Abstract
Model antigens are frequently introduced into pathogens to study determinants that influence T-cell responses to infections. To address whether an antigen's subcellular location influences the nature and magnitude of antigen-specific T-cell responses, we generated Plasmodium berghei parasites expressing the model antigen ovalbumin (OVA) either in the parasite cytoplasm or on the parasitophorous vacuole membrane (PVM). For cytosolic expression, OVA alone or conjugated to mCherry was expressed from a strong constitutive promoter (OVAhsp70 or OVA::mCherryhsp70); for PVM expression, OVA was fused to HEP17/EXP1 (OVA::Hep17hep17). Unexpectedly, OVA expression in OVAhsp70 parasites was very low, but when OVA was fused to mCherry (OVA::mCherryhsp70), it was highly expressed. OVA expression in OVA::Hep17hep17 parasites was strong but significantly less than that in OVA::mCherryhsp70 parasites. These transgenic parasites were used to examine the effects of antigen subcellular location and expression level on the development of T-cell responses during blood-stage infections. While all OVA-expressing parasites induced activation and proliferation of OVA-specific CD8+ T cells (OT-I) and CD4+ T cells (OT-II), the level of activation varied: OVA::Hep17hep17 parasites induced significantly stronger splenic and intracerebral OT-I and OT-II responses than those of OVA::mCherryhsp70 parasites, but OVA::mCherryhsp70 parasites promoted stronger OT-I and OT-II responses than those of OVAhsp70 parasites. Despite lower OVA expression levels, OVA::Hep17hep17 parasites induced stronger T-cell responses than those of OVA::mCherryhsp70 parasites. These results indicate that unconjugated cytosolic OVA is not stably expressed in Plasmodium parasites and, importantly, that its cellular location and expression level influence both the induction and magnitude of parasite-specific T-cell responses. These parasites represent useful tools for studying the development and function of antigen-specific T-cell responses during malaria infection.
INTRODUCTION
T cells play a central role in the immune response to malaria and can help to control blood-stage infections (1, 2). For example, in human and rodent malaria infections, effector CD4+ T cells promote antiparasitic antibody production and regulate macrophage-based antiparasitic effector responses (1, 2). However, it is also clear that proinflammatory T-cell responses, if not regulated appropriately or if present in the wrong environment, can contribute to the development of immunopathology during malaria infection (3, 4). Thus, understanding how malarial proteins are recognized by the immune system to initiate adaptive T-cell responses and identifying the antigen-specific T-cell responses involved in protection and pathology during infection have significant importance for vaccine development and for identification of predictive immunological biomarkers for severe malarial disease.
Difficulties in identifying endogenous T-cell epitopes within blood-stage malaria parasites have hampered the investigation of parasite-specific adaptive T-cell responses, necessitating the generation and use of transgenic parasites expressing model antigens. Transgenic Plasmodium parasites expressing ovalbumin (OVA) in the cytoplasm have been used successfully to examine parasite-specific CD8+ responses during both blood and liver stages of infection (5–7). These parasites do not, however, induce strong OVA-specific CD4+ T-cell responses in vivo (8). One potential explanation for the dichotomy in the ability of these parasites to prime OVA-specific CD4+ T-cell and OVA-specific CD8+ T-cell responses is that different antigen-processing and -presenting pathways exist for the presentation of antigens by major histocompatibility complex (MHC) class I and MHC class II molecules (9) and that OVA expressed from the cytoplasmic location does not effectively enter the MHC class II antigen-processing pathway. In support of this, it has been reported for a variety of different models, such as Trypanosoma cruzi and Toxoplasma gondii, that secreted proteins induce stronger T-cell activation than cytoplasmic proteins (10, 11). These data indicate that the subcellular location of antigen expression significantly influences how it is recognized by the immune system during protozoan infections. These observations likely have relevance to endogenous malarial proteins, as it has been shown that not all endogenous Plasmodium antigens induce strong T-cell responses but that a select number of malarial antigens are preferentially recognized by the immune system and initiate superior T-cell responses (2, 12).
In the present study, we directly compared the extents to which the expression level of a protein and its subcellular location in blood-stage malaria parasites influence the development of antigen-specific T-cell responses. We generated transgenic Plasmodium berghei parasites expressing OVA either in the cytoplasm, under the control of the heat shock protein 70 (HSP70) promoter, or on the parasitophorous vacuole membrane (PVM), through fusion of OVA to the PVM protein EXP1/HEP17 (exported protein 1, hepatocyte erythrocyte protein 17 kDa) (13–15). We found that while both cytoplasmic and PVM-anchored OVA could activate OVA-specific CD8+ T cells (OT-I) and CD4+ T cells (OT-II), OVA fused to the PVM induced the strongest antigen-specific T-cell responses. This was despite OVA being expressed at higher levels in parasites when it was present in the parasite cytoplasm. These results demonstrate that both secreted and intracellular antigens can be cross-presented within the immune system and that the subcellular location of antigens affects parasite-specific T-cell responses. These data increase our understanding of the parasite-specific features that influence activation and expansion of T cells during a malaria infection. The transgenic OVA-expressing parasites described in this study are therefore valuable reagents for examining site-specific immune responses and immunopathology during a malarial infection.
MATERIALS AND METHODS
Experimental animals and reference P. berghei lines.
For generation of the transgenic parasite lines, female Swiss OF1 mice (6 to 8 weeks old; Charles River/Janvier) were used. For the induction of experimental cerebral malaria (ECM) and the immunological assays, the following mouse strains were used: C57BL/6 (CD45.2+), RAG-1 OT-I × Pep3 (CD45.1+) F1 mice, and RAG-1 OT-II × Pep3 (CD45.1+) F1 mice. All mice (6 to 10 weeks old) were purchased from Charles River, United Kingdom, or bred in-house at the University of Manchester, United Kingdom.
All animal experiments performed at the Leiden University Medical Centre (LUMC) were approved by the Animal Experiments Committee of LUMC (approval 12042). The Dutch Experiments on Animal Act was established under European guidelines (EU directive 86/609/EEC, regarding the protection of animals used for experimental and other scientific purposes). Animal experiments performed at the University of Manchester (UoM) were approved following local ethical review by the UoM Animal Procedures and Ethics Committee and were performed in strict accordance with the UK Home Office Animals (Scientific Procedures) Act 1986 (approved Home Office project license 70/7293).
To generate the transgenic lines, two reference P. berghei lines were used. These lines are gene insertion/marker out (GIMO) mother lines of P. berghei ANKA (GIMOPbANKA; line 1596cl1) (16) and P. berghei NK65 (GIMOPbNK65; line 1995cl2) (described below) that were generated to rapidly introduce transgenes without drug-selectable markers (see below). In addition, we used the reference cl15cy1 line of P. berghei ANKA (17) and the reference NK65 Edinburgh line.
Generation of GIMOPbNK65 mother line.
The GIMOPbNK65 line (line 1995cl2) was generated similarly to the GIMOPbANKA line (16). Briefly, the DNA construct pL1603 (16) was used to introduce a positive/negative selectable marker (SM) cassette (hdhfr::yfcu) by double-crossover homologous recombination into the “silent” 230p gene locus (PBANKA_030600) of NK65 Edinburgh parasites. The hdhfr::yfcu marker is expressed under the control of the eef1α promoter and is a fusion gene of the positive SM human dihydrofolate reductase gene (hdhfr) and a negative SM, which is a fusion gene of the yeast cytosine deaminase and uridyl phosphoribosyl transferase genes (yfcu). Transfection, selection, and cloning of mutants were performed by standard procedures described for transfection of P. berghei (17). Correct integration of the constructs was verified by diagnostic PCR analysis and Southern blot analysis of pulsed-field gel electrophoresis (PFGE)-separated chromosomes as described previously (16).
Generation of different transgenic P. berghei (ANKA and NK65) lines expressing OVA.
To generate transgenic mutants expressing OVA under the control of the strong, constitutive hsp70 promoter, we generated two DNA constructs. For the first construct (pL1812), the complete coding sequence (CDS) of OVA was amplified from plasmid pENTRY201-OVA (kindly provided by K. Franken, Department of Infectious Diseases, Leiden University Medical Center) by using the primers 6464 and 6465 (see Table S1 in the supplemental material for primer sequences). This fragment was subsequently subcloned into BamHI/SgrAI restriction sites of construct pL1694, thereby replacing mCherry with OVA between the hsp70 promoter and the hsp70 3′-untranslated region (3′-UTR). pL1812 was linearized with the restriction enzyme SacII prior to transfection. For the second construct (pL1838), the CDS (without the stop codon) of OVA was PCR amplified from plasmid pENTRY201-OVA by using primers 6466 and 6467 (see Table S1) and cloned into XhoI/BamHI restriction sites of pL1809, resulting in placement of OVA between the hsp70 promoter region and the mCherry CDS. pL1838 was linearized with the restriction enzymes HindIII and NdeI prior to transfection.
To generate a transgenic mutant with OVA fused to HEP17 (PBANKA_092670) (15), the DNA construct pL1884 was generated (see Fig. S1 in the supplemental material). First, the 5′-UTR of hep17 (1.3 kb upstream of the start codon) and the signal peptide (SP) sequence of hep17 (bp 1 to 81) were amplified from wild-type P. berghei ANKA genomic DNA by use of primers 6659 and 6660 and then subcloned into the pCR2.1-TOPO vector (TOPO TA cloning kit; Invitrogen). Second, the following two DNA sequences were subsequently cloned into this vector: (i) the rest of the hep17 CDS after the SP (bp 82 to 726), along with the ∼700-bp 3′-UTR, which was amplified using primers 6661 and 6662; and (ii) the CDS of OVA, which was amplified from pENTRY201-OVA by use of primers 6920 and 6467. These two sequences were cloned into BamHI/KpnI and NdeI/BamHI sites, respectively, resulting in an intermediate construct encoding a fusion protein of OVA::HEP17. Third, the 5′- and 3′-targeting regions of 230p (PBANKA_030600) were amplified from wild-type P. berghei ANKA genomic DNA by use of primers 6838 and 6839 and primers 5587 and 6840, respectively, and then subcloned into the above-described intermediate vector, using XhoI/EcoRI and KpnI sites, respectively, resulting in the final construct pL1884 (see Table S1 for primer sequences). This construct was linearized using SacII sites before transfection (see Fig. S1).
Transfection was performed using parasites of the P. berghei mother lines GIMOPbANKA (line 1596cl1) (16) and GIMOPbNK65 (line 1995cl2) (see above), and transfected parasites were selected using the GIMO method of negative selection as described previously (16). Negative selection was performed by treating mice infected with transfected parasites with 5-fluorocytosine (5FC) in the drinking water (2 mg/ml). Clonal parasite lines were obtained by the method of limiting dilution (17). Correct integration of DNA constructs was verified by Southern analyses of chromosomes separated by pulsed-field gel electrophoresis (17). Hybridization was performed with a mixture of two probes: a control probe recognizing p25 on chromosome 5 and an hdhfr probe recognizing the hdhfr::yfcu SM (16). See Table S1 in the supplemental material for PCR primers used to generate the different probes.
Northern and Western analyses of OVA expression in transgenic parasite lines.
Transcription was analyzed by standard Northern blot analyses. Total RNAs were isolated from in vitro-cultured and purified schizonts (17) of P. berghei ANKA (cl15cy1) and the different transgenic lines. Northern blots were hybridized with probes specific for the ova CDS, which was PCR amplified from plasmid pENTRY201-OVA (see above) by using primers 6920 and 6467 (see Table S1 in the supplemental material). As a loading control, Northern blots were hybridized with the oligonucleotide probe L644R, which recognizes the large-subunit (LSU) rRNA gene unit (18). For Western blot analysis of OVA expression, total protein extracts from in vitro-cultured and purified mature schizonts and from cultured trophozoites and immature schizonts obtained from synchronized infections in mice (19) were separated by 12% SDS-PAGE and transferred to nitrocellulose membranes by electroblotting. OVA expression was detected by incubation of membranes with polyclonal rabbit IgG anti-OVA antibody (kindly provided by M. Camps, Department of Immunohematology and Blood Transfusion, LUMC), followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Invitrogen). Polyclonal goat IgG anti-DsRed (mCherry) antibodies (Santa Cruz Biotechnology) were used to confirm OVA::mCherry expression, followed by HRP-conjugated rabbit anti-goat IgG secondary antibody (Invitrogen). Immunostained protein complexes were visualized by enhanced chemiluminescence (Amersham). As a loading control, the membranes were stained with anti-PbHSP70 antibody (20), followed by incubation with HRP-conjugated goat anti-mouse IgG secondary antibody. The relative signal intensities of bands on Northern and Western blots were quantified using ImageJ software and graphed using GraphPad Prism.
Immunofluorescence analysis of cellular localization of OVA.
For immunofluorescence assays (IFA) of blood-stage parasites, parasites were cultured under standard in vitro culture conditions (17), except for a lower culture temperature (34°C). Infected erythrocytes collected from these cultures contained both maturing trophozoites and schizonts. The infected erythrocytes on slides were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min and permeabilized with 0.5% Triton X-100 in PBS for 10 min. The parasites were stained with anti-OVA or anti-EXP1 (21) antibodies, followed by Alexa Fluor 594–anti-rabbit or Alexa Fluor 488–anti-chicken antibodies (Invitrogen). Nuclei were stained with Hoechst 33342 (2 μmol/liter; Sigma, The Netherlands) for 15 min. Slides were mounted in Vectashield (Vector Laboratories Inc.) and examined using a DM-IRBE Flu Leica fluorescence microscope.
For immunofluorescence analysis of in vitro exoerythrocytic-form (EEF) parasites, 5 × 104 sporozoites were added to a monolayer of Huh7 cells on coverslips in 24-well plates in complete RPMI 1640 medium supplemented with 10% (vol/vol) fetal bovine serum (FBS), 1% (vol/vol) penicillin-streptomycin, and 1% (vol/vol) GlutaMAX (Invitrogen) and were maintained at 37°C with 5% CO2. Forty-eight hours after infection, cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 in PBS, blocked with 10% FBS in PBS, and subsequently stained with primary and secondary antibodies for 2 h and 1 h, respectively. Primary antibodies used were anti-PbEXP1 (raised in chickens [21]) and anti-UIS4 (raised in rabbits [20]), with both detecting the PVM-resident proteins; anti-PbHSP70 (raised in mice [20]), detecting the cytoplasmic heat shock protein 70 (PBANKA_081890); and anti-OVA antibodies (raised in rabbits; see above). Anti-mouse, -chicken, and -rabbit secondary antibodies, conjugated to Alexa Fluor 488 or 594, were used for visualization (Invitrogen). Nuclei were stained with Hoechst 33342. Cells were mounted in Vectashield and examined using a DM-IRBE Flu Leica fluorescence microscope.
Blood-stage infections and induction of ECM.
Cryopreserved OVAhsp70, OVA::mCherryhsp70, OVA::Hep17hep17, and GIMOANKA parasites were passaged once through C57BL/6 mice before being used to infect experimental animals. Mice were infected by intravenous (i.v.) injection of 104 parasitized red blood cells (pRBCs). Peripheral parasitemia was monitored by examination of Giemsa-stained thin smears of tail blood. The induction and severity of ECM were assessed using the following well-defined grading system (22): 1, no signs; 2, ruffled fur and/or abnormal posture; 3, lethargy; 4, reduced responsiveness to stimulation and/or ataxia and/or respiratory distress/hyperventilation; and 5, prostration and/or paralysis and/or convulsions. All animals were euthanized when observed at stage 4 or 5. Stages 2 and 3 were classified as prodromal signs of ECM, and stages 4 and 5 were classified as ECM. Animals in stage 4 or 5 invariably show signs of cerebral pathology, including blocked vessels, hemorrhages, edema, perivascular cuffing, and disruption and damage to cerebral vasculature endothelial linings, following histological examination (22).
Flow cytometry.
Spleens were removed from naive and malaria-infected mice on day 4 and day 6 or 7 postinfection (p.i.). Single-cell suspensions were generated by homogenizing tissue through a 70-μm cell sieve (BD Biosciences). Brains were chopped into small pieces, aspirated through a 10-ml syringe, and incubated in Hanks balanced salt solution (HBSS) containing 2% fetal calf serum (FCS) with collagenase (final concentration, 1 mg/ml) (Sigma) for 45 min on a tube roller at room temperature. The resulting suspension was filtered through a 70-μm cell sieve, layered on a 30% Percoll gradient, and centrifuged at 2,000 × g for 10 min. The supernatant was discarded and the pellet collected. For both the spleen and brain preparations, RBCs were lysed using RBC lysing buffer (BD Biosciences). Absolute cell numbers were determined by microscopy, using a hemocytometer, and live/dead cell differentiation was performed using the trypan blue exclusion cell viability assay (Sigma).
Identification by flow cytometry of adoptively transferred OT-I and OT-II cells and the activation of endogenous (CD45.1−) and transferred (CD5.1+) T cells was performed by surface staining with the following antibodies: anti-mouse CD45.1 (A20), anti-mouse CD4 (GK1.5), anti-mouse CD8 (53-6.7), anti-mouse CD69 (H1.2F3), anti-mouse CD25 (PC61.5), anti-mouse CD44 (IM7), and anti-mouse CD62L (MEL-14). All antibodies were obtained from eBioscience or Biolegend. To determine the proliferative status of the T-cell populations, the cells were first stained with antibodies to surface markers, washed, and subsequently fixed and permeabilized by incubation with Foxp3 fixation/permeabilization buffer (eBioscience). The cells were then washed and incubated with anti-mouse Ki67 (SolA15) for 30 min before flow cytometry analysis.
All flow cytometry analyses were performed using an LSR II flow cytometer (BD Systems, United Kingdom). Subsequent data analyses were performed using FlowJo software (Treestar Inc., OR). Fluorescence-minus-one controls were utilized to validate flow cytometric data.
Adoptive transfer of OVA-specific T cells.
Splenic single-cell suspensions were prepared from naive RAG-1 OT-I × Pep3 (C57BL/6 CD45.1+) F1 mice and RAG-1 OT-II × Pep3 F1 mice, as described above. Splenic CD8+ and CD4+ T lymphocytes were negatively selected from RAG-1 OT-I × Pep3 (C57BL/6 CD45.1+) F1 mice and RAG-1 OT-II × Pep3 F1 mice, respectively, by using anti-phycoerythrin (anti-PE) midiMACS beads following surface staining with PE-labeled anti-mouse CD19 (eBio1D3), F4/80 (BM8), or anti-MHC-II (M5/114.15.2) and either anti-CD4 (GK1.5) or anti-CD8 (53-6.7), respectively, according to the manufacturer's instructions (Miltenyi Biotec). A total of 1 × 104 RAG-1 OT-I × Pep3 CD8+ T cells and 2.5 × 105 RAG-1 OT-II × Pep3 CD4+ T cells were adoptively transferred, together, into recipient mice by intravenous injection on the day before (−1) infection with the different P. berghei ANKA transgenic and wild-type lines. The purity and phenotypes of positively selected T-cell populations were assessed by flow cytometry prior to adoptive transfer. CD4+ and CD8+ T cells were approximately 95% T-cell receptor αβ positive (TCRαβ+) and CD3+ and were predominantly naive (CD44− CD62L+).
Statistical analysis.
Statistical significance was determined using one-way analysis of variance (ANOVA) with Tukey's post hoc analysis. All statistical analyses were performed using GraphPad Prism. In all cases, results were classified as significantly different if the P value was <0.05.
RESULTS
Generation of different transgenic P. berghei parasite lines expressing OVA.
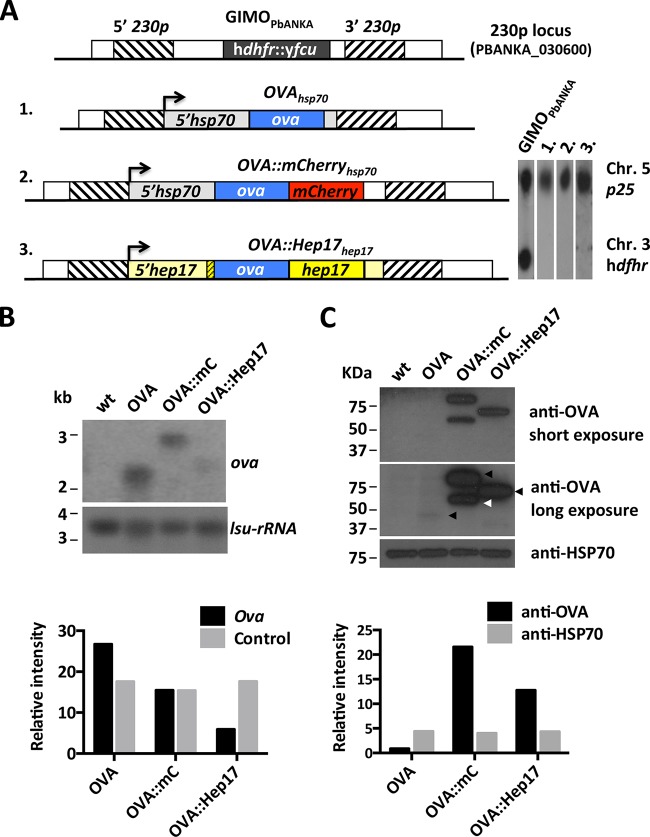
Using the GIMO transfection method, we generated three different transgenic P. berghei ANKA lines, each expressing the reference antigen chicken OVA (Fig. 1A). In the first two lines, OVAhsp70 and OVA::mCherryhsp70 parasites, OVA is expressed under the control of the strong and constitutive hsp70 promoter (PBANKA_071190). When transgenes such as the green fluorescent protein (GFP) and mCherry genes are carried under the control of this promoter, the result is strong protein expression in the parasite cytosol (23–25). The OVAhsp70 line expresses unmodified full-length OVA, whereas the OVA::mCherryhsp70 line encodes full-length OVA that is C-terminally tagged with the fluorescent protein mCherry. In the third line, the OVA::Hep17hep17 line, full-length OVA is fused to HEP17/EXP1 (PBANKA_092670) (13, 14), which is a protein present on the PVM (21). The PVM is the structure that separates the parasite from the inside of the infected hepatocyte or red blood cell (26). In this transgenic line, the expression of this fusion protein (OVA::HEP17) is under the control of the 5′-UTR (promoter) and 3′-UTR (terminator) regions of the hep17 gene. To ensure correct trafficking of OVA to the PVM, we placed the full-length CDS of OVA directly after the signal peptide of hep17 and before the remainder of the hep17 CDS (see Fig. S1 in the supplemental material).
FIG 1.
Different transgenic P. berghei ANKA lines expressing OVA. (A) (Left) Schematic representation of the 230p locus in the reference GIMOPbANKA mother line and in 3 OVA-expressing transgenic lines. GIMOPbANKA has the positive and negative selectable marker cassette (hdhfr::yfcu; black box) inserted into 230p. The OVA expression cassettes were introduced into 230p by double-crossover homologous recombination at the target regions (hatched boxes), using the method of GIMO transfection. Line 1 (1988cl1; OVAhsp70) encodes full-length ovalbumin (ova; blue box) under the control of the 5′ (promoter)- and 3′ (terminator)-UTRs of the hsp70 gene (gray boxes). Line 2 (2027cl1; OVA::mCherryhsp70) encodes OVA which is C-terminally fused to mCherry (red box) under the control of the 5′-UTR of hsp70 and the 3′-UTR of dhfr/ts (white box). Line 3 (2030cl1; OVA::Hep17hep17) encodes OVA flanked by the N-terminal signal peptide of hep17 (bp 1 to 81; yellow hatched box) and the remainder of the hep17 open reading frame (bp 82 to 726; yellow box), under the control of the 5′- and 3′-UTRs of the hep17 gene (light yellow boxes). The direction of transcription is indicated by the arrows. (Right) Southern analyses of PFGE-separated chromosomes confirmed the integration of the constructs into the 230p locus on chromosome 3 (Chr), resulting in the removal of the hdhfr::yfcu selection cassette. Hybridization was performed with a mixture of two probes: a control probe recognizing p25 on chromosome 5 and an hdhfr probe recognizing hdhfr::yfcu. (B) Northern blot analysis of OVA-transgene transcription in purified schizonts of the 3 transgenic lines. Blots were hybridized using a PCR probe recognizing ova (primers 6466 and 6467). As a loading control, the oligonucleotide probe L644R, which recognizes the large-subunit (lsu) rRNA gene, was used. Quantification of the hybridization signals showed that OVAhsp70 (OVA) and OVA::mCherryhsp70 (OVA::mC) parasites had comparable levels of ova transcripts, while in OVA::Hep17hep17 (OVA::Hep17) parasites the transcript levels were lower. The relative intensities of the hybridization signals were quantified using ImageJ. wt, wild-type P. berghei ANKA parasites. (C) Western analysis of OVA-transgene expression levels in purified schizonts of the 3 transgenic lines (arrowheads on the long-exposure image indicate the OVA products of expected sizes). Blots were stained with anti-OVA antibodies. Anti-HSP70 antibody staining was used as a loading control. Quantification of the staining signals showed hardly detectable OVA expression in OVAhsp70 parasites. In OVA::mCherryhsp70 parasites, an OVA product of the expected size (2nd black arrow) was detected, in addition to a smaller (truncated) product (white arrow). The relative intensities of OVA signals (long exposure) were quantified using ImageJ.
In addition to the OVA-expressing P. berghei ANKA lines, we generated two transgenic lines in the GIMO mother line of P. berghei NK65 (GIMONK65). These two NK65 lines also express OVA under the control of the hsp70 promoter and were generated using the same constructs as those used to generate P. berghei ANKA OVAhsp70 and OVA::mCherryhsp70 (see Fig. S2A in the supplemental material). Genotyping by Southern analysis of PFGE-separated chromosomes of all these transgenic lines confirmed that all the different OVA constructs were integrated correctly into the 230p locus after GIMO transfection (Fig. 1A; see Fig. S2B).
Expression and subcellular location of OVA in different transgenic P. berghei lines.
Northern and Western analyses were performed to analyze the expression level of OVA antigen in equivalent numbers of infected RBCs of the different transgenic lines. Abundant OVA transcripts were present in OVAhsp70 and OVA::mCherryhsp70 blood-stage ANKA and NK65 parasites (Fig. 1B; see Fig. S2C in the supplemental material). OVA transcript levels were substantially lower in the OVA::Hep17hep17 (expressed under the control of the hep17 promoter) parasites than in the other 2 lines expressing OVA under the control of the hsp70 promoter (Fig. 1B). Unexpectedly, Western analysis using antibodies against OVA revealed very low levels of OVA protein in the OVAhsp70 line compared with the OVA::mCherryhsp70 ANKA line (Fig. 1C). This very low but specific level of OVA expression was observed in 2 independent P. berghei ANKA OVAhsp70 lines (see Fig. S2E), and moreover, it was also reproduced in the equivalent NK65 parasites (see Fig. S2D). These observations indicate that unconjugated/unmodified cytosolic OVA is unstable (and/or rapidly degraded) in blood-stage P. berghei parasites. The OVA::mCherryhsp70 parasites expressed high levels of the full-length OVA::mCherry fusion protein (ca. 75 kDa) and also (less of) a truncated form (ca. 55 kDa) of the OVA::mCherry protein recognized by anti-OVA antibodies (Fig. 1C). When we used anti-mCherry antibodies against protein material derived from OVA::mCherryhsp70 parasites, we observed both the full-length fusion protein and a weaker, ∼20-kDa band, indicating that the truncation of OVA::mCherry fusion protein was occurring due to a specific cleavage within the mCherry reporter protein and that the T-cell epitopes within OVA remained intact (see Fig. S2F). In the OVA::Hep17hep17 parasites, we observed a single protein product of the expected size (62 kDa), corresponding to the OVA::HEP17 fusion (Fig. 1C). The OVA protein levels in purified mature schizonts of OVA::Hep17hep17 parasites were lower than those in mature schizonts of OVA::mCherryhsp70 parasites but still significantly higher than those observed in OVAhsp70 parasites (Fig. 1C). To analyze OVA expression levels during blood-stage development, we collected different (synchronized) blood stages of OVA::Hep17hep17 and OVA::mCherryhsp70 parasites. Western analyses of these samples revealed that OVA expression in both lines was present in trophozoites and that it increased as the parasite matured into schizonts. Moreover, in all stages, OVA expression was greater in OVA::mCherryhsp70 than in OVAhsp70 parasites (see Fig. S2G).
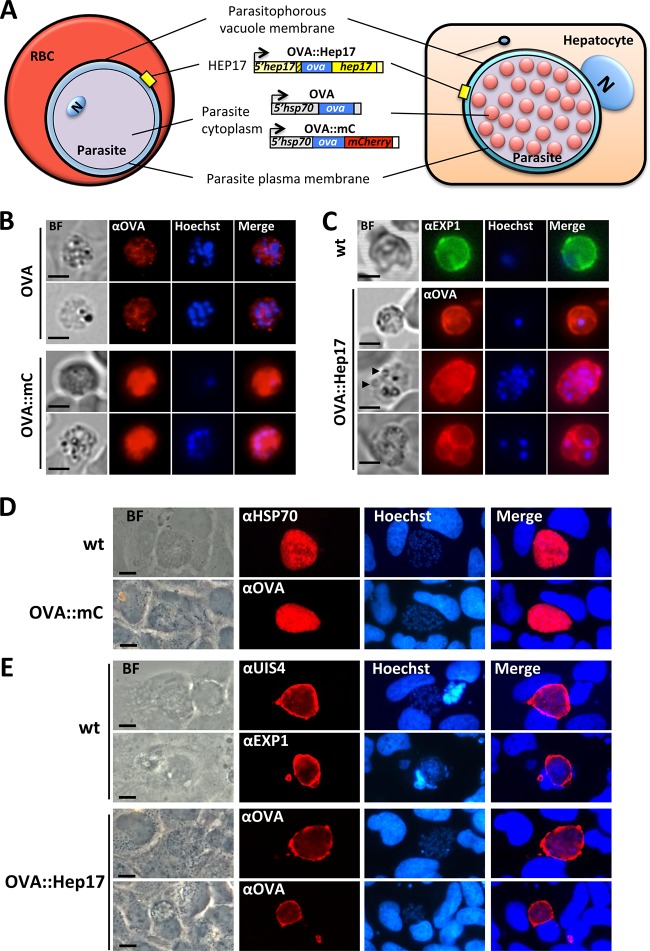
We next performed immunofluorescence analyses to determine the subcellular locations of OVA in the different lines. These analyses confirmed the low levels of OVA protein expression in OVAhsp70 parasites compared to the other two lines (Fig. 2B and C). In addition, they revealed, as expected, a cytosolic location of OVA in both OVAhsp70 and OVA::mCherryhsp70 blood-stage parasites (Fig. 2A and B). In OVA::Hep17hep17 blood-stage parasites, a clear circumferential staining was observed in both trophozoites and schizonts, indicative of a PVM location (Fig. 2A and C) and similar to the staining of the PVM protein HEP17/EXP1 (PBANKA_092670) in wild-type blood-stage parasites (Fig. 2C). The circumferential staining in schizonts with fully formed merozoites strongly suggested that OVA was not located at the parasite plasma membrane, although we cannot exclude its presence in the parasitophorous vacuole (PV). Consequently, to confirm the PVM subcellular location of OVA in the OVA::Hep17 line, we analyzed its expression in liver-stage parasites. Plasmodium liver-stage parasites are larger, and the boundaries between the different membranes that surround the parasite in the host cell are more clearly defined and detailed (15). As such, immunofluorescence analyses of the subcellular locations of different PVM-located proteins have been performed previously with P. berghei liver-stage parasites (15) (Fig. 2A). OVA was clearly localized in the parasite cytosol of maturing OVA::mCherryhsp70 liver-stage parasites (at 48 h post-sporozoite infection), with anti-OVA antibody staining closely resembling that of the cytoplasmic P. berghei protein HSP70 (Fig. 2D). In contrast, in maturing OVA::Hep17hep17 liver-stage parasites, anti-OVA antibody staining provided characteristic PV/PVM staining, closely resembling the staining of the PVM proteins HEP17/EXP1 (PBANKA_092670) and UIS4 (PBANKA_050120) (Fig. 2E). In combination, our analyses demonstrate strong OVA expression in the OVA::mCherryhsp70 and OVA::Hep17hep17 parasites, with OVA located (in both blood and liver stages) in the parasite cytoplasm in the OVA::mCherryhsp70 line and on the PVM in the OVA::Hep17hep17 line.
FIG 2.
Subcellular locations of OVA in different transgenic P. berghei ANKA lines. Transgenic P. berghei parasites expressed full-length OVA either under the control of the constitutive hsp70 promoter, unconjugated or fused to mCherry (OVA and OVA::mC, respectively), or under the control of the hep17 promoter, where OVA was fused to the PVM protein HEP17/EXP1 (OVA::Hep17). (A) Schematics showing the expected localization of OVA in the 3 transgenic lines during blood-stage (RBC) and liver-stage (hepatocyte) development. N, nucleus (parasite nucleus in the RBC and hepatocyte nucleus in the liver cell). (B) Immunofluorescence analysis of blood-stage (schizont) parasites stained with anti-OVA antibodies (red) shows a low-level cytoplasmic expression in OVA parasites, whereas in OVA::mC parasites, a very strong cytoplasmic expression of OVA is observed. Trophozoites and schizonts are shown (single and multinucleated cells, respectively). Nuclei were stained with Hoechst 33342 (blue). (C) In OVA::Hep17 parasites, anti-OVA staining reveals a circumferential pattern at the periphery of the parasites, indicative of localization at the PVM, which is very similar to the PVM pattern observed for wild-type (wt) blood-stage parasites by use of antibodies against HEP17/EXP1. A trophozoite (single nuclei) and a fully segmented schizont (with individual merozoites indicated by arrowheads) are shown surrounded by OVA, with a pattern indicative of the PVM. A multiply infected erythrocyte (bottom panels) is also shown, with each individual parasite inside the red blood cell also surrounded by an OVA-stained structure, again indicating the PVM signal. (D) Immunofluorescence analysis of cultured Huh7 hepatocytes infected with OVA::mC (at 40 h post-sporozoite infection [hpi]), using anti-OVA antibodies (red), reveals a cytoplasmic expression for OVA. Intracellular wild-type parasites (at 40 hpi) stained with anti-HSP70 antibody show a similar cytoplasmic localization pattern. (E) Immunofluorescence analysis of cultured Huh7 hepatocytes infected with OVA::Hep17 parasites at 48 hpi, using anti-OVA antibodies (red), reveals a clear circumferential staining around the periphery of the parasites. This staining pattern is very similar to the PVM pattern observed for wild-type liver-stage parasites (also at 48 hpi) by using antibodies against the PVM markers EXP1 and UIS4 (PBANKA_050120). Nuclei were stained with Hoechst 33342 (blue). BF, bright field. Bars = 5 μm (B and C) and 10 μm (D and E).
Induction of OVA-specific T-cell responses in the spleen during blood-stage infections with the different OVA-expressing P. berghei ANKA lines.
To address how the magnitude and location of OVA expression in malaria parasites influence the development of antigen-specific T-cell responses during a blood-stage infection, we adoptively transferred both CD45.1+ OT-I and OT-II cells into congenic (CD45.2+) C57BL/6 mice 1 day prior to infection with the various OVA-expressing transgenic parasite lines. To control for non-antigen-specific bystander activation of OT-I and OT-II cells, we also infected recipient mice with the non-OVA-expressing GIMOANKA (wild-type) parasites. The peripheral parasitemia and the kinetics and incidence of ECM development were largely similar in the various infections (see Fig. S3A and B in the supplemental material), suggesting that the courses of blood-stage infections with the different parasite lines were comparable.
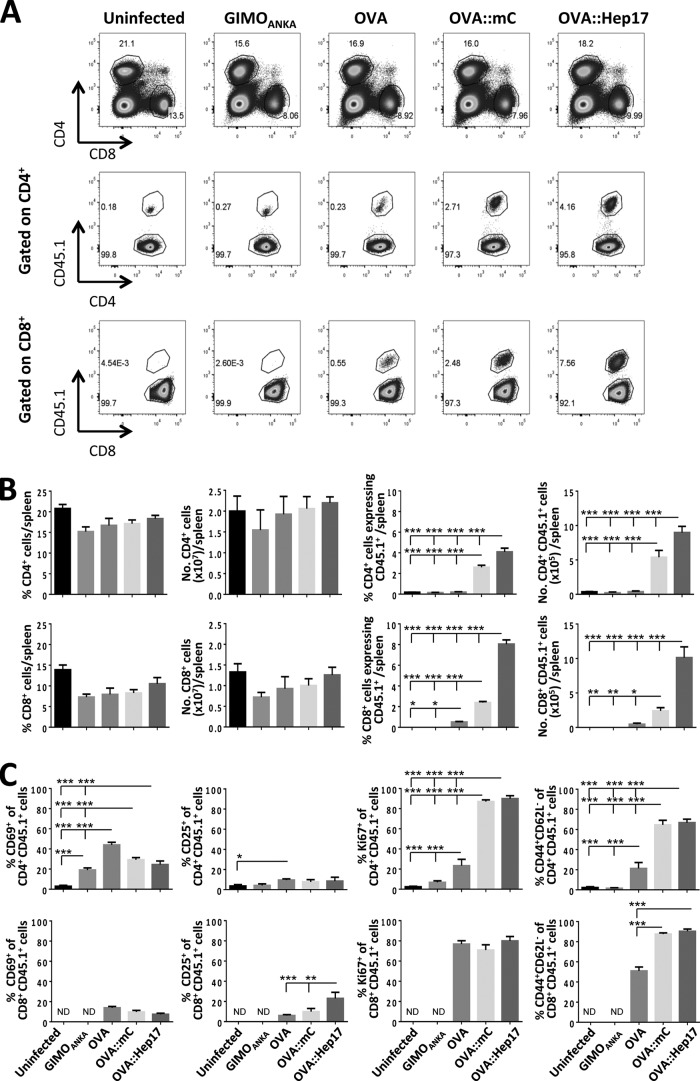
To assess the early stages of OVA-specific T-cell activation during infection with the different OVA-expressing parasite lines, we first examined the splenic T-cell response to infection on day 4 of infection. The spleen is the major site of T-cell priming during the early stages of blood-stage malaria infection (27). The adoptively transferred OVA-specific T cells were distinguished from endogenous T cells based upon CD45.1+ expression, and OT-I and OT-II cells were differentially identified based upon their CD8 and CD4 receptor expression, respectively (see Fig. S4A in the supplemental material). In preliminary experiments, we determined that adoptively transferred OT-I and OT-II cells do not downregulate the CD8 and CD4 receptors during the course of blood-stage P. berghei infections in C57BL/6 mice (results not shown). On day 4 of infection, we observed OT-II cells, but not OT-I cells, in spleens of all groups of mice (see Fig. S4A and B). The OT-II cells had not yet expanded in mice infected with the transgenic OVA-expressing parasites, in contrast to the case in mice infected with GIMOANKA or in uninfected mice (see Fig. S4A and B). Interestingly, however, significantly higher frequencies of splenic OT-II cells expressed the activation markers CD69 (early lymphocyte activation) and CD25 (alpha chain of the interleukin-2 [IL-2] receptor) and the proliferation marker Ki67 in the OVA::mCherryhsp70- and OVA::Hep17hep17-infected mice than in the OVAhsp70- and GIMOANKA-infected mice (see Fig. S4C). Moreover, early changes in CD44 (adhesion molecule and marker of effector T-cell maturation) and CD62L (L-selectin, involved in cell adhesion) expression by splenic OT-II cells (forming effector CD44+ CD62L− cells) were observed in the mice infected with OVA::mCherryhsp70 and OVA::Hep17hep17 parasites but not in the mice infected with OVAhsp70 parasites (see Fig. S4C). These observations indicate that OVA::mCherryhsp70 and OVA::Hep17hep17 parasites, but not OVAhsp70 parasites, promote early activation of splenic OVA-specific CD4+ T cells. As OT-I cells were not identified in the spleen on day 4 of infection, due to the lack of expansion from the relatively small population of transferred OT-I cells, it was not possible to determine their activation status at this early time point.
We next quantified the expansion and numbers of OVA-specific T cells in the spleen on day 6 of infection, when T cells have acquired full effector status during P. berghei infection (28), and at the time of ECM development (see Fig. S3B in the supplemental material). Adoptively transferred OT-II cells were again identified in the spleens of all groups of mice on day 6 of infection. In addition, OT-I cells were clearly visible in the spleens of OVAhsp70-, OVA::mCherryhsp70-, and OVA::Hep17hep17-infected mice at this later time point (Fig. 3A). Importantly, the highest frequencies and numbers of OT-I and OT-II cells were observed in the spleens of mice infected with OVA::Hep17hep17 parasites, compared with mice infected with GIMOANKA, OVAhsp70, and OVA::mCherryhsp70 parasites (Fig. 3A and B). Unsurprisingly, based upon the low level of OVA protein expression (Fig. 1C), the lowest frequencies and numbers of splenic OT-I and OT-II cells (out of the three groups of OVA-expressing parasite infections) were observed in mice infected with OVAhsp70 parasites (Fig. 3B).
FIG 3.
The location of OVA in blood-stage parasites influences the development of OVA-specific T-cell responses in the spleen. A total of 10,000 naive CD45.1+ OT-I and 250,000 naive CD45.1+ OT-II cells were adoptively transferred into CD45.2+ C57BL/6 mice 1 day prior to infection (104 pRBCs i.v.) with different P. berghei ANKA lines (GIMOANKA [control], OVAhsp70 [OVA], OVA::mCherryhsp70 [OVA::mC], and OVA::Hep17hep17 [OVA::Hep17]). (A) Representative flow cytometric plots of total and donor CD4+ and CD8+ T cells in spleens of mice infected with different P. berghei ANKA lines at day 6 after infection. Hierarchal gating was performed on total splenic CD4+ and CD8+ T cells (top row), host CD45.1− CD4+ T cells and donor OVA-specific CD45.1+ CD4+ OT-II cells (middle row), and host CD45.1− CD8+ T cells and donor OVA-specific CD45.1+ CD8+ OT-I cells (bottom row). (B) Percentages and absolute numbers (means and standard errors of the means [SEM]) of total and donor (OT-I and OT-II) T cells in spleens of mice (n = 4) infected with different P. berghei ANKA lines at day 6 after infection. The magnitudes of the total splenic T-cell responses were comparable in mice infected with the different parasite lines (left 4 graphs). Stronger OVA-specific T-cell responses were observed in mice infected with OVA::Hep17 parasites than in mice infected with OVA::mC and OVA parasites (right 4 graphs). The results are representative of 2 separate experiments. ND, not detected; *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Percentages and absolute numbers (means and SEM) of donor (OT-I and OT-II) T cells in spleens of mice (n = 4) infected with different P. berghei ANKA lines at day 6 after infection. The activation of CD4+ CD45.1+ OT-II cells (top row) and CD8+ CD45.1+ OT-I cells (bottom row) was determined by examining the expression of CD69, CD25, Ki67, CD44, and CD62L. The results are representative of 2 separate experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (by one-way ANOVA with Tukey's post hoc analysis).
We next expanded upon the above analyses and examined the activation status and maturation of the splenic OVA-specific T-cell response on day 6 of infection with the different OVA-expressing parasites. Significantly higher frequencies of mature effector (CD44+ CD62L−) OT-II and OT-I cells were observed in the spleens of mice infected with OVA::Hep17hep17 and OVA::mCherryhsp70 parasites than in those of mice infected with OVAhsp70 parasites (Fig. 3C); however, interestingly, the maturation statuses of the OT-I and OT-II cells, as defined by the CD44+ CD62L− phenotype, were largely similar in spleens of mice infected with OVA::Hep17hep17 and OVA::mCherryhsp70 parasites (Fig. 3C). The expression levels of Ki67, CD25, and CD69 in OT-II and OT-I cells were also largely similar in the spleens of mice infected with OVA::Hep17hep17 and OVA::mCherryhsp70 parasites, with the exception of CD25 expression on OT-I cells, which was higher with OVA::Hep17hep17 parasites than with OVA::mCherryhsp70 parasites (Fig. 3C). Thus, these results suggest that the location of OVA expression by blood-stage malaria parasites influences the size of the antigen-specific T-cell population, while the magnitude of OVA expression within blood-stage malaria parasites influences the maturation kinetics of the antigen-specific response.
Importantly, the kinetics, activation, and magnitudes of the endogenous (polyclonal) splenic T-cell responses (both CD4+ and CD8+ T cells) were largely comparable in mice infected with the different OVA-expressing and non-OVA-expressing parasites (see Fig. S5 in the supplemental material). This shows that different OVA-expressing and non-OVA-expressing parasites do not inherently differ in the capacity to initiate and maintain parasite-specific T-cell responses and verifies that the disparities in splenic OT-I and OT-II responses observed in mice infected with the different parasite lines resulted from differences in the magnitude and location of OVA expression.
Development of OVA-specific T-cell responses in the brain during blood-stage infections with different OVA-expressing P. berghei ANKA lines.
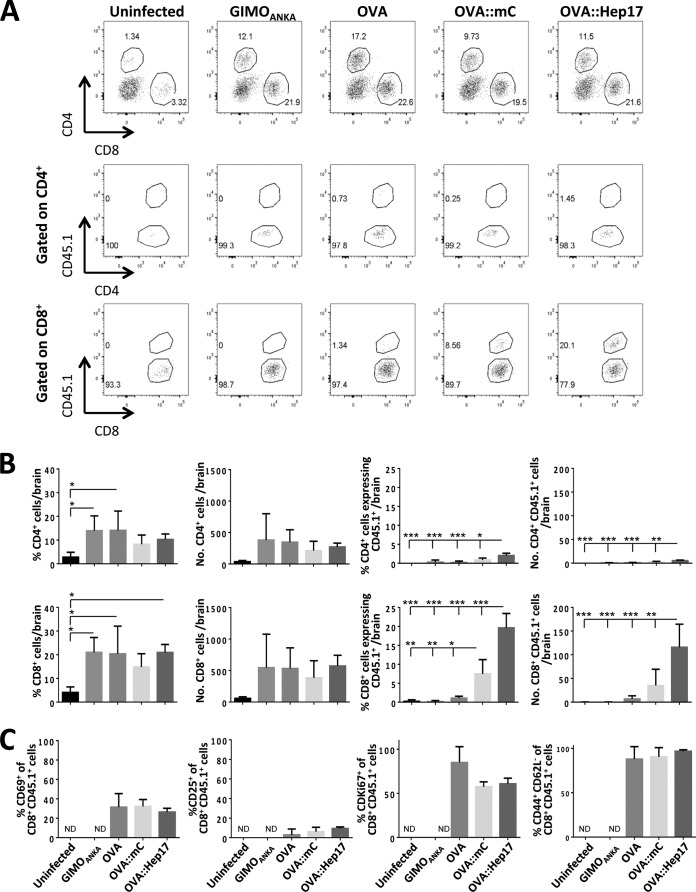
ECM in P. berghei ANKA-infected mice is a T-cell-driven neuropathology characterized by migration to and accumulation of pathogenic CD4+ and CD8+ T cells in the brain (29, 30). To address whether differences in the location and magnitude of malarial antigen expression in blood-stage parasites significantly affect the functionality of the antigen-specific T-cell response during infection, we therefore quantified the accumulation and activation status of OT-I and OT-II cells within the brains of mice infected with the different OVA-expressing parasite lines. As expected, we observed significant increases in the frequencies and numbers of total (transferred CD45.1+ and recipient CD45.1−) CD4+ T cells and CD8+ T cells in the brains of mice showing signs of ECM (day 6 after infection) compared with those for naive mice (Fig. 4A and B). Importantly, however, the frequencies, numbers, and activation of total and endogenous (CD45.1−) CD4+ T cells and CD8+ T cells were not significantly different within the brains of mice infected with the different parasite lines on day 6 of infection (Fig. 4A and B; see Fig. S6 in the supplemental material). These data verify that there were no differences in generalized brain-derived T-cell migratory cues or activating signals in mice infected with the different OVA-expressing and non-OVA-expressing parasites.
FIG 4.
The location of OVA in blood-stage parasites influences the magnitude of the OVA-specific T-cell response in the brain during ECM. A total of 10,000 naive CD45.1+ OT-I and 250,000 naive CD45.1+ OT-II cells were adoptively transferred into CD45.2+ C57BL/6 mice 1 day prior to infection (104 pRBCs i.v.) with different P. berghei ANKA lines (GIMOANKA [control], OVAhsp70 [OVA], OVA::mCherryhsp70 [OVA::mC], and OVA::Hep17hep17 [OVA::Hep17]). (A) Representative flow cytometric plots of total and donor CD4+ and CD8+ T cells in brains of mice infected with different P. berghei ANKA lines at day 6 after infection. Hierarchal gating was performed on total brain CD4+ and CD8+ T cells (top row), host CD45.1− CD4+ T cells and donor OVA-specific CD45.1+ CD4+ OT-II cells (middle row), and host CD45.1− CD8+ T cells and donor OVA-specific CD45.1+ CD8+ OT-I cells (bottom row). (B) Percentages and absolute numbers (means and SEM) of total and donor (OT-I) T cells in brains of mice (n = 4) infected with different P. berghei ANKA lines at day 6 after infection. The magnitudes of the total intracerebral T-cell responses were comparable in mice infected with the different parasite lines (left 4 graphs). Stronger OVA-specific T-cell responses were observed in mice infected with OVA::Hep17 parasites than in mice infected with OVA::mC and OVA parasites (right 4 graphs). The results are representative of 2 separate experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Percentages and absolute numbers (means and SEM) of donor (OT-I) T cells in brains of mice (n = 4) infected with different P. berghei ANKA lines at day 6 after infection. The activation of CD8+ CD45.1+ OT-I cells was determined by examining the expression of CD69, CD25, Ki67, CD44, and CD62L. The results are representative of 2 separate experiments. ND, not detected; *, P < 0.05; **, P < 0.01; ***, P < 0.001 (by one-way ANOVA with Tukey's post hoc analysis).
Populations of OT-I and OT-II cells were observed in the brains of mice infected with all three OVA-expressing parasites (on day 6 of infection). However, significantly higher frequencies and numbers of OT-I and OT-II cells were found in the brains of mice infected with OVA::Hep17hep17 parasites than in those of mice infected with OVA::mCherryhsp70 and OVAhsp70 parasites (Fig. 4A and B). Moreover, consistent with the data for the spleen, significantly fewer OT-I and OT-II cells were found in the brains of mice infected with the OVAhsp70 parasites than in those of mice infected with the OVA::Hep17hep17 parasites (Fig. 4A and B). Note that despite the heterogeneity in recruitment/accumulation of OT-I cells to the brains of mice infected with the different transgenic OVA-expressing parasites, the intracerebral OT-I cells displayed comparable activation profiles, as determined by CD69, CD25, Ki67, CD44, and CD62L expression in the different groups of mice, at the time of ECM development (day 6 of infection) (Fig. 4C). The low level of OT-II cell recruitment and accumulation within the brains of infected mice precluded the assessment of their activation status. In combination, our results show that the magnitude and location of OVA expression within blood-stage malaria parasites influence the level, but not the activation state, of the cerebral antigen-specific T-cell response that develops during ECM.
DISCUSSION
For other infections, such as T. cruzi, T. gondii, and Leishmania infections, it has been shown that the subcellular location of antigens is a defining characteristic that dictates the development of antigen-specific T-cell responses (10, 11, 31). In the case of T. gondii, a model antigen that is secreted by the parasite initiates superior antigen-specific CD4+ and CD8+ T-cell responses compared to those induced by a model antigen that is restricted to the parasite cytosol (11, 32). In this study, we similarly found that the location of model antigen expression by blood-stage malaria parasites influences how the antigen is recognized by the immune system. Specifically, we determined that OVA expressed on the PVM (fused to HEP17/EXP1) induced stronger splenic OVA-specific CD4+ and CD8+ T-cell responses during blood-stage infection than those obtained with OVA located in the cytoplasm. This was despite OVA being expressed at higher absolute levels in the cytoplasm of OVA::mCherryhsp70 parasites than the PVM-associated OVA levels in the OVA::Hep17hep17 parasites. The OVA::Hep17hep17 parasites also promoted the highest levels of OT-I and OT-II cell migration to and accumulation within the brain during ECM development, compared with OVAhsp70 and OVA::mCherryhsp70 parasites. It is very unlikely that the difference in the magnitudes of OVA-specific T-cell responses to OVA::Hep17hep17 parasites and OVA::mCherryhsp70 parasites was due to an indirect effect of the different OVA-processing and epitope generation methods. We expressed full-length OVA (385 amino acid residues) in the transgenic parasites, with the OT-II and OT-I epitopes being located well within the protein (at residues 323 to 339 and 257 to 264, respectively), and we showed that OVA remained intact in all lines. Therefore, the amino acids flanking the epitopes, which provide the specificity for enzymatic cleavage, remain unaffected by fusion of OVA to either mCherry or HEP17.
Importantly, we found that the polyclonal endogenous T-cell responses, in terms of priming within the spleen and migration to and accumulation within the brain, were comparable during infection with the different parasite lines. This implies that the splenic and brain environments, as well as central nervous system migratory cues, are comparable during infections with the different OVA-expressing parasites, thus supporting the conclusion that the variations in OVA-specific T-cell responses during the infections with the different OVA-expressing parasites were directly determined by the nature of OVA expression, not by other inherent differences in the capacity of the parasites to influence T-cell activity. Consequently, while it has been reported that OT-I cells can be recruited to the brain during infections with OVA-expressing P. berghei ANKA parasites (5, 6), our study is the first to show that the level of antigen-specific T-cell recruitment to the brain can be governed largely by the magnitude of splenic T-cell priming to a specific antigen rather than by secondary differences in brain-localized T-cell migratory cues.
Why the subcellular location of a protein within blood-stage malaria parasites is a determining characteristic that influences the induction of an antigen-specific T-cell response during malaria infection is not immediately obvious. In contrast to T. gondii, Leishmania (31), and Mycobacterium tuberculosis (33), which can invade and reside within professional antigen-presenting cells, the malaria merozoite invades circulating RBCs and, within these cells, creates and resides in a PV (15). As mature RBCs neither display MHC-I and MHC-II molecules nor have antigen-processing machinery, an adaptive T-cell response can theoretically be generated only when classical DCs phagocytose infected RBCs or free merozoites. However, an explanation is that the location of protein expression within blood-stage parasites influences their incorporation into microparticles that are derived from infected RBCs. It is known that such microparticles are produced when schizonts mature and that these microparticles influence the activation of innate immune responses (34–36). The current understanding suggests that at schizogony, both PV and RBC membranes rupture, releasing microparticles containing RBC and PV membrane-associated proteins. In contrast, parasite proteins that are protected by the parasite plasma membrane, such as those located in the parasite cytosol, are less likely to become incorporated into such microparticles. Thus, OVA expressed on the PVM (OVA::Hep17hep17 parasites) is potentially recognized by the immune system in a different manner and at a different level than OVA expressed in the parasite cytoplasm (OVA::mCherryhsp70 and OVAhsp70 parasites). It would therefore be interesting to generate further transgenic lines expressing OVA in other subcellular locations (e.g., secreted into the RBC cytoplasm or on the RBC membrane) and to characterize their capacity to stimulate T-cell and/or antigen-presenting cell activation.
Notably, we unexpectedly found that very weak OVA protein expression occurred in OVAhsp70 parasites, in which unconjugated OVA was produced in the cytosol. This was despite strong OVA transcription levels in these parasites. When OVA was fused to mCherry and expressed under the control of the same promoter in OVA::mCherryhsp70 parasites, we observed strong OVA expression, and the levels reflected the transcription levels. Thus, our results show that unconjugated cytosolic OVA is unstable in blood-stage malaria parasites, which is an important finding with implications for researchers involved in making transgenic reporter parasites. The exact reason for unconjugated OVA instability is unclear; other unconjugated heterologous proteins (such as fluorescent and luminescent proteins) are highly expressed in transgenic Plasmodium parasites using the hsp70 promoter (37). It is possible that unconjugated OVA, which is a phosphorylated glycoprotein (38), is rapidly degraded because it is incorrectly posttranslationally modified in Plasmodium, whereas stability is conferred to OVA when it is fused to another protein. Irrespective of the exact reasons for the dichotomous expression of OVA in these two parasite lines, when we examined the T-cell responses to the parasites, we observed that OVAhsp70 parasites induced significantly inferior OT-I and OT-II immune responses compared with those of OVA::mCherryhsp70 parasites. Thus, these data indicate that the absolute expression level of an antigen, in addition to its location, is an important factor in determining antigen-specific immune responses during malaria infection.
In summary, these results demonstrate that the subcellular location of a model antigen in a transgenic parasite influences its effectiveness in initiating different T-cell responses. The transgenic P. berghei ANKA and NK65 reporter parasites we have generated are useful tools for further study of how parasite antigen expression and location can modulate site-specific immune responses in both the blood and liver stages of infection. Indeed, in future work, comparisons of OVA-transgenic parasites that induce ECM (P. berghei ANKA) and those that do not (i.e., P. berghei NK65) can be used to study the pathways orchestrating development and differentiation of pathogenic T-cell responses during malaria infection. Additionally, P. berghei NK65 lines can be used to examine how the location and magnitude of OVA expression differentially influence antigen-specific T-cell responses in more chronic malarial infections. More generally, the OVA-transgenic parasite lines described here may provide information on the types of endogenous malaria proteins that are most likely to be recognized by the immune system, potentially informing decisions about which malarial proteins should be incorporated into vaccines.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jai Ramesar for technical support. We thank Kees Franken (Infectious Diseases, Leiden University Medical Center, LUMC) for providing plasmid pENTRY201-OVA, Marcel Camps (Immunohematology and Blood Transfusion, LUMC) for anti-OVA serum, and Maria Mota (Instituto de Medicina Molecular, University of Lisbon, Portugal) for kindly providing us with the anti-P. berghei HSP70 antibodies.
J.-W. Lin was supported by the China Scholarship Council-Leiden University Joint Programme, and C. J. Janse was supported by a grant from the European Community's Seventh Framework Programme (FP7/2007–2013), under grant agreement 242095.
Footnotes
Published ahead of print 25 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01940-14.
REFERENCES
- 1.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. 2008. Immunity to malaria: more questions than answers. Nat. Immunol. 9:725–732. 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 2.Hafalla JC, Silvie O, Matuschewski K. 2011. Cell biology and immunology of malaria. Immunol. Rev. 240:297–316. 10.1111/j.1600-065X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- 3.Finney OC, Riley EM, Walther M. 2010. Regulatory T cells in malaria—friend or foe? Trends Immunol. 31:63–70. 10.1016/j.it.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Spence PJ, Langhorne J. 2012. T cell control of malaria pathogenesis. Curr. Opin. Immunol. 24:444–448. 10.1016/j.coi.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Miyakoda M, Kimura D, Yuda M, Chinzei Y, Shibata Y, Honma K, Yui K. 2008. Malaria-specific and nonspecific activation of CD8+ T cells during blood stage of Plasmodium berghei infection. J. Immunol. 181:1420–1428. 10.4049/jimmunol.181.2.1420. [DOI] [PubMed] [Google Scholar]
- 6.Lundie RJ, de Koning-Ward TF, Davey GM, Nie CQ, Hansen DS, Lau LS, Mintern JD, Belz GT, Schofield L, Carbone FR, Villadangos JA, Crabb BS, Heath WR. 2008. Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8alpha+ dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 105:14509–14514. 10.1073/pnas.0806727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura K, Kimura D, Matsushima Y, Miyakoda M, Honma K, Yuda M, Yui K. 2013. CD8+ T cells specific for a malaria cytoplasmic antigen form clusters around infected hepatocytes and are protective at the liver stage of infection. Infect. Immun. 81:3825–3834. 10.1128/IAI.00570-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundie RJ, Young LJ, Davey GM, Villadangos JA, Carbone FR, Heath WR, Crabb BS. 2010. Blood-stage Plasmodium berghei infection leads to short-lived parasite-associated antigen presentation by dendritic cells. Eur. J. Immunol. 40:1674–1681. 10.1002/eji.200939265. [DOI] [PubMed] [Google Scholar]
- 9.Vyas JM, Van der Veen AG, Ploegh HL. 2008. The known unknowns of antigen processing and presentation. Nat. Rev. Immunol. 8:607–618. 10.1038/nri2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg N, Nunes MP, Tarleton RL. 1997. Delivery by Trypanosoma cruzi of proteins into the MHC class I antigen processing and presentation pathway. J. Immunol. 158:3293–3302. [PubMed] [Google Scholar]
- 11.Gregg B, Dzierszinski F, Tait E, Jordan KA, Hunter CA, Roos DS. 2011. Subcellular antigen location influences T-cell activation during acute infection with Toxoplasma gondii. PLoS One 6:e22936. 10.1371/journal.pone.0022936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howland SW, Poh CM, Gun SY, Claser C, Malleret B, Shastri N, Ginhoux F, Grotenbreg GM, Renia L. 2013. Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol. Med. 5:916–931. 10.1002/emmm.201202273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons D, Woollett G, Bergin-Cartwright M, Kay D, Scaife J. 1987. A malaria protein exported into a new compartment within the host erythrocyte. EMBO J. 6:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kara UA, Stenzel DJ, Ingram LT, Kidson C. 1988. The parasitophorous vacuole membrane of Plasmodium falciparum: demonstration of vesicle formation using an immunoprobe. Eur. J. Cell Biol. 46:9–17. [PubMed] [Google Scholar]
- 15.Graewe S, Rankin KE, Lehmann C, Deschermeier C, Hecht L, Froehlke U, Stanway RR, Heussler V. 2011. Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog. 7:e1002224. 10.1371/journal.ppat.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JW, Annoura T, Sajid M, Chevalley-Maurel S, Ramesar J, Klop O, Franke-Fayard BM, Janse CJ, Khan SM. 2011. A novel ‘gene insertion/marker out' (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS One 6:e29289. 10.1371/journal.pone.0029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janse CJ, Ramesar J, Waters AP. 2006. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat. Protoc. 1:346–356. 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 18.van Spaendonk RM, Ramesar J, van Wigcheren A, Eling W, Beetsma AL, van Gemert GJ, Hooghof J, Janse CJ, Waters AP. 2001. Functional equivalence of structurally distinct ribosomes in the malaria parasite, Plasmodium berghei. J. Biol. Chem. 276:22638–22647. 10.1074/jbc.M101234200. [DOI] [PubMed] [Google Scholar]
- 19.Janse CJ, Waters AP. 1995. Plasmodium berghei: the application of cultivation and purification techniques to molecular studies of malaria parasites. Parasitol. Today 11:138–143. 10.1016/0169-4758(95)80133-2. [DOI] [PubMed] [Google Scholar]
- 20.Mueller AK, Camargo N, Kaiser K, Andorfer C, Frevert U, Matuschewski K, Kappe SH. 2005. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl. Acad. Sci. U. S. A. 102:3022–3027. 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT. 2006. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313:1287–1290. 10.1126/science.1129720. [DOI] [PubMed] [Google Scholar]
- 22.Villegas-Mendez A, Greig R, Shaw TN, de Souza JB, Gwyer Findlay E, Stumhofer JS, Hafalla JC, Blount DG, Hunter CA, Riley EM, Couper KN. 2012. IFN-gamma-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J. Immunol. 189:968–979. 10.4049/jimmunol.1200688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prevost MC, Ishino T, Yuda M, Menard R. 2008. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3:88–96. 10.1016/j.chom.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Ishino T, Orito Y, Chinzei Y, Yuda M. 2006. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Mol. Microbiol. 59:1175–1184. 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- 25.Kooij TW, Rauch MM, Matuschewski K. 2012. Expansion of experimental genetics approaches for Plasmodium berghei with versatile transfection vectors. Mol. Biochem. Parasitol. 185:19–26. 10.1016/j.molbiopara.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Lingelbach K, Joiner KA. 1998. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J. Cell Sci. 111:1467–1475. [DOI] [PubMed] [Google Scholar]
- 27.Ferrer M, Martin-Jaular L, De Niz M, Khan SM, Janse CJ, Calvo M, Heussler V, del Portillo HA. 2014. Imaging of the spleen in malaria. Parasitol. Int. 63:195–205. 10.1016/j.parint.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Villegas-Mendez A, de Souza JB, Murungi L, Hafalla JC, Shaw TN, Greig R, Riley EM, Couper KN. 2011. Heterogeneous and tissue-specific regulation of effector T cell responses by IFN-gamma during Plasmodium berghei ANKA infection. J. Immunol. 187:2885–2897. 10.4049/jimmunol.1100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renia L, Potter SM, Mauduit M, Rosa DS, Kayibanda M, Deschemin JC, Snounou G, Gruner AC. 2006. Pathogenic T cells in cerebral malaria. Int. J. Parasitol. 36:547–554. 10.1016/j.ijpara.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 30.de Souza JB, Hafalla JC, Riley EM, Couper KN. 2010. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137:755–772. 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 31.Bertholet S, Goldszmid R, Morrot A, Debrabant A, Afrin F, Collazo-Custodio C, Houde M, Desjardins M, Sher A, Sacks D. 2006. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J. Immunol. 177:3525–3533. 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 32.Pepper M, Dzierszinski F, Crawford A, Hunter CA, Roos D. 2004. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infect. Immun. 72:7240–7246. 10.1128/IAI.72.12.7240-7246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Einarsdottir T, Lockhart E, Flynn JL. 2009. Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect. Immun. 77:4621–4630. 10.1128/IAI.00415-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couper KN, Barnes T, Hafalla JC, Combes V, Ryffel B, Secher T, Grau GE, Riley EM, de Souza JB. 2010. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 6:e1000744. 10.1371/journal.ppat.1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantel PY, Hoang AN, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, Ghiran I, Toner M, Irimia D, Ivanov AR, Barteneva N, Marti M. 2013. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe 13:521–534. 10.1016/j.chom.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mantel PY, Marti M. 2014. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell. Microbiol. 16:344–354. 10.1111/cmi.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hliscs M, Nahar C, Frischknecht F, Matuschewski K. 2013. Expression profiling of Plasmodium berghei HSP70 genes for generation of bright red fluorescent parasites. PLoS One 8:e72771. 10.1371/journal.pone.0072771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki T, Kitajima K, Emori Y, Inoue Y, Inoue S. 1997. Site-specific de-N-glycosylation of diglycosylated ovalbumin in hen oviduct by endogenous peptide: N-glycanase as a quality control system for newly synthesized proteins. Proc. Natl. Acad. Sci. U. S. A. 94:6244–6249. 10.1073/pnas.94.12.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.