MALDI) and tandem mass spectrometry (MS/MS), as previously described (24). Fractions in which the fatty acid binding protein was identified were pooled, exhaustively desalted against PBS, concentrated by ultrafiltration using an YM-10 membrane (which excludes components with molecular masses lower than 10 kDa), and stored at −20°C.
Immunoreactivity of the purified Fh12 protein.
Enzyme-linked immunosorbent assay (ELISA) and Western blotting techniques were used to assess the immunoreactivity of the purified F12 protein following established protocols. The purified protein was tested against a pool of sera from people with chronic fascioliasis, an antiserum prepared against F. hepatica tegument extract (24), a specific polyclonal antibody obtained against ESPs (25), and an specific polyclonal antibody raised against Fh15, the recombinant form of FABP (26).
Human MDM culture.
Blood was collected from healthy subjects using lithium heparin-coated Vacutainers. The Institutional Review Board (IRB) of the University of Puerto Rico School of Medicine approved the studies (protocol H14122), and subjects gave written informed consent before collection of blood samples. The heparinized blood was layered on Ficoll-Histopaque (BD Pharmingen) and subjected to centrifugation at 1,500 × g for 30 min. The white layer of peripheral blood mononuclear cells (PBMCs) was gently aspirated and aseptically transferred into sterile tubes. The cell suspension was washed and cultured in sterile RPMI 1640 supplemented with 20 mM l-glutamine, 10% fetal bovine serum (FBS), and antibiotics (1 ml penicillin and streptomycin/100 ml of medium) (Sigma). After assessment of cell viability by trypan blue staining, PBMCs were seeded at a density of 4 × 106 cells/well in supplemented RPMI 1640 and cultured in 6-well culture plates. After 3 h of incubation at 37°C, nonadherent cells were removed by washing with warm RPMI. Adherent cells were highly enriched for macrophages (>95%) as assessed by fluorescence-activated cell sorting staining with the macrophage marker F4/80 (BD Pharmingen, San Diego, CA). Adherent cells were removed with cold PBS and a scraper and were seeded at a density of 106 cells/ml. Cells were cultured for 6 days, with the medium changed every second day. The cells were treated with lipopolysaccharide (LPS) (15 μg/ml), Fh12 (1, 5 and 10 μg/ml), or a mixture of LPS and Fh12 for 48 h. Cells treated with interleukin-4 (IL-4) (10 ng/ml) as reported in elsewhere (27) were used as control of alternative activation of macrophages. Supernatants were collected and analyzed for nitric oxide (NO). Cells (mostly monocyte-derived macrophages [MDM]) were washed with RPMI, removed with a scraper, and analyzed for intracellular arginase activity by a colorimetric method.
NO.
Supernatants were tested for nitric oxide using the Griess reagent system (Promega). Briefly, 50 μl of supernatant was added to each well of a 96-well plate, and then 50 μl of sulfanimide solution was added and the plate was incubated for 10 min in the dark at room temperature. Immediately after, 50 μl of N-napthylethylenediamine dihydrochloride (NED) solution was added, and the plate was incubated at room temperature. Absorbance measurements were taken at 540, nm and NO concentrations were determined using a standard curve prepared from a stock solution of 100 μM nitrate.
Arginase activity.
Cells were lysed using 100 μl of radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-HCl [pH 7.4], containing 0.15 mM pepstatin-A, 0.2 mM leupeptin, and 0.4% [wt/vol] Triton X-100) followed by incubation on a rocking platform for 1 h and centrifugation at 20,000 × g at 4°C for 10 min. Supernatants (40 μl) from cell lysates were mixed with 10 μl of 5× arginine substrate in a 96-well plate, and the reaction mixture was incubated for 2 h at 37°C. Two hundred 200 microliters of urea reagent was then added to each well, and the solution was incubated for 60 min at room temperature. Absorbance measurements were taken at 430 nm. The levels of urea in the samples were determined by using a standard curve. One unit of enzyme activity was defined as the quantity of enzyme required to convert 1 μM l-arginine to ornithine and urea per minute, at pH 9.5 and 37°C, per million cells.
RNA extraction, RT, and quantitative real-time PCR analysis.
RNA was extracted from Fh12- and LPS-stimulated cells using RNeasy columns from Qiagen, following the manufacturer's instructions. One microgram of total RNA was treated with DNase I (Ambion, Inc.). For reverse transcription (RT)-PCR, first-strand cDNA was produced with oligo(dT) primers from 1 μg total RNA using avian myeloblastosis virus reverse transcriptase (reverse transcription system; Syd Labs Inc., Malden, MA, USA) at 42°C for 15 min, followed by 95°C for 3 min. A 1-μl aliquot of the resultant cDNA was amplified using primers specific for the genes for NOS2, Arg-1, eosinophil chemotactic factor chitinase-3-like protein (CHI3L1), IL-12A, tumor necrosis factor alpha (TNF-α), IL-1β, IL-10, and β-actin (ACTB) as a housekeeping gene (Table 1). The amplification involved 40 cycles, with 1 cycle consisting of 15 s of denaturation at 95°C, 1 min of annealing of primers at 55°C, and 1 min of elongation at 55°C. Real-time PCR was performed with an Agilent MX300 cycler and a SYBR green I Master kit (Syd Labs). For each primer set, the primer concentration was optimized, and dissociation curves were generated to verify the amplification of a single PCR product. The relative expression of the genes was calculated using the 2−ΔΔCT formulas and ACTB as a normalizer. Cells stimulated with PBS were used as a negative control. Values are reported as the mean of two replicates. The standard deviation (SD) of each mean is shown as error bars in each graph.
TABLE 1.
Primers used in real-time RT-PCR experiments
| Primer | Direction | Sequence |
|---|---|---|
| NOS2 | Forward | 5′-TCA CCT ACC ACA CCC GAG AT-3′ |
| Reverse | 5′-TTC AGG CTG TTG AGC CAT GT-3′ | |
| Arg-1 | Forward | 5′-GAG AGC TCA AGT GCA GCA AAG-3′ |
| Reverse | 5′-AAG CAG ACC AGC CTT TCT CAA-3′ | |
| CHIL31 | Forward | 5′-TCC CTC TCC TAT AAG CAA GAA-3′ |
| Reverse | 5′-CTG GAA TTC CCG GTC CTG AG-3′ | |
| IL12a | Forward | 5′-TCC AGA AGG CCA GAC AAA CTC-3′ |
| Reverse | 5′-AAT GGT AAA CAG GCC TCC ACT-3′ | |
| TNF-α | Forward | 5′-TGG GAT CAT TGC CCT GTT GAG-3′ |
| Reverse | 5′-TCT AAG CTT GGG TTC CGA CC-3′ | |
| IL-1βB | Forward | 5′-GCT CGC CAG TGA AAT GAT GG-3′ |
| Reverse | 5′-GTC CTG GAA GGA GCA CTT CAT-3′ | |
| IL-10 | Forward | 5′-AGG GCA CCC AGT CTG AGA AC-3′ |
| Reverse | 5′-CAC TCT GCT GAA GGC ATC TCG-3′ | |
| ACTB | Forward | 5′-ACA GAG CCT CGC CTT TGC CGA T-3′ |
| Reverse | 5′-TTG CAC ATG CCG GAG CCG TT-3′ |
Cell viability.
MDM were seeded into 96-well plates (2 × 105 cells/ml) and treated with LPS (15 μg/ml), Fh12 (5 μg/ml), or IL-4 (10 ng/ml) for 24 h or 48 h. Following incubation, cell viability was assessed by adding 50 μl XTT {sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sulfonic acid hydrate} labeling reagent (Roche Life Science, USA) to each well. Following an incubation of 6 h at 37°C, the absorbance of each well was read at 480 nm.
Statistical analysis.
All experiments were repeated three times and the results expressed as the mean value ± standard deviation. For comparisons of values for two groups, the unpaired Student t test was used, and when four groups were compared, the analysis of variance (ANOVA) test was used. Statistical analyses were done using GraphPad Prism software (Prism 6). For all tests, a P value of <0.05 was deemed significant.
RESULTS
Purification of native F. hepatica FABP.
A number of protocols have been designed to purify native FABPs from humans and parasites (28–30). In this study, Fh12 was purified from soluble extracts of adult flukes following a preoptimized protocol that combines separation by molecular sieving chromatography and two consecutive rounds of preparative isoelectric focusing (IEF) using ampholyte mixes with a wide range of pH values (23). After separating the F. hepatica extract using Sephadex G-50 chromatography, two major peaks were recovered. The first peak contained proteins with molecular masses of ≥30 kDa. The second peak contained proteins in the range of 2 × 104 to 1.2 × 103 kDa. Fractions from the second peak were pooled and fractionated by preparative IEF steps. To ensure the availability of sufficient protein for subsequent separation and analysis, a large volume of protein solution (peak 2, >800 mg) was processed. Initial experiments showed that using a large quantity of protein resulted in protein precipitation during preparative IEF. Therefore, three consecutive preparative IEF runs were performed, using 300 mg of protein per run. The 20 fractions collected per run were analyzed by SDS-PAGE, and the patterns of fractions from the three runs were found to be similar among all fractions. Identically numbered fractions from each of the three runs were pooled. Thus, 20 fractions were obtained (fractions 1 to 20). After analyzing by 12.5% SDS-PAGE, seven fractions with isoelectric points (pIs) of 4.61, 5.11, 5.24, 5.33, 5.44, 5.72, and 5.9 were selected because they contained the 12-kDa polypeptide, as reported previously (23). In the selected fractions a faint slightly smaller band was also observed. MALDI-MS/MS analysis of either the 12-kDa band or the smaller band excised directly from gel confirmed the presence of fatty acid binding protein, indicating that despite using a protease cocktail in the extract preparation, some minor protein degradation occurred during the purification process. Selected IEF fractions were pooled, exhaustively desalted against PBS, and concentrated by ultrafiltration using an YM-10 membrane. The resulting purified protein was termed Fh12 (Fig. 1). Purified Fh12 was highly reactive against an anti-Fh15, immune serum as shown by ELISA, as well as against human sera obtained from patients with chronic fascioliasis. Moreover, Fh12 was also highly reactive against anti-ESP and anti-FhTg antisera (Fig. 2A). Consistent with the ELISA results, the 12-kDa polypeptide band was also identified when Fh12 was analyzed by Western blotting against the anti-Fh15, anti-FhTg, and anti-ESP sera (Fig. 2B).
FIG 1.
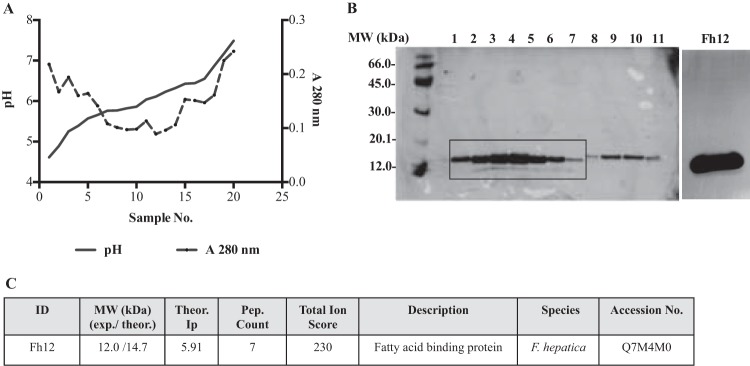
Purification of the native 12-kDa Fasciola hepatica fatty acid binding protein (Fh12). After molecular sieving chromatography using a Sephadex G-50 column, peak 2, which contains proteins of 12.0 to 20.0 kDa, was dialyzed against 1% glycine and subjected to successive fractionations by preparative isoelectric focusing (IEF) using ampholyte mixtures of pH 3 to 10 and pH 5 to 7 at a ratio of 1:4. Individual IEF fractions were harvested, and their pH and absorbance at 280 nm were measured. (A) Typical curve of pH versus A280 obtained in each IEF separation. (B) IEF fractions were analyzed by 12% SDS-PAGE, and the proteins were visualized by silver staining. Fractions 1 to 7, with a pH range of 4.2 to 5.9, were excised from the gel, analyzed by MALDI-MS/MS, pooled, and named Fh12. (C) The presence of fatty acid binding protein was identified by MALDI-MS/MS in the pooled fractions.
FIG 2.
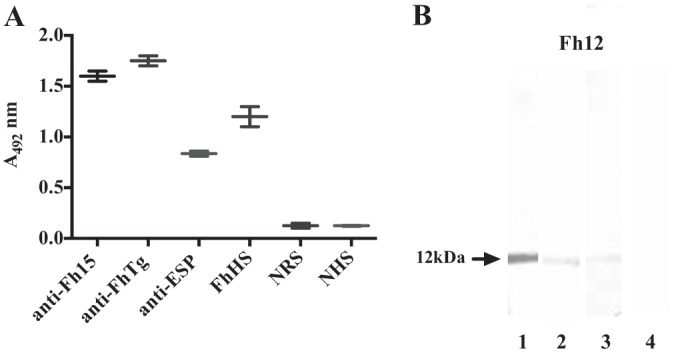
Immunoreactivity of native Fh12 as determined by ELISA and Western blotting. Purified F. hepatica fatty acid binding protein (Fh12) was assessed against different antisera raised in rabbits. (A) The ELISA plates were coated with 15 μg/ml Fh12. Sera were diluted 1:400 in PBS containing 0.05% Tween 20 (PBST), and the anti-rabbit IgG or anti-human IgG peroxidase conjugates were used diluted 1:5,000 in PBST. Fh12 was tested against an antiserum prepared against recombinant F. hepatica FABP (anti-Fh15), an antiserum prepared against F. hepatica tegumental antigen (anti-FhTg), an antiserum against F. hepatica excretory-secretory products (anti-ESP), 6 human sera obtained from patients with chronic fascioliasis (FhHS), 6 negative rabbit sera (NRS), and 6 sera from healthy subjects (NHS). The experiment was replicated three times and the results expressed as the mean absorbance at 492 nm ± standard deviation (SD). (B) Purified Fh12 was analyzed by 12.5% SDS-PAGE and electrotransfer to a nitrocellulose membrane, which was cut into strips that were incubated with anti-Fh15 (lane 1), anti-FhTg (lane 2), anti-ESP antiserum (lane 3), and negative rabbit serum (lane 4) diluted 1:100 in PBST. After successive washes to eliminate excess antibody and addition of the anti-rabbit IgG peroxidase conjugate (diluted 1:2,000 in PBST), immunoblots were revealed by incubation with diaminobenzidine as a chromogenic substrate. The arrow indicates the presence of the 12-kDa polypeptide detected in each immunoblot.
Alternative activation of macrophages by native 12-kDa F. hepatica FABP.
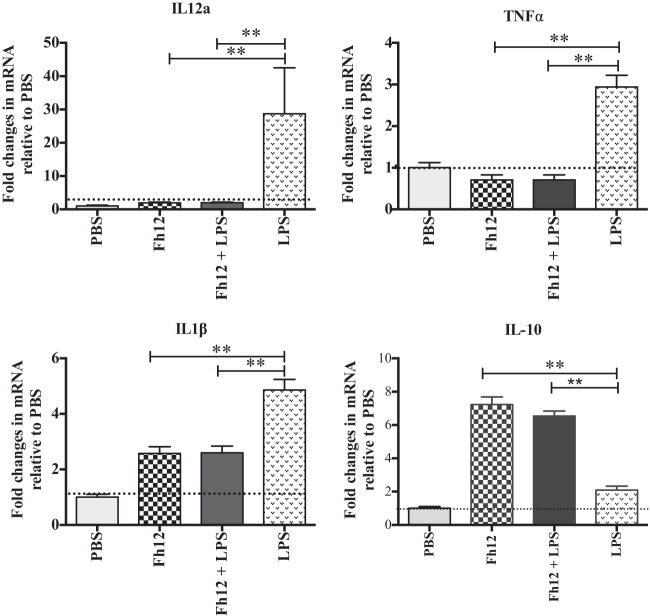
MDM from naive donors exposed to different concentrations of the native 12-kDa F. hepatica FABP (Fh12) expressed differential expression of Arg-1, which reached maximal values at an Fh12 concentration of 5 μg/ml (data not shown). Therefore, subsequent experiments were performed using 5 μg/ml as the optimal Fh12 concentration. Cells that were exposed to Fh12 alone or in combination with LPS expressed significantly high levels of Arg-1 and eosinophil chemotactic factor chitinase-3-like protein (CHI3L1) accompanied by downregulation of NOS2, which were not significantly different from those stimulated by IL-4, which is considered a potent activator of alternatively activated macrophages (AAMϕ) (27) (Fig. 3). The inverse relationship observed indicates a differential regulation of Arg-1 and NOS2 in human MDM and demonstrates the ability of Fh12 to stimulate the AAMϕ. Consistent with these observations cells stimulated with Fh12 also showed high levels of arginase activity that peaked (3U/106 cells) at 48 h after stimulation. NO was undetectable in Fh12-stimulated cells at time points from 24 to 72 h after stimulation. In contrast, the stimulation of cells with LPS resulted in significantly high levels of NO (35 μM) that peaked at 24 h after stimulation (P < 0.001), whereas arginase activity at any time point was undetectable. It was interesting to note that when cells were treated with Fh12 prior to LPS stimulation, the levels of NO dropped drastically and become undetectable at 24 h poststimulation (Fig. 4). It was also found that treatment of MDM with Fh12 resulted in the suppression of IL-12A, TNF-α, and IL-1β (P ≤ 0.01), which are cytokines highly associated with the inflammatory response induced by LPS, and an increase in the expression levels of IL-10, which is an anti-inflammatory cytokine (Fig. 5).
FIG 3.
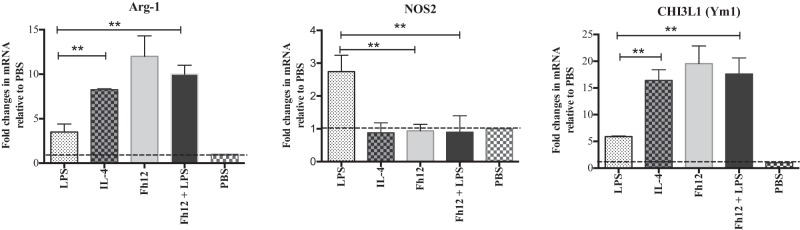
Fh12 induces alternative activation of human monocyte-derived macrophages. Monocyte-derived macrophages (MDM) from naive donors were isolated and cultured in triplicate in 96-well plates (106 cells/well) at 37°C with 5% CO2. Cells were stimulated with LPS (15 μg/ml), Fh12 (5 μg/ml), or a mixture of LPS and Fh12 for 48 h. Cells stimulated with IL-4 (10 ng/ml) were used as control of alternative activation. MDM were removed with a scraper, and RNA was extracted. Quantitative PCR was used to determine the expression levels of the nitric oxide synthase-2 (NOS2), Arg-1, and CHI3L1 genes in MDM treated with LPS, IL-4, Fh12, or Fh12 plus LPS. Results are expressed as fold changes relative to the PBS treatment and represent the means ± SD from a minimum of three experiments, each in triplicate. **, P < 0.005 compared with the PBS control group.
FIG 4.
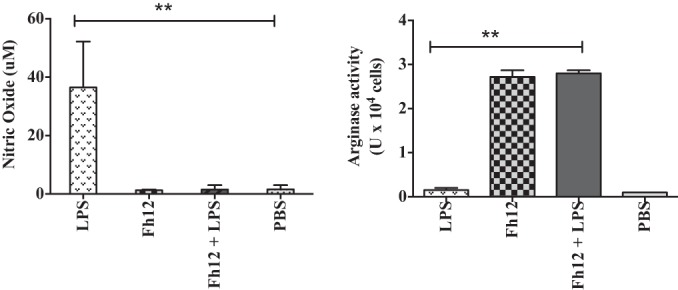
Fh12 reduces production of nitric oxide from macrophages. Monocyte-derived macrophages (MDM) from naive donors were isolated and cultured in triplicate in 96-well plates (106 cells/well) at 37°C with 5% CO2. Cells were stimulated with LPS (15 μg/ml) or Fh12 (5 μg/ml) prior to or after LPS stimulation for 48 h, after which the supernatants were removed and centrifuged at 1,000 × g. Levels of nitric oxide (NO) measured in the cell supernatant were found to be significantly diminished in cells treated with Fh12. Adherent cells (MDM) were removed with a scraper and analyzed for intracellular arginase activity by a colorimetric method. The arginase activity was found to be significantly increased in cells that were treated with Fh12 prior to or after LPS stimulation. Results represent the means ± SD from a minimum of three experiments, each in triplicate. **, P < 0.005 compared with the PBS control group.
FIG 5.
Fh12 downregulates the expression of proinflammatory markers typically induced by LPS. Monocyte-derived macrophages from human donors were treated with Fh12 (5 μg/ml) and further stimulated with LPS (15 μg/ml) for 24 h. Expression of IL-12a, TNF-α, IL-1β, and IL-10 was analyzed by quantitative PCR. IL-12a, TNF-α, and IL-1β were found to be downregulated in cells that were treated with Fh12, while IL-10 was found to be upregulated. Results are expressed as fold changes relative to PBS treatment and represent the means ± SD from a minimum of three experiments, each in triplicate. **, P < 0.001 compared with the PBS control group.
Given such a potent influence on the expression of inflammatory cytokines and other inflammatory markers from MDM, we measured the influence of Fh12 on cell viability using the XTT assay. The results demonstrated that treatment of MDM for 24 to 48 h with LPS, IL-4, or Fh12 did not inhibit cell growth or viability (Fig. 6). Treatment of MDM with medium or Fh12 plus LPS gave similar results in all experiments (data not shown). These results demonstrated that the differences in the cytokine pattern expression produced by Fh12 might not be attributable to cell death.
FIG 6.
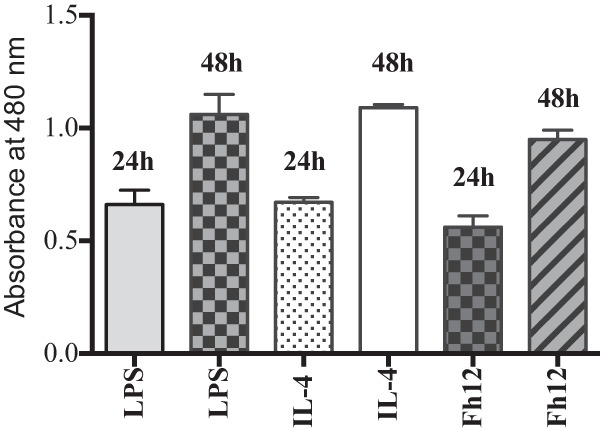
Treatment of human MDM with LPS or IL-12 for 48 h does not affect viability or growth of cells. Monocyte-derived macrophages from human donors were treated with Fh12 (5 μg/ml), LPS (15 μg/ml), or IL-4 (10 ng/ml) for 24 to 48 h. Cell viability in the experimental groups was assessed using an XTT assay.
DISCUSSION
Although FABPs are cytosolic proteins, several isoforms of these molecules have been found in the excretory-secretory products (ESPs) of F. hepatica (3, 4) and extracellular vesicles (31), which is believed to be one of the ways that many parasite proteins are excreted. Moreover, our research group recently demonstrated that FABP is one of the most prominent components of an F. hepatica tegumental coat antigen (FhTg) that was found to be highly reactive with sera from patients with chronic fascioliasis (24). Consistent with these results, the native F. hepatica FABP (Fh12) purified in this study was found to be highly reactive against human sera obtained from patients with chronic fascioliasis and against antisera raised against specific tegumental coat antigens and excretory-secretory products of F. hepatica. These results suggest that the protein purified in this study has antigenic properties similar to those that are naturally produced by the parasite and secreted through the tegument.
Since the fatty acid binding protein (Fh12) is a component of the external tegumental coat of the parasite and is secreted through the tegument, this protein could have a role in the interaction with the Toll-like receptors of antigen-presenting cells (APC). Macrophages are antigen-presenting cells that are essential for the interaction between the innate and acquired immune systems and are critical mediators of most chronic inflammatory diseases. The activity of macrophages is regulated by specific signals that induce their development into discrete phenotypes, which differ in terms of receptor expression, effector function, and cytokine secretion. The best-characterized phenotype is the classically activated macrophages (CAMϕ), which develop in response to proinflammatory cytokines (such as gamma interferon [IFN-γ]), microbial infection, or bacterial products such as lipopolysaccharide (LPS). In contrast, macrophages activated by the interleukin-4 (IL-4)-dependent signal pathway are classified as alternatively activated macrophages (AAMϕ) (14). The effects of F. hepatica tegumental antigen and excretory-secretory products on macrophages derived from mice, cattle, and ovines have been well documented (14, 16, 19, 20, 32, 33). Moreover, recombinant versions of the parasite peroxiredoxin have been shown to induce the alternative activation of macrophages, as indicated by an increase in arginase activity in murine (14, 19) and ruminant (16, 20) models. Recently, a homogenate of adult flukes has also been shown to induce arginase activity in bovine macrophages derived from nonvaccinated animals and cultured in vitro (34). AAMϕ have also been shown to occur in animal models of Schistosoma mansoni (35), Trichinella spiralis (36), and Taenia crassiseps (37) infection. The results of this study are the first demonstrating the response of human-derived macrophages to an F. hepatica molecule. Our results expand the available knowledge on macrophage activation by helminthes.
A key result from this study is that Fh12 leads to significant increases in arginase activity in human MDM, as observed by the upregulation of Arg-1, which is characteristic of AAMϕ. In support of these results, we also found that the chitinase-3-like protein (CHI3L1) gene was upregulated in cells stimulated with Fh12. It has been suggested that CHI3L1 expressed by macrophages plays a role in Th2 differentiation, and it is typically expressed in the setting of AAMϕ (38). Macrophages metabolize l-arginine by two mechanisms, using either inducible nitric oxide synthase (NOS2) or arginase (Arg-1). The differential regulation of these enzymes is known to correspond to either classical or alternative macrophage activation (39). NOS2 is used as marker of classical activation (CAMϕ), while Arg-1 and CHI3L1 are used to identify AAMϕ (34). CAMϕ are usually associated with the Th1 responses and therefore fight bacterial infections via free radical production. AAMϕ are linked to Th2 responses, which occur during asthma, fungal, and parasitic infections. The differential regulation of these two macrophage phenotypes has been shown to correlate with the Th1/Th2 cytokine balance and T cell subsets (40). Th2-dominated responses have been observed in F. hepatica infection in mice, humans, and ruminant hosts. Antibody production is dominated by the immunoglobulin G1 isotype (24, 41, 42). In experimentally infected cattle, IFN-γ production and cellular proliferation are detected immediately after infection with the F. hepatica antigen. However, in response to this stimulus, cellular proliferation and IFN-γ production decrease as infection progresses, and when animals enter the chronic stage of infection, these responses are no longer detectable (43).
Our results also suggest that AAMϕ generated by in vitro exposure to Fh12 display a phenotype that may either modulate or simply become nonresponsive to another activating molecule. Incubation of MDM with Fh12 prior stimulation with LPS, a potent macrophage activator that signals through TLR4 to produce NO (44), resulted in poor production of NO and in the upregulation of Arg-1. Consistent with these observations, TNF-α and IL-1β, which are important mediators of inflammation, were also found to be downregulated by normal MDMs treated with Fh12 in the presence of LPS, while IL-10 was found to be overexpressed; this clearly is evidence of the anti-inflammatory potential of Fh12. Moreover, the observation that the IL-12A gene was also downregulated is consistent with the downregulation of NOS2A/NOS2 induced by Fh12. It is well known that NOS2 is required for the signaling process of this cytokine in innate immunity. A decrease in inflammatory cytokines is in keeping with a helminth's need to suppress inflammatory processes (45), and since IL-12 is an important polarizing cytokine known to drive Th1 differentiation (46), this suppression may result in the Th1 suppression observed during F. hepatica coinfections. Studies have demonstrated that animals infected with F. hepatica exhibit alternatively activated macrophages (21), and this effect is mimicked by excretory-secretory products (ESPs) (14, 16, 19), which are composed of almost 80% cathepsin peptidases. Fatty acid binding proteins (FABPs) are in relatively less abundance in the ESPs (4). However, FABPs have been implicated in parasite protection from the host immune system and may play a part in the detoxification of reactive oxygen intermediates (47). Based on the results obtained in this study demonstrating that an acid FABP isoform (Fh12) is able to induce the alternative activation of macrophages and exert a strong anti-inflammatory effect, it is feasible to conclude that it also contributes to the immunomodulatory effect of ESPs.
Our results suggest that the mechanism by which Fh12 signals is capable of interfering with the LPS signaling pathway. This interference may occur by a number of different mechanisms; for instance, the alternative activation by Fh12 may alter the expression levels of TLR4. In a different mechanism, Fh12 may block the binding of LPS to the lipid binding protein (LBP) or may interact directly with TLR4, CD14, or MD2 coreceptors, leaving them unavailable for additional signaling. We have also observed that cells stimulated with LPS and treated shortly thereafter with Fh12 also exhibited high levels of Arg-1 and produced low levels of NO and inflammatory cytokines (data not shown). This suggests that human MDM may possess some degree of plasticity in their response to signals promoting an alternative activation, as has been reported by other investigators (48). However, the conditions under which this may occur have yet to be elucidated. The exact mechanism by which Fh12 interacts with the TLR4 is under investigation. However, since many other parasite molecules also signal through TLR4 to produce similar effects, it is possible that Fh12 could have a role similar to those of other excretory-secretory products or tegumental antigens. Moreover, since fatty acid binding proteins are proteins shared with other helminthes, it is likely that this protein also participates in the macrophage activation in other helminthic infections. This observation will be relevant in the efforts to elucidate the Fh12 mechanism of activation and in deciphering its role in Th2 induction and immune responses.
In conclusion, we have presented evidence that F. hepatica fatty acid binding protein is capable of modulating human MDM and of generating macrophages activated by an alternative phenotype. Fh12 inhibits the LPS-mediated induction of the inflammatory cytokines TNF-α and IL-1β; this observation could have pharmacological implications. This study represents an analysis at one time point. Further studies will be necessary to explore the interaction of Fh12 with human macrophages at different time points, before and after stimulation with LPS and other macrophage activators, in in vivo experiments. Our finding that Fh12 is another molecule involved in directing macrophage activation represents an important step in elucidating a mechanism of activation and in deciphering the role of Fh12 in Th2 induction in F. hepatica infections.
ACKNOWLEDGMENTS
This work was supported by a SCORE grant (1SC1AI096108-01A2) and an MBRS-RISE grant (R25GM061838-13) from the National Institutes of Health, USA.
The content of this paper is solely the responsibility of the authors and does not represent the official views of the NIH.
We thank Lizzie Santiago-Santiago for editing the manuscript.
Footnotes
Published ahead of print 15 September 2014
REFERENCES
- 1.McManus DP, Dalton JP. 2006. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica and Fasciola gigantica. Parasitology 133(Suppl):S43–S61. 10.1017/S0031182006001806. [DOI] [PubMed] [Google Scholar]
- 2.Mas-Coma S, Esteban JG, Bargues MD. 1999. The Northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Trop. Med. Int. Health 4:454–467. 10.1046/j.1365-3156.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 3.Hacariz O, Sayers G, Baykal AT. 2012. A proteomic approach to investigate the distribution and abundance of surface and internal Fasciola hepatica proteins during the chronic stage of natural liver fluke infection in cattle. J. Proteome Res. 11:3592–3604. 10.1021/pr300015p. [DOI] [PubMed] [Google Scholar]
- 4.Jefferies JR, Campbell AM, van Rossum AJ, Barrett J, Brophy PM. 2001. Proteomic analysis of Fasciola hepatica excretory-secretory products. Proteomics 1:1128–1132. [DOI] [PubMed] [Google Scholar]
- 5.Buffoni L, Zafra R, Perez-Ecija A, Martinez-Moreno FJ, Martinez-Galisteo E, Moreno T, Perez J, Martinez-Moreno A. 2010. Immune response of goats immunised with glutathione S-transferase and experimentally challenged with Fasciola hepatica. Parasitol. Int. 59:147–153. 10.1016/j.parint.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Casanueva R, Hillyer GV, Ramajo V, Oleaga A, Espinoza EY, Muro A. 2001. Immunoprophylaxis against Fasciola hepatica in rabbits using a recombinant Fh15 fatty acid-binding protein. J. Parasitol. 87:697–700. 10.1645/0022-3395(2001)087[0697:IAFHIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.De Bont J, Claerebout E, Riveau G, Schacht AM, Smets K, Conder G, Brake DA, Capron A, Vercruysse J. 2003. Failure of a recombinant Schistosoma bovis-derived glutathione S-transferase to protect cattle against experimental Fasciola hepatica infection. Vet. Parasitol. 113:135–144. 10.1016/S0304-4017(02)00450-8. [DOI] [PubMed] [Google Scholar]
- 8.Jayaraj R, Piedrafita D, Dynon K, Grams R, Spithill TW, Smooker PM. 2009. Vaccination against fasciolosis by a multivalent vaccine of stage-specific antigens. Vet. Parasitol. 160:230–236. 10.1016/j.vetpar.2008.10.099. [DOI] [PubMed] [Google Scholar]
- 9.Law RH, Smooker PM, Irving JA, Piedrafita D, Ponting R, Kennedy NJ, Whisstock JC, Pike RN, Spthill TW. 2003. Cloning and expression of the major secreted cathepsin B-like protein from juvenile Fasciola hepatica and analysis of immunogenicity following liver fluke infection. Infect. Immun. 71:6921–6932. 10.1128/IAI.71.12.6921-6932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Fernandez AR, Nogal-Ruiz JJ, Lopez-Aban J, Ramajo V, Oleaga A, Manga-Gonzalez Y, Hillyer GV, Muro A. 2004. Vaccination of mice and sheep with Fh12 FABP from Fasciola hepatica using the new adjuvant/immunomodulator system ADAD. Vet. Parasitol. 126:287–298. 10.1016/j.vetpar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Mendes RE, Perez-Ecija RA, Zafra R, Martinez-Moreno A, Dalton JP, Mulcahy G, Perez J. 2010. Evaluation of local immune response to Fasciola hepatica experimental infection in the liver and hepatic lymph nodes of goats immunized with Sm14 vaccine antigen. Mem. Inst. Oswaldo Cruz 105:698–705. 10.1590/S0074-02762010000500017. [DOI] [PubMed] [Google Scholar]
- 12.Muro A, Ramajo V, Lopez J, Simon F, Hillyer GV. 1997. Fasciola hepatica: vaccination of rabbits with native and recombinant antigens related to fatty acid binding proteins. Vet. Parasitol. 69:219–229. 10.1016/S0304-4017(96)01131-4. [DOI] [PubMed] [Google Scholar]
- 13.Preyavichyapugdee N, Sahaphong S, Riengrojpitak S, Grams R, Viyanant V, Sobhon P. 2008. Fasciola gigantica and Schistosoma mansoni: vaccine potential of recombinant glutathione S-transferase (rFgGST26) against infections in mice. Exp. Parasitol. 119:229–237. 10.1016/j.exppara.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly S, O'Neill SM, Sekiya M, Mulcahy G, Dalton JP. 2005. Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect. Immun. 73:166–173. 10.1128/IAI.73.1.166-173.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowling DJ, Hamilton CM, Donnelly S, La Course J, Brophy PM, Dalton J, O'Neill SM. 2010. Major secretory antigens of the helminth Fasciola hepatica activate a suppressive dendritic cell phenotype that attenuates Th17 cells but fails to activate Th2 immune responses. Infect. Immun. 78:793–801. 10.1128/IAI.00573-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn RJ, Irwin JA, Olivier M, Sekiya M, Dalton JP, Mulcahy G. 2007. Alternative activation of ruminant macrophages by Fasciola hepatica. Vet. Immunol. Immunopathol. 120:31–40. 10.1016/j.vetimm.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Robinson MW, Donnelly S, Hutchinson AT, To J, Taylor NL, Norton RS, Perugini MA, Dalton JP. 2011. A family of helminth molecules that modulate innate cell responses via molecular mimicry of host antimicrobial peptides. PLoS Pathog. 7:e1002042. 10.1371/journal.ppat.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thivierge K, Cotton S, Schaefer DA, Riggs MW, To J, Lund ME, Robinson MW, Dalton JP, Donnelly SM. 2013. Cathelicidin-like helminth defence molecules (HDMs): absence of cytotoxic, anti-microbial and anti-protozoan activities imply a specific adaptation to immune modulation. PLoS Negl. Trop. Dis. 7:e2307. 10.1371/journal.pntd.0002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnelly S, Stack CM, O'Neill SM, Sayed AA, Williams DL, Dalton JP. 2008. Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB J. 22:4022–4032. 10.1096/fj.08-106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn RJ, Mulcahy G. 2008. Possible role for Toll-like receptors in interaction of Fasciola hepatica excretory/secretory products with bovine macrophages. Infect. Immun. 76:678–684. 10.1128/IAI.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. 2007. Alternatively activated macrophages in helminth infections. Curr. Opin. Immunol. 19:448–453. 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulcahy G, O' Connor F, Clery D, Hogan SF, Dowd AJ, Andrews SJ, Dalton JP. 1999. Immune responses of cattle to experimental anti-Fasciola hepatica vaccines. Res. Vet. Sci. 67:27–33. 10.1053/rvsc.1998.0270. [DOI] [PubMed] [Google Scholar]
- 23.Espino AM, Rodriguez Medina JR, Hillyer GV. 2001. Isolation and immunological characterization of fatty acid binding protein isoforms from Fasciola hepatica. J. Parasitol. 87:1028–1033. 10.1645/0022-3395(2001)087[1028:IAICOF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Morales A, Espino AM. 2012. Evaluation and characterization of Fasciola hepatica tegument protein extract for serodiagnosis of human fascioliasis. Clin. Vaccine Immunol. 19:1870–1878. 10.1128/CVI.00487-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espino AM, Dumenigo BE, Fernandez R, Finlay CM. 1987. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory-secretory products. Am. J. Trop. Med. Hyg. 37:605–608. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Perez J, Rodriguez-Medina JR, Garcia-Blanco MA, Hillyer GV. 1992. Fasciola hepatica: molecular cloning, nucleotide sequence, and expression of a gene encoding a polypeptide homologous to a Schistosoma mansoni fatty acid-binding protein. Exp. Parasitol. 74:400–407. 10.1016/0014-4894(92)90202-L. [DOI] [PubMed] [Google Scholar]
- 27.Vannier E, Miller LC, Dinarello CA. 1992. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc. Nat. Acad. Sci. U. S. A. 89:4076–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SH, Bae YA, Yang HJ, Shin JH, Diaz-Camacho SP, Nawa Y, Kang I, Kng Y. 2012. Structural and binding properties of two paralogous fatty acid binding proteins of Taenia solium metacestode. PLoS Negl. Trop. Dis. 6:e1868. 10.1371/journal.pntd.0001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen SU, Spener F. 1993. Fatty acid-binding protein from rat heart is phosphorylated on Tyr19 in response to insulin stimulation. J. Lipid Res. 34:1355–1366. [PubMed] [Google Scholar]
- 30.Xu LZ, Sanchez R, Sali A, Heintz N. 1996. Ligand specificity of brain lipid-binding protein. J. Biol. Chem. 271:24711–24719. 10.1074/jbc.271.40.24711. [DOI] [PubMed] [Google Scholar]
- 31.Marcilla A, Trelis M, Cortes A, Sotillo J, Cantalapiedra F, Minguez MT, Valero ML, Sanchez del Pino MM, Munoz-Antoli C, Toledo R, Bernal D. 2012. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One 7:e45974. 10.1371/journal.pone.0045974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn RJ, Mannion C, Golden O, Hacariz O, Mulcahy G. 2007. Experimental Fasciola hepatica infection alters responses to tests used for diagnosis of bovine tuberculosis. Infect. Immu. 75:1373–1381. 10.1128/IAI.01445-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hacariz O, Sayers G, Mulcahy GA. 2011. Preliminary study to understand the effect of Fasciola hepatica tegument on naive macrophages and humoral responses in an ovine model. Vet. Immunol. Immunopathol. 139:245–249. 10.1016/j.vetimm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Golden O, Flynn RJ, Read C, Sekiya M, Donnelly SM, Stack C, Dalton JP, Mulcahy G. 2010. Protection of cattle against a natural infection of Fasciola hepatica by vaccination with recombinant cathepsin L1 (rFhCL1). Vaccine 28:5551–5557. 10.1016/j.vaccine.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegman A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunology 20:623–635. 10.1016/S1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 36.Dzik JM, Golos B, Jagielska E, Zielinski Z, Walajtys-Rode E. 2004. A non-classical type of alveolar macrophage response to Trichinella spiralis infection. Parasite Immunol. 26:197–205. 10.1111/j.0141-9838.2004.00700.x. [DOI] [PubMed] [Google Scholar]
- 37.Terrazas LI, Montero D, Terrazas CA, Reyes JL, Rodriguez-Sosa M. 2005. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int. J. Parasitol. 35:1349–1358. 10.1016/j.ijpara.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Arora M, Chen L, Paglia M, Gallagher I, Allen JE, Vyas YM, Ray A, Ray P. 2006. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc. Nat. Acad. Sci. U. S. A. 103:7777–7782. 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35. 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 40.Hesse M, Modollel M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. 2001. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J. Immunol. 167:6533–6544. 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 41.Clery D, Torgerson P, Mulcahy G. 1996. Immune responses of chronically infected adult cattle to Fasciola hepatica. Vet. Parasitol. 62:71–82. 10.1016/0304-4017(95)00858-6. [DOI] [PubMed] [Google Scholar]
- 42.O'Neill SM, Brady MT, Callanan JJ, Mulcahy G, Joyce P, Mills KH, Dalton JP. 2000. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 22:147–155. 10.1046/j.1365-3024.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- 43.Clery DG, Mulcahy G. 1998. Lymphocyte and cytokine responses of young cattle during primary infection with Fasciola hepatica. Res. Vet. Sci. 65:169–171. 10.1016/S0034-5288(98)90171-0. [DOI] [PubMed] [Google Scholar]
- 44.Dobrovolskaia MA, Vogel SN. 2002. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 4:903–914. 10.1016/S1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 45.Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, Liew FY. 2005. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted products ES-62. J. Immunol. 174:284–293. 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 46.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146. 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 47.Robinson MW, Menon R, Donnelly SM, Dalton JP, Ranganathan S. 2009. An integrated transcriptomics and proteomics analysis of the secretome of the helminth pathogen Fasciola hepatica: proteins associated with invasion and infection of the mammalian host. Mol. Cell Proteomics 8:1891–1907. 10.1074/mcp.M900045-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. 2005. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 175:342–349. 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]