Abstract
Streptococcus pneumoniae adherence to human epithelial cells (HECs) is the first step in pathogenesis leading to infections. We sought to determine the role of human antibodies against S. pneumoniae protein vaccine candidates PhtD, PcpA, and Ply in preventing adherence to lung HECs in vitro and mouse nasopharyngeal (NP) colonization in vivo. Human anti-PhtD, -PcpA, and -Ply antibodies were purified and Fab fragments generated. Fabs were used to test inhibition of adherence of TIGR4 and nonencapsulated strain RX1 to A549 lung HECs. The roles of individual proteins in adherence were tested using isogenic mutants of strain TIGR4. Anti-PhtD, -PcpA, and -Ply human antibodies were assessed for their ability to inhibit NP colonization in vivo by passive transfer of human antibody in a murine model. Human antibodies generated against PhtD and PcpA caused a decrease in adherence to A549 cells (P < 0.05). Anti-PhtD but not anti-PcpA antibodies showed a protective role against mouse NP colonization. To our surprise, anti-Ply antibodies also caused a significant (P < 0.05) reduction in S. pneumoniae colonization. Our results support the potential of PhtD, PcpA, and Ply protein vaccine candidates as alternatives to conjugate vaccines to prevent non-serotype-specific S. pneumoniae colonization and invasive infection.
INTRODUCTION
Streptococcus pneumoniae is an important human pathogen that colonizes the nasopharynx (NP) and causes local (acute otitis media [AOM] and sinusitis) and severe (pneumonia, sepsis, and meningitis) invasive infections (1–3). Adherence of S. pneumoniae to NP epithelial cells is a first step in its pathogenesis, and its attachment to lung epithelial cells is required to establish pneumonia (2, 4). Several pneumococcal cell surface proteins contribute to adherence of S. pneumoniae to epithelial cells, including choline-binding protein A (CbpA), histidine triad protein (PhtD), and pneumococcal adhesion and virulence protein (PavA) (4, 5). Antibodies directed against pneumococcal adhesin proteins might protect at the human mucosal surface by preventing S. pneumoniae attachment and subsequent NP colonization. Current licensed pneumococcal capsular polysaccharide vaccines are effective at reducing carriage and preventing invasive disease caused by the pneumococcal vaccine serotypes included in the vaccines. Unfortunately, these polysaccharide vaccines are ineffective against all circulating serotypes, and consequently an increase in NP carriage by strains of S. pneumoniae expressing other serotypes has been observed (6). Therefore, efforts are being made to investigate S. pneumoniae protein-based candidate vaccines that are common to all serotypes. A number of proteins, including a nontoxic, genetically modified cholesterol-binding cytotoxin known as pneumolysin (PlyD1), choline-binding proteins (PspA, CbpA, and PcpA), and histidine triad proteins (PhtD), are in various stages of development and clinical trials as alternate vaccine candidates against S. pneumoniae (7, 8). Our group is working with three of these vaccine candidate proteins, PhtD, PcpA, and PlyD1. We know that children, who are naturally exposed to S. pneumoniae during NP colonization and during AOM, generate antibody responses against PhtD, PcpA, and PlyD1 (9, 10). In a recent study, we demonstrated that both infant and adult mice vaccinated with a trivalent formulation of PhtD, PcpA, and PlyD1 are protected against lethal pneumonia infection (11).
How antibodies directed to PhtD, PcpA, and Ply would play a role in the S. pneumoniae pathogenesis process is still unclear. Pht proteins (PhtA, PhtB, PhtD, and PhtE) belong to a well-conserved surface exposed protein family characterized mainly by a histidine triad motif (5). Several functions have been proposed for these proteins, including promoting adherence to host cell surfaces (9), protection against complement deposition (12), and scavenging of zinc ions (13). PhtD protein has been shown to elicit protection in a mouse model against S. pneumoniae systemic infection caused by different serotypes (11, 14). Human anti-PhtD antibodies have been detected in children during NP colonization and invasive diseases caused by S. pneumoniae, indicating that PhtD is expressed and recognized by the host immune system during infection (10, 15, 16).
Pneumococcal choline-binding protein A (PcpA) is distinct from other S. pneumoniae choline-binding proteins (CbpA and PspC) (17). The pcpA gene has been shown to be conserved among 25 different strains examined (18), and PcpA protein is surface exposed (18). PcpA is not required for NP colonization of the mouse (18, 19) but is indispensable for murine lung infection (20). PcpA is under the control of a manganese (Mn)-dependent regulator, PsaR, and the Mn concentration in the NP of mice has been shown to result in downregulation of pcpA expression (19). The antibody response elicited by recombinant PcpA vaccination has been shown to provide protection against systemic infection (18) but not against colonization (21) in mice. However, we have shown that commensal NP colonization of children results in production of mucosal and serum antibodies (16, 22, 23), strongly suggesting that expression of PcpA occurs in the NP of children, unlike mice.
Pneumolysin (Ply) is a cholesterol-dependent cytolysin virulence factor localized to the cell wall of S. pneumoniae (24) and is surface accessible based on cellular hemolytic activity and proteinase K treatment of intact cells (25). Ply is released from the cell in a LytA-independent manner, and ply-deficient mutants demonstrate attenuated infection and NP colonization in mice (26–28). Ply is cytotoxic to ciliated bronchial epithelial cells and causes alveolar edema and hemorrhage during pneumococcal pneumonia (29, 30). The cytotoxic effect of Ply can directly inhibit phagocyte and immune cell function (31). Ply has been suggested to play a role separate from its hemolytic activity in the early development of pneumococcal biofilms (32).
Our previous work demonstrated the role of PhtD, PhtE, and PcpA in adherence of S. pneumoniae to mucosal epithelium in vitro (9, 33). In this report, we show a direct role of human antibodies directed against PhtD and PcpA in blocking S. pneumoniae adherence to human lung epithelial cells, and we demonstrate a significant reduction in NP colonization of mice after passive transfer of natural human anti-PhtD and anti-Ply specific antibodies.
MATERIALS AND METHODS
Antigens and A549 cells.
Recombinant pneumococcal histidine triad protein (PhtD), choline-binding protein (PcpA), and a genetic derivative of pneumolysin (PlyD1) were provided by Sanofi Pasteur. The mutations in PlyD1 are T65C, G293C, and C428A (34). PlyD1 lacks hemolytic activity and induces neutralizing antibodies against the wild-type toxin (35).
Recombinant P6 protein of nontypeable Haemophilus influenzae (NTHI), used as a control antigen in some experiments, was a gift from Lea Vacca Michel (Rochester Institute of Technology, Rochester, NY). Human lung epithelial cells (A549) were purchased from American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco's minimal essential medium (DMEM; Life Technologies, Grand Island, NY) with 10% fetal bovine serum (FBS; Atlanta Biologicals, GA) and 1% penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO).
Bacterial strains, labeling, and adherence assays with mutants.
Streptococcus pneumoniae wild-type strain TIGR4 (serotype 4), used in this study, and its isogenic PhtD, PcpA, and PhtABD mutants were received from Sanofi Pasteur. Methods for generating these mutants were described previously (9, 33). A noncapsular strain (RX1) was also used in the study. Wild-type TIGR4 and its isogenic mutants were grown in manganese-depleted Todd-Hewitt yeast (THY) broth (9) to an optical density at 600 nm (OD600) of 0.6 (5 × 108 bacteria/ml). Bacteria were pelleted by centrifugation and washed twice with phosphate-buffered saline (PBS). Bacteria were fluorescently labeled with PKH2 green dye (Sigma-Aldrich) according to the manufacturer's instructions. Briefly, 1 μl of 4 × 10−6 M PKH2 dye was mixed in 4 ml of diluent, and 500 μl of that mix was added to 1 × 109 bacteria, mixed by pipetting, and incubated at 25°C for 5 min. The labeling reaction was stopped with 1% bovine serum albumin (BSA) (Sigma-Aldrich), and bacteria were washed 3 times with PBS by centrifugation at 6,000 × g for 1 min. After the final wash, labeled bacteria were resuspended in PBS and used for the assay. Viability of S. pneumoniae was confirmed by growth on blood agar plates. A flow cytometry-based assay was used to detect S. pneumoniae adherence to A549 cells by the method described by Khan et al. (33). We used single fluorescence to label the bacteria, leaving the epithelial cells unlabeled.
Purification of human anti-PhtD, -PcpA, and -Ply antibodies.
Anti-pneumococcal protein antibodies were purified from human sera following natural exposure to S. pneumoniae by means of an affinity column. The beads were prepared by coupling recombinant PhtD, PcpA, or PlyD1 to CN-Br-activated Sepharose 4B (GE Health Care, Uppsala, Sweden) according to the manufacturer's instructions. Immunoglobulins (IgGs) were purified from human sera using a Melon Gel IgG spin purification kit (Thermo Scientific, Waltham, MA) according to the manufacturer's instructions. To purify human anti-PhtD, -PcpA, and -Ply specific antibodies, purified human IgGs were pooled and loaded onto the respective pneumococcal protein-coupled beads, the column was washed with binding buffer (100 mM sodium phosphate and 150 mM NaCl), bound antibodies were eluted with 0.1 M glycine (pH 2.5), and the eluent was immediately exchanged against PBS. For control studies, an irrelevant antibody (anti-P6, directed to Haemophilus influenzae outer membrane protein P6) was also purified from human sera. Purity of the anti-PhtD preparation was checked using enzyme-linked immunosorbent assay (ELISA) to screen different pneumococcal antigens (PhtE, PcpA, Ply, LytB, and PspA). There was no significant cross-reactivity detected among those antibodies except for low-level reactivity against PhtE antigen. Mitchell et al. (36) have shown that Ply is capable of nonspecifically binding the Fc region of IgG. We tested our isolated human antibodies to Ply against whole-cell lysates of TIGR4 and NW550V3, a clinical isolate, in Western blot analysis. We did not observe binding to other nonpneumolysin proteins (data not shown) supporting that any effect of human anti-PlyD1 antibodies against S. pneumoniae is directed against Ply. Similarly, Virolainen et al. (37) did not detect nonspecific antibodies to Ply in their study.
Generation of Fab fragments from anti-PhtD, -PcpA, and -Ply antibodies.
Purified anti-PhtD, -PcpA, and -Ply specific antibodies were concentrated using a Vivaspin 20 Ultra centrifugal filter (molecular weight cutoff [MWCO] of 10; GE Health Care, United Kingdom). The purity of IgG was confirmed on a 4-to-15% gradient SDS-PAGE gel (Bio-Rad, Hercules, CA) and quantitated by NanoDrop (Thermo Scientific). IgG Fab fragments were prepared as described earlier from our laboratory (33). Briefly, 2 mg/ml papain (Sigma) was preactivated in Fab digestion buffer (Pierce Fab Micro preparation kit; Thermo Scientific) plus 20 mM l-cysteine for 10 min at 37°C. Antibodies were added to the papain mixture and incubated for 6 h at 37°C. The mixture was passed over a protein A column (Pierce) to remove undigested IgG and Fc fragments with elution buffer. Fab fractions were concentrated using Vivaspin 20, and purity was confirmed on an SDS-PAGE gel (see Fig. S1 in the supplemental material).
Adherence assay with antigen-specific Fab fragments.
A549 cells were grown in a humidified incubator at 37°C with 5% CO2 and harvested using 0.25% trypsin-EDTA. After harvesting, A549 cells were centrifuged at 500 × g for 7 min at room temperature, washed three times with fresh medium, and adjusted to 1 × 106 cells/ml. In 96-well plates, A549 cells were added at 1 × 105/well and infected with PKH2-stained TIGR4 (multiplicity of infection [MOI] = 200). To study the effect of antigen-specific Fab antibodies in blocking adherence of TIGR4 to A549 cells, 1 μg of antibody was added to each well. The plates were incubated statically at 37°C for 1 h and then pelleted at 500 × g for 7 min. The cell pellets were washed with PBS three times, and after a final wash, each sample was resuspended in 200 μl of PBS without Ca2+ or Mg2+. Experiments were performed in triplicate and repeated at 2 different times. Different concentrations of Fab antibodies (0.1 to 2 μg) were also used in order to determine the optimal concentration for preventing bacterial adherence. Adherence assay with antigen-specific Fab fragments was also repeated using unlabeled TIGR4 strain by the CFU counting (CFU/ml) method. Briefly, 1 μg antigen-specific Fab antibodies were pretreated with TIGR4 (MOI = 200) before being added to 1 × 105 A549 cells and tested under the experimental conditions described above. TIGR4 cells were plated onto blood agar plates at different dilutions, and CFU were determined. As a control, 1 μg of noncorrelated Fab antibodies against P6 of NTHI was also included in the CFU assay.
Animals and passive transfer of human anti-pneumococcal protein antibodies.
C57BL/6 mice (5 weeks old, female) were purchased from Jackson Laboratory (Bar Harbor, ME). Mouse handling and experiment procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Rochester General Hospital (RGH). Preliminary experiments were done to establish the NP colonization dose of wild-type TIGR4, and 5 × 106 CFU successfully established NP colonization that persisted for up to 2 weeks after intranasal inoculation. On day 0, mice (n = 5/group) were anesthetized using Isoflurane (1%), and 50 μl of anti-PhtD, -PcpA, and -Ply antibodies (10 μg or 25 μg in PBS) were administered intravenously (lateral tail veins). Anti-P6 antibodies were administered to mice as control. Mice were subsequently given 5 × 106 CFU of TIGR4 intranasally in 10 μl PBS (5 μl/naris) for colonization. On day 3, all different treatment groups of mice were euthanized by CO2 inhalation, and 500 μl of PBS was instilled retrogradely through the transected trachea for collecting the nasal wash. The first 7 drops of the nasal wash (∼0.1 ml) were collected onto a sterile petri dish, and serial 10-fold dilutions were subsequently plated on blood agar plates with gentamicin and incubated overnight at 37°C and 5% CO2. The colonies were enumerated after incubation to estimate the level of colonization in CFU/mouse. This procedure was sufficient to detect the majority of S. pneumoniae associated with NP colonization; further homogenates of dissected ventral maxillary turbinates in sterile PBS yielded fewer CFU when cultured.
Statistical analysis.
All statistical analysis was performed using GraphPad Prism 6. An unpaired Student's t test was used to compare groups, and a P value of <0.05 was considered significant. Analysis of variance (ANOVA) with Dunnett's correction for multiple comparisons was also performed to compare the effects of anti-Fab on adherence of TIGR4 and A549 cells.
RESULTS
TIGR4 PcpA and Pht mutants have reduced adherence to human lung epithelial cells.
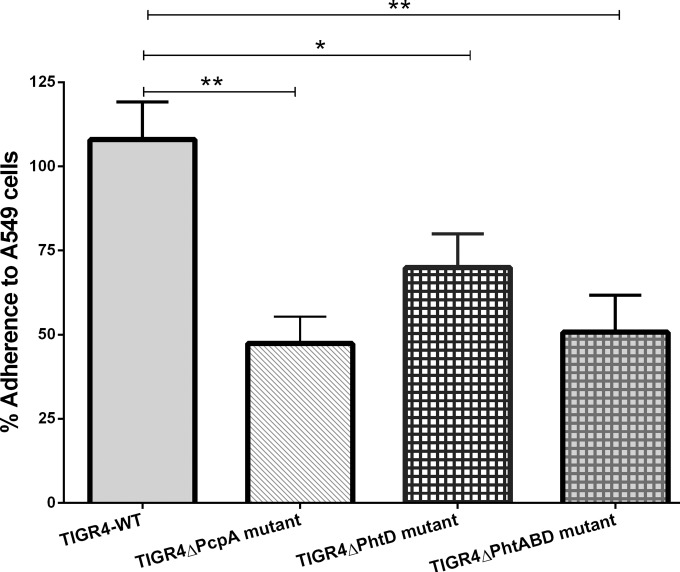
To study the contribution of PcpA, PhtD, and PhtABD proteins to adherence to human lung epithelial cells, TIGR4 and its isogenic mutants, the TIGR4ΔPcpA insertion-duplication mutant (19), TIGR4ΔPhtD, and a TIGR4ΔPhtABD triple mutant (9), were incubated with A549 cells for 1 h. The adherence of wild-type TIGR4 to A549 cells was set as maximal adherence (100%), and the reduction in adherence of the mutants compared to that of TIGR4 is shown as percentage adherence compared to that of the wild type (Fig. 1). All the mutants showed a significant reduction in adherence to A549 cells compared to wild-type TIGR4. Although PcpA and PhtABD mutants showed the greatest reduction in adherence (53% and 50%, respectively), there was no significant difference between these mutants and the PhtD mutant, which showed a 30% reduction. These data suggest that PcpA and PhtD play a role in adherence to human lung epithelia.
FIG 1.
Adherence of TIGR4 wild type (TIGR4-WT) and its isogenic PcpA, PhtD, and PhtA, -B, and -D mutants on A549 cell line (lung epithelial cells). The mean adherence of TIGR4-WT on A549 cells was normalized, and the percent adherence of TIGR4 and its mutants was calculated compared to mean adherence of TIGR4-WT. Data are means with standard errors from two experiments done in triplicate. **, mean P value < 0.01; *, mean P value < 0.05 (adherence of TIGR4-WT versus that of its isogenic mutants).
Human anti-PhtD and -PcpA specific IgGs block pneumococcal adherence to A549 cells.
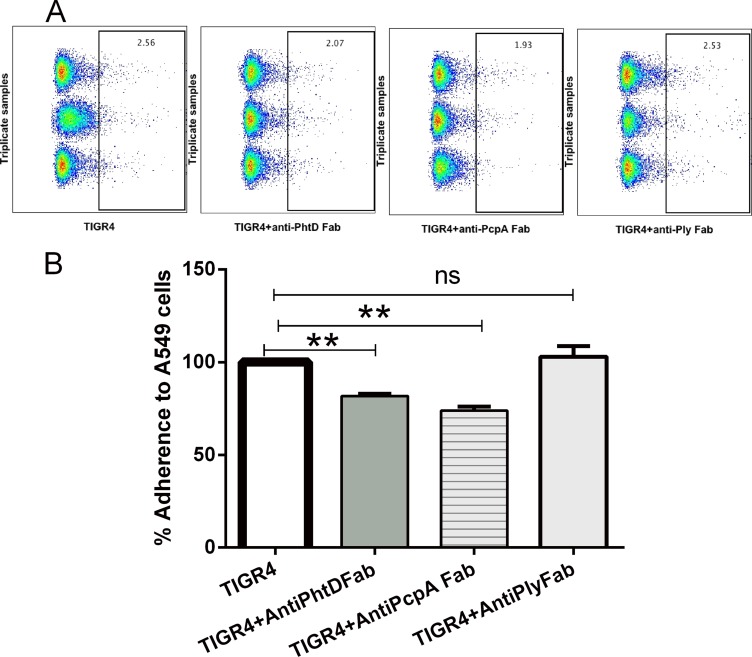
Previous experiments in our laboratory have demonstrated that human IgG antibodies cause pneumococcal aggregation, as detected by flow cytometry and confocal microscopy (33), which may be clinically relevant but confounds measurements of the impact of antibody on specific adherence. Therefore, we purified antigen-specific Fabs to investigate the specific role of antibodies generated against PhtD, PcpA, and Ply in blocking the adherence of S. pneumoniae to A549 cells. Various amounts (0.1, 0.25, 0.5, 1, and 2 μg) of anti-PhtD, -PcpA and -Ply specific Fab fragments were tested. The optimum inhibition of TIGR4 binding to A549 cells was obtained with 0.5 μg or 1 μg of Fab. Fab at 2 μg gave results similar to those obtained with 1 μg (data not shown). Flow cytometry data of TIGR4 binding to A549 along with data for TIGR4 treated with anti-PhtD, anti-PcpA, or anti-Ply specific Fab are shown in Fig. 2A. Bar graphs of the data (Fig. 2B) show a 20 to 30% reduction of S. pneumoniae binding to A549 cells with anti-PhtD and -PcpA Fabs. Anti-Ply specific Fab did not affect adherence of TIGR4 to A549 cells, indicating that human anti-PhtD and anti-PcpA play a functional role in blocking lung epithelial cell adherence.
FIG 2.
Effect of Anti-PhtD, -PcpA, and -Ply specific Fabs on pneumococcal adherence to A549. (A) Flow cytometry data showing the actual percent adherence of labeled bacteria to A549 cells. Gated boxes represent concatenated data of triplicates with labeled TIGR4 as the control and pretreated TIGR4 with anti-PhtD, -PcpA, and -Ply Fabs. (B) Decrease in adherence of TIGR4 to A549 cells with treatment of 1 μg of anti-PhtD, -PcpA, and -Ply Fabs. The mean adherence of TIGR4 on A549 cells was normalized, and the percent adherence of TIGR4 with the Fabs was compared to that with TIGR4 alone. Data are means with standard deviations from two experiments done in triplicate. **, P < 0.01; ns, not significant.
Since polysaccharide capsules have been shown to interfere with adherence (38), a noncapsulated strain (RX1) was tested with anti-PhtD, -PcpA, and -Ply specific Fabs. Enhanced reduction in adherence was observed using RX1 (P < 0.001) with anti-PhtD- and anti-PcpA-treated Fab but no effect with the anti-Ply Fab (see Fig. S2 in the supplemental material). To investigate whether there might be additive/synergistic effects when Fabs were combined, adherence experiments were repeated using both TIGR4 and RX1 strains (Fig. 3). The mixed-Fab treatment resulted in a mean 34% decrease in adherence for TIGR4 and a 42% decrease for RX1. This slight increase in blocking adherence of the S. pneumoniae strains to A549 cells with the mixed Fabs suggests a marginal additive/synergistic effect under the conditions of the experiment. The effect of antigen-specific Fab on A549 adherence was also studied by the CFU counting method and showed levels of reduction (see Fig. S4 in the supplemental material) similar to those observed in fluorescence-activated cell sorting (FACS) analysis. Anti-Ply specific Fab and anti-P6 Fab of NTHI did not affect adherence of TIGR4 to A549 cells, while anti-PhtD, anti-PcpA, and mixed Fab showed 25 to 60% reduction in adherence.
FIG 3.
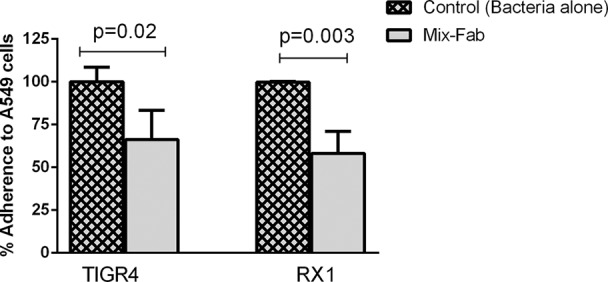
Decrease in adherence of strains TIGR4 and RX1 to A549 cells with treatment of 1.5 μg of mixed Fabs (0.5 μg of each anti-PhtD, -PcpA. and -Ply Fabs). The mean adherence of TIGR4 and RX1 alone on A549 cells was normalized, and the percent adherence of TIGR4 and RX1 with mixed Fab treatment compared to that with no treatment is shown. Data are means with standard deviations from triplicates.
Passive transfer of purified anti-pneumococcal protein antibodies reduces NP TIGR4 colonization.
The biological functionality of naturally acquired human anti-PhtD, -PcpA, and -Ply antibodies to NP colonization was evaluated in mice by passive transfer of IgG affinity-purified human antibody and study of subsequent intranasal colonization of wild-type TIGR4. In this experiment, we used an irrelevant human antibody (P6 of NTHI) as the control. On day 3 after immunization/inoculation, the number of S. pneumoniae organisms in the nasal wash was determined. Figure 4 shows a significant (P < 0.05) decrease in TIGR4 colonization in mice passively receiving anti-PhtD but not those receiving anti-PcpA. Surprisingly, there was a significant (P < 0.05) decrease in TIGR4 colonization in mice passively receiving anti-Ply IgG. A lower dose of 10 μg/mouse of anti-PhtD showed a significant reduction in S. pneumoniae colonization (see Fig. S3 in the supplemental material), while similar lower doses of anti-PcpA and -Ply did not reduce colonization (data not shown).
FIG 4.
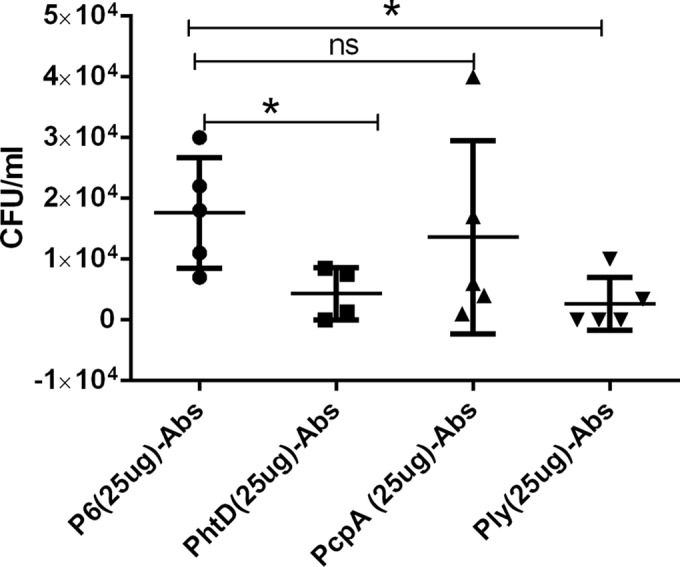
Effect of passive transfer of human anti-PhtD, -PcpA, and -Ply IgG antibodies in a mouse pneumococcal NP colonization model. A significant decrease in S. pneumoniae colonization was observed with 25 μg of anti-PhtD and -Ply antibodies compared to nonspecific control antibodies against P6 of NTHI.
DISCUSSION
Streptococcus pneumoniae NP colonization is the first step in pathogenesis leading to local invasive disease such as acute otitis media, sinusitis, and pneumonia. Several cell wall-associated proteins (PsaA, CbpA, Pht, PavA, and PsrR) have been shown to be involved in NP colonization (4, 5). Previous work from our laboratory has shown a potential role for PhtD, PhtE, and PcpA proteins in adherence to human NP epithelial cells. Recently, work by Kallio et al. (39) corroborated our results, suggesting a role for Pht proteins in adherence of S. pneumoniae to mucosal surfaces. Here we demonstrated for the first time that (i) there is a direct effect of human antibodies directed to PhtD and PcpA in preventing adherence of S. pneumoniae to human lung epithelial cells and (ii) passive transfer of purified human antibodies against PhtD and Ply reduces the NP colonization by S. pneumoniae in a mouse model.
Our group previously showed a reduction in S. pneumoniae adherence to human NP epithelial cells using human Fabs directed to PcpA, PhtD, and PhtE (9, 33). Other groups have established additional S. pneumoniae proteins involved in adherence to human epithelial cells, such as CbpA (40), PhtD (39), and PsaA (surface protein A) (41). Our study is unique in the design of the adherence assay using human Fabs from natural immunization and purified antigen-specific IgG to evaluate the role of specific antibodies in the S. pneumoniae adherence process.
Pht proteins play a major role in adherence of S. pneumoniae to the human respiratory epithelium (39). The results from our study using an S. pneumoniae mutant lacking PhtA, -B, and -D support the role of Pht proteins in adherence to human lung epithelial cells. Pht proteins are important virulence factors involved in NP colonization and in causing pneumonia in animal models. Because they facilitate the earliest stage of the S. pneumoniae infection process (5, 12, 20), these proteins have been identified as excellent potential vaccine antigens.
S. pneumoniae usually undergoes phase variation during pathogenesis, which involves a change in capsule thickness as well as expression of different surface proteins that may affect bacterial adherence to epithelium (42–45). In our study, we used TIGR4, a capsule type 4 strain, as well as RX1, a noncapsulated strain, to demonstrate the contributions of PhtD and PcpA to A549 adherence by S. pneumoniae. As expected, we observed enhanced adherence of the unencapsulated strain (see Fig. S2 in the supplemental material) compared to TIGR4, consistent with other studies showing that capsule thickness (transparent versus opaque strains), serotype, and genetic background contribute to differences in S. pneumoniae adherence to epithelial cells (39, 46, 47).
Several studies have explored the use of different S. pneumoniae surface proteins as vaccine candidates in mouse immunization challenge models (35, 48). While this was informative, a question remained as to whether the protection observed was due to mouse-specific antibodies and therefore inherent in an artificial immunization model. We addressed this question by using purified human anti-PhtD, -PcpA, and -Ply antibodies from naturally exposed human subjects and showed that after transfer these human antibodies were able to confer protection against NP colonization in mice. The decrease in murine NP bacterial load with human anti-PhtD antibodies indicates that naturally raised human antibodies can be functional to reduce the number of colonizing S. pneumoniae and therefore relevant to developing a PhtD-based vaccine. Godfroid et al. also showed a role for anti-PhtD human antibody in protection against lethal intranasal challenge in a mouse model (49); however, they did not use Fabs. Vaccination of rhesus macaques with PhtD has been shown to elicit protection against pneumonia (50) and is currently in human clinical trials (51).
To our surprise, antibodies specific to Ply, which do not have a role in blocking adherence of S. pneumoniae to human lung epithelial cells, showed the greatest level of protection against NP colonization in mice. Shak et al. have demonstrated a role for Ply in biofilm formation, independent of its hemolytic activity, which is important during both NP colonization and disease (32). In addition, maternal antibodies against Ply have been shown to delay S. pneumoniae carriage in infants (52). Our data support their work by showing a role for Ply in NP colonization in mice. Antibodies against Ply might increase host ability to reduce the numbers of S. pneumoniae effectively colonizing the NP, as sublytic concentrations of Ply induce human neutrophil respiratory burst, chemotaxis, bactericidal activity, and production of lymphokines and immunoglobulins (53). A recent study by Neill et al. (54) showed a role for Ply in NP colonization by altering the balance of pro- and anti-inflammatory-cytokine production in the upper airways.
We did not observe any effect of anti-PcpA antibodies in preventing NP colonization in mice. Expression of PcpA is regulated by PsaR (a metal-dependent regulator), which negatively affects the expression of various surface proteins, including PspA (18). Johnston et al. reported that the expression of PcpA in the NP of mice is suppressed due to high Mn concentrations in mouse nasal secretions (19). The lack of NP colonization protection using passively transferred anti-PcpA antibodies might be due to the absence of PcpA expression on the bacterial cell surface in the mouse NP. We have measured Mn concentrations in the NP secretions of children and adults when they were healthy and during a viral upper respiratory infection (URI). We found that dilution of Mn likely occurs during a viral URI, since Mn concentrations are often undetectable, whereas levels are detectable and higher when human adults and children are healthy (R. Kaur, J. R. Casey, and M. E. Pichichero, unpublished results).
In conclusion, our findings support a functional role of anti-PhtD and anti-PcpA antibodies in blocking pneumococcal adherence to human lung epithelial cells and a role of anti-PhtD and anti-Ply in adherence to the mouse NP during attempted NP colonization. The results of our study support clinical testing of a trivalent vaccine consisting of PhtD, PcpA, and PlyD1 as an alternative or adjunct to S. pneumoniae serotype-dependent protein-polysaccharide conjugate vaccines.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jessica Klapa and Ted Nicolosi for their help in animal handling. We also thank Robert Zagursky for his critical reading and valuable discussions.
This work was supported by Sanofi Pasteur.
Footnotes
Published ahead of print 22 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02124-14.
REFERENCES
- 1.Balakrishnan I, Crook P, Morris R, Gillespie SH. 2000. Early predictors of mortality in pneumococcal bacteraemia. J. Infect. 40:256–261. 10.1053/jinf.2000.0653. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144–154. 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 3.Pichichero ME. 2013. Otitis media. Pediatr. Clin. North Am. 60:391–407. 10.1016/j.pcl.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Kadioglu A, Weiser JN, Paton JC, Andrew PW. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288–301. 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 5.Plumptre CD, Ogunniyi AD, Paton JC. 2013. Surface association of Pht proteins of Streptococcus pneumoniae. Infect. Immun. 81:3644–3651. 10.1128/IAI.00562-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973. 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauksens K, Nilsson AC, Caubet M, Pascal TG, Van Belle P, Poolman JT, Vandepapeliere PG, Verlant V, Vink PE. 2014. Randomized controlled study of the safety and immunogenicity of pneumococcal vaccine formulations containing PhtD and detoxified pneumolysin with alum or adjuvant system AS02V in elderly adults. Clin. Vaccine Immunol. 21:651–660. 10.1128/CVI.00807-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darrieux M, Goulart C, Briles D, Leite LC. 2013. Current status and perspectives on protein-based pneumococcal vaccines. Crit. Rev. Microbiol. 10.3109/1040841X.2013.813902. [DOI] [PubMed] [Google Scholar]
- 9.Khan MN, Pichichero ME. 2012. Vaccine candidates PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. Vaccine 30:2900–2907. 10.1016/j.vaccine.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur R, Casey JR, Pichichero ME. 2011. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. The Pediatric infectious disease journal. 30:645–650. 10.1097/INF.0b013e31821c2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhoeven D, Xu Q, Pichichero ME. 2014. Vaccination with a Streptococcus pneumoniae trivalent recombinant PcpA, PhtD and PlyD1 protein vaccine candidate protects against lethal pneumonia in an infant murine model. Vaccine 32:3205–3210. 10.1016/j.vaccine.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Ogunniyi AD, Grabowicz M, Mahdi LK, Cook J, Gordon DL, Sadlon TA, Paton JC. 2009. Pneumococcal histidine triad proteins are regulated by the Zn2+-dependent repressor AdcR and inhibit complement deposition through the recruitment of complement factor H. FASEB J. 23:731–738. 10.1096/fj.08-119537. [DOI] [PubMed] [Google Scholar]
- 13.Loisel E, Chimalapati S, Bougault C, Imberty A, Gallet B, Di Guilmi AM, Brown J, Vernet T, Durmort C. 2011. Biochemical characterization of the histidine triad protein PhtD as a cell surface zinc-binding protein of pneumococcus. Biochemistry 50:3551–3558. 10.1021/bi200012f. [DOI] [PubMed] [Google Scholar]
- 14.Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, Dagan R, Brewah YA, Barren P, Lathigra R, Langermann S, Koenig S, Johnson S. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 69:949–958. 10.1128/IAI.69.2.949-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmlund E, Quiambao B, Ollgren J, Jaakkola T, Neyt C, Poolman J, Nohynek H, Kayhty H. 2009. Antibodies to pneumococcal proteins PhtD, CbpA, and LytC in Filipino pregnant women and their infants in relation to pneumococcal carriage. Clin. Vaccine Immunol. 16:916–923. 10.1128/CVI.00050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pichichero ME, Kaur R, Casey JR, Xu Q, Almudevar A, Ochs M. 2012. Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum. Vaccines Immunother. 8:799–805. 10.4161/hv.19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks-Walter A, Briles DE, Hollingshead SK. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533–6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glover DT, Hollingshead SK, Briles DE. 2008. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect. Immun. 76:2767–2776. 10.1128/IAI.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston JW, Briles DE, Myers LE, Hollingshead SK. 2006. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect. Immun. 74:1171–1180. 10.1128/IAI.74.2.1171-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hava DL, Camilli A. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389–1406. 10.1046/j.1365-2958.2002.03106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Beato AR, Lopez R, Garcia JL. 1998. Molecular characterization of PcpA: a novel choline-binding protein of Streptococcus pneumoniae. FEMS Microbiol. Lett. 164:207–214. 10.1111/j.1574-6968.1998.tb13087.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu Q, Pichichero ME. 2014. Co-colonization by Haemophilus influenzae with Streptococcus pneumoniae enhances pneumococcal-specific antibody response in young children. Vaccine 32:706–711. 10.1016/j.vaccine.2013.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q, Casey JR, Pichichero ME. 2014. Higher natural acquisitive mucosal antibody levels to pneumococcal vaccine candidate proteins correlate with reduced acute otitis media caused by Streptococcus pneumoniae in young children, abstr G295c In Abstr. 54th Intersci. Conf. Antimicrob. Agents Chemother http://www.icaac.org/. [Google Scholar]
- 24.Marriott HM, Mitchell TJ, Dockrell DH. 2008. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 8:497–509. 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 25.Price KE, Greene NG, Camilli A. 2012. Export requirements of pneumolysin in Streptococcus pneumoniae. J. Bacteriol. 194:3651–3660. 10.1128/JB.00114-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balachandran P, Hollingshead SK, Paton JC, Briles DE. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108–3116. 10.1128/JB.183.10.3108-3116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quin LR, Moore QC, III, McDaniel LS. 2007. Pneumolysin, PspA, and PspC contribute to pneumococcal evasion of early innate immune responses during bacteremia in mice. Infect. Immun. 75:2067–2070. 10.1128/IAI.01727-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paton JC, Andrew PW, Boulnois GJ, Mitchell TJ. 1993. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu. Rev. Microbiol. 47:89–115. 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 29.Paton JC, Ferrante A. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41:1212–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman C, Munro NC, Jeffery PK, Mitchell TJ, Andrew PW, Boulnois GJ, Guerreiro D, Rohde JA, Todd HC, Cole PJ, et al. 1991. Pneumolysin induces the salient histologic features of pneumococcal infection in the rat lung in vivo. Am. J. Respir. Cell Mol. Biol. 5:416–423. 10.1165/ajrcmb/5.5.416. [DOI] [PubMed] [Google Scholar]
- 31.Alexander JE, Lock RA, Peeters CC, Poolman JT, Andrew PW, Mitchell TJ, Hansman D, Paton JC. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683–5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shak JR, Ludewick HP, Howery KE, Sakai F, Yi H, Harvey RM, Paton JC, Klugman KP, Vidal JE. 2013. Novel role for the Streptococcus pneumoniae toxin pneumolysin in the assembly of biofilms. mBio 4:e00655-13. 10.1128/mBio.00655-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan MN, Sharma SK, Filkins LM, Pichichero ME. 2012. PcpA of Streptococcus pneumoniae mediates adherence to nasopharyngeal and lung epithelial cells and elicits functional antibodies in humans. Microbes Infect. 14:1102–1110. 10.1016/j.micinf.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oloo EO, Yethon JA, Ochs MM, Carpick B, Oomen R. 2011. Structure-guided antigen engineering yields pneumolysin mutants suitable for vaccination against pneumococcal disease. J. Biol. Chem. 286:12133–12140. 10.1074/jbc.M110.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salha D, Szeto J, Myers L, Claus C, Sheung A, Tang M, Ljutic B, Hanwell D, Ogilvie K, Ming M, Messham B, van den Dobbelsteen G, Hopfer R, Ochs MM, Gallichan S. 2012. Neutralizing antibodies elicited by a novel detoxified pneumolysin derivative, PlyD1, provide protection against both pneumococcal infection and lung injury. Infect. Immun. 80:2212–2220. 10.1128/IAI.06348-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell TJ, Andrew PW, Saunders FK, Smith AN, Boulnois GJ. 1991. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol. Microbiol. 5:1883–1888. 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 37.Virolainen A, Jero J, Kayhty H, Karma P, Eskola J, Leinonen M. 1995. Nasopharyngeal antibodies to pneumococcal pneumolysin in children with acute otitis media. Clin. Diagn. Lab. Immunol. 2:704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talbot UM, Paton AW, Paton JC. 1996. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect. Immun. 64:3772–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kallio A, Sepponen K, Hermand P, Denoel P, Godfroid F, Melin M. 2014. Role of Pht proteins in attachment of Streptococcus pneumoniae to respiratory epithelial cells. Infect. Immun. 82:1683–1691. 10.1128/IAI.00699-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo R, Mann B, Lewis WS, Rowe A, Heath R, Stewart ML, Hamburger AE, Sivakolundu S, Lacy ER, Bjorkman PJ, Tuomanen E, Kriwacki RW. 2005. Solution structure of choline binding protein A, the major adhesin of Streptococcus pneumoniae. EMBO J. 24:34–43. 10.1038/sj.emboj.7600490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajam G, Anderton JM, Carlone GM, Sampson JS, Ades EW. 2008. Pneumococcal surface adhesin A (PsaA): a review. Crit. Rev. Microbiol. 34:163–173. 10.1080/10408410802383610. [DOI] [PubMed] [Google Scholar]
- 42.Overweg K, Pericone CD, Verhoef GG, Weiser JN, Meiring HD, De Jong AP, De Groot R, Hermans PW. 2000. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect. Immun. 68:4604–4610. 10.1128/IAI.68.8.4604-4610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiser JN, Markiewicz Z, Tuomanen EI, Wani JH. 1996. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect. Immun. 64:2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JO, Weiser JN. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368–377. 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 45.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819–829. 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 46.Berry AM, Paton JC. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero-Steiner S, Pilishvili T, Sampson JS, Johnson SE, Stinson A, Carlone GM, Ades EW. 2003. Inhibition of pneumococcal adherence to human nasopharyngeal epithelial cells by anti-PsaA antibodies. Clin. Diagn. Lab. Immunol. 10:246–251. 10.1128/CDLI.10.2.246-251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tai SS. 2006. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit. Rev. Microbiol. 32:139–153. 10.1080/10408410600822942. [DOI] [PubMed] [Google Scholar]
- 49.Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. 2011. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect. Immun. 79:238–245. 10.1128/IAI.00378-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denoel P, Philipp MT, Doyle L, Martin D, Carletti G, Poolman JT. 2011. A protein-based pneumococcal vaccine protects rhesus macaques from pneumonia after experimental infection with Streptococcus pneumoniae. Vaccine 29:5495–5501. 10.1016/j.vaccine.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seiberling M, Bologa M, Brookes R, Ochs M, Go K, Neveu D, Kamtchoua T, Lashley P, Yuan T, Gurunathan S. 2012. Safety and immunogenicity of a pneumococcal histidine triad protein D vaccine candidate in adults. Vaccine 30:7455–7460. 10.1016/j.vaccine.2012.10.080. [DOI] [PubMed] [Google Scholar]
- 52.Francis JP, Richmond PC, Pomat WS, Michael A, Keno H, Phuanukoonnon S, Nelson JB, Whinnen M, Heinrich T, Smith WA, Prescott SL, Holt PG, Siba PM, Lehmann D, van den Biggelaar AH. 2009. Maternal antibodies to pneumolysin but not to pneumococcal surface protein A delay early pneumococcal carriage in high-risk Papua New Guinean infants. Clin. Vaccine Immunol. 16:1633–1638. 10.1128/CVI.00247-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubins JB, Paddock AH, Charboneau D, Berry AM, Paton JC, Janoff EN. 1998. Pneumolysin in pneumococcal adherence and colonization. Microb. Pathog. 25:337–342. 10.1006/mpat.1998.0239. [DOI] [PubMed] [Google Scholar]
- 54.Neill DR, Coward WR, Gritzfeld JF, Richards L, Garcia-Garcia FJ, Dotor J, Gordon SB, Kadioglu A. 2014. Density and duration of pneumococcal carriage is maintained by transforming growth factor beta1 and T regulatory cells. Am. J. Respir. Crit. Care Med. 189:1250–1259. 10.1164/rccm.201401-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.