Abstract
Streptococcus mutans is a major etiologic agent of dental caries, a prevalent worldwide infectious disease and a serious public health concern. The surface-localized S. mutans P1 adhesin contributes to tooth colonization and caries formation. P1 is a large (185-kDa) and complex multidomain protein considered a promising target antigen for anticaries vaccines. Previous observations showed that a recombinant P1 fragment (P139–512), produced in Bacillus subtilis and encompassing a functional domain, induces antibodies that recognize the native protein and interfere with S. mutans adhesion in vitro. In the present study, we further investigated the immunological features of P139–512 in combination with the following different adjuvants after parenteral administration to mice: alum, a derivative of the heat-labile toxin (LT), and the phase 1 flagellin of S. Typhimurium LT2 (FliCi). Our results demonstrated that recombinant P139–512 preserves relevant conformational epitopes as well as salivary agglutinin (SAG)-binding activity. Coadministration of adjuvants enhanced anti-P1 serum antibody responses and affected both epitope specificity and immunoglobulin subclass switching. Importantly, P139–512-specific antibodies raised in mice immunized with adjuvants showed significantly increased inhibition of S. mutans adhesion to SAG, with less of an effect on SAG-mediated bacterial aggregation, an innate defense mechanism. Oral colonization of mice by S. mutans was impaired in the presence of anti-P139–512 antibodies, particularly those raised in combination with adjuvants. In conclusion, our results confirm the utility of P139–512 as a potential candidate for the development of anticaries vaccines and as a tool for functional studies of S. mutans P1.
INTRODUCTION
Streptococcus mutans is a Gram-positive bacterium and an established etiological agent of human dental caries, a transmissible, chronic, nonlethal infectious disease with a worldwide distribution (1, 2). Adherence of S. mutans to the tooth surface involves two stages: a sucrose-independent stage and a sucrose-dependent stage (1, 3). The initial sucrose-independent step is mediated by a reversible interaction between a large (185-kDa) bacterial surface protein, P1 (also referred to as antigen I/II [Ag I/II], antigen B, or PAc), and a high-molecular-weight salivary glycoprotein, called gp340, adsorbed to the tooth enamel (4, 5). Ag I/II family molecules are present on virtually all oral streptococci and have also been identified in other Streptococcus species (6–8). Based on its primary sequence, P1 demonstrates several distinct features: a secretion signal sequence (amino acids [aa] 1 to 38); the N-terminal pre-A region (aa 39 to 185); a series of three alanine-rich tandem repeats called the A region (aa 186 to 464); a variable region, or V region, where strain-strain differences are clustered (aa 679 to 823); a series of three tandem proline-rich repeats, or the P region (aa 840 to 963); and C-terminal anchoring and trans-membrane sequences including an LPXTG sortase motif (aa 1486 to 1561) (9–12). Monoclonal antibodies (MAbs) have been used to identify functional segments within P1 (9, 13, 14). The crystal structure of much of the protein has been resolved, revealing a unique architecture. The A region forms an α-helix that intertwines with the helical P region to form a highly elongated 50-nm fibrillar stalk with a globular domain that includes the V region at the tip and three globular DE (immunoglobulin motif) variant IgG-like domains encompassing the carboxy terminus at the base (15, 16). The N terminus of the protein has not yet been crystallized but is known to interact with the C terminus and to contribute to the overall folding, stability, and function of the full-length molecule (17). Two adhesive domains have been identified and localized in the modeled structure near or within the globular regions located at the ends of the helical stalk (15, 16).
The P1 protein of S. mutans interacts in different ways with soluble, and tooth attached, forms of human salivary agglutinin (SAG), a multimeric protein complex (18). The binding of bacteria to either soluble or immobilized SAG determines whether the bacteria will be aggregated and ingested or will remain in the oral cavity and adhere to the tooth surface. When immobilized, SAG serves as a substrate for the adherence of S. mutans and subsequent biofilm formation leading to the onset of the tooth decay process (19, 20). In contrast, aggregation by fluid-phase SAG represents an innate host defense mechanism (18).
Since P1 contributes to the cariogenicity of S. mutans, antibodies against P1 functional domains can block bacterial adherence, thereby disrupting colonization of the oral cavity (21–23). Indeed, a recombinant P1 fragment, corresponding to the A region or the saliva-binding region (SBR), expressed in Escherichia coli has been successfully used, after genetic fusion with cholera toxin, to induce antibodies and T cells with potential protective effects against dental caries under experimental conditions (23, 24). Recently, we have shown that another P1-derived fragment generated in Bacillus subtilis, encompassing amino acids 39 to 512 and named P139–512, is immunogenic in mice and induces antibodies that interfere with S. mutans adhesive properties in vitro (25). We furthermore demonstrated that a mucosal delivery system based on genetically modified B. subtilis spores that express P139–512 in vivo induced specific antibodies in serum and saliva that interfered with S. mutans adhesion to abiotic surfaces without preventing bacterial aggregation (26). These findings highlight the need for an understanding of the immunological, structural, and functional characteristics of P139–512 as an alternative to the full-length protein as a target antigen to generate protective immunity against dental caries. In addition, the observation that systemic IgG enters the oral cavity via the gingival crevice and confers protection to dental caries suggests that parenteral routes should also be tested for potential anticaries vaccines (21, 27–29).
Adjuvants are present in most vaccine formulations, particularly those containing purified proteins, also named acellular vaccines. Aluminum salts (alum) are added as adjuvants in most presently used vaccines (30). However, several alternative compounds, including molecules of microbial origin, have received growing interest as potential vaccine adjuvants, such as derivatives of heat-labile toxin (LT) produced by some enterotoxigenic Escherichia coli strains (31) and flagellin (FliC) of Salmonella enterica serovar Typhimurium, a Toll-like receptor 5 (TLR5) agonist capable of triggering the innate immune system (32).
In the present study, we evaluated the immunological features and potential protective effects of P139–512 produced in B. subtilis strains. The recombinant protein allowed us to define further the epitope specificity of several different P1-specific MAbs known to interfere with the adhesive functions of S. mutans. In addition, parenteral coadministration of P139–512 and different adjuvants (alum, flagellin, or an LT derivative) permitted us to characterize the serum antibody responses with regard to specificity and subclass distribution as well as potential anticaries protective effects, including adherence inhibition in vitro and bacterial colonization in vivo. Our results demonstrate that P139–512 expressed in B. subtilis represents a promising antigen for the development of anticaries vaccines and a useful reagent for functional, immunological, and structural studies of the S. mutans P1 protein.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
S. mutans strains (UA159, NG8, or P1-deficient mutant strain PC3370) were grown in Todd-Hewitt broth or in brain heart infusion (BHI) broth, each supplemented with 0.3% yeast extract, at 37°C in 5% CO2 (5, 33, 34). E. coli (CG14) and B. subtilis (1012 or LDV701) strains were grown aerobically at 37°C with constant shaking in Luria-Bertani (LB) broth (29, 35). Cultures were supplemented with antibiotics as needed.
Purification of P1-derived fragments and protein adjuvants.
Expression and purification of full-length P1 (CG14), truncated P1 fragments (P139–512) (NR21, LT1, and MA41), and the adjuvant proteins (LTK4R and FliC) in E. coli or B. subtilis strains were performed according to previously described protocols (36–38). The LT derivative LTK4R shows reduced toxicity compared to the reference LT form (38), while recombinant FliC represents the phase 1 flagellin type (named FliCi) originally expressed by S. Typhimurium LT2 (39).
P1-specific monoclonal antibodies.
P1-specific MAbs (3-8D, 1-6F, 4-9D, and 4-10A) were obtained from hybridomas previously generated at the University of Florida (40). The binding characteristics and functional activity of these antibodies were reported previously (41–43).
Reactivity of anti-P1 MAbs with P139–512 determined by ELISA.
Enzyme-linked immunosorbent assay (ELISA) plates were coated with 200 ng/well of recombinant soluble or heat-denatured (100°C for 10 min) P139–512 in carbonate-bicarbonate buffer (pH 9.6), and the reactions were performed with MAbs 3-8D, 1-6F, 4-9D, and 4-10A. Binding of MAbs to the immobilized antigen was detected by using peroxidase-conjugated anti-mouse IgG followed by development with 0.1 M O-phenylenediamine dihydrochloride (OPD; Sigma-Aldrich) and 0.012% H2O2 diluted in phosphate citrate buffer (0.01 M; pH 5.0). The reactions were stopped with 2 M H2SO4, and the absorbance at 492 nm was measured in an ELISA plate reader (Multiskan EX; ThermoLabsystems, Finland). All samples were assayed in duplicate, and dilution curves were prepared for each sample.
Western blot analysis of P139–512 with anti-P1 MAbs.
Recombinant P139–512 purified from B. subtilis was subjected to electrophoresis on a polyacrylamide gel (7.5%) and transferred onto nitrocellulose membranes. The membranes were blocked with phosphate-buffered saline (PBS)–Tween 20 (0.3%) and then incubated with anti-P1 MAb ascites fluids at a dilution of 1/3,000. The membranes were washed and incubated with an anti-mouse IgG conjugate labeled with peroxidase (Southern Biotechnology Associates) (1/3,000), followed by development with a solution of 4-chloro-1 naphthol. A positive-control reaction was carried out with control serum raised in mice immunized with anti-P139–512 and Freund's adjuvant (25).
Interaction between P139–512 and immobilized SAG and inhibition of S. mutans adherence to SAG by anti-P139–512 antibodies determined by surface plasmon resonance.
Interaction of P139–512 with and inhibition of adherence of S. mutans NG8 to immobilized SAG were determined by surface plasmon resonance (SPR) using a BIAcore model 3000 instrument (GE Healthcare, Salt Lake City, UT, USA). Human SAG was prepared as described previously and immobilized on CM5 chips (GE Healthcare) (∼1,500 resonance units [RU]), according to previously described methods (42). For protein interaction experiments, aliquots (10 μl) containing 500 ng of P139–512 were injected onto the chip surface and subsequently eluted with regeneration buffer (PBS-Tween [0.3%] with EDTA [10 mM], NaCl [100 mM], and NaOH [100 mM]) at the end of each assay. The signal from the control surface (FC1) (not coated with SAG) was subtracted from that of the test surface (FC2) (coated with SAG) to produce a sensorgram (change in resonance units [ΔRU]). For inhibition of adherence of S. mutans NG8, serum samples (pools) from the different immunization groups were used at a dilution of 1/100. The ΔRU was used to calculate the percentage of inhibition of adhesion {adhesion inhibition = 100 − [(ΔRU of S. mutans NG8 incubated with test serum × 100)/ΔRU of S. mutans NG8]}. Sera from sham-treated mice were used as a negative control, and values for these sera were subtracted from the final absorbance value as the background. Serum samples collected after the third dose were used in the assays. The final values represent results of three independent experiments. Data were analyzed by using BIAE valuation software (v4.2; BIAcore, University of Florida, USA).
Immunization regimen and sample collection.
All experiments involving animals were performed with prior approval from the Committee on the Ethical Use of Laboratory Animals of the Institute of Biomedical Sciences of São Paulo University (protocol no. 013) and in accordance with guidelines for the care and use of laboratory animals of the National Committee on the Ethics of Research (CONEP). Groups of five female BALB/c mice, aged 6 to 8 weeks, were immunized with three subcutaneous (s.c.) doses given at intervals of 15 days. Each dose consisted of 10 μg of P139–512 protein (per animal) admixed with 12.5 μg of aluminum hydroxide (alum), 1 μg of LTK4R, or 5 μg of FliC. Control groups were sham immunized with PBS. Mice were bled by submandibular puncture on days 0, 14, 29, 44, 60, and 112. Samples were held at room temperature for 30 min and then incubated for another 30 min at 4°C. Following centrifugation (6,000 × g for 30 min at 4°C), sera were stored in aliquots at −20°C until use.
Determination of anti-P1 IgG responses, antibody avidity, and reactivity of anti-P139–512 antibodies with P1-derived fragments by ELISA.
Serum samples were evaluated for their level of anti-P1 or anti-P139–512 total IgG or subclass titers (IgG1, IgG2a, and IgG2b), avidity, and recognition of P1 fragments by ELISA. To measure IgG responses, plates were coated with recombinant full-length P1 (CG14) or P139–512 (200 ng/well), and the assays were performed as previously described (25). The absorbance values obtained for samples collected from sham-treated mice were subtracted as the background. Serial dilution curves were drawn for each sample, and the endpoint titers were calculated as the reciprocal values of the last dilution with an optical density of 0.05. The avidity of serum antibody binding to P139–512 was determined by elution of ammonium thiocyanate, as previously described (44). After incubation with primary antibodies (all serum samples were normalized to an optical density at 492 nm [OD492] of 1.0), plates were washed, and increasing concentrations of ammonium thiocyanate (0 to 8 M) diluted in PBS were added to the wells and incubated for 15 min. The concentration of ammonium thiocyanate required to dissociate 50% of the bound antibodies was determined. Percent binding of antibodies was determined by the use of the following formula: (OD492 in the presence of ammonium thiocyanate × 100)/OD492 in the absence of ammonium thiocyanate. The assay was performed with sera collected after the third vaccine dose. All samples were assayed in duplicate, and the experiments were performed independently at least twice with pooled sera from different immunization groups. To assess antibody specificity, the reactivity of sera from mice immunized with anti-P139–512 with and without adjuvant against truncated recombinant P1 fragments (P139–512, MA41, LT1, and NR21, as described above) was measured by ELISA (Fig. 1a).
FIG 1.
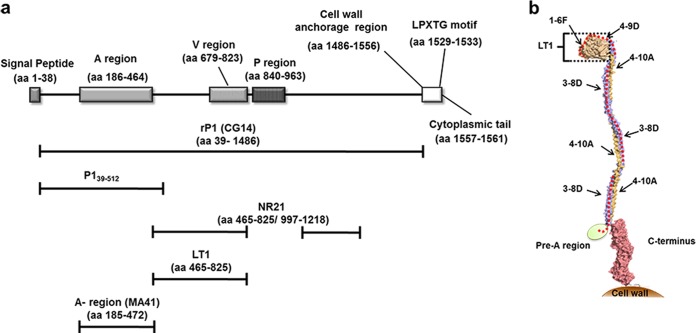
Schematic representation of functional and structural domains of the S. mutans P1 protein. (a) Linear representation of the protein sequence with the indicated functional/structural domains. The corresponding amino acid positions are shown in parentheses. Bars placed below the schematic indicate the recombinant proteins used in the present study: CG14 (recombinant full-length P1 sequence [rP1]), P139–512, MA41 (A region), LT1 (globular segment intervening between the A and P regions), and NR21 (fusion polypeptide lacking the P region). (b) Tertiary structural model of the S. mutans P1 protein. The location of P139–512 is marked by a red dotted line. The location of the LT1 polypeptide is indicated by a bracket. The approximate positions of experimentally determined epitopes recognized by the P1-specific MAbs (1-6F, 4-9D, 4-10A, and 3-8D) are indicated by arrows. The crystal structure of the N terminus has not yet been determined and is represented as an ellipse. It is known to interact with the C terminus to form a stable complex.
Evaluation of antibody binding to P1 present on the surface of S. mutans by fluorescence-activated cell sorting (FACS).
Binding assays were performed according to a previously described protocol (45). Briefly, S. mutans UA159 cells were grown in Todd-Hewitt broth supplemented with 0.3% yeast extract (THYE) to an OD600 of 0.5, washed once, and suspended in an equal volume of PBS. Bacteria were incubated for 30 min at 37°C with serum pools from each group (third dose) diluted 1/100. After additional washing, the bacteria were incubated with 100 μl of anti-mouse IgG conjugated to fluorescein isothiocyanate (FITC) (MP Biomedicals) diluted 1:2,000. Finally, the samples were analyzed by using a FACScalibur instrument (BD Biosciences). Sera from sham-treated mice were used as negative controls, and only sera from the third dose of the different immunization groups were evaluated. All samples were assayed in duplicate, and the experiments were performed independently at least twice. Ten thousand gated events were acquired, and the median bacterial fluorescence intensity is shown for each sample.
Assessment of inhibition of SAG-mediated S. mutans aggregation.
Assessment of the inhibition of SAG-mediated aggregation of S. mutans NG8 was performed according to a previously described protocol (46). The serum pools were tested at a final dilution of 1/100. The change in absorbance (ΔAbs600 nm) was used to calculate the percentage of inhibition of aggregation {aggregation inhibition = 100 − [(ΔAbs600 nm of S. mutans NG8 incubated with test serum × 100)/S. mutans NG8 ΔAbs600 nm)]}. Values for samples from sham-treated mice were used as background controls, and values were subtracted from the final absorbance values for samples collected from the different immunization groups. Serum samples collected after the third dose were used in the assays. The final values represent results of three independent experiments performed in duplicate.
Serum neutralization of oral colonization of mice by S. mutans.
The antiadhesive function of anti-P139–512 antibodies under in vivo conditions was determined by using a serum neutralization model developed in our laboratory. Briefly, 1 day before inoculation of bacteria, BALB/c mice (n = 5), aged 6 to 8 weeks, were given water containing 1% sucrose ad libitum, as described previously (47). The next day, the mice were anesthetized with ketamine (80 mg/kg of body weight) and xylazine hydrochloride (8 mg/kg of body weight), and the oral cavity of each animal was cleaned with chlorhexidine (0.12%) by using a sterile swab. Samples of endogenous oral microbiota were collected and plated onto LB and Mitis-Salivarius (MS) agar before and after treatment. After the oral cavity was washed with 1× PBS (pH 7.0), an aliquot (20 μl) of clarified saliva, prepared as described previously (48), was applied onto the tooth surfaces of each mouse by using a micropipette tip, and the animal was kept immobile for 30 s. Next, excess saliva was removed with a swab; immediately afterwards, an aliquot (20 μl) containing 3 × 109 CFU of S. mutans strain NG8 was applied to each mouse with a micropipette tip, and the animal was again kept immobile for 30 s. Bacteria were prewashed with TBSC (10 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2 [pH 7.6]) and suspended in TBSC for application. All procedures were performed while the animals were anesthetized. Animals remained anesthetized for 90 min. Oral colonization with S. mutans was determined 6 h after the mice returned from anesthesia by brushing the tooth surfaces with a sterile swab, resuspending the recovered bacteria in 1 ml of 1× PBS (pH 7.0), and plating samples onto MS agar plates containing bacitracin (0.2 U/ml) and sucrose (5%). Plates were incubated for 48 h at 37°C under anaerobic conditions, at which time CFU were counted. To evaluate antibody-dependent neutralization, prior to administration, bacteria were incubated with pooled sera from the various immunization groups for 30 min at 37°C. Pooled sera were diluted 1/100 in TBSC. Positive and negative controls included untreated S. mutans strain NG8 and the spaP-negative derivative PC3370, which is devoid of P1 (5), respectively.
Statistical analyses.
The results were analyzed with the GraphPad Prism 5 program and were expressed as means ± standard errors of the means (SEM). Statistically significant differences were determined by two-way analysis of variance (ANOVA) and Bonferroni's multiple-comparison posttests.
RESULTS
Epitopes recognized by P1-specific antiadhesive MAbs are preserved within P139–512.
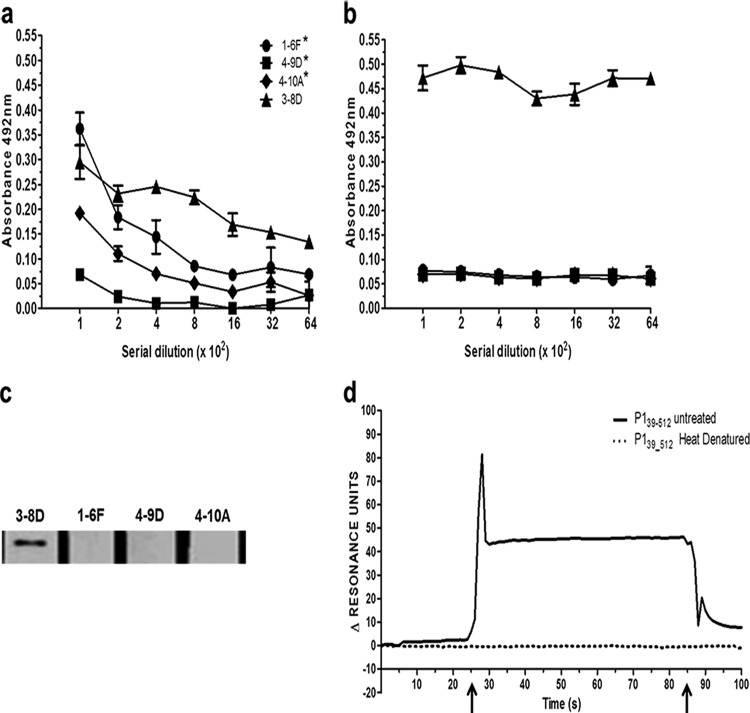
Recombinant P139–512 expressed in B. subtilis strain LDV701 encompasses 148 amino acids and contains the entire N terminus, the A region (aa 186 to 464), and the first 49 amino acids of the post-A-region sequence (25). To evaluate whether known epitopes were conserved within the recombinant protein, we reacted P139–512 with several previously characterized P1-specific MAbs (1-6F, 4-9D, 4-10A, and 3-8D) (40–43). These MAbs were selected because they recognize epitopes that have been mapped to the N-terminal and central portions of the P1 protein (Fig. 1b). Three of these MAbs (1-6F, 4-9D, and 4-10A) were demonstrated previously to inhibit P1-mediated S. mutans adhesion to abiotic surfaces (42, 43). P139–512 was also subjected to a heat denaturation treatment to evaluate the presence of conformational epitopes recognized by the MAbs. The results presented in Fig. 2 show that all four anti-P1 MAbs recognized the untreated P139–512 antigen to some degree when tested by ELISA (Fig. 2a). Heat denaturation ablated recognition by MAbs 1-6F, 4-9D, and 4-10A but increased the reactivity of MAb 3-8D (Fig. 2b), which reacts within the A region and does not bind well to the intact native protein (9, 13, 14). Only MAb 3-8D reacted with denatured P139–512 in Western blots (Fig. 2c). Taken together, these results indicate that P139–512 preserves several different structural epitopes contained within the full-length native S. mutans protein. Unmasking of the 3-8D epitope by denaturation further confirms that untreated P139–512 achieves a native-like conformation, since this epitope is only partially accessible in the context of the folded molecule.
FIG 2.
Recombinant P139–512 retains functional epitopes of the native S. mutans P1 protein. (a) Reactivity of P139–512 with MAbs that recognize epitopes localized to the N-terminal half of P1. Plates were coated with soluble P139–512 produced in recombinant B. subtilis strain LDV701 and reacted with 2-fold serial dilutions (beginning at 1:100) of MAb 1-6F, 4-9D, 4-10A, or 3-8D by ELISA. Asterisks indicate the anti-P1 MAbs that are strong inhibitors of S. mutans adherence. (b) Same as panel a but with reactions carried out with heat-denatured P139–512 as the immobilized antigen. (c) Reactivity of P139–512 with anti-P1 MAbs by Western blotting. Recombinant P139–512 was separated on a 7.5% SDS denaturing gel, blotted onto nitrocellulose, and then reacted with each MAb followed by goat horseradish peroxidase-conjugated anti-mouse IgG. (d) Binding of P139–512 to immobilized SAG monitored by surface plasmon resonance (BIAcore). The chip surface was coated with SAG and reacted with untreated or heat-denatured recombinant P139–512. Arrows indicate the beginning and end of the injection period.
Recombinant P139–512 binds to immobilized SAG.
We next assessed the functional properties of P139–512 by interactions with SAG. As can be seen in Fig. 2d, P139–512 binds to SAG immobilized on a BIAcore chip, as demonstrated by SPR. Heat denaturation of P139–512 eliminated its binding to immobilized SAG, suggesting that the SAG-binding activity is heat sensitive and dependent on structural aspects of the protein.
Characterization of antibody responses in mice immunized with P139–512 in combination with different adjuvants.
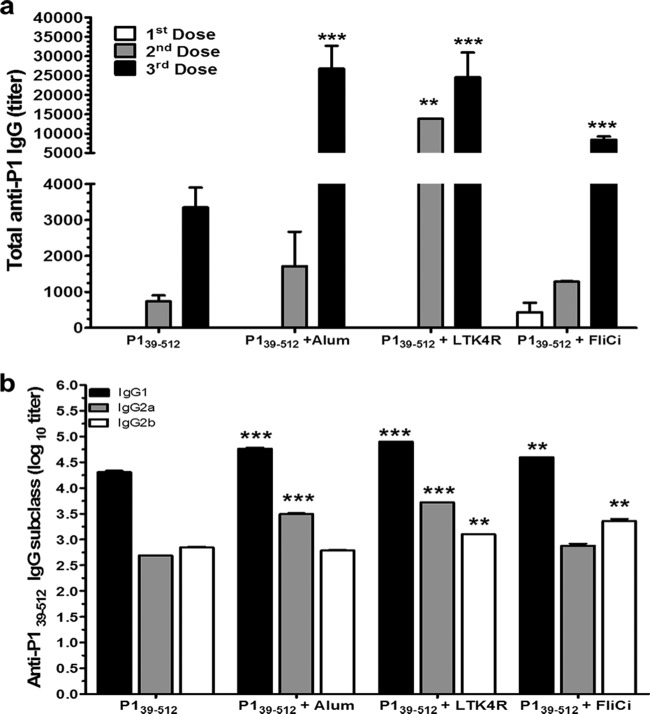
In the next step, we evaluated whether the immunogenicity of P139–512 could be enhanced by s.c. administration to BALB/c mice in combination with three different vaccine adjuvants: alum, an LT derivative, and a Salmonella flagellin (FliC) (Fig. 3). Coadministration of adjuvants, particularly alum and LT, significantly increased the systemic IgG responses against full-length P1. After the third dose, titers were 2.6 × 104 (P139–512 plus alum), 2.4 × 104 (P139–512 plus LT), and 8.4 × 103 (P139–512 plus FliC), which were higher than that achieved in mice immunized with nonadjuvanted P139–512 (titer of 3.3 × 103) (Fig. 3a). Immunized mice maintained high anti-P1 serum IgG levels for at least 3 months after the last vaccine dose (data not shown). Analysis of the serum IgG subclass responses showed that immunization with P139–512 resulted in a balanced Th1/Th2 response (Fig. 3b). Mice immunized with P139–512 combined with adjuvants showed not only increased amounts of IgG1 but also larger amounts of IgG2a (alum and LT) and/or IgG2b (LT and FliC). Both IgG2a and IgG2b, but not IgG1, have been correlated with the antiadhesive activity of P1-specific antisera (49), suggesting that the efficacy of the antibodies would be affected by the incorporation of adjuvants. As expected, parenteral immunization of mice with the different vaccine formulations did not induce significant amounts of secretory IgA in saliva or feces (data not shown).
FIG 3.
Induction of P1-specific serum IgG in mice immunized s.c. with P139–512 admixed with different adjuvants. (a) P1-specific serum IgG responses in BALB/c mice immunized with 3 vaccine doses containing 10 μg/dose of P139–512 alone or coadministered with aluminum hydroxide (alum) (12.4 μg/dose), LT (1 μg/dose), or Salmonella flagellin (FliC) (5 μg/dose). Serum samples were collected 2 weeks after each dose. ELISAs were performed on pooled samples by using purified full-length recombinant P1 as the immobilized antigen on the plate. (b) Anti-P139–512 IgG subclass responses measured in pooled sera of vaccinated mice following the third vaccine dose. Data are based on two independently performed immunization experiments and are expressed as means ± standard errors (**, P < 0.01; ***, P < 0.001).
Effect of adjuvants on the avidity of anti-P139–512 antibodies and on the recognition of P1 on the surface of S. mutans.
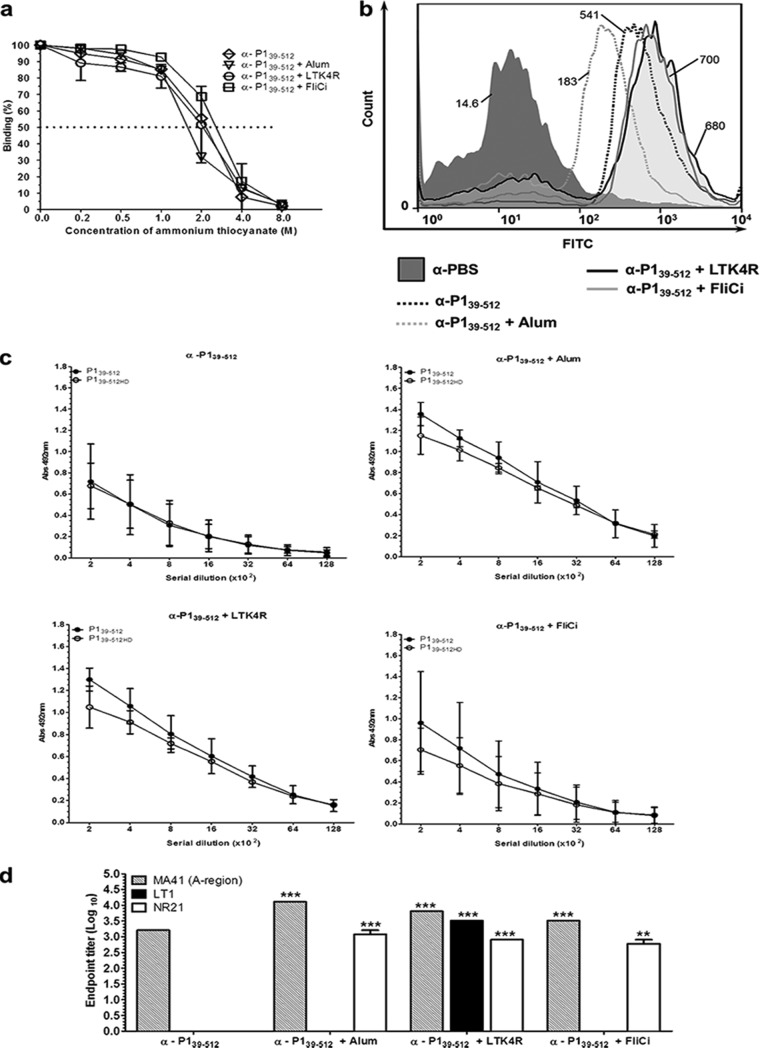
To determine whether the incorporation of adjuvants into the vaccine formulations altered the overall avidity of the induced antibodies, an ELISA-based ammonium thiocyanate dissociation assay was performed. There were no significant differences in the measured avidities of the anti-P139–512 antibodies raised in the different vaccination groups (Fig. 4a). We also measured the ability of the anti-P139–512 antibodies to bind to S. mutans whole cells, since this would be an important property of an effective vaccine. As illustrated in Fig. 4b, P1-specific antibodies from all four immunization groups bound to the surface of S. mutans cells from strain UA159. Antibodies raised in mice immunized with P139–512 admixed with alum displayed lower reactivity with S. mutans cells than did antibodies in samples collected from animals immunized with P139–512 only. In contrast, antibodies in sera collected from animals immunized with LT and FliC were more reactive with S. mutans than were those in the sera from mice immunized with P139–512. Similar results were observed with strain NG8 (data not shown), which is expected, since the P1 proteins from both strains share 97% identity, and in the relevant sequence (aa 39 to 512), this identity increases to 99%.
FIG 4.
Determination of avidity, reactivity with the S. mutans cell surface, and epitope specificity of antibodies raised in mice immunized with P139–512 and the different vaccine adjuvants. (a) Antibody avidity was assessed by normalizing pooled serum samples from each group to achieve an absorbance of 1 on ELISA plates coated with P139–512 and tested for dissociation with increasing amounts of ammonium thiocyanate, as described in Materials and Methods. The dotted line indicates 50% dissociation of antigen-antibody complexes. (b) Determination of reactivity of anti-P139–512 antibodies with native P1 protein on the surface of S. mutans cells by FACS analysis. Immunization groups are indicated. The median bacterial fluorescence intensity is indicated. (c) Determination of reactivity of serum samples with recombinant P139–512 protein by ELISA. P139–512 was used as a solid-phase antigen in two forms: untreated or heat denatured (P139–512HD). Immunization groups are indicated at the top. (d) Reactivity of anti-P139–512 antibodies with other truncated P1 fragments (Fig. 1a). Recombinant proteins representing the A region (MA41), the segment intervening between the A- and P-repeat regions (LT1), and a polypeptide representing LT1 fused to post-P-region sequence (NR21) were used as solid-phase-bound antigens in ELISAs. All experiments were performed with serum pools collected from mice following the third vaccine dose. Results are represented as endpoint titer values. Statistically significant differences were determined in comparison to serum samples from mice immunized with P139–512 without adjuvant (**, P < 0.01; ***, P < 0.001).
Adjuvant coadministration influences the specificity of P1-specific antibodies.
In the next step, we tested whether adjuvant incorporation affects the epitope specificity of antibodies induced against P139–512. As shown in Fig. 4c, sera from mice vaccinated with the recombinant protein alone demonstrated similar reactivity with untreated and heat-denatured P139–512, suggesting that these antibodies were not directed against conformational determinants. Conversely, antibodies raised in animals immunized with P139–512 in combination with the tested adjuvants reacted more strongly with the nondenatured form of the protein. This observation suggests that coadministration of adjuvants contributed to the formation of additional antibodies against conformational determinants retained in P139–512.
In order to further investigate changes in epitope specificity of antibodies generated in mice immunized with P139–512 in combination with the adjuvants, we measured the reactivity of serum samples with several available P1-derived fragments that overlap the P139–512 sequence (Fig. 1a). The MA41 fragment corresponds to the A region (aa 185 to 472). It is recognized by MAb 3-8D (14), a weak inhibitor of immobilized SAG adherence (48). The LT1 fragment (aa 465 to 825) corresponds to the globular region of P1 that intervenes between the alanine- and proline-rich repeat segments. LT1 is recognized by MAb 1-6F, an antibody that is highly inhibitory of bacterial adherence to SAG (42, 49). The NR21 fragment (aa 465 to 825 fused to aa 987 to 1218) lacks the P region and was generated as a tool for previous epitope mapping studies (43). It is also recognized by the adherence-inhibiting MAb 1-6F. As expected, serum samples from all four groups were reactive with MA41. Coadministration of adjuvants significantly increased serum reactivity against MA41. In contrast, only serum samples from mice immunized with P139–512 admixed with alum, LTK4R, or FliC, but not those from mice immunized with P139–512 alone, recognized NR21. Only sera from mice immunized with P139–512 and LTK4R recognized LT1 (Fig. 4d). The gain of reactivity of antibodies against LT1, but not against NR21, in mice immunized with P139–512 plus LTK4R suggests that at least one epitope may be masked within the longer NR21 polypeptide. Given the highly complex structure of P1, it is not surprising that certain epitopes may or may not be formed or exposed depending on their context. Collectively, our results strongly suggest that incorporation of adjuvants within the vaccine formulation broadens the epitope specificity of the induced antibodies, including that against determinants recognized by MAbs known to inhibit the adhesive function of S. mutans.
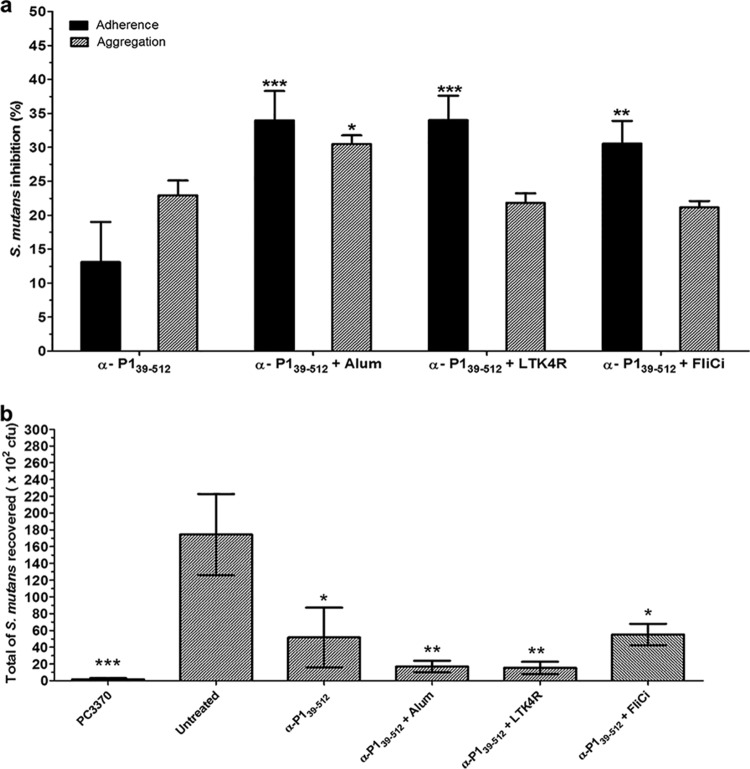
Coadministration of adjuvants with P139–512 enhances the inhibition of S. mutans adhesion to immobilized SAG.
Next, serum samples collected from immunized mice were tested for inhibition of S. mutans adherence to immobilized SAG as well as for inhibition of bacterial aggregation in the presence of soluble SAG (Fig. 5a). Sera from mice immunized with P139–512 admixed with alum, LT, or FliC all showed a significant improvement in their ability to inhibit bacterial adherence compared to those from mice immunized with P139–512 alone. Sera from mice immunized with P139–512 admixed with alum, but not those admixed with LTK4R or FliC, exhibited a moderate increase in inhibition of SAG-mediated bacterial aggregation, although this was not as pronounced as the increase in adherence inhibition observed for this group.
FIG 5.
Blockage of the adhesive functions of S. mutans with anti-P139–512 antibodies. (a) Inhibition of SAG-mediated adhesion and aggregation of S. mutans cells by anti-P139–512 antibodies. Determination of adherence inhibition was carried out by surface plasmon resonance analysis. The percentage of adherence inhibition by immune serum samples from the indicated groups was determined by using the values obtained for S. mutans without serum as the reference. The percentage of inhibition of bacterial aggregation was calculated after incubation for 1 h in the presence of fluid-phase SAG and immune serum in comparison to S. mutans cells incubated without serum. Nonimmune mouse serum was used as a negative control, and the values were subtracted as the background. The results are based on data from at least in two independently performed experiments, and data represent means ± SEM. Statistically significant differences were determined in comparison to serum samples from mice immunized with P139–512 without adjuvant. (b) Inhibition of initial colonization of murine tooth surfaces with S. mutans by anti-P139–512 antibodies. BALB/c mice were pretreated with human saliva and then inoculated with S. mutans NG8 cells previously incubated with sera from mice immunized with P139–512 with and without adjuvants. Tooth swabs were collected 6 h after inoculation and plated onto Mitis-Salivarius medium plates containing bacitracin (0.2 U/ml) and sucrose (5%). S. mutans without preincubation with serum (untreated) and a P1-deficient mutant strain (PC3370) were used as positive and negative controls, respectively. Assays were performed with serum samples collected after the third dose. All sera were diluted 1/100. Data are expressed as the means ± SEM. Statistically significant differences were determined in comparison to the untreated group (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Adjuvants enhance the neutralizing capacity of anti-P139–512 antibodies in vivo.
Considering that P1 is involved in the initial stages of colonization by S. mutans, we developed a murine oral colonization model to evaluate the protective potential of anti-P1 antibodies in vivo. First, we confirmed that early colonization of the tooth surface by S. mutans was dependent on the presence of P1. The wild-type strain, which produces P1, was detectable at significantly higher levels (∼1.7 × 104 CFU) 6 h after inoculation than the spaP-negative mutant (PC3370) (∼1.6 × 102 CFU), which lacks P1 (Fig. 5b). Also, mouse oral colonization by S. mutans was dependent on the presence of human saliva (data not shown). Next, to determine if antibodies raised against P139–512 alone, or admixed with adjuvants, could prevent colonization in this model, we preincubated S. mutans with sera collected from mice in the different immunization groups prior to introduction of the bacteria into the oral cavities of naive mice. S. mutans colonization was significantly reduced by pretreatment of bacteria with sera from all four groups, with the greatest reductions being seen with sera collected from mice immunized with P139–512 combined with alum or LTK4R. Sera from mice immunized with FliCi combined with P139–512 reduced S. mutans colonization to the same extent as did sera from mice immunized with P139–512 alone. These results indicate that antibodies raised in mice following immunization with P139–512, particularly in combination with alum or LTK4R, disrupt P1-dependent oral colonization of S. mutans in a murine model.
DISCUSSION
P1 is a widely studied antigen capable of triggering protective immunity against S. mutans infection and caries formation (21–23). Despite much promise, an effective vaccine against tooth decay is not yet available; therefore, identification of recombinant antigens capable of eliciting antibodies optimal for the blockage of bacterial adhesion to the tooth surface is critical. The S. mutans P1 protein is an exceptionally large and structurally complex protein for which a detailed framework of the key epitopes involved in bacterial adhesion is still incomplete. Generation of a recombinant P1 fragment preserving relevant immunological features of the native protein is an essential step for the development of an effective anticaries vaccine.
In the present study, we investigated functional, conformational, and immunological features of P139–512, a recombinant protein generated in B. subtilis cells that encompasses the saliva-binding region of the S. mutans P1 protein and adjacent sequences. Our results demonstrated that P139–512 displays conformational epitopes similar to those present in the native protein P1 and also retains the ability to bind to human SAG. In addition, parenteral immunization of mice with P139–512 in combination with adjuvants not only affected the amounts of anti-P1 antibodies raised in vaccinated animals but also changed the IgG subclass differentiation and epitope specificity of the generated antibodies, particularly against conformational epitopes. More importantly, anti-P139–512 antibodies interfered with the adhesion of S. mutans to immobilized SAG, both in vitro and in vivo, and its protective potential was enhanced by the inclusion of adjuvants in the immunization schedule. Altogether, these results indicate that P139–512 preserves relevant features of the native S. mutans protein and should be regarded as a promising candidate for anticaries vaccine development.
The S. mutans P1 adhesin comprises a novel three-dimensional structure, and the appropriate folding of the protein is critical to its cell surface localization and function (16, 17, 48, 50). Our results indicate that even in the absence of the P region, the recombinant polypeptide P139–512 exhibits conformational features that mimic those of the native protein. This conclusion is based on the fact that the recombinant protein was recognized by three different anti-P1 MAbs, namely, 1-6F, 4-9D, and 4-10A, all of which recognize complex antigenic determinants of the P1 protein and inhibit bacterial adherence (41–43, 48, 49). Recognition by these antibodies, and the ability of P139–512 to interact with immobilized SAG, was lost after thermal denaturation of the protein, suggesting that epitope formation and functionality are dependent on structural features of the polypeptide. In contrast, the 3-8D epitope contained within the A region (9) is thought to be linear and is partially masked within the full-length molecule (14). Similarly, our results support the proposed nature of this epitope and demonstrate that it is also partially masked within the recombinant P139–512 protein, as evidenced by the increased reactivity of 3-8D after heat denaturation of this polypeptide.
Together, our data indicate that recombinant P139–512 produced in B. subtilis displays important conformational neutralizing epitopes and, therefore, sufficiently preserves the natural structure of the native protein. Furthermore, the N-terminal SAG-adhesive site of P1 appears to be located within an area spanning the third alanine-rich repeat and immediately adjacent to the post-A-region sequence (aa 386 to 512), since these residues are shared between P139–512 and the previously crystallized A3VP1 polypeptide (aa 386–874), which also binds immobilized SAG (15). In fact, Okuda and collaborators have also shown that a small peptide (aa 390 to 400) from Streptococcus gordonii is able to bind to salivary receptors and to prevent S. gordonii and S. mutans binding to saliva-coated hydroxyapatite (s-HA) in an in vitro assay (51). Together with our previous observations, the present data indicate that P139–512 preserves key epitopes recognized by antibodies capable of inhibiting P1-mediated adherence of S. mutans to SAG-coated surfaces.
The performance of subunit vaccines relies on the right combination of adjuvants, which enhance both the quantity and the quality of an induced immune response. Taking this into consideration, we evaluated the impact of three different adjuvants on the immunogenicity of P139–512 with regard to serum IgG titers, IgG subclass, antigen avidity, epitope specificity, and functional activity of induced antibodies. As expected, incorporation of adjuvants significantly augmented the total quantity of P1-specific IgG antibodies. In addition, incorporation of alum and LTK4R resulted in increases in antigen-specific IgG2a levels, while LKT4R and FliC promoted increases in IgG2b levels. Since the IgG2a and IgG2b subclasses have previously been correlated with an ability to neutralize the adhesive properties of S. mutans (41, 48), the increased amounts produced in mice immunized in conjunction with LT or flagellin would be expected to have a positive impact on the inhibition of bacterial adherence to SAG.
Another notable difference observed for the immune responses elicited in mice immunized with adjuvanted vaccine formulations was the altered epitope specificity of the induced antibodies. This was particularly evident in the recognition of heat-labile conformational epitopes associated with bacterial adherence and recognized by previously characterized MAbs capable of blocking the adhesion of S. mutans. In the absence of adjuvants, antibodies raised in mice immunized with recombinant P139–512 recognized predominantly heat-stable nondenaturable linear epitopes. Incorporation of adjuvants resulted in increased amounts of antibodies reacting with polypeptide MA41, corresponding to the A region. In addition, all of the adjuvants also increased reactivity against NR21, a fusion polypeptide that was generated in previous studies to assess the contribution of the P region to the adhesive function of P1 and that is recognized by MAb 1-6F but not the other MAbs in our panel (41). The increased reactivity against NR21 is consistent with the observed increases in IgG2a and IgG2b levels and the improved ability of the adjuvanted sera to inhibit S. mutans adherence to immobilized SAG. In previous studies, we found that 1-6F-like antibodies belong primarily to the IgG2a and IgG2b subclasses (36, 48). In addition, mice immunized with P139–512 in conjunction with LTK4R, but not the other combinations, also developed antibodies that recognize the LT1 polypeptide. The conformation-dependent adherence-inhibiting MAb 1-6F recognizes both LT1 and NR21, supporting the positive role of adjuvants in the generation of functional antibodies. Our results suggest that the adjuvant effect of heat-labile toxins favors the generation of antibodies against conformational determinants relevant for the function of the P1 adhesin. Previous studies employing the saliva-binding region (SBR) of P1 have also reported positive results when the immunogen was administered in combination with different LT derivatives, either as an isolated polypeptide or as a chimeric protein genetically fused to the B subunit of cholera toxin (52, 53).
Host defense against oral infection by S. mutans requires the induction of neutralizing antibodies that prevent or reduce bacterial adhesion to the enamel surface. As a mediator of sucrose-independent attachment, P1 is an important target of such antibodies (21, 22, 27, 52–55). To infer the potential protective effects conferred by immunization with P139–512, we determined if serum samples collected from vaccinated mice would reduce the initial adhesion of S. mutans to the dental surface of nonimmunized mice. Our results clearly demonstrated that anti-P139–512 antibodies raised in vaccinated mice interfere with this process. The inclusion of adjuvants, specifically alum and LTK4R, made the induction of antibodies capable of inhibiting initial oral colonization of mice by a cariogenic S. mutans strain even more effective. Thus, immunization with P139–512, especially when coadministered with adjuvants, represents a promising strategy for the generation of neutralizing antibodies that act both in vitro and in vivo to reduce S. mutans adherence, including adherence to saliva-coated teeth. Furthermore, our method to assess initial oral colonization of mice by S. mutans may be a useful and facile tool for those who are interested in evaluating the efficacy of anti-S. mutans strategies.
In conclusion, our results indicate that P139–512 contains relevant target epitopes and induces adherence-inhibiting neutralizing antibodies that are effective in vivo. We provide evidence that adjuvants modulate the immune system to improve the quantity, and particularly the quality, of the host antibody response against S. mutans P1. Since serum IgG, which enters the oral cavity via the gingival crevice (21, 27–29), as well as salivary IgA (2, 4) contribute to immune protection against caries, the search for vaccine formulations allowing concomitant activation of a mucosal response is a priority. In addition, it may be possible to improve efficacy further by incorporation of relevant C-terminal target sequences that correspond to the second known adhesive domain of P1 (15, 16, 37), in conjunction with appropriate adjuvants and delivery vectors.
ACKNOWLEDGMENTS
This study was supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) (grants 2012/51189-3 and 2013/06671-4), CNPq (Conselho Nacional de Pesquisas), USP through the Vaccine Research Core Group (NPV), and the National Institute for Dental and Craniofacial Research (grant R01 DE13882).
We thankfully acknowledge the invaluable technical assistance of Monica R. de Jesus, Naína I. Garcia, Eduardo G. Martins, Monika Oli, Jason Obirek, and Marjorie Chow.
Footnotes
Published ahead of print 15 September 2014
REFERENCES
- 1.Hamada S, Slade HD. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taubman MA, Nash DA. 2006. The scientific and public-health imperative for a vaccine against dental caries. Nat. Rev. Immunol. 6:555–563. 10.1038/nri1857. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J. Dent. Res. 90:294–303. 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 4.Oho T, Yu H, Yamashita Y, Koga T. 1998. Binding of salivary glycoprotein-secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans. Infect. Immun. 66:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley P, Brady L. 1999. Virulence of a spaP mutant of Streptococcus mutans in a gnotobiotic rat model. Infect. Immun. 67:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma JK, Kelly CG, Munro G, Whiley RA, Lehner T. 1991. Conservation of the gene encoding streptococcal antigen I/II in oral streptococci. Infect. Immun. 59:2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Green NM, Sitkiewicz I, Lefebvre RB, Musser JM. 2006. Identification and characterization of an antigen I/II family protein produced by group A Streptococcus. Infect. Immun. 74:4200–4213. 10.1128/IAI.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkinson HF, Demuth DR. 1997. Structure, function and immunogenicity of streptococcal antigen I/II polypeptides. Mol. Microbiol. 23:183–190. 10.1046/j.1365-2958.1997.2021577.x. [DOI] [PubMed] [Google Scholar]
- 9.Brady LJ, Piacentini DA, Crowley PJ, Bleiweis AS. 1991. Identification of monoclonal antibody-binding domains within antigen P1 of Streptococcus mutans and cross-reactivity with related surface antigens of oral streptococci. Infect. Immun. 59:4425–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischetti VA, Pancholi V, Schneewind O. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 4:1603–1605. 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 11.Murakami Y, Yamashita Y, Nakano Y, Ito HO, Yu H, Koga T. 1997. Role of the charged tail in localization of a surface protein antigen of Streptococcus mutans. Infect. Immun. 65:1531–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ton-That H, Marraffini LA, Schneewind O. 2004. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta 1694:269–278. 10.1016/j.bbamcr.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 13.van Dolleweerd CJ, Kelly CG, Chargelegue D, Ma JK. 2004. Peptide mapping of a novel discontinuous epitope of the major surface adhesin from Streptococcus mutans. J. Biol. Chem. 279:22198–22203. 10.1074/jbc.M400820200. [DOI] [PubMed] [Google Scholar]
- 14.McArthur WP, Rhodin NR, Seifert TB, Oli MW, Robinette RA, Demuth DR, Brady LJ. 2007. Characterization of epitopes recognized by anti-Streptococcus mutans P1 monoclonal antibodies. FEMS Immunol. Med. Microbiol. 50:342–353. 10.1111/j.1574-695X.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 15.Larson MR, Rajashankar KR, Patel MH, Robinette RA, Crowley PJ, Michalek S, Brady LJ, Deivanayagam C. 2010. Elongated fibrillar structure of a streptococcal adhesin assembled by the high-affinity association of alpha- and PPII-helices. Proc. Natl. Acad. Sci. U. S. A. 107:5983–5988. 10.1073/pnas.0912293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson MR, Rajashankar KR, Crowley PJ, Kelly C, Mitchell TJ, Brady LJ, Deivanayagam C. 2011. Crystal structure of the C-terminal region of Streptococcus mutans antigen I/II and characterization of salivary agglutinin adherence domains. J. Biol. Chem. 286:21657–21666. 10.1074/jbc.M111.231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heim KP, Crowley PJ, Brady LJ. 2013. An intramolecular interaction involving the N terminus of a streptococcal adhesin affects its conformation and adhesive function. J. Biol. Chem. 288:13762–13774. 10.1074/jbc.M113.459974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakobphol A, Xu F, Hoang VM, Larsson T, Bergstrom J, Johansson I, Frängsmyr L, Holmskov U, Leffler H, Nilsson C, Borén T, Wright JR, Strömberg N, Fisher SJ. 2000. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J. Biol. Chem. 275:39860–39866. 10.1074/jbc.M006928200. [DOI] [PubMed] [Google Scholar]
- 19.Stenudd C, Nordlund A, Ryberg M, Johansson I, Kallestal C, Stromberg N. 2001. The association of bacterial adhesion with dental caries. J. Dent. Res. 80:2005–2010. 10.1177/00220345010800111101. [DOI] [PubMed] [Google Scholar]
- 20.Loimaranta V, Jakubovics NS, Hytonen J, Finne J, Jenkinson HF, Stromberg N. 2005. Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties. Infect. Immun. 73:2245–2252. 10.1128/IAI.73.4.2245-2252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehner T, Russell MW, Caldwell J, Smith R. 1981. Immunization with purified protein antigens from Streptococcus mutans against dental caries in rhesus monkeys. Infect. Immun. 34:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, Hein MB, Lehner T. 1998. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat. Med. 4:601–606. 10.1038/nm0598-601. [DOI] [PubMed] [Google Scholar]
- 23.Hajishengallis G, Russell WM, Michalek SM. 1998. Comparison of an adherence domain and a structural region of Streptococcus mutans antigen I/II in protective immunity against dental caries in rats after intranasal immunization. Infect. Immun. 66:1740–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toida N, Hajishengallis G, Wu HY, Russell W. 1997. Oral immunization with the saliva-binding region of Streptococcus mutans AgI/II genetically coupled to cholera toxin B subunit elicits T-helper-cell responses in gut-associated lymphoid tissues. Infect. Immun. 65:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavares MB, Silva BM, Cavalcante RCM, Souza RD, Luiz WB, Paccez JD, Crowley PJ, Brady LJ, Ferreira LCS, Ferreira RCC. 2010. Induction of neutralizing antibodies in mice immunized with an amino-terminal polypeptide of Streptococcus mutans P1 protein produced by a recombinant Bacillus subtilis strain. FEMS Immunol. Med. Microbiol. 59:131–142. 10.1111/j.1574-695X.2010.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batista MT, Souza RD, Paccez JD, Luiz WB, Ferreira EL, Cavalcante RCM, Ferreira RCC, Ferreira LCS. 2014. Gut adhesive Bacillus subtilis spores as a platform for mucosal delivery of antigens. Infect. Immun. 82:1414–1423. 10.1128/IAI.01255-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Challacombe SJ, Russell MW, Hawkes J. 1978. Passage of intact IgG from plasma to the oral cavity via crevicular fluid. Clin. Exp. Immunol. 34:417–422. [PMC free article] [PubMed] [Google Scholar]
- 28.Lehner T, Challacombe SJ, Caldwell J. 1980. Oral immunization with Streptococcus mutans in rhesus monkeys and the development of immune response and dental caries. Immunology 41:857–864. [PMC free article] [PubMed] [Google Scholar]
- 29.Tenovuo J, Lehtonen OP, Aaltonen AS. 1990. Caries development in children in relation to the presence of mutans streptococci in dental plaque and of serum antibodies against whole cells and protein antigen I/II of Streptococcus mutans. Caries Res. 24:59–64. 10.1159/000261240. [DOI] [PubMed] [Google Scholar]
- 30.Kool M, Fierens K, Lambrecht BN. 2012. Alum adjuvant: some of the tricks of the oldest adjuvant. J. Med. Microbiol. 61:927–934. 10.1099/jmm.0.038943-0. [DOI] [PubMed] [Google Scholar]
- 31.da Hora VP, Conceição FR, Dellagostin OA, Doolan DL. 2011. Non-toxic derivatives of LT as potent adjuvants. Vaccine 29:1538–1544. 10.1016/j.vaccine.2010.11.091. [DOI] [PubMed] [Google Scholar]
- 32.Mizel SB, Bates JT. 2010. Flagellin as an adjuvant: cellular mechanisms and potential. J. Immun. 185:5677–5682. 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Lau PCY, Lee JH, Ellen RP, Cvitkovitch DG. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183:897–908. 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ajdić D, McShan WM, McLaughlin RE, Savić G, Chang J, Carson MB, Primeaux C, Tian R, Kenton S, Jia H, Lin S, Qian Y, Li S, Zhu H, Najar F, Lai H, White J, Roe B, Ferretti JJ. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 99:14434–14439. 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wehrl W, Niederweis M, Schumann W. 2000. The FtsH protein accumulates at the septum of Bacillus subtilis during cell division and sporulation. J. Bacteriol. 182:3870–3873. 10.1128/JB.182.13.3870-3873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braga CJM, Massis LM, Sbrogio-Almeida ME, Alencar BCG, Bargieri DY, Boscardin SB, Rodrigues MM, Ferreira LCS. 2010. CD8+ T cell adjuvant effects of Salmonella FliCd flagellin in live vaccine vectors or as purified protein. Vaccine 28:1373–1382. 10.1016/j.vaccine.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Robinette RA, Oli MW, McArthur WP, Brady LJ. 2011. A therapeutic anti-Streptococcus mutans monoclonal antibody used in human passive protection trials influences the adaptive immune response. Vaccine 29:6292–6300. 10.1016/j.vaccine.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues JF, Mathias-Santos C, Sbrogio-Almeida ME, Amorim JH, Cabrera-Crespo J, Balan A, Ferreira LCS. 2011. Functional diversity of heat-labile toxins (LT) produced by enterotoxigenic Escherichia coli: differential enzymatic and immunological activities of LT1 (hLT) and LT4 (pLT). J. Biol. Chem. 286:5222–5233. 10.1074/jbc.M110.173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massis LM, Braga CJM, Sbrogio-Almeida ME, Lauand C, Newton SMC, Klebba PE, Ferreira LCS. 2008. Anti-flagellin antibody responses elicited in mice orally immunized with attenuated Salmonella enterica serovar Typhimurium vaccine strains. Mem. Inst. Oswaldo Cruz 103:606–610. 10.1590/S0074-02762008000600017. [DOI] [PubMed] [Google Scholar]
- 40.Ayakawa GY, Boushell LW, Crowley PJ, Erdos GW, McArthur WP, Bleiweis AS. 1987. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect. Immun. 55:2759–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodin NR, Van Tilburg ML, Oli MW, McArthur WP, Brady LJ. 2004. Further characterization of immunomodulation by a monoclonal antibody against Streptococcus mutans antigen P1. Infect. Immun. 72:13–21. 10.1128/IAI.72.1.13-21.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oli MW, McArthur WP, Brady LJ. 2006. A whole cell BIAcore assay to evaluate P1-mediated adherence of Streptococcus mutans to human salivary agglutinin and inhibition by specific antibodies. J. Microbiol. Methods 65:503–511. 10.1016/j.mimet.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Robinette RA, Oli MW, McArthur WP, Brady LJ. 2009. Beneficial immunomodulation by Streptococcus mutans anti-P1 monoclonal antibodies is Fc independent and correlates with increased exposure of a relevant target epitope. J. Immun. 183:4628–4638. 10.4049/jimmunol.0803300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bargieri DY, Rosa DS, Lasaro MAS, Ferreira LCS, Soares IS, Rodrigues MM. 2007. Adjuvant requirement for successful immunization with recombinant derivatives of Plasmodium vivax merozoite surface protein-1 delivered via the intranasal route. Mem. Inst. Oswaldo Cruz 102:313–317. 10.1590/S0074-02762007005000039. [DOI] [PubMed] [Google Scholar]
- 45.Darrieux M, Miyaji EN, Ferreira DM, Lopes LM, Lopes APY, Ren B, Briles DE, Hollingshead SK, Leite LCC. 2007. Fusion proteins containing family 1 and family 2 PspA fragments elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect. Immun. 75:5930–5938. 10.1128/IAI.00940-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brady LJ, Piacentini DA, Crowley PJ, Oyston PC, Bleiweis AS. 1992. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect. Immun. 60:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito T, Maeda T, Senpuku H. 2012. Roles of salivary components in Streptococcus mutans colonization in a new animal model using NOD/SCID.e2f1-/- mice. PLoS One 7:e32063. 10.1371/journal.pone.0032063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rundegren JL, Arnold RR. 1987. Bacteria-agglutinating characteristics of secretory IgA and a salivary agglutinin. Adv. Exp. Med. Biol. 216B:1005–1013. [PubMed] [Google Scholar]
- 49.Oli MW, Rhodin N, Mcarthur WP, Brady LJ. 2004. Redirecting the humoral immune response against Streptococcus mutans antigen P1 with monoclonal antibodies. Infect. Immun. 72:6951–6960. 10.1128/IAI.72.12.6951-6960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crowley PJ, Seifert TB, Isoda R, van Tilburg M, Oli MW, Robinette RA, McArthur WP, Bleiweis AS, Brady LJ. 2008. Requirements for surface expression and function of adhesin P1 from Streptococcus mutans. Infect. Immun. 76:2456–2468. 10.1128/IAI.01315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okuda K, Hanada N, Usui Y, Takeuchi H, Koba H, Nakao R, Watanabe H, Senpuku H. 2010. Inhibition of Streptococcus mutans adherence and biofilm formation using analogues of the SspB peptide. Arch. Oral Biol. 55:754–762. 10.1016/j.archoralbio.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Nawar HF, Arce S, Russell MW, Connell TD. 2007. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect. Immun. 75:621–633. 10.1128/IAI.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao W, Zhao Z, Russell MW. 2011. Characterization of antigen-presenting cells induced by intragastric immunization with recombinant chimeric immunogens constructed from Streptococcus mutans AgI/II and type I or type II heat-labile enterotoxins. Mol. Oral Microbiol. 26:200–220. 10.1111/j.2041-1014.2011.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehner T, Caldwell J, Smith R. 1985. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect. Immun. 50:796–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma JK, Lehner T. 1990. Prevention of colonization of Streptococcus mutans by topical application of monoclonal antibodies in human subjects. Arch. Oral Biol. 35:115S–122S. 10.1016/0003-9969(90)90140-6. [DOI] [PubMed] [Google Scholar]