Abstract
The complex segmented genome of Borrelia burgdorferi is comprised of a linear chromosome along with numerous linear and circular plasmids essential for tick and/or mammalian infectivity. The pathogenic necessity for specific borrelial plasmids has been identified; most notably, infections of the tick vector and mammalian host both require linear plasmid 25 (lp25). Genes carried on lp25, specifically bptA and pncA, are postulated to play a role for B. burgdorferi to infect and persist in Ixodes ticks. In this study, we complemented an lp25-deficient borrelial strain with pncA alone or pncA accompanied by bptA to evaluate the ability of the complemented strains to restore larval colonization and persistence through transstadial transmission relative to that of wild-type B. burgdorferi. The acquisition of the complemented strains by tick larvae from infected mice and/or the survival of these strains was significantly decreased when assayed by cultivation and quantitative PCR (qPCR). Only 10% of the pncA-complemented strain organisms were found by culture to survive 17 days following larval feeding, while 45% of the pncA- and bptA-complemented strain organisms survived, with similar results by PCR. However, neither of the complemented B. burgdorferi strains was capable of persisting through the molt to the nymphal stage as analyzed by culture. qPCR analyses of unfed nymphs detected B. burgdorferi genomes in several nymphs at low copy numbers, likely indicating the presence of DNA from dead or dying cells. Overall, the data indicate that pncA and bptA cannot independently support infection, suggesting that lp25 carries additional gene(s) or regulatory elements critical for B. burgdorferi survival and pathogenesis in the Ixodes vector.
INTRODUCTION
Borrelia burgdorferi, a spirochetal bacteria and the etiological agent of Lyme borreliosis, is transmitted to humans by Ixodes ticks via blood meal acquisition, causing a multistage infection that can be resolved with antibiotic treatment if properly diagnosed during the early stages of the disease (1–4). As the predominant tick-borne disease in the United States, an estimated 300,000 patients are diagnosed with Lyme disease each year according to a recent study by the CDC (5). The initial stage of the infection is localized to the site of the tick bite, where in 70% of cases a characteristic rash called erythema migrans can develop along with general flulike symptoms. When left untreated, the pathogen disseminates throughout the body, localizing to specific tissues, some of which are purportedly immunoprotective niches, and establishes late-stage disease. B. burgdorferi colonizes Ixodes larvae during the initial blood meal on an infected reservoir, typically small rodents or birds, and attaches to the tick midgut through the adherence of borrelial outer surface protein A (OspA) to the tick receptor TROSPA (6). The Ixodes larvae molt and mature into nymphs when a second blood meal occurs, continuing the pathogenic cycle of B. burgdorferi by transferring the organism to a reservoir or human host (4, 7). It is critical for B. burgdorferi to be able to colonize and persist in the various microenvironments and developmental stages of the tick to ultimately cause mammalian infection (8).
B. burgdorferi contains a unique segmented genome with a linear chromosome and numerous plasmids, both circular (cp) and linear (lp) (9). Essential virulence determinants are carried on the borrelial plasmids, indicating the pathogenic importance of maintaining this genomic composition (10, 11). Complex regulation of chromosomal and plasmid-borne genes are required in the tick vector or mammalian host in response to environmental cues, including temperature, pH, O2, CO2, and other unknown host factors (12–22). Previous studies have shown lp25 and lp28-1 to be necessary for establishing infection and maintaining persistence, respectively, in the murine experimental model (23–26). Plasmid lp25 is also required for colonization and persistence in the Ixodes vector (27, 28). This essential plasmid carries numerous open reading frames (ORFs) that are less than 200 bp, with many annotated as pseudogenes. Plasmid lp25 possesses a restriction modification system, encoded by bbe02, that limits the ability to genetically modify B. burgdorferi in vitro and is relieved when bbe02 is absent or disrupted (29, 30). Purser et al. identified lp25-encoded nicotinamidase, encoded by bbe22 (pncA), as the essential element for mammalian infection (31). Contradicting reports have disputed the ability of pncA to restore tick infectivity in the absence of lp25 (26–28). In addition, BptA is an lp25-encoded gene product that has been shown to be required for full mammalian infectivity and maintenance of borrelial infection in ticks, but its function is yet unknown (32).
The restoration of genes necessary for tick and mammalian infection in a B. burgdorferi lp25-deficient background would generate an infectious strain with an improved transformation efficiency rate (due to the absence of bbe02) and one that would efficiently complete an experimental enzootic cycle, which is important for creating genetic knockout mutations essential for defining gene function. Previous studies have indicated that pncA and bptA are important for the colonization and infection of the tick vector (27, 28, 32). Here, we tested the ability of pncA alone or pncA and bptA together to restore colonization and persistence in Ixodes scapularis larvae and nymphs in an lp25-deficient strain. Our findings indicate that neither pncA alone nor pncA together with bptA is adequate to fully restore colonization of larvae and to contribute to persistence through the tick life cycle.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Borrelia burgdorferi B31 derivative strains clonal MSK5 (wild type), ML23 lacking lp25 [here lp25(−)], and ML23/pBBE22 were used in this study and grown under microaerobic conditions at 32°C in BSK-II medium supplemented with 6% normal rabbit serum, as previously described (24, 33). When selective pressure was required, B. burgdorferi was grown in the presence of kanamycin (300 μg/ml). The Institute Biosafety Committee at Texas A&M University approved the use of infectious B. burgdorferi described in the study.
Escherichia coli Top10 cells were utilized for cloning steps and transformed with PCR-amplified product cloned into pCR8/GW/TOPO (Life Technologies). E. coli cultures were grown under aeration at 37°C in LB medium with the appropriate antibiotic selection: spectinomycin at 100 μg/ml or kanamycin at 50 μg/ml.
Genetic engineering of B. burgdorferi.
A borrelial shuttle vector derived from pBBE22 (31) was constructed, carrying both the bbe22 region and bbe16, as follows: LR Clonase sequences were cloned into the multicloning site (MCS) of pBBE22 to result in the destination vector, pBBE22gate (34). The bbe16 gene with the native promoter was PCR amplified with primers previously described (32) using Supermix High Fidelity (Life Technologies), cloned into pCR8/GW/TOPO (Life Technologies), and designated pJH419. The bbe16 region was subcloned into destination vector pBBE22gate by LR recombinase, resulting in pJH420. B. burgdorferi strain ML23, lacking lp25, was made competent and electrotransformed with pJH420 as previously described (34). Transformants were isolated in complete BSK-II with kanamycin (300 μg/ml).
Tick feeding and culturing.
C3H/HeJ mice were infected via subcutaneous needle inoculation with 1 × 104 organisms (strains MSK5, ML23/pBBE22, or ML23/pJH420). One mouse was infected with MSK5, and 2 mice each were infected with ML23/pBBE22 and ML23/pJH420. Mice were assayed for infection at 21 days postinoculation by serology (immunoblotting against whole-cell B. burgdorferi lysates) and culture of ear biopsy specimens in BSK-II supplemented with antibiotics and amphotericin B as described previously (35). B. burgdorferi-infected mice were infested with approximately 150 to 300 larvae each, and replete larvae were collected daily, stored at 21°C in a saturated humidity chamber, and allowed to molt to the nymphal stage (about 6 to 8 weeks).
Replete larvae (17 days after repletion) and molted unfed nymphs (2.5 months after larval repletion) were assessed for B. burgdorferi infectivity by culturing in BSK-II medium as follows. Ticks were surface sterilized by placement in 3% hydrogen peroxide for 3 min, followed by submersion in 70% ethanol for 10 min. Ticks were wicked of excess ethanol on filter paper, crushed with sterile forceps, placed into BSK-II medium supplemented with antibiotics and amphotericin B, and incubated at 34°C in capped tubes in a 5% CO2 incubator. After 4 to 7 days of incubation, culture samples were observed by dark-field microscopy for borrelial growth. Negative culture samples were maintained and observed for 4 weeks before being discarded. Experimental protocols involving mice were approved by the Institutional Animal Care and Use Committee at the Division of Vector-borne Diseases, CDC, Fort Collins, CO.
PCR of B. burgdorferi from ticks.
Fed larvae (17 days postrepletion) and unfed molted nymphs from the larval feed (approximately 2.5 months postrepletion) were processed for total DNA purification. Individual fed larvae were macerated in a 2-ml screw-cap microcentrifuge tube containing sterile 2.0-mm zirconia beads (0.75 g beads/tube) (Biospec Products, Inc., Bartlesville, OK) and 250 μl 10 mM Tris, pH 8.0. The tube was vortexed on a Disruptor Genie (Scientific Industries, Bohemia, NY) at 2,900 rpm for 2 min at room temperature. Individual unfed nymphs were macerated in 10 mM Tris (pH 8.0) using Ten Broeck grinders and transferred to 2-ml screw-cap microcentrifuge tubes. Following tick disruption, each tube was frozen at −20°C.
B. burgdorferi genomic equivalents per single Ixodes scapularis were determined by quantitative PCR (qPCR) using TaqMan Universal PCR Mastermix with flaB primers and probe using 2 μl of macerated supernatant with an Applied Biosystems ABI 7500 real-time PCR system (Life Technologies) as previously described (36). Three technical replicates were performed for each macerated supernatant. PCR screening for the presence of pBBE22 or pJH420 in 4 μl of macerated tick supernatant was performed with Phusion DNA polymerase (NEB) and pncA primers (CATTCGAAGTGGTATTACACAAAGG and CAAGGGATATTGCCTAATATAGC). PCR product was amplified with 1 cycle at 98°C for 1 min followed by 35 cycles at 98°C for 10 s, 62°C for 30 s, and 72°C for 1 min. The final cycle was 72°C for 10 min.
Western immunoblot analysis.
Borrelial cells were pelleted, and cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein was transferred to a polyvinylidene difluoride (PVDF) membrane for Western analysis as previously described (12, 22, 37). Production of BBE16 and flagellar protein was evaluated using antiserum described in reference 38 and antiserum obtained from Affinity BioReagents, Golden, CO, respectively, followed by incubation with anti-mouse horseradish peroxidase (HRP)-conjugated antibody. The Western Lightning ECL Reagent Plus detection system (PerkinElmer) was used for visualization of membrane-bound immune complexes.
Statistical analyses.
Fisher's exact probability 2-tailed test was utilized to evaluate the significance between borrelial cultivation data and qPCR. qPCR data sets of B. burgdorferi strains were analyzed by the Mann-Whitney two-tailed test using GraphPad software to compare the statistical differences of two independent groups.
RESULTS
Complementation of lp25(−) borrelial strain with pncA and bptA.
Ixodes ticks require the presence of genes carried on lp25 for colonization and persistence, but the specific required ORFs have yet to be clearly elucidated (26, 28). Previous work suggested that pncA and bptA, borne on lp25, play a role in borrelial infection of the Ixodes vector, and therefore a shuttle vector was generated to complement an lp25(−) strain with pncA and bptA (27, 32). bptA with its native promoter was PCR amplified and cloned into pCR8/GW/TOPO, resulting in pJH419. An LR Clonase reaction was performed to recombine bptA into pBBE22gate (34), which carries pncA, designated pJH420, resulting in a shuttle vector that has the potential to restore infectivity in the mammalian and tick cycle when present in lp25-lacking B. burgdorferi (Fig. 1A). ML23, a B. burgdorferi B31 lp25(−) derivative, was complemented with either pBBE22 (33) or pJH420 to evaluate the role of pncA or the combination of pncA and bptA in Ixodes. ML23/pJH420 transformants were screened by PCR for bptA, which is absent in ML23/pBBE22 (Fig. 1B). MSK5 and ML23, the parental derivative of ML23/pJH420, served as positive and negative controls for pncA and bptA, respectively. The presence of pncA was confirmed in ML23/pBBE22 and ML23/pJH420 (Fig. 1B). Additionally, the presence of BptA production in ML23/pJH420 was assessed by Western analysis and normalized to levels of FlaB (Fig. 1C). The borrelial plasmid content of transformants was assessed by PCR, and all plasmids (except cp9) were confirmed (data not shown). We were able to obtain a multicopy complementation of pncA and bptA in an lp25(−) borrelial strain in order to evaluate these genes in I. scapularis.
FIG 1.
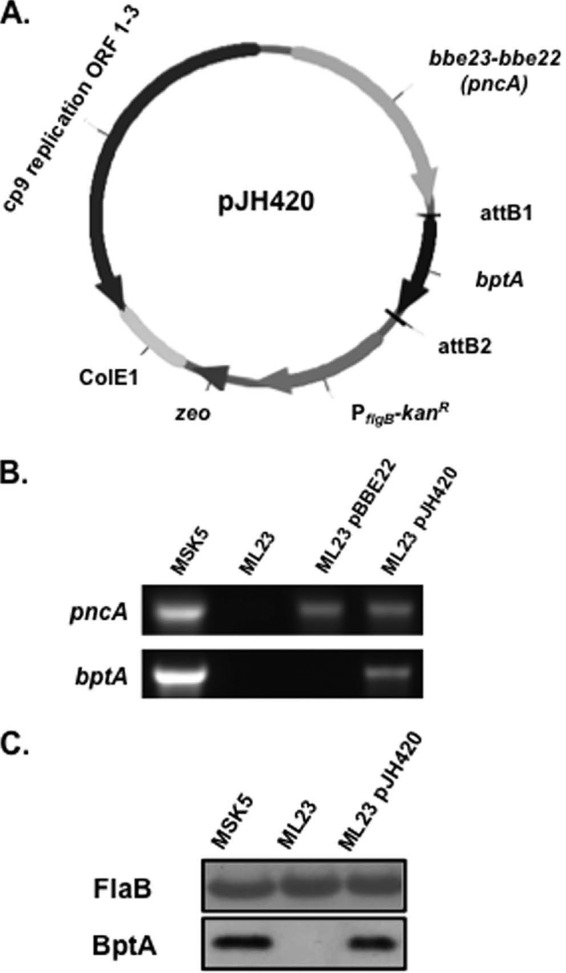
bptA and pncA complementation of B. burgdorferi lacking lp25. (A) The borrelial shuttle vector pJH420 carries pncA and bptA with each gene's native promoter. The plasmid backbone is pBBE22, which includes bbe23 upstream of bbe22, with a modified MCS including LR Clonase sites. (B) PCR verification of pncA and bptA in ML23/pJH420 transformant. MSK5, the wild-type strain containing all plasmids, and ML23, an lp25-deficient derivative, serve as positive and negative controls, respectively, for pncA and bptA. ML23/pBBE22 and ML23/pJH420 were screened for genes pncA and bptA. (C) Western blot analysis of BptA production in the wild-type (MSK5), lp25(−) (ML23), and ML23/pJH420 strains. Borrelial cell lysates were immunoblotted and probed with antisera to the antigen indicated on the left. B. burgdorferi FlaB served as a loading control.
bptA and/or pncA complementation does not completely restore I. scapularis larval colonization.
An essential step in the pathogenesis of B. burgdorferi is the ability to colonize larval ticks, and this phenotype is lost in lp25-deficient strains (26–28). To evaluate the ability of lp25-borne pncA and bptA to restore infectivity, uninfected larval ticks were fed on mice inoculated with MSK5 (wild type), ML23/pBBE22 (pncA), or ML23/pJH420 (pncA and bptA), allowing the acquisition and colonization to occur by the natural route of infection (Fig. 2). Infection of mice was confirmed by seroconversion and by ear biopsy specimen culture 2 weeks postinoculation (data not shown). Larval ticks fed to repletion on one MSK5-infected mouse and two ML23/pBBE22- or ML23/pJH420-infected mice. Seventeen days after feeding, 9 or 10 larval ticks were taken from each mouse for culture and 9 or 10 larval ticks for qPCR analysis to assess qualitatively and quantitatively the ability of the strains to colonize. Macerated fed larval ticks were transferred to fresh medium for outgrowth of borrelial strains. Infectious MSK5 was recovered from 100% of larval ticks, while 10% and 45% of ML23/pBBE22 and ML23/pJH420 organisms, respectively, were culture positive (Table 1). There is a significant reduction in the recovery of viable B. burgdorferi from larvae infected with ML23/pBBE22 and ML23/pJH420 relative to MSK5 (P = 5 × 10−6 and 0.01, respectively). The combination of pncA and bptA improved the overall number of colonized ticks compared to pncA complementation alone. This result suggested that pncA alone, or together with bptA, was not able to completely restore larval colonization in the lp25-deficient background.
FIG 2.
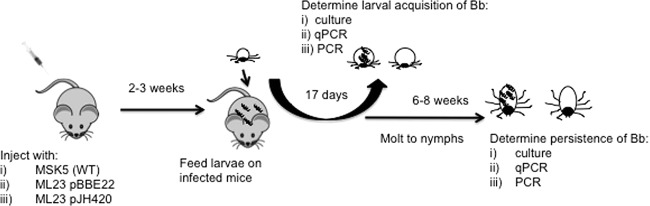
Experimental design of tick infection and genetic analysis. C3H/HeJ mice were infected with 104 cells of MSK5 (WT), ML23/pBBE22 (with pncA complementation), or ML23/pJH420 (with pncA and bptA complementation) by needle inoculation. Larval ticks were allowed to feed to repletion 2 to 3 weeks postinfection. Ten ticks were taken per mouse per assay to determine acquisition of borrelial strains by cultivation, qPCR, and PCR. Additional ticks were allowed to molt to the nymphal stage for analysis of borrelial persistence.
TABLE 1.
Cultivation and PCR evaluation of B. burgdorferi-infected Ixodes ticks
| Tick stage | B. burgdorferi strain | No. of ticks positive/total no. (%) |
||
|---|---|---|---|---|
| Culture | qPCR | pncA complementation | ||
| Larva | MSK5 | 9/9 (100) | 9/9 (100) | 8/9 (89) |
| ML23/pBBE22 | 2/20a (10) | 8/20a (40) | 6/20a (30) | |
| ML23/pJH420 | 9/20a (45) | 8/20a (40) | 6/20a (30) | |
| Nymph | MSK5 | 10/10a (100) | 10/10 (100) | 9/10 (90) |
| ML23/pBBE22 | 0/20a (0) | 4/20a (20) | 1/20a (5) | |
| ML23/pJH420 | 0/20a (0) | 5/20a (25) | 0/20a (0) | |
Total for ticks from mouse 1 and mouse 2.
In addition to culturing, the ability of the strains to colonize larval ticks was qualitatively and quantitatively assessed by qPCR with a separate cohort of larvae, using a chromosomal flagellar gene, flaB, with three technical repeats and averages calculated for each tick (Table 1 and Fig. 3A). qPCR indicated that 40% of ML23/pBBE22- and ML23/pJH420-infected larvae contained detectable borrelial DNA. However, Fisher analysis of larval culture and qPCR indicated no significant difference between these two methodologies for ML23/pBBE22 or ML23/pJH420, which resulted in P values of 0.065 and 1, respectively. Although not statistically significant, increased detection of colonization of ML23/pBBE22 by qPCR compared to cultivation may be due to the presence of remnant DNA from nonviable B. burgdorferi remaining in the macerated tick. The average bacterial load of wild-type B. burgdorferi (MSK5) was 7.27 × 105 copies of flaB per larva, which was significantly (P > 0.0001) greater than the 2.75 × 103 and 3.1 × 103 average copies of flaB per tick observed with ML23/pBBE22 and ML23/pJH420, respectively, representing a ≥234-fold reduction in colonization. These results demonstrate that complementation with pncA alone or pncA and bptA was unable to restore a colonization phenotype in B. burgdorferi lacking lp25 to the levels observed for wild-type borrelial strains.
FIG 3.
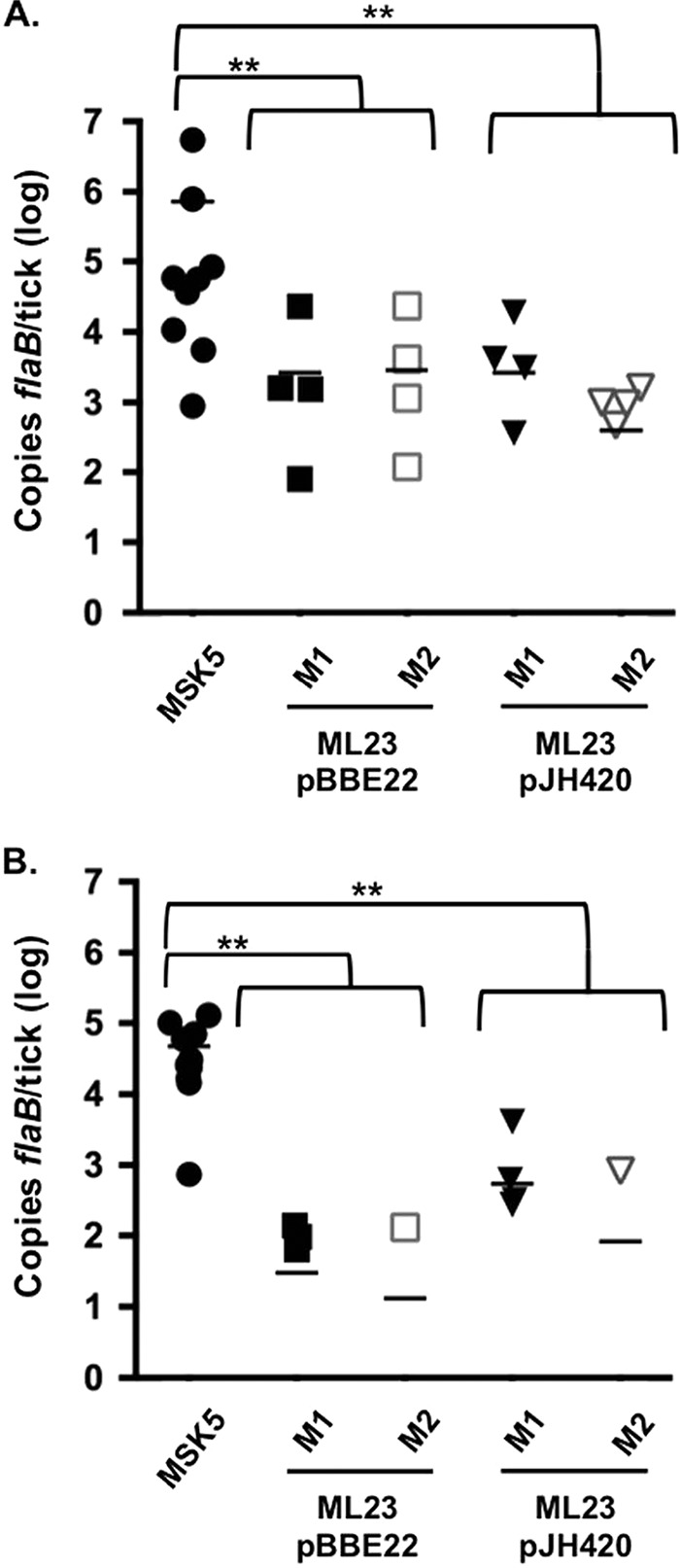
Quantitative evaluation of borrelial load per tick. Ten ticks per mouse were macerated in 10 mM Tris (pH 8.0) for qPCR assessment of B. burgdorferi cells. Larval (A) or nymph (B) macerated supernatant was evaluated for bacterial load by determining the number of flaB genomic copies in each tick. M1 and M2 represent ticks gathered from mouse 1 and mouse 2, respectively. Bar, average of 10 ticks. **, P > 0.0001 relative to MSK5 (WT).
Persistence of B. burgdorferi lacking lp25 is not maintained through molting with pncA alone or with bptA complementation.
B. burgdorferi transstadial transmission from the larval to nymphal stage of the Ixodes life cycle is critical for the continuation of pathogenesis into the mammalian host (31). Larvae infected with MSK5, ML23/pBBE22, or ML23/pJH420 were allowed to molt into nymphs prior to cultivation or molecular assessment of borrelial infection (Fig. 2). All wild-type MSK5-infected nymphs resulted in positive cultures, while no ML23/pBBE22- or ML23/pJH420-infected nymphs displayed borrelial outgrowth, demonstrating that neither pncA nor combined pncA and bptA complementation of an lp25(−) strain supported persistence of viable B. burgdorferi in nymphs (Table 1). A separate collection of ML23/pBBE22 and ML23/pJH420 nymphal ticks were evaluated for bacterial DNA by qPCR after molting (Fig. 3B). Complementation with pncA alone resulted in the detection of borrelial genomic DNA in 4 of 20 ticks, and the inclusion of bptA with pncA for complementation of ML23 detected genomic B. burgdorferi flaB in 5 of 20 ticks (Table 1). qPCR of ML23/pBBE22 demonstrated an average detection of 21.6 copies of flaB per tick, which was significantly decreased relative to MSK5-infected nymphs at 4.73 × 104 genomic copies (P < 0.0001) (Fig. 3B). ML23/pJH420 genomic analysis revealed an average of 308.5 copies of flaB per tick, which was significantly lower than that obtained with MSK5 (P < 0.0001) (Fig. 3B). There was no significant difference between the two complemented borrelial strains. Although qPCR was more sensitive than culture in detecting organisms, positive results can include remnant DNA from dead or dying cells. The large decrease in borrelial DNA in nymphs with the complemented strains suggests that these organisms were not capable of survival, which was consistent with the culture results.
pncA and bptA are maintained on shuttle vectors throughout the Ixodes cycle.
The cloning of a gene essential for mammalian or tick infectivity on a shuttle vector should serve as an in vivo selective pressure to maintain the plasmid. A similar “marker” is not known at this time for the Ixodes infectious life cycle. To ensure that the lack of infectivity observed for ML23/pBBE22 and ML23/pJH420 in Ixodes ticks was not due to the loss of the complement shuttle vector, tick macerates were qualitatively evaluated for the presence of pncA, which is carried by wild-type lp25, pBBE22, and pJH420 (Table 1). pncA was detected by PCR in 89%, 30%, and 30% of MSK5-, ML23/pBBE22-, and ML23/pJH420-infected larvae, respectively, at levels similar to that observed for flaB by qPCR. Nymph macerates had 90%, 5%, and 0% detection of pncA for MSK5, ML23/pBBE22, and ML23/pJH420, respectively. The detection of ML23/pJH420 pncA is less than observed for flaB with qPCR and may be due to decreased sensitivity of conventional PCR (Table 1). These results suggest that the shuttle vector was maintained in the populations tested and was not the reason for the loss of B. burgdorferi at either stage of the Ixodes cycle, which was instead due to the inability of pncA and bptA to restore borrelial infectivity.
DISCUSSION
B. burgdorferi is a genetically complex pathogen consisting of a linear chromosome and numerous plasmids, both circular and linear, that carry essential virulence determinants for the tick and mammalian cycle (7, 9, 39). The loss of lp25 alone results in a borrelial strain that is unable to infect the tick vector or mammalian model while increasing the in vitro transformation efficiency. This is due to the lp25 gene bbe02, which encodes a restriction modification system that inhibits the generation of genetically modified borrelial strains in the infectious background (4, 23–28, 30). Therefore, trans-complementation of genes required for tick and mammalian infectivity in an lp25-deficient strain results in an infectious B. burgdorferi strain that can be more easily transformed and provides in vivo selective pressure to maintain a shuttle vector. Previous studies identified pncA as solely responsible for restoration of experimental mammalian infection on lp25 since its presence on a borrelial shuttle vector restored virulence to B. burgdorferi strains lacking lp25 (31). Despite insight on the mammalian side, it remains to be elucidated which lp25 genes are required for colonization and persistence in the tick vector. The lp25 plasmid is essential for infectivity of Ixodes ticks, but the specific requirement of pncA for colonization or persistence in the arthropod vector is unclear to date. A recent study showed that bptA (bbe16) is essential for tick persistence, suggesting that it may function similarly to pncA for the mammalian cycle, i.e., bbe16 could restore tick infectivity without the presence of other lp25 genes (32).
In this study, we evaluated the role of pncA alone or in combination with bptA to colonize larval ticks by the natural route of infection and ability to persist through molting in nymphs. The presence of pncA in all tested B. burgdorferi strains, i.e., MSK5, ML23/pBBE22, and ML23/pJH420, allowed the use of natural infections through the feeding of ticks to repletion on needle-inoculated mice instead of requiring artificial infection of the Ixodes vector. The complementation of pncA in an lp25-deficient strain failed to result in the recovery of viable B. burgdorferi in larvae or nymphs in amounts similar to wild-type levels (Table 1). Viable ML23/pBBE22, carrying pncA, was recovered from 10% of larval ticks 17 days after repletion, but none were recovered from unfed nymphs following the molt (Table 1). Molecular assessment utilizing qPCR detected fewer larvae containing ML23/pBBE22 borrelial DNA, with a 60% reduction compared to wild-type-infected larvae, demonstrating the ability of the pncA complement strain to enter the tick but not to thrive in a fashion similar to that of the wild type (Table 1 and Fig. 3A). Furthermore, a significantly lower number of genomic B. burgdorferi ML23/pBBE22 copies per larva was observed, >100-fold lower than for wild-type MSK5 (Fig. 3A). Molting into the nymph life cycle stage further reduced the detectable genomic copies of ML23/pBBE22 to 20% of ticks with a 24-fold reduction of organisms per tick relative to the wild type (Table 1 and Fig. 3B). These results suggest that pncA alone does not support colonization of larval or transstadial transmission in Ixodes, indicating that additional lp25-borne genetic elements are required for these steps in B. burgdorferi pathogenesis.
Revel et al. indicated that bbe16, designated bptA, was important for B. burgdorferi persistence in ticks (32). In this study, we asked if pncA and bptA, independent of other lp25 genes, could complement an lp25 deficiency and promote larval and nymphal infection. Molecular analysis of pncA and bptA complementation in larvae and nymphs yielded results similar to those obtained with complementation by pncA alone (Fig. 3 and Table 1). Recovery of viable B. burgdorferi carrying pncA and bptA from larval ticks, at 45%, was improved relative to recovery with pncA alone, which stood at 10%, but was considerably lower than the 100% recovery of the wild-type strain by cultivation (Table 1). This advantage given with the addition of bptA is short-lived and completely lost during transstadial transmission, resulting in the lack of viable pathogen recovered via cultivation, suggesting that distinct genes function for distinct stages in the tick life cycle to establish colonization or persist for long-term infection.
The pncA gene, encoding a nicotinamidase, was examined for its ability to restore tick infectivity in two studies with differing conclusions (27, 28). Grimm et al. observed reduced colonization using larvae immersion infected with an lp25(−) strain, subsequently fed on naive mice, that were later cleared after molting into the nymph stage (27). trans-complementation of the lp25(−) strain with bbe22 restored larval colonization by immersion to levels similar to those of an lp25-complemented strain observed by immunofluorescence assay (IFA) and plating, but it failed to reach complete infectivity following the immersion method as we observed with our wild-type B31-derived B. burgdorferi MSK5. These investigators complemented an lp25(−) strain with lp25 encoding gentamicin resistance to represent wild-type B. burgdorferi, as it contained all the borrelial plasmids, but wild-type B. burgdorferi lacking molecular modification was not included as a control in the study. These investigators observed approximately 75% infectivity with either pncA- or lp25-complemented immersion-infected larvae. After molting, the pncA complement was not detectable by plating, while 40% recovery was observed from unfed nymphs infected with the lp25-complemented strain. This group concluded that pncA alone was sufficient to restore tick colonization but also suggested that additional genetic elements contribute to survival and replication.
Strother and de Silva utilized the mammalian infectivity of a pncA-complemented lp25(−) strain to examine the role of this gene through the natural route of transmission (28). The absence of lp25 resulted in a complete lack of infection in larvae after feeding, but the complementation of pncA improved to levels slightly lower than those of wild-type-infected ticks. The number of nymphs that remained infected with pncA-complemented B. burgdorferi dramatically declined relative to larvae and wild-type-infected nymphs. Strother and de Silva evaluated the difference between immersion and capillary infection with lp25-deficient and pncA-complemented borrelial strains and observed increased levels of infection relative to naturally infected ticks with both methodologies, which may explain the differences between their findings and those observed by Grimm et al. (27, 28). The findings from our study are consistent with those from Strother and de Silva, although our study observed lower levels of larvae and nymph infectivity with pncA complementation, which may be due to differences in trans-complementation vectors or the use of modified B31 strains (28). Overall, our study and the Strother and de Silva paper have a similar conclusion, and we have corroborated their finding that pncA is not able to completely restore infectivity in Ixodes ticks through the life cycle and appears to provide a limited contribution to colonization (28).
The Grimm et al. study did not report assays of their strains for stability of the pncA gene complemented on the shuttle plasmid during the tick transstadial cycle to ensure that the phenotype was due to the inability of pncA to restore infectivity and not due to plasmid loss because of lack of selective pressure in vivo (27). Admittedly, this is difficult to track when borrelial populations do not survive the tick environment. The facts that our complemented strains were able to infect mice and that borrelial DNA from larvae and nymphs contained pncA provided evidence for the presence of pBBE22 and pJH420 in the borrelial populations through the tick cycle. Strother and de Silva also noted the stability of their pncA-carrying vector in mice (28). Tilly et al. reported the loss of an ospC-carrying borrelial shuttle vector during mammalian infection, indicating that the shuttle vector will not be maintained without the appropriate in vivo selective pressure (40).
Another lp25-carried putative lipoprotein gene, bptA (bbe16), was found to contribute to virulence in the mammalian host and was required for persistence in Ixodes nymphs (32). Revel et al. insertionally disrupted bptA with an antibiotic cassette in B. burgdorferi 297, and nonclonal populations were injected into mice. The 50% infective dose (ID50) of the bptA mutant was increased 12.4 times in comparison to wild-type B. burgdorferi. Despite the reduced mammalian bacterial load by the bptA mutant, larval ticks were infected to levels similar to those of the wild type when allowed to feed to repletion. A notable decrease in bptA mutant-infected ticks occurred after molting into nymphs, suggesting that the gene functions in persistence and complementation of the bptA mutant with a wild-type copy of the gene reversed the defective phenotype. It is important to note that the bptA-complemented strain in the Revel et al. study was lp25(+) (32). Our data did not indicate an improvement in persistence with bptA complementation in conjunction with pncA in the lp25(−) strain. These results are consistent with the findings from Revel et al., i.e., bptA contributes to Ixodes persistence, but bptA cannot independently support long-term tick infection, and it follows that additional lp25-borne genetic components are required for persistent infection in the tick (32).
To fully elucidate the role of lp25 in the Ixodes vector, a comprehensive study of the genetic elements that it carries is necessary. The annotation of lp25 consists of 31 predicted ORFs that are comprised mainly of small ORFs and several pseudogenes (31, 41). In addition to pncA and bptA, a restriction modification system is encoded by bbe02, which when absent or disrupted improves the transformation efficiency of B. burgdorferi for genetic modification (30). The bbe31 gene encodes an outer surface lipoprotein that has been shown to be upregulated under fed-nymph conditions and has been suggested to play a role in the transmission of B. burgdorferi from the midgut to the hemolymph (42). Numerous paralogous gene members are carried on lp25, suggesting that redundant function is represented elsewhere in the borrelial genome. Recent characterization of borrelial small RNA (sRNA), dsrA, and chaperone Hfq as regulators of gene expression opens the possibility of additional sRNA in the B. burgdorferi genome, particularly on lp25, but to date none have been identified (43, 44). At this time, it is unclear if the clearance of lp25-deficient B. burgdorferi is due to the loss of a functional virulence determinant, such as an adhesin, to absence of a key player in a genetic regulatory pathway, or to the inability to evade the tick immune response.
The role of lp25-borne genetic elements in the Ixodes vector is not fully understood. We have shown that pncA and bptA are involved in colonization of larvae in a limited capacity, which is insufficient for persistence transstadially. This study suggests that expression of distinct lp25-borne gene(s) or regulatory elements contribute to Ixodes colonization and persistence in addition to pncA and bptA. Future studies will comprehensively evaluate lp25 for the identification of genomic regions, elements, and/or ORFs essential for Ixodes colonization and persistence that may serve as potential targets to prevent or inhibit tick infection with B. burgdorferi.
ACKNOWLEDGMENTS
J.A.H. thanks Jon Skare for mentoring and support over the last decade. She also recognizes David McMurray for the thoughtful advice and various means of support. We thank Michael Norgard for generously sharing antiserum against BptA and Toni Patton for assistance with tick feeds.
Work in the Hyde lab is funded by PHS grants AI103627 and AI101740.
Footnotes
Published ahead of print 22 September 2014
REFERENCES
- 1.Steere AC, Coburn J, Glickstein L. 2004. The emergence of Lyme disease. J. Clin. Invest. 113:1093–1101. 10.1172/JCI200421681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadelman RB, Wormser GP. 1998. Lyme borreliosis. Lancet 352:557–565. 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 3.Stanek G, Strle F. 2003. Lyme borreliosis. Lancet 362:1639–1647. 10.1016/S0140-6736(03)14798-8. [DOI] [PubMed] [Google Scholar]
- 4.Brisson D, Drecktrah D, Eggers CH, Samuels DS. 2012. Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 46:515–536. 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin. Infect. Dis. 59:676–681. 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, Desilva AM, Bao F, Yang X, Pypaert M, Pradhan D, Kantor FS, Telford S, Anderson JF, Fikrig E. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457–468. 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10:87–99. 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kung F, Anguita J, Pal U. 2013. Borrelia burgdorferi and tick proteins supporting pathogen persistence in the vector. Future Microbiol. 8:41–56. 10.2217/fmb.12.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb J-F, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586. 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 10.Kenedy MR, Lenhart TR, Akins DR. 2012. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol. Med. Microbiol. 66:1–19. 10.1111/j.1574-695X.2012.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norris SJ. 2012. How do lyme borrelia organisms cause disease? The quest for virulence determinants. Open Neurol. J. 6:119–123. 10.2174/1874205X01206010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyde JA, Trzeciakowski JP, Skare JT. 2007. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189:437–445. 10.1128/JB.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Invest. 101:2240–2250. 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks CS, Hefty PS, Jolliff SE, Akins DR. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371–3383. 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll JA, Cordova RM, Garon CF. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 68:6677–6684. 10.1128/IAI.68.12.6677-6684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll JA, Garon CF, Schwan TG. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour A, Jasinskas A, Benach J, Katona L, Radolf J, Caimano M, Skare J, Swingle K, Akins D, Schwartz I. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689–1705. 10.1128/IAI.71.4.1689-1705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revel AT, Talaat AM, Norgard MV. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. U. S. A. 99:1562–1567. 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwan TG. 2003. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem. Soc. Trans. 31:108–112. [DOI] [PubMed] [Google Scholar]
- 20.Stevenson B, Schwan TG, Rosa PA. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470–1479. 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- 22.Seshu J, Boylan JA, Gherardini FC, Skare JT. 2004. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi. Infect. Immun. 72:1580–1586. 10.1128/IAI.72.3.1580-1586.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labandeira-Rey M, Seshu J, Skare JT. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608–4613. 10.1128/IAI.71.8.4608-4613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labandeira-Rey M, Skare JT. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446–455. 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 97:13865–13870. 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimm D, Eggers CH, Caimano MJ, Tilly K, Stewart PE, Elias AF, Radolf JD, Rosa PA. 2004. Experimental assessment of the roles of linear plasmids lp25 and lp28-1 of Borrelia burgdorferi throughout the infectious cycle. Infect. Immun. 72:5938–5946. 10.1128/IAI.72.10.5938-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm D, Tilly K, Bueschel DM, Fisher MA, Policastro PF, Gherardini FC, Schwan TG, Rosa PA. 2005. Defining plasmids required by Borrelia burgdorferi for colonization of tick vector Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 42:676–684. 10.1603/0022-2585(2005)042[0676:DPRBBB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Strother KO, de Silva A. 2005. Role of Borrelia burgdorferi linear plasmid 25 in infection of Ixodes scapularis ticks. J. Bacteriol. 187:5776–5781. 10.1128/JB.187.16.5776-5781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawabata H, Norris SJ, Watanabe H. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147–7154. 10.1128/IAI.72.12.7147-7154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrenz MB, Kawabata H, Purser JE, Norris SJ. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 70:4798–4804. 10.1128/IAI.70.9.4798-4804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753–764. 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 32.Revel AT, Blevins JS, Almazan C, Neil L, Kocan KM, de la Fuente J, Hagman KE, Norgard MV. 2005. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc. Natl. Acad. Sci. U. S. A. 102:6972–6977. 10.1073/pnas.0502565102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Hook M, Skare JT. 2006. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol. Microbiol. 59:1591–1601. 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- 34.Hyde JA, Weening EH, Skare JT. 2011. Genetic transformation of Borrelia burgdorferi. Curr. Protoc. Microbiol. Chapter 12:Unit 12C.4. 10.1002/9780471729259.mc12c04s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinsky RJ, Piesman J. 1989. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J. Clin. Microbiol. 27:1723–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilmore RD, Howison RR, Schmit VL, Nowalk AJ, Clifton DR, Nolder C, Hughes JL, Carroll JA. 2007. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice. Infect. Immun. 75:2753–2764. 10.1128/IAI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Höök M, Skare JT. 2008. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect. Immun. 76:5694–5705. 10.1128/IAI.00690-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyde JA, Weening EH, Chang M, Trzeciakowski JP, Höök M, Cirillo JD, Skare JT. 2011. Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol. Microbiol. 82:99–113. 10.1111/j.1365-2958.2011.07801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuels DS. 2011. Gene regulation in Borrelia burgdorferi. Annu. Rev. Microbiol. 65:479–499. 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- 40.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554–3564. 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516. 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Zhang Y, Adusumilli S, Liu L, Narasimhan S, Dai J, Zhao YO, Fikrig E. 2011. Molecular interactions that enable movement of the Lyme disease agent from the tick gut into the hemolymph. PLoS Pathog. 7:e1002079. 10.1371/journal.ppat.1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lybecker MC, Samuels DS. 2007. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64:1075–1089. 10.1111/j.1365-2958.2007.05716.x. [DOI] [PubMed] [Google Scholar]
- 44.Lybecker MC, Abel CA, Feig AL, Samuels DS. 2010. Identification and function of the RNA chaperone Hfq in the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 78:622–635. 10.1111/j.1365-2958.2010.07374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


