Abstract
The golden hamster (Mesocricetus auratus) is a susceptible model to Leishmania (Viannia) spp.; however, available studies employ different infection protocols, which account for clinical and pathological presentation differences. Herein, L. (V.) braziliensis preparations were standardized to contain 104, 105, or 106 parasites to determine an optimal inoculum that ensured cutaneous lesions without causing a disseminated infection in hamsters. Lesion development was followed for 105 days by size measurements, and skin, draining lymph node, spleen, and sera were investigated to check parasite load, spleen visceralization, cytokine expression, histopathological changes, and anti-Leishmania IgG levels. The lesion emergence time was inversely proportional to the parasite concentration in the inocula. Animals infected by 104 parasites presented nodular lesions, while those infected with 106 parasites often exhibited ulcerated lesions. The differences in the final lesion sizes were observed between 104 and 105 inocula or 104 and 106 inocula. High IFNG expression, anti-Leishmania IgG levels, and parasite load occurred independently of the inoculum used. A mild inflammatory skin involvement was observed in animals infected with 104 parasites, while extensive tissue damage and parasite spleen visceralization occurred with 105 and 106 parasites. These results indicate that inocula with different concentrations of parasites generate differences in the time of lesion emergence, clinical presentation, and systemic commitment, despite high and similar IFNG expression and parasite load. This suggests that a modulation in the immune response to different parasite numbers occurs in an early phase of the infection, which could dictate the establishment and magnitude of the chronic phase of the disease.
INTRODUCTION
Leishmaniasis has several characteristics that are responsible for the different clinical forms observed over the course of an infection in humans. An important factor is the diversity of the species that cause disease, which includes clonal differences within the same species that lead to clinical variants (1–3). Another determinant is the absolute parasite numbers that infect the host, which can influence the infection outcome in combination with the immunological and genetic characteristics of the host (4, 5).
Parasites from the Viannia subgenus, Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis, are the most widespread species in the Americas that cause cutaneous leishmaniasis. Most of the knowledge regarding the immunopathogenesis of L. braziliensis infection comes from studies performed in human patients and asymptomatic individuals (2, 6, 7). Despite the impact of American tegumentary leishmaniasis (ATL), few experimental studies have been developed for L. braziliensis infections (8, 9). This can be attributable mostly to the resistance of common laboratory mice strains to infection by these Leishmania species (10, 11). BALB/c mice have been widely used to study Old World cutaneous leishmaniasis, but long-term lesions do not develop when they are infected with L. braziliensis (8, 12). The lack of an adequate experimental model to reproduce the human L. braziliensis infection is a limiting factor for the development of biological and pharmacological approaches to address ATL.
Golden hamsters have proven to be an excellent model for cutaneous leishmaniasis given their high susceptibility to the Viannia species and the ability to reproduce many of the clinical and histopathological characteristics of human cutaneous leishmaniasis (13–15). Considering that hamsters present an outbred genetic background, it is expected that individual characteristics have an important role in different clinical outcomes of the disease, in such a way that they may reproduce immune responses observed in the human disease. Despite these advantages, few studies have involved L. braziliensis infection in the hamster model, and the protocols vary among them in terms of isolate and inoculum size (13, 16, 17). However, even when an infection is established with the same parasite numbers and L. braziliensis strain, the lesion development is variable. Moreover, although high inocula such as 106 parasites warrant lesion development, they also lead to visceralization, an occurrence that is not observed in human ATL (15). It is known that the biological characteristics of the parasites used in infections, such as the passage number in vitro, growth phase, developmental stage, or the metacyclic form index, can impact the clinical course of the experimental infections with Leishmania (18, 19). In both mice and hamsters, another factor that influenced lesion onset and size was the absolute parasite numbers in the inoculum (16, 20).
In the present study, we standardized conditions for the generation of inocula with different parasite numbers in order to investigate the parasite concentration that more closely reproduces the cutaneous leishmaniasis observed in human and the immunopathological aspects associated with these infections in the hamster model. We had hypothesized that different parasite numbers in the inoculum would induce different clinical presentations and tissue damage degrees and also lead to spleen visceralization differences. For this study, hamsters were infected with 104, 105, and 106 L. braziliensis parasites in a well-defined inoculum condition and were evaluated by clinical and immunopathological alterations.
We showed that in the chronic phase, the animals that were infected with a lower parasite inoculum (104) developed a disease phenotype that produced smaller lesions and less histopathological damage, although there was no difference in terms of tissue parasite load, IgG levels, or gamma interferon (IFN-γ) and interleukin 10 (IL-10) gene expression in comparison with that in animals infected with the 105 or 106 parasite inoculum.
MATERIALS AND METHODS
Animals and ethics statements.
Adult female outbred golden hamsters (Mesocricetus auratus) (6 to 10 weeks old), weighing 80 to 90 g, obtained from the animal facilities of the Fundação Oswaldo Cruz, were used. Sixty infected animals were separated into three groups in four independent experiments (n = 5 animals per group) according to the inoculum size, and 10 uninfected animals were used as the control. This study was approved by the Ethics Committee on Animal Use (CEUA) of Fundação Oswaldo Cruz—FIOCRUZ, with protocol number LW 11/11.
Parasites for infection and immunological studies.
Leishmania (Viannia) braziliensis (MCAN/BR/98/R619) was maintained in Schneider's Drosophila medium (Sigma Chemical Co., USA) supplemented with 20% fetal bovine serum (Life Technologies, Brazil), l-glutamine (1 mM; Life Technologies, Brazil), and antibiotics (200 U/ml penicillin and 200 μg/ml streptomycin; Sigma Chemical Co., USA). Parasites in the stationary growth phase from the third in vitro passage were washed in sterile phosphate-buffered saline (PBS) and counted on a hemocytometer. Inocula were prepared in a total volume of 30 μl of PBS containing 1 × 104, 1 × 105, or 1 × 106 parasites for intradermal inoculation into the dorsal hind paw of hamsters. Standardization of the inoculum included using the same parasite frozen stock to prepare cultures, culturing for three passages, determination of the percentage of metacyclic forms, determined by the complement lysis test as previous described (19), and measuring the pH of the culture medium by using a pH indicator strip (Merck, Darmstadt, Germany). The percentage of metacyclic forms ranged from 44% to 51.6%, and the pH ranged from 5.5 to 6.5. For immunological studies, Leishmania (Viannia) braziliensis (MHOM/BR/75/2903) parasites from stationary growth phase (Lb-Ag) were prepared as previous described (21) and kept at −20°C in a final concentration of 1 mg/ml.
Clinical evaluation of Leishmania (Viannia) braziliensis infection.
The clinical evolution of lesions was monitored weekly from day 10 up to 105 days postinfection, by measuring the paw dorsal-ventral thickness with a digital thickness gauge (Mitutoyo America Corporation, São Paulo, Brazil) and expressing the data in millimeters. The lesion size was determined by the difference between the thickness of the infected and the noninfected paws of the same animal. Discrepancies in lesion sizes were determined by the variance coefficient (VC = [standard deviation/mean] × 100). Clinical aspects of the lesions were qualified as nodular lesions, nodular and ulcerated lesions, or ulcerated ones. The macroscopic aspect of paws was also registered by digital photographs.
Quantification of parasite load from infected skin and popliteal lymph node.
Parasite loads from fragments of infected skin and adjacent lymph nodes were determined by a limiting dilution assay (LDA) as previously described (22). Briefly, fragments were placed onto a stainless steel screen containing 1 ml of Schneider's medium (Sigma, USA). The tissue was macerated with a pistil. Twenty microliters of the cell suspension was diluted into 180 μl of supplemented Schneider's medium in a Nunclon Delta 96-well microwell plate (Thermo Scientific, Denmark) in quadruplicate. From the first well, serial dilutions were made (dilution factor of 1:10). The plates were then incubated at 26°C and evaluated weekly for the presence of parasites over a period of 30 days. Results were obtained from the average from the last four wells where viable parasites were observed divided by the weight of fragments and then expressed as the number of parasites per gram of tissue.
Quantification of anti-Leishmania antibodies.
The levels of anti-Leishmania IgG were determined in plasma samples through an enzyme-linked immunosorbent assay (ELISA) as previously described (21) with some adaptations. Briefly, L. braziliensis parasite (MHOM/BR/1975/M2903) soluble antigen (40 μg/ml of PBS) was added (50 μl/well) to a polystyrene flat-bottom microtiter plate (Nunc-Immuno plate; Roskilde, Denmark) and incubated overnight in a humidified chamber. After plates were washed, 50 μl of plasma samples (diluted 1:5,000) from infected and uninfected (negative controls, n = 5) animals was added in duplicate, and the plate was incubated at room temperature. Horseradish peroxidase-labeled goat anti-hamster IgG (1:5,000) was used as a detector system (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The results were expressed as ELISA index (EI), obtained by the mean of sample absorbance divided by the mean of negative-control absorbance. The cutoff was determined by the receiver operator characteristic curve (ROC) method, based on the best relationship between sensibility and specificity.
Macroscopic analysis of spleens.
In order to evaluate the enlargement of spleens as a result of the presence of nodules in the parenchyma, which represents visceralization of Leishmania, spleens were submitted to a macroscopic inspection and weighed on a precision balance.
Skin and spleen histopathological analysis.
Fragments from the skin of the infected paws and spleens were fixed in 10% buffered formalin and processed for paraffin embedding. Sections of 4-μm thickness were stained with hematoxylin and eosin and then observed by light microscopy (Nikon Eclipse E600 microscope; Tokyo, Japan). Images were captured by a CoolSNAP-ProcCF camera and displayed by ImagePro Plus 4.5.1.29 (Media Cybernetics, Maryland, USA). Results from the skin histopathological analysis were expressed by score criteria as previously described (23), based on a semiquantitative analysis that evaluated the intensity of each histopathological feature: granuloma extension, presence of vacuolated macrophages, Leishmania amastigotes, Schaumann bodies, and necrosis. The scoring ranged from no observation (score = 0) to slight (score = 1), moderate (score = 2), or intense (score = 3) observation. Final results were given as means from the four independent experiments per inoculum, each one representing the sum of individual animals' scores. Dissemination of parasites from the inoculation site to spleens was confirmed by visualization of Leishmania or histopathological alterations (presence of granuloma) through histopathological analysis of spleens.
Tissue cytokine mRNA expression by real-time RT-PCR.
The samples (skin of paws and popliteal lymph nodes) were collected in RNAlater (Ambion, Life Technologies, Carlsbad, CA, USA) and frozen at −20°C until use. Total RNA was extracted from 20 to 30 mg of tissue using the RNeasy kit (Qiagen, Austin, TX, USA). All RNA samples were treated with RQ1 RNase-free DNase (Promega Corporation, Madison, WI, USA), quantified using the Pico 200 microliter spectrophotometer (Picodrop Ltd., Saffon Walden, United Kingdom), and kept at −80°C until they were ready to be used. RNA (0.8 μg) was reverse transcribed using the high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA, USA). For real-time PCR assays of each target gene, 2 μl cDNA was used to a final reaction volume of 15 μl, in duplicate, using 7.5 μl of TaqMan universal PCR mastermix (Applied Biosystems, USA) in ABI Prism 7500 Fast real-time PCR equipment (Applied Biosystems, USA). The sequences and concentrations of primers and probes for the hamster target genes as well as reverse transcription-quantitative PCR (RT-qPCR) cycling conditions used in this study were previously described (24). Results were calculated by relative quantification using the comparative threshold cycle method (ΔΔCT), as previously described (25), having as the endogenous control the expression of the GAPDH golden hamster constitutive gene (5′ to 3′, forward, GTGGAGCCAAGAGGGTCATC; reverse, GGTTCACACCCATCACAAACAT; probe, 5′-FAM-TCTCCGCACCTTCTGCTGATGCC-3′-TAMRA (GenBank accession no. DQ403055.1), where FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine. Subsequently, we calculated the ΔΔCT based on the calibrator, represented by uninfected animals. Final results were expressed by 2−ΔΔCT, representing the number of times that there was a change in cytokine gene expression in relation to the calibrator (fold change).
Statistical analysis.
The results were expressed as the means ± standard deviations and medians with interquartile ranges. Statistical tests were performed with GraphPad Prism software version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Comparison between experimental groups was performed using one-way analysis of variance (ANOVA). A parametric or nonparametric test was selected according the distribution of the raw data, followed by a posttest analysis for multiple groups as appropriate. Correlation analysis was performed based on Spearman's or Pearson's rank correlation test, according to data distribution. Significant differences were considered when P values were <0.05.
RESULTS
The Leishmania (Viannia) braziliensis parasite concentration in the inoculum influenced the skin lesion clinical course.
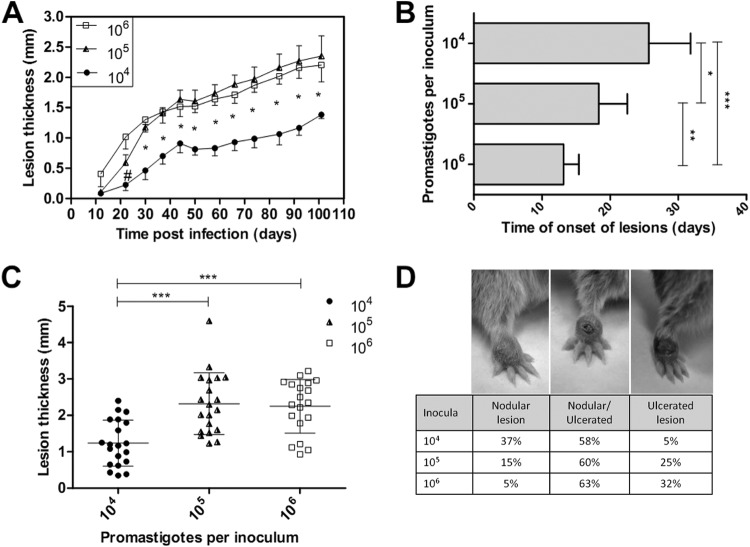
The lesion development kinetics over 105 days revealed a chronic and progressive course of evolution. None of the infected animals evolved with a spontaneous resolution of their lesions. The lesion sizes in the animals infected with 104 parasites were significantly smaller than those in animals inoculated with 105 and 106 parasites in all measurements that were conducted over the entire clinical observation period (P < 0.05). These two last groups showed similar lesion sizes at most points of the kinetic curve (Fig. 1A). The parasite inoculum concentration directly influenced the lesion time onset with an inverse relationship (Fig. 1B). Clinical detection of lesions was observed later in the animals infected with 104 parasites (mean of 25.7 ± 6.1 days postinfection) than in the animals infected with 105 (mean = 18.3 ± 4.2 days postinfection) or 106 (mean = 13.2 ± 2.2 days postinfection) parasites. Significant differences were observed between the 104 and 105 groups (P < 0.05), between the 105 and 106 groups (P < 0.01), and between the 104 and 106 groups (P < 0.0001). Additionally, the mean lesion size at the endpoint was significantly lower in the animals infected with 104 parasites (1.24 ± 0.63 mm; median = 1.18 mm; n = 20 animals; P < 0.0001) than in those infected with 105 parasites (2.32 ± 0.85 mm; median = 2.22 mm; n = 20 animals) or 106 parasites (2.25 ± 0.74 mm; median = 2.35 mm; n = 19 animals) (Fig. 1C).
FIG 1.
Evaluation of the golden hamster skin lesions that were infected with Leishmania (Viannia) braziliensis at three different inoculum concentrations (104, 105, and 106 parasites). The lesion sizes were measured by evaluating the dorsal-ventral thickness differences between the infected and uninfected paws. These data are expressed in millimeters. (A) Skin lesion development kinetics through the 105 days following the infections. The graph represents four independent experiments (median and standard error; n = 59 animals). (B) The onset of clinical skin lesions for each inoculum concentration, given in days postinfection (mean ± SD); (C) final skin lesion measurements 105 days after infection (mean ± SD; n = 59 animals; P < 0.0001); (D) final lesion clinical aspect images 105 days after infection. Left, a nodular lesion; middle, a nodular and an ulcerated lesion; right, an ulcerated lesion. #, a significant difference was observed only between the 104 and 106 parasite-infected groups (P < 0.05). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The clinical aspects of the lesions from the animals infected with 104 parasites differed from those in animals infected with 105 and 106 parasites. The animals infected with 104 parasites exhibited a higher percentage of nodular lesions than the latter groups, whereas 105 and 106 parasite-infected animals exhibited more nodular and ulcerated lesions, which was considered a more destructive outcome (Fig. 1D). Notably, all the animals inoculated with either 105 or 106 parasites presented clinical lesions, and only one animal that was infected with 104 parasites (5%) did not present any visible signs of a skin lesion.
The variability pattern for the lesion sizes was determined by a variance coefficient that was calculated between each experiment for a given group. From the four experiments in which the animals were infected with 104 parasites, the lesion sizes presented a heterogeneous pattern (VC > 30%). This pattern was observed in two experiments that used 105 and 106 parasite inocula, while the other two experiments in each group showed an intermediary pattern (VC between 15% and 30%). None of the experimental comparisons displayed a homogenous pattern (VC < 15%) for either of the inoculum groups.
Skin lesions and lymph nodes presented high parasite loads that were independent of the inoculum concentration of Leishmania (Viannia) braziliensis.
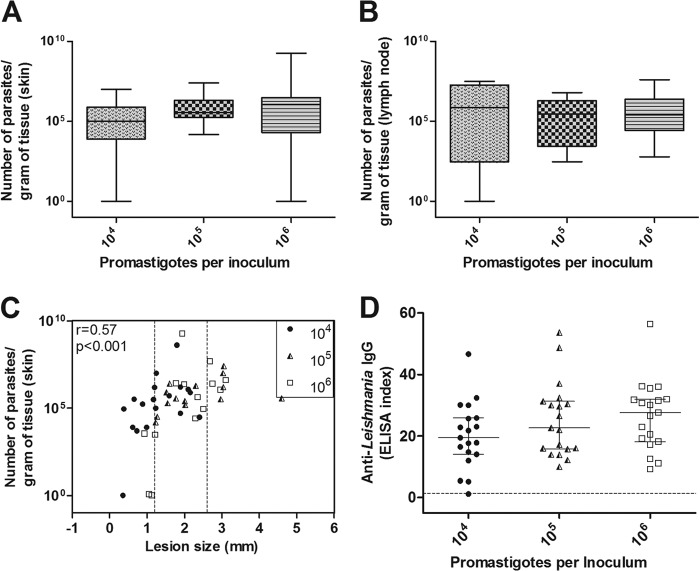
Although there was an observed trend for increased skin and popliteal lymph node parasite loads according to inocula, it was not statistically significant. The animals infected with 104 parasites presented with skin lesions that had a median of 1 × 105 parasites/g, whereas the animals infected with 105 parasites had 3.6 × 105 parasites/g, and in those infected with 106 parasites, the observed median was 11.0 × 105 parasites/g (Table 1, Fig. 2A). The adjacent lymph nodes also did not present a significant difference between the median values of the three groups: 104 = 7.4 × 105 parasites/g, 105 = 2.9 × 105 parasites/g, and 106 = 2.6 × 105 parasites/g (Table 1, Fig. 2B). The skin parasite load, however, was positively correlated with the lesion size (r = 0.57, P < 0.0001). Of note in Fig. 2C, a region is shown that is delineated by dotted lines in which 58.7% of the animals were infected with either of the three inocula presented and had lesions with a measured size between 1.2 and 2.6 mm (interquartile range, 25% to 75%), which was independent of the inoculum and final parasite load.
TABLE 1.
Summary of the clinical, parasitological, and immunological feature numerical data of the golden hamsters at 105 days postinfection with different Leishmania (Viannia) braziliensis parasite numbersa
| Control or parasite no. | Skin lesion parasite load (no. of parasites/g of tissue) | Lymph node parasite load (no. of parasites/g of tissue) | Spleen wt (g) | Fold change |
% spleen visceralization of Leishmania | |||
|---|---|---|---|---|---|---|---|---|
| Skin IFN-γ expression | Lymph node IFN-γ expression | Skin IL-10 expression | Lymph node IL-10 expression | |||||
| Control | 0.240 (0.204–0.265) | 1.0 (0.1–3.1) | 1.5 (0.9–1.6) | 1.0 (0.2–2.7) | 0.9 (0.7–2.3) | |||
| 104 | 1 × 105 (0.08 × 105–7.7 × 105) | 7.4 × 105 (0.003 × 105–190 × 105) | 0.289 (0.257–0.361) | 229 (137.8–1,158) | 39.3 (28.6–55.9) | 7.3 (1.9–14) | 1.2 (0.7–2.5) | 16 (4/25) |
| 105 | 3.6 × 105 (1.8 × 105–20 × 105) | 2.9 × 105 (0.028 × 105–19 × 105) | 0.326 (0.273–0.697) | 723.6 (357.4–1,502) | 30.2 (20.6–45) | 3.7 (2.4–8.8) | 2.6 (0.57–5.5) | 23.3 (7/30) |
| 106 | 11.0 × 105 (0.2 × 105–30 × 105) | 2.6 × 105 (0.27 × 105–24 × 105) | 0.380 (0.270–0.547) | 454.8 (247–975) | 41.1 (24.1–46.9) | 5.3 (1.6–12.8) | 3.2 (2.0–8.2) | 69.2 (18/26) |
Data are median (interquartile ranges) and percentage (no. of affected animals/no. of total animals analyzed) values.
FIG 2.
The parasite load and anti-Leishmania immunoglobulin levels in the golden hamsters that were infected with different Leishmania (Viannia) braziliensis concentrations (104, 105, and 106 parasites) after 105 days of infection. The skin parasite (A) and lymph node parasite (B) loads are shown. (C) The correlation between skin parasite loads and lesion sizes (the medians and interquartile ranges are shown). The dotted lines represent intersection regions in which the lesions that were generated by the three different inocula measured between 1.2 and 2.6 mm (58.7% of the total animals). (D) Anti-Leishmania IgG serum levels. The dotted line represents the cutoff value. Each point represents one animal, and the error bars represent the medians and interquartile ranges (r = correlation coefficient; p = significance level).
Different Leishmania (Viannia) braziliensis numbers in inoculum produced similar anti-Leishmania species IgG levels.
All of the animal groups presented high anti-Leishmania IgG levels that were independent of different parasite inoculum concentrations, with no significant statistical difference between them. The animals that developed small lesions or did not develop lesions had low IgG levels or produced no IgG, respectively. A weak but positive correlation was observed between lesion sizes and anti-Leishmania IgG levels (r = 0.31, P = 0.02; data not shown). The average antibody levels (ELISA index) for the 104, 105, and 106 parasite-infected groups were 20.1 ± 10.8 (median = 19.4), 25.2 ± 12.1 (median = 22.7), and 26.6 ± 11.2 (median = 27.6), respectively (Fig. 2D).
The spleen weights varied with the highest parasite inoculum concentration and were correlated with lesion severity.
As spleen enlargement is a common finding observed in L. braziliensis-infected hamsters (15), which show the presence of nodules in the parenchyma of the organ, we evaluated spleen weights at the endpoint of infection. The animals infected with 104 parasites did not exhibit significant spleen enlargement (median of 0.289 g) in comparison with that of uninfected animals (median of 0.240 g). The spleen weights from both 105 (median of 0.326 g) and 106 (median of 0.380 g) parasite-infected animals were higher than those of the controls animals (P < 0.05). Notably, seven out of 19 animals in both groups presented spleen weights equal to or larger than 0.500 g. A positive correlation between spleen weight and lesion size was observed (r = 0.57, P < 0.0001) (Table 1, Fig. 3A and B).
FIG 3.
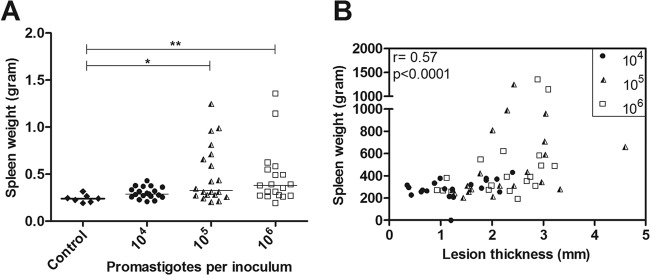
The spleen weights in grams from the golden hamsters that were infected with Leishmania (Viannia) braziliensis at three different inoculum concentrations (104, 105, and 106 parasites) after 105 days of infection. (A) The spleen weights in the control and in the three inoculum groups. The horizontal bars represent the medians. *, P < 0.05; **, P < 0.01. (B) Skin lesion sizes positively correlated with spleen weight (Spearman correlation). Each point represents one animal (r = correlation coefficient; p = significance level; *, P < 0.05; **, P < 0.01).
Infections with different L. (V.) braziliensis inoculum concentrations were characterized by high IFN-γ expression in skin and lymph node.
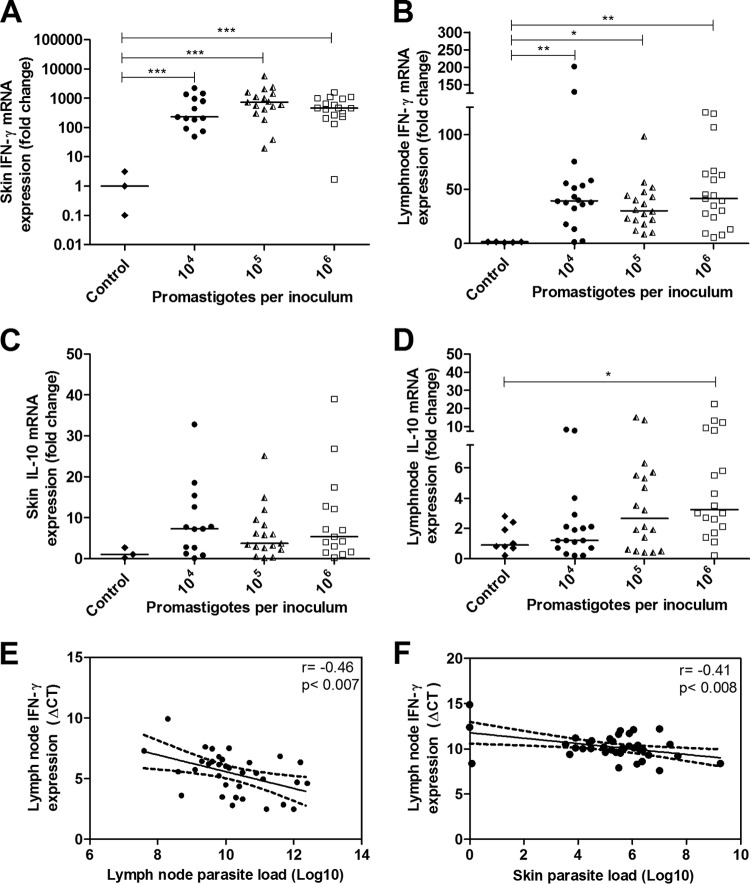
High IFN-γ expression was observed in the skin of the L. braziliensis-infected animals compared with that of the control group (P < 0.0001), independent of the inoculum concentrations, and no significant difference was observed between the three infected groups (Table 1, Fig. 4A). The adjacent lymph nodes also presented high IFN-γ expression levels compared with those of the uninfected control group (P < 0.01 with the 105 group and P < 0.0001 with the 104 and 106 groups) (Table 1, Fig. 4B); however, there were no significant differences between the groups. IFN-γ expression in the lymph nodes was at least 10 times lower than the IFN-γ expression in the skin. The expression of IL-10 in the skin of animals infected with 104, 105, and 106 parasites was similar to that in the uninfected control group (Table 1, Fig. 4C). The lymph nodes from the 106 parasite-infected group had a 3.5-fold increase in IL-10 levels compared with those of the control group (P < 0.05) (Table 1, Fig. 4D). A negative correlation was observed between the lymph node IFN-γ ΔCT and the lymph node parasite load (r = −0.46, P < 0.007) (Fig. 4E) and skin parasite load (r = −0.41, P < 0.008) (Fig. 4F), which indicated that as parasite loads in the lymph nodes and skin increased, IFN-γ expression in lymph nodes also increased.
FIG 4.
Cytokine mRNA expression as determined by RT-qPCR in the organs of golden hamsters infected with different Leishmania (Viannia) braziliensis parasite concentrations (104, 105, 106 parasites) at 105 days postinfection. The control group represents the uninfected animals. The results are expressed as relative fold changes between the experimental samples and the skin or lymph nodes of a control animal to which the value 1 was arbitrarily assigned. IFN-γ mRNA expression in skin lesions (A) and lymph nodes (B) and IL-10 mRNA expression in skin lesions (C) and lymph nodes (D) are shown. Pearson's correlations between the parasite load and IFN-γ expression in the lymph nodes (E) and the parasite load in skin and the IFN-γ expression in the lymph node (F) are also displayed. Each point represents one animal, and the error bars represent the medians and interquartile ranges. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
A high parasite inoculum concentration contributed to a high degree of tissue damage and was associated with spleen visceralization of dermotropic Leishmania.
The pathological parameters of the Leishmania infections and tissue damage were semiquantitatively evaluated and ranked with scores as shown in Fig. 5A. Significant differences were observed between the groups infected with 104 and 105 parasites (P < 0.05) and between the groups infected with 104 and 106 parasites (P < 0.05) (Fig. 5A). A positive correlation was observed between lesion sizes and histopathological scores, in which a higher degree of tissue damage was associated with a greater lesion size (Fig. 5B). Additionally, an association between the skin parasite load and histopathological features was also observed (r = 0.47, P < 0.008) (data not shown).
FIG 5.
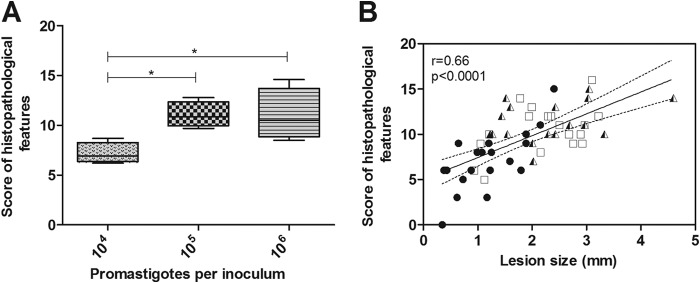
Skin lesion histopathological analyses of golden hamsters infected with different Leishmania (Viannia) braziliensis parasite concentrations (104, 105, 106 parasites) at 105 days postinfection. (A) Histopathological features measured by scores in the animal groups infected with 104, 105, and 106 parasites (medians and interquartile ranges) are shown. (B) A correlation between the skin histopathological feature scores and the lesion sizes of the animals infected with 104, 105, and 106 parasites. Each point represents one animal. The correlation graph shows a fit line with a confidence curve. r = correlation coefficient; p = significance level; *, P < 0.05.
All infected animals displayed a similar histopathological pattern, which was represented by a granulomatous reaction that was surrounded and/or interspaced by neutrophils, eosinophils, lymphocytes, plasma cells, and the occasional presence of small foci of fibrinoid necroses. The Leishmania intensity was scarce in the majority of the animals. The main difference, at the histopathological level, between the three different inocula was the degree of tissue damage, which was represented by an extension of the granulomatous inflammatory infiltrates and necroses. This was usually proportionally more important in the higher inocula. Parameters such as the granuloma extension (Fig. 6A and B), vacuolated macrophage intensity (Fig. 6C), presence of amastigotes (Fig. 6C), and the presence of Schaumann bodies (Fig. 6D) were considered pathological alterations due to the Leishmania infections.
FIG 6.
Photomicrography of organs from golden hamsters infected with different Leishmania (Viannia) braziliensis concentrations (104, 105, and 106 parasites) at 105 days postinfection. The following are displayed: a localized dermal granuloma that is representative of a 104 parasite-infected animal (hematoxylin and eosin; ×10 magnification) (A), a dermal spread granulomatous reaction that is representative of a 106 parasite-infected animal (arrow, necrosis; arrowhead, Schaumann bodies) (hematoxylin and eosin; ×20 magnification) (B), macrophage vacuoles with amastigotes (arrow) inside a dermal granuloma of a 106 parasite-infected animal (hematoxylin and eosin; ×100 magnification) (C), Schaumann bodies inside a dermal granuloma (arrows) of a 106 parasite-infected animal (hematoxylin and eosin; ×10 magnification) and Schaumann bodies inside a dermal multinucleated giant cell of a 106 parasite-infected animal (inset, ×40 magnification) (D), granulomas in the spleen parenchyma (arrows) (hematoxylin and eosin; ×10 magnification) that are representative of macroscopic splenic nodules of a 106 parasite-infected animal (inset, arrowhead) (E), and splenic vacuolated macrophages that contain Leishmania, which are representative of a 106 parasite-infected animal (arrow) (hematoxylin and eosin; ×100 magnification) (F).
The histopathological analysis of the spleens of the highest inoculum-infected animals showed, compared with the lower inoculum (see Fig. S1 in the supplemental material), enlarged organs with nodules in the parenchyma (inset in Fig. 6E) and extensive areas with granulomas (Fig. 6E) that sometimes had vacuolated macrophages with intracytoplasmic Leishmania (Fig. 6F arrow). Leishmania visceralization was observed in 16% of the 104 (4/25), in 23.3% of the 105 (7/30), and in 69.2% of the 106 (18/26) parasite-infected animals (Table 1).
DISCUSSION
It is known that the parasite number in the infection dose is essential to disease outcomes in both murine (26–28) and hamster (16) models of ATL. The variability of the final lesion sizes and spleen visceralization observed in hamsters infected with 106 L. braziliensis parasites (15) prompted us to ask if such a high inoculum could interfere with the generation of an effective model system that tests the protective effects of vaccine candidates and the effectiveness of new drugs under development. In the present study, we used three different L. braziliensis inocula in a standardized protocol to reduce the variability that is inherent to the hamster outbred genetic background and to test the inoculum that ensures infection but does not lead to an exacerbated disease.
The lesion time onset in the L. braziliensis-infected hamsters that were studied was inversely proportional to the parasite inoculum concentration (104, 105, and 106), as was observed in previous studies (13, 16). However, at the infection endpoint, after 105 days, we observed that 105 and 106 parasite inocula caused the same lesion sizes and that these were significantly larger than lesions in the hamsters infected with 104 parasites. These results were also reported in an L. major infection mouse model (20). Nevertheless, the consequences of each inoculum in the clinical and immunopathologic evolution of lesions produced by L. braziliensis in a hamster model of infection are not yet known.
The initial immune response elicited by an infection can be dependent upon the parasite inoculum concentration, which can influence the establishment of the chronic phase. It has been hypothesized that a threshold limit of dermally infected macrophages or of parasites released has not been reached in the early infection phase (known as the silent phase) in animals infected with lower inocula. It may render a quiescent development of parasites that are restricted to macrophages at the inoculation site without stimulation of IL-12 and IFN-γ production by lymph node T cells, which leads to lesion development (29). This phenomenon could explain the longer onset of lesions in the 104 parasite-infected animals observed in the present work. From this same point of view, the 105 and 106 parasite-infected animals could have reached this threshold limit earlier, which would elicit cytokine production and lead to earlier lesion development. The lesion size differences observed in the present work with the variance coefficients were also observed in inbred mouse models (20, 30), suggesting that the outbred genetic background of the hamster model is not the main factor that contributes to this variability.
Although the lesion onset was more precocious in the animals that were infected with higher parasite concentration inocula, no differences in the final parasite load (i.e., the chronic phase of infection) were observed between the three groups. The same behavior was observed in the L. major mouse model, in which infections with low and high Leishmania doses led to an equivalent parasite load during the chronic infection phase (28). It is possible that an excess of parasites could imply an increased Leishmania extracellular death, because the threshold of macrophage infection was achieved.
Despite the similarity in the parasite load endpoint between the three inocula, the clinical aspects of lesions and the histopathological features varied in animals infected with the lower inoculum compared to those in animals infected with the highest inoculum. The animals infected with 104 parasites presented a higher percentage of nodular lesions than the 105 and 106 parasite-infected groups, which presented a higher percentage of nodular and ulcerated lesions. Accordingly, a reduced skin inflammatory infiltrate was observed in the 104 parasite-infected animal group in comparison with that of the 105 or 106 group. In agreement, mice infected with low and high L. major parasite numbers resulted in minor and severe skin pathological damage, respectively (28). These results suggest that parasite replication during the silent infection phase could also dictate the magnitude of the inflammatory reaction in the infection site. Afterward, the parasite-specific effector response, which was triggered by the highest inoculum (105 or 106), should sustain an unregulated inflammatory response until the chronic phase occurs. Conversely, it is possible that the longer clinical lesion onset and the less severe tissue damage observed in animals infected with 104 parasites can be explained by a delay in the capacity for the parasites to reach the replication threshold and, consequently, recruit fewer inflammatory cells to the inoculation site.
Another possible explanation for the differences in tissue damage and clinical presentation by the different parasite inoculum concentrations could be the cytokine profile. Previous reports regarding human L. braziliensis infections demonstrated a positive correlation between large and ulcerated lesions with IFN-γ and tumor necrosis factor (TNF) levels, which suggests that these cytokines contribute to tissue injury (31). Recent results also have shown that tissue damage in cutaneous lesions can be attributed to CD8+ granzyme B+ T cells, while CD4+ T cells that produced IFN-γ did not correlate with lesion size but did correlate with parasite killing (32). In our hamster model, however, high IFN-γ levels were expressed during the infection endpoint, independent of the inoculum concentration used, lesion size, or clinical presentation. Notably, no detectable IFN-γ expression was observed in a unique animal that was infected with 104 parasites that did not present a lesion, which reinforces a possible role for IFN-γ in lesion development.
The higher IFN-γ expression along with the presence of parasites gave rise to the hypothesis that IFN-γ was not sufficient to control parasite replication. In hamster visceral leishmaniasis, a diminished capacity for IFN-γ to activate macrophages was attributed to limited binding of IFN-γ to the IFN-γ receptor, which was compensated by increased IFN-γ expression (33, 34). Later studies have shown that this impairment in parasite killing was not due to IFN-γ inactivity but to a diminished NO production due to low inducible nitric oxide synthase (iNOS) expression and activity, despite a strong observed Th1 response (35, 36). A nonpolarized mixed type 1 and type 2 cytokine pattern, which was conferred by high IFN-γ, IL-12p40, IL-4, IL-10, IL-13, and IL-21 levels, was detected in the skin of animals infected with L. panamensis during the early infection phase (24). The coexpression of type 1 and type 2 cytokines was also observed in chronic lesions of hamsters infected with L. panamensis (14). This inflammatory response can contribute to skin tissue damage; however, the role of other cytokines has to be taken into account. Another explanation for the extensive tissue damage can be low expression or no modulation of IL-10 mRNA, which has been observed in the majority of tissues of all animals and leads to a decreased ability to downregulate IFN-γ mRNA. In human leishmaniasis patients, a high IFN-γ/IL-10 ratio is associated with more severe clinical forms of the disease, such as mucosal leishmaniasis (37, 38).
Visceralization of dermotropic Leishmania spp. distant from the inoculation site is considered a common finding in hamsters and mice that are experimentally infected with inocula that contain a high parasite concentration (13, 15, 17, 39, 40). This also correlated with lesion size and consequently with infection severity (15). In the animals that were inoculated with 104 parasites, the involvement of the spleen was observed only in 16% of animals, compared to 70% for animals infected with 106 parasites.
In the present work, well-organized granulomas that contained amastigotes were easily detectable in the spleens of the Leishmania-infected hamsters. We hypothesized that these granulomas have a well-organized formation but are not functional (41), at least in this infection phase, because the amastigotes were not completely cleared. This finding reinforces the presence of the Th1 response that was generated by the hamster, because granuloma formation requires IL-12, IFN-γ, and TNF (42). Granuloma formation is also possibly associated with deficient NO generation, which is required to kill parasites within granulomas. Additionally, this is known to occur in hamster models (36).
A positive correlation between anti-Leishmania IgG and lesion size is a common finding in dermotropic Leishmania species infections in hamster models (14, 15) and in humans (43) and could be considered a biomarker of the active cutaneous disease. Herein, we also observed that the disease was accompanied by high anti-Leishmania IgG levels, although there were no differences between the inoculum groups. Because the measurements of antibody levels were made at the infection endpoint, the antibody production and seroconversion kinetics were unknown. Notably, hamsters infected with L. infantum presented increased anti-Leishmania IgG levels that were associated with time following infections (44). Thus, further studies may help to explain whether the onset of lesions is associated with B lymphocyte activation and antibody production.
In summary, different parasite inoculum concentrations influenced disease onset, clinical presentation, final lesion size, and the degree of tissue damage but did not lead to differences in the final parasite load, cytokine expression, or anti-Leishmania IgG levels between the three inocula. These observations indicate that in the early infection phase, during the innate immune response, the differences observed between the different parasite inoculum concentrations dictate the establishment and magnitude of the adaptive immune response, which thus determines the chronic disease phase. The inoculum with 104 parasites generated more benign lesions and a less systemic commitment than the higher-parasite-number inocula, which was more consistent with ATL presentation in humans.
Supplementary Material
ACKNOWLEDGMENTS
David William Provance provided the English review and Pedro E. Cabello provided the statistical analysis.
R.P.R.-R., E.F.P., and A.M.D.-C. participated in the conception and design of the study, analysis, and interpretation of data.
Financial support was provided by FAPERJ, CAPES, and CNPq.
Footnotes
Published ahead of print 6 October 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02083-14.
REFERENCES
- 1.Schriefer A, Schriefer AL, Góes-Neto A, Gumarães LH, Carvalho LP, Almeida RP, Machado PR, Lessa HÁ de Jesus AR, Riley LW, Carvalho EM. 2004. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American cutaneous leishmaniasis. Infect. Immun. 72:508–514. 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa JM, Saldanha AC, Nascimento D, Sampaio G, Carneiro F, Lisboa E, Silva ML, Barral A. 2009. Clinical modalities, diagnosis and therapeutic approach of the tegumentary leishmaniasis in Brazil. GM Bahia 79:70–83. [Google Scholar]
- 3.Cupolillo E, Brahim LR, Toaldo CB, de Oliveira-Neto MP, de Brito ME, Falqueto A, de Farias Naiff M, Grimaldi G., Jr 2003. Genetic polymorphism and molecular epidemiology of Leishmania (Viannia) braziliensis from different hosts and geographic areas in Brasil. J. Clin. Microbiol. 41:3126–3132. 10.1128/JCM.41.7.3126-3132.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutinho S, Pirmez C, Mendonça S, Conceição-Silva F, Dórea R. 1987. Pathogenesis in immunopathology of leishmaniasis. Mem. Inst. Oswaldo Cruz 82:214–228. 10.1590/S0074-02761987000500005. [DOI] [Google Scholar]
- 5.Jeronimo SM, Duggal P, Ettinger NA, Nascimento ET, Monteiro GR, Cabral AP, Pontes NN, Lacerda HG, Queiroz PV, Gomes CE, Pearson RD, Blackwell JM, Beaty TH, Wilson ME. 2007. Genetic predisposition to self-curing infection with the protozoan Leishmania chagasi: a genomewide scan. J. Infect. Dis. 196:1261–1269. 10.1086/521682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7:581–596. 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho LP, Passos S, Schriefer A, Carvalho EM. 2012. Protective and pathologic immune response in human tegumentary leishmaniasis. Front. Immunol. 3:301. 10.3389/fimmu.2012.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Moura TR, Novais FO, Oliveira F, Clarêncio J, Noronha A, Barral A, Brodskyn C, de Oliveira CI. 2005. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect. Immun. 73:5827–5834. 10.1128/IAI.73.9.5827-5834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salay G, Dorta ML, Santos NM, Mortata RA, Brodskyn C, Oliveira CI, Barbiéri CL, Rodrigues MM. 2007. Testing of four Leishmania vaccine candidates in a mouse model of infection with Leishmania (Viannia) braziliensis, the main causative agent of cutaneous leishmaniasis in the New World. Clin. Vaccine Immunol. 14:1173–1181. 10.1128/CVI.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neal RA, Hale C. 1983. A comparative study of susceptibility of inbred and outbred mouse strains compared with hamsters to infection with New World leishmaniasis. Parasitology 87:7–13. 10.1017/S0031182000052379. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira CI, Teixeira MJ, Gomes R, Barral A, Brodskyn C. 2004. Animal models for infectious diseases caused by parasites: leishmaniasis. Drug Discov. Today 1:81–86. 10.1016/j.ddmod.2004.07.005. [DOI] [Google Scholar]
- 12.Dekrey GK, Lima HC, Titus RG. 1998. Analysis of the immune responses of mice infected with Leishmania braziliensis. Infect. Immun. 66:827–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahl L, Byram J, David J, Comerford S, Von Lichtenberg F. 1991. Leishmania (Viannia) braziliensis: comparative pathology of golden hamsters infected with isolates from cutaneous and mucosal lesions of patients residing in Tres Bracos, Bahia, Brazil. Am. J. Trop. Med. Hyg. 44:218–232. [DOI] [PubMed] [Google Scholar]
- 14.Osorio Y, Melby PC, Pirmez C, Chandrasekar B, Guarín N, Travi BL. 2003. The site of cutaneous infection influences the immunological response and clinical outcome of hamsters infected with Leishmania panamensis. Parasite Immunol. 25:139–148. 10.1046/j.1365-3024.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 15.Gomes-Silva A, Valverde JG, Ribeiro-Romão RP, Plácido-Pereira RM, Da-Cruz AM. 2013. Golden hamster (Mesocricetus auratus) as an experimental model for Leishmania (Viannia) braziliensis infection. Parasitology 140:771–779. 10.1017/S0031182012002156. [DOI] [PubMed] [Google Scholar]
- 16.Wilson H, Dieckmann B, Childs B. 1979. Leishmania braziliensis and Leishmania mexicana: experimental cutaneous infection in golden hamsters. Exp. Parasitol. 47:270–283. 10.1016/0014-4894(79)90079-1. [DOI] [PubMed] [Google Scholar]
- 17.Almeida M, Cuba-Cuba C, Moraes M, Miles M. 1996. Dissemination of Leishmania (Viannia) braziliensis. J. Comp. Pathol. 115:311–316. 10.1016/S0021-9975(96)80088-0. [DOI] [PubMed] [Google Scholar]
- 18.Rey JA, Travi BL, Valencia AZ, Saraiva NG. 1990. Infectivity of the subspecies of the Leishmania braziliensis complex in vivo and in vitro. Am. J. Trop. Med. Hyg. 43:623–631. [DOI] [PubMed] [Google Scholar]
- 19.Gamboa D, Torres K, De Doncker S, Zimic M, Arevalo J, Dujardin JC. 2008. Evaluation of an in vitro and in vivo model for experimental infection with Leishmania (Viannia) braziliensis and Leishmania (V.) peruviana. Parasitology 135:319–326. 10.1017/S0031182007003848. [DOI] [PubMed] [Google Scholar]
- 20.Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmannt H. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257:539–542. [DOI] [PubMed] [Google Scholar]
- 21.Gomes-Silva A, Souza MA, Afonso-Cardoso SR, Andrade LR, Dietze R, Lemos E, Belli A, Favoreto Júnior S, Ferreira MS. 2008. Serological reactivity of different antigenic preparations of Leishmania (Leishmania) amazonensis and the Leishmania braziliensis complex. Rev. Soc. Bras. Med. Trop. 41:135–141. 10.1590/S0037-86822008000200001. [DOI] [PubMed] [Google Scholar]
- 22.Titus RG, Marchand M, Boon T, Louis JA. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545–555. 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Zhao Y, Chen X, Jiang B, Sun D. 2013. The protective effect of recombinant Lactococcus lactis oral vaccine on a Clostridium difficile-infected animal model. BMC Gastroenterol. 13:117. 10.1186/1471-230X-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espitia CM, Zhao W, Saldarriaga O, Osorio Y, Harrison LM, Cappello M, Travi BL, Melby PC. 2010. Duplex real-time reverse transcriptase PCR to determine cytokine mRNA expression in a hamster model of New World cutaneous leishmaniasis. BMC Immunol. 22:11–31. 10.1186/1471-2172-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408. 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Doherty M, Coffman R. 1996. Leishmania major: effect of infectious dose on T cell subset development in BAL/c mice. Exp. Parasitol. 84:124–135. 10.1006/expr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 27.Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, Ribeiro J, Sacks DL. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941–1953. 10.1084/jem.188.10.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimblin N, Peters N, Debrabant A, Secundino N, Egen J, Lawyer P, Fay MP, Kamhawi S, Sacks D. 2008. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc. Natl. Acad. Sci. U. S. A. 105:10125–10130. 10.1073/pnas.0802331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969–977. 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- 30.Pereira CG, Silva AL, de Castilhos P, Mastrantonio EC, Souza RA, Ribeiro-Romão RP, Rezende RJ, Pena JD, Beletti ME, Souza MA. 2009. Different isolates from Leishmania braziliensis complex induce distinct histopathological features in a murine model of infection. Vet. Parasitol. 165:231–240. 10.1016/j.vetpar.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Antonelli LRV, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. 2005. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol. Lett. 101:226–230. 10.1016/j.imlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Santos CS, Boaventura V, Ribeiro Cardoso C, Tavares N, Lordelo MJ, Noronha A, Costa J, Borges VM, Oliveira CI, Van Weyenbergh J, Barral A, Barral-Netto M, Brodskyn CI. 2013. CD8(+) granzyme B(+) mediated tissue injury VS. CD4(+)IFN-γ(+)-mediated parasite killing in human cutaneous leishmaniasis. J. Investig. Dermatol. 133:1533–1540. 10.1038/jid.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melby PC, Tryon VV, Chandrasekar B, Freeman GL. 1998. Cloning of Syrian hamster (Mesocricetus auratus) cytokine cDNAs and analysis of cytokine mRNA expression in experimental visceral leishmaniasis. Infect. Immun. 66:2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W, Valencia AZ, Melby PC. 2006. Biological activity of hamster interferon-gamma is modulated by the carboxyl-terminal tail. Cytokine 34:243–251. 10.1016/j.cyto.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Melby CP, Chandrasekar B, Zhao W, Coe E. 2001. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J. Immunol. 166:1912–1920. 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- 36.Perez L, Chandrasekar B, Saldarriaga O, Zhao W, Arteaga L, Travi B, Melby P. 2006. Reduced nitric oxide synthase 2 (NOS2) promoter activity in the Syrian hamster renders the animal functionally deficient in NOS2 activity and unable to control an intracellular pathogen. J. Immunol. 176:5519–5528. 10.4049/jimmunol.176.9.5519. [DOI] [PubMed] [Google Scholar]
- 37.Gomes-Silva A, Bittar RC, Nogueira RS, Amato VS, Mattos MS, Oliveira-Neto MP, Coutinho SG, Da-Cruz A. 2007. Can interferon-γ and interleukin 10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin. Exp. Immunol. 149:440–444. 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novoa R, Bacellar O, Nascimento M, Cardoso TM, Ramasawmy R, Oliveira WN, Schriefer A, Carvalho EM. 2011. IL-17 and regulatory cytokines (IL-10 and IL-27) in L. braziliensis infection. Parasite Immunol. 33:132–136. 10.1111/j.1365-3024.2010.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abreu-Silva AL, Calabrese KS, Cupolilo SMN, Cardoso FO, Souza CSF, Gonçalves da Costa SC. 2004. Histopathological studies of visceralized Leishmania (Leishmania) amazonensis in mice experimentally infected. Vet. Parasitol. 121:179–187. 10.1016/j.vetpar.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Soliman MFM. 2006. The persistence, dissemination and visceralization tendency of Leishmania major in Syrian hamsters. Acta Trop. 97:146–150. 10.1016/j.actatropica.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, Zubairi S, Engwerda CR. 2004. The immunopathology of experimental visceral leishmaniasis. Immunol. Rev. 201:239–253. 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 42.Murray HW. 2001. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Path. 82:249–267. 10.1046/j.1365-2613.2001.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomes-Silva A, Pereira-Carvalho R, Fagundes-Silva GA, Oliveira-Neto MP, Da-Cruz AM. 2009. Homeostasis of specific immune response in clinically cured cutaneous leishmaniasis subjects due to Leishmania (Viannia) braziliensis. Rev. Soc. Bras. Med. Trop. 42:147–150. [Google Scholar]
- 44.Requena JM, Soto M, Doria MD, Alonso C. 2000. Immune and clinical parameters associated with Leishmania infantum infection in the golden hamster model. Vet. Immunol. Immunopathol. 76:269–271. 10.1016/S0165-2427(00)00221-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.