Abstract
Amphibians are suffering unprecedented global declines. A leading cause is the infectious disease chytridiomycosis caused by the chytrid fungus Batrachochytrium dendrobatidis. Chytridiomycosis is a skin disease which disrupts transport of essential ions leading to death. Soluble factors produced by B. dendrobatidis impair amphibian and mammalian lymphocytes in vitro, but previous studies have not shown the effects of these inhibitory factors in vivo. To demonstrate in vivo inhibition of immunity by B. dendrobatidis, a modified delayed-type-hypersensitivity (DTH) protocol was developed to induce innate and adaptive inflammatory swelling in the feet of Xenopus laevis by injection of killed bacteria or phytohemagglutinin (PHA). Compared to previous protocols for PHA injection in amphibians, this method induced up to 20-fold greater inflammatory swelling. Using this new protocol, we measured DTH responses induced by killed bacteria or PHA in the presence of B. dendrobatidis supernatants. Swelling induced by single injection of PHA or killed bacteria was not significantly affected by B. dendrobatidis supernatants. However, swelling caused by a secondary injection of PHA, was significantly reduced by B. dendrobatidis supernatants. As previously described in vitro, factors from B. dendrobatidis appear to inhibit lymphocyte-mediated inflammatory swelling but not swelling caused by an inducer of innate leukocytes. This suggests that B. dendrobatidis is capable of inhibiting lymphocytes in a localized response to prevent adaptive immune responses in the skin. The modified protocol used to induce inflammatory swelling in the present study may be more effective than previous methods to investigate amphibian immune competence, particularly in nonmodel species.
INTRODUCTION
Amphibians are declining at a rate faster than any other vertebrate taxon (1, 2). Infectious diseases, particularly chytridiomycosis, are major contributors to amphibian declines (3–5; reviewed in references 6, 7, and 8). Chytridiomycosis is a lethal skin infection of amphibians caused by the chytrid fungus Batrachochytrium dendrobatidis (9–11) and has been responsible for over 200 mass mortality events and an increasing threat of extinctions in many parts of the world (7, 12). The presence or absence of skin defenses predicts whether an amphibian species or population will persist with B. dendrobatidis infection (13–15; reviewed in reference 16). Despite the fact that immune responses may play a major role in survival of amphibians susceptible to chytridiomycosis, very little is known about the interactions between the amphibian immune system and B. dendrobatidis within the skin.
Adaptive immune responses, particularly those mediated by T lymphocytes, are essential for the clearance of fungal pathogens (reviewed in reference 17). Immunity to the potentially lethal amphibian-infecting chytrid fungus, B. dendrobatidis, also appears to require lymphocyte responses (15, 18). B. dendrobatidis probably resists host immunity in the skin by producing inhibitory factors that impair infiltrating lymphocytes. These factors inhibit in vitro responses of both amphibian and mammalian lymphocytes but do not appear to impair the viability or defenses of amphibian phagocytes (19). This immune evasion strategy allows B. dendrobatidis to infect immunocompetent hosts and helps to explain why species lacking innate mucosal immune defenses are so susceptible to chytridiomycosis (reviewed in reference 16).
Due to the current limitations in molecular tools for studies of amphibian immunology and the absence of genetic manipulation in B. dendrobatidis, the in vivo interactions between B. dendrobatidis and host cells are difficult to investigate. Xenopus laevis is one of the best models for studies of amphibian immunology, and yet many of the reagents necessary to determine which cell populations and cytokines are important in immunity to chytridiomycosis (20) are not available. Therefore, investigations of amphibian immunity must rely on more classical immunological techniques. Amphibian leukocytes respond to mitogens (21) or bacterial products (19, 22) similar to other vertebrates. Phytohemagglutinin (PHA), phorbol-12-myristate 13-acetate, concanavalin A, and killed bacteria are mitogenic for amphibian B and T lymphocytes (19, 21). Also, the intraperitoneal injection of killed Escherichia coli into X. laevis induces infiltration of macrophages and neutrophils into the peritoneum (19, 23).
PHA injection is a common method used to investigate immunocompetence in nonmodel vertebrates. In birds, PHA is injected subcutaneously in avian patagia (wing-webs), and inflammation is measured by swelling at the site of injection (24). PHA induces robust T cell proliferation in vitro in amphibians (19, 21) and can also induce in vivo inflammatory swelling in adults, metamorphs, and tadpoles (25–28). In vivo, PHA induces swelling through the recruitment of granulocytes, phagocytes, thrombocytes, and lymphocytes (24, 27). Leukocyte recruitment typically begins with infiltration of neutrophils/heterophils and macrophages that are later followed by lymphocytes. Significant lymphocyte (primarily T cell) recruitment and activation induced by PHA typically requires a second injection of PHA (27, 29). Evaluation of T cell responses using PHA injection in amphibians probably requires two PHA injections because the inflammation of the primary response is overwhelmingly comprised of innate phagocytes and granulocytes.
In adult frogs, investigators have injected PHA into several different sites, including the toe (25), the thigh (26), and toe webbing (27). In these studies, subcutaneous injection of PHA induced less than a millimeter of swelling compared to buffer controls, with most recorded differences being about 0.1 mm. Such small differences require very precise measuring tools and handling, and they may not represent a biologically significant response. In pilot experiments with a large (>100 g) X. laevis, we observed no noticeable or measurable difference in swelling after subcutaneous PHA injection in different locations of the foot. Therefore, the technique was modified for the present study by the injection of PHA deeper into the foot tissue.
To determine the effects of B. dendrobatidis inhibitory factors on amphibian innate or adaptive immune responses in vivo, this modified method of foot injection was used. A primarily innate immune response was induced with killed E. coli, known to stimulate phagocyte infiltration into the peritoneum (19, 23). Injection of PHA was chosen as a way to induce mixed innate and adaptive immune responses. Here we show that immune inhibitory factors produced by B. dendrobatidis inhibited adaptive immune responses induced locally in the foot after an intraperitoneal priming injection and a second PHA injection in the foot but did not impair swelling caused by a single injection of killed E. coli or PHA into the foot. These data support our previous in vitro observations that B. dendrobatidis produces soluble factors that inhibit lymphocyte responses but do not appear to inhibit innate phagocyte responses (19).
MATERIALS AND METHODS
Animals.
Outbred X. laevis from Xenopus I (Dexter, MI) or Nasco (Fort Atkinson, WI) were kept in polystyrene containers at a density of six to eight frogs per 16 liters of dechlorinated tap water. Females ranging in size between 110 and 225 g were used for foot injection experiments. During foot injection experiments, frogs were housed individually in polystyrene containers so that the experiments could be measured in a blinded fashion. Before foot injection and measurements, frogs were anesthetized in ethyl-m-aminobenzoate methanesulfonate salt (MS-222; MP Biomedicals LLC, Solon, OH) at 5 g/liter until all movement ceased. Frogs were then immediately placed in dechlorinated water to wash off the anesthetic. After measurements were taken, frogs were carefully observed in fresh dechlorinated water until they were able to make voluntary motions. Due to possible harmful effects of multiple exposures to MS 222, individuals were anesthetized three or fewer times. All animal procedures were approved by the Institutional Animal Care and Use Committee of Vanderbilt University School of Medicine.
Induction of inflammatory swelling in the foot region.
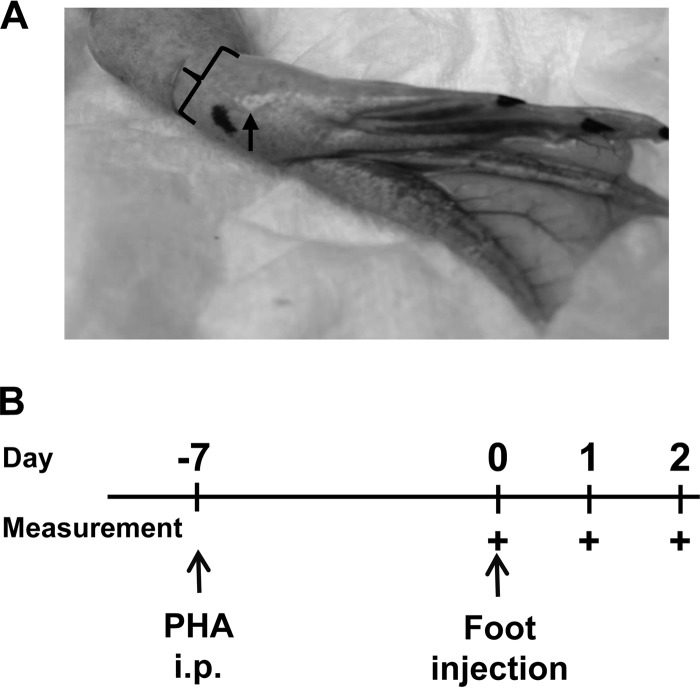
The site and depth of injection were determined based on pilot injections of PHA-P (Sigma, St. Louis, MO) in amphibian phosphate-buffered saline (APBS) (18) at 2 mg/ml or killed bacteria (E. coli, 1010 killed CFU/ml) into several sites in the foot from the ankle to the toes. Subcutaneous injection yielded insignificant swelling. Deeper, intramuscular injections in the foot region caused more swelling than injections in the ankle or toes. After this optimization, injections were made intramuscularly into the feet of X. laevis in the middle of the plantar side of the foot (Fig. 1A) using 1-ml syringes with 25 or 30 gauge needles. Dimensions of the foot were measured to the nearest 0.1 mm using a Mitutoyo plastic digital caliper (model 4LB11; Mitutoyo USA, Morgan Precision Tools, Aurora, IL). The foot width (in the plane of the foot) and foot thickness (perpendicular to the plane of the foot) were measured. The dimensions of each foot were measured at least three times at each time point, and these measurements were averaged to determine a more accurate measurement of foot dimension.
FIG 1.
Experimental design for injection of feet with inducers of inflammatory swelling. (A) X. laevis feet were injected intramuscularly in the middle of the foot on the plantar side (arrow). Measurements of the foot thickness (bracket) and width (perpendicular to the thickness) were recorded with a caliper. (B) Schematic of foot injections. Foot injections occurred on day 0 with either APBS, PHA alone, killed E. coli alone, PHA and B. dendrobatidis supernatant (Bd Sup), or killed E. coli and B. dendrobatidis supernatant. Intraperitoneal (i.p.) injection with PHA only occurred for defined experiments in which frogs were primed with PHA. The time points at which feet were measured are indicated (+).
Foot perimeter (P) was calculated using the foot width and thickness measurements to calculate the perimeter of an ellipse using one-half of the foot width (a) and one-half of the foot thickness (b) as follows:
The peak of inflammatory swelling induced by PHA in previous amphibian studies occurs at about 24 h (Fig. 1B) (25–27). Therefore, foot dimensions were measured before injection and 24 h after injection in all experiments. Foot dimensions were also measured at 48 h after injection to follow the kinetics of PHA-induced swelling. The percent increase in foot size was determined by dividing the foot dimension at 24 or 48 h postinjection by the foot dimension from the same foot before injection. Because the natural foot width and thickness had a range of ∼3.5 mm between largest and smallest frogs, the percent increase was corrected for the variation in size of individual frogs.
Injection treatments were randomly assigned by a coin flip to the right or left foot after the initial measurements were taken. Based on the coin flip, one foot received the control treatment and the other received the experimental treatment. Before measurements were taken at 24 and 48 h postinjection, frogs were randomly reassigned new identification so that the person measuring the foot dimensions was blinded to the injection treatment. The identity of frogs was revealed after measurements were recorded.
Coinjection with B. dendrobatidis supernatants.
B. dendrobatidis supernatants were prepared from isolate JEL197 as previously described (19). Briefly, B. dendrobatidis cells were centrifuged, washed with sterile glass distilled water, resuspended at 107 matured cells (all cells beyond zoospore stage) per ml in sterile distilled water, and incubated at 21°C for 24 h in large flasks. Cells were centrifuged, and supernatants were passed through 0.2-μm-pore-size filters (Fisher, Waltham, MA) to remove any cells. Supernatants were lyophilized and resuspended in APBS to be injected with inducers of inflammatory swelling. Supernatants were injected into the feet at a concentration of 10× of the original supernatant concentration before lyophilization.
To quantify PHA-induced inflammatory swelling, PHA-P in APBS at 2 mg/ml or APBS buffer alone was injected in a volume of 100 μl into the right or left foot of six individual X. laevis. At 7 days before foot injection with PHA and B. dendrobatidis supernatant, X. laevis were or were not primed with an intraperitoneal injection of 100 μl of 1 mg/ml PHA in APBS (Fig. 1B). When PHA (1 mg/ml) was injected with B. dendrobatidis supernatants (10×), 200 μl of either PHA alone or PHA with B. dendrobatidis supernatant (10×) were injected into the feet of 6 (without PHA priming) or 12 (with PHA priming) individual X. laevis.
A culture of heat-killed E. coli was prepared as previously described (19). Briefly, E. coli (strain DH 5α) was grown to a concentration of approximately 5.7 × 108 CFU/ml. The cells were boiled for 1 h in a water bath, washed twice, and resuspended in sterile APBS to 1010 killed CFU/ml. To verify that injection of heat-killed E. coli induces inflammatory swelling, 200 μl of either APBS or E. coli in APBS were injected into the feet of six individual X. laevis. When heat-killed E. coli were injected with B. dendrobatidis supernatants, 200 μl of either killed E. coli alone or killed E. coli with B. dendrobatidis supernatant (10×) were injected into the feet of 12 individual X. laevis.
Correlation of B. dendrobatidis infection with spleen size.
Spleens were previously harvested from X. laevis to obtain a lymphocyte population to investigate the effects of B. dendrobatidis on amphibian lymphocytes (19). The individuals used in this experiment were males and females ranging in mass between 50 and 250 g. The number of splenocytes from each individual spleen was counted after splenocytes were enriched by centrifugation over a Ficoll-Hypaque cushion (Sigma). Individuals not screened for B. dendrobatidis infection (n = 75) were used to confirm that spleen size does significantly correlate with total mass in X. laevis. Because larger individuals tend to have larger spleens, the number of splenocytes was normalized by dividing the total number of splenocytes by the mass, in mg, of the frog. To keep track of the B. dendrobatidis infection status in the colony, a number of these frogs (n = 61) were swabbed before euthanasia and spleen harvesting. DNA was extracted from these swabs to determine the number of zoospore equivalents on the skin according to the methods of Boyle et al. (30) and Hyatt et al. (31) by quantitative PCR. Swabbing procedure, PCR conditions, and standard curve calculation were as described by Ramsey et al. (18). Zoospore equivalents (z) determined by quantitative PCR were log transformed to calculate B. dendrobatidis infection load (B. dendrobatidis load) using the following equation B. dendrobatidis load = log10(z + 1).
Statistics.
The increase in size due to inflammatory swelling from the two compared treatments in foot injection experiments was analyzed by using two-tailed, paired Student's t test because each individual received both treatments but in different feet. A Bonferroni correction was made because each experiment contained three-foot dimension measurements, so the alpha for statistical significance was set to 0.017. To determine whether there was a correlation between infection load and spleen size (relative number of splenocytes) a regression analysis was used. A P value of <0.05 was considered statistically significant.
RESULTS
Injection of PHA and killed E. coli to induce inflammatory swelling.
Before injection with PHA or killed E. coli, the average foot width among all X. laevis individuals in all experiments (42 frogs, 84 feet) was 11.65 ± 0.08 mm (mean ± the standard error of the mean [SEM]), and the average foot thickness was 11.21 ± 0.08 mm (mean ± the SEM). Comparisons were made between left and right feet irrespective of treatment, and there were no significant differences between the left feet and right feet for any experiment at any time point (data not shown).
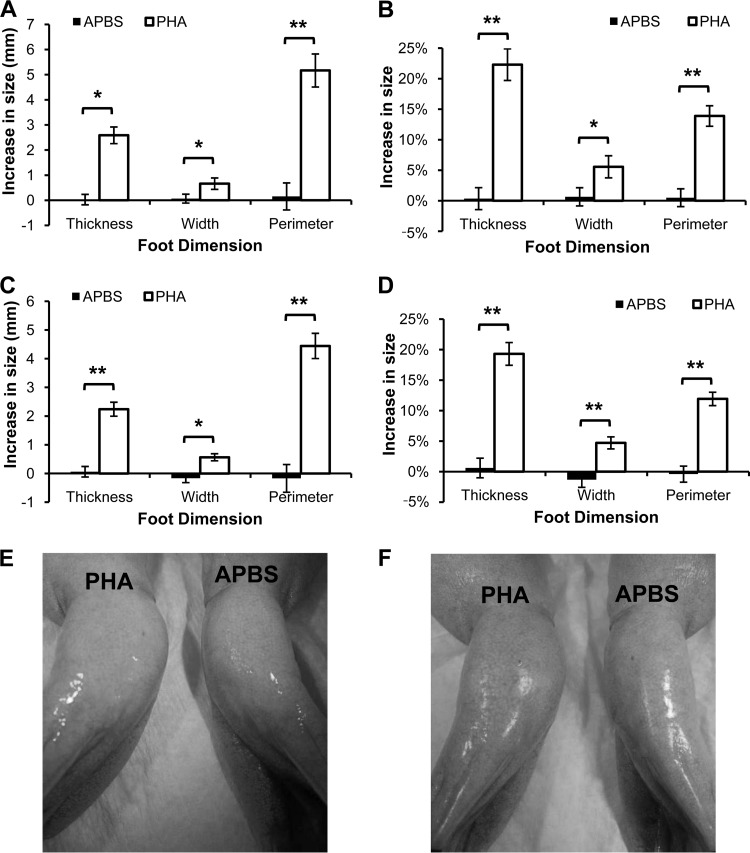
Intramuscular PHA injection into the foot area caused significantly greater swelling in all dimensions compared to the injection of APBS vehicle control (Fig. 2A and C). Swelling induced by PHA increased the width of the foot by about 5% and the thickness by about 20% (Fig. 2B and D). PHA induced a large increase in the foot size 24 h after injection (Fig. 2A and B), which was not significantly changed after 48 h (Fig. 2C and D); however, the difference in swelling between PHA and APBS treatments was more significant at 48 h than at 24 h. Feet injected with PHA were visibly larger than feet injected with APBS for all individuals (Fig. 2E and F).
FIG 2.
Intramuscular injection of phytohemagglutinin (PHA) into the foot of X. laevis induces inflammatory swelling after 24 h (A, B, and E) and 48 h (C, D, and F). PHA injections induced significantly greater swelling than buffer (APBS) controls (*, P < 0.01; **, P < 0.001 [paired Student's t test; alpha set to 0.017 for multiple tests]). The data show the mean (± the SEM) increase in actual size (A and C) or percent increase (B and D) in foot size compared to each foot's measurement before injection from both feet of six frogs. Representative photographs of individuals at 24 h (E) or 48 h (F) after injection of APBS in the left foot and PHA into the right foot (pictures show ventral side of frogs).
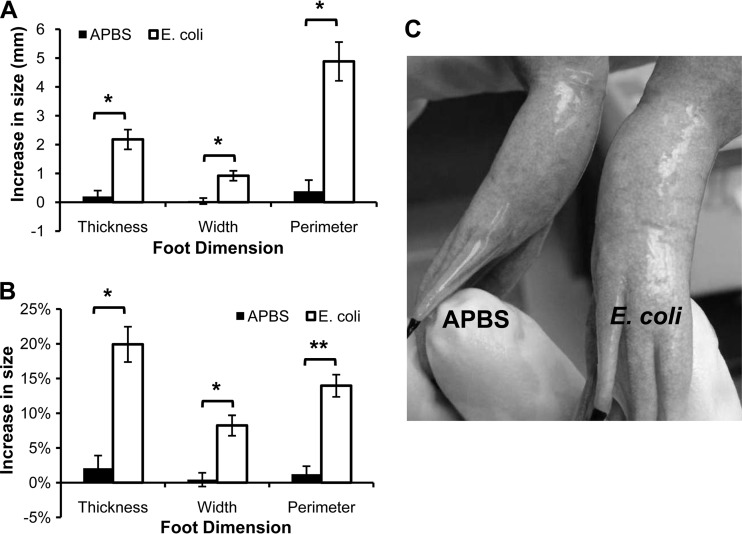
X. laevis feet injected with heat-killed E. coli showed profound swelling 24 h after injection, and control feet injected with APBS buffer did not show a significant change in foot size in comparison with the size determined before injection (Fig. 3). Injection of killed E. coli caused a significant increase in the size of the feet (Fig. 3A). This increase represented an increase in the foot width by about 8% and foot thickness by 20% after E. coli injection (Fig. 3B). As with injection with PHA, killed E. coli induced a visible increase in foot size compared to the APBS control (Fig. 3C).
FIG 3.
Intramuscular injection of killed E. coli into the foot of X. laevis induces inflammatory swelling after 24 h. E. coli injections induced significantly greater swelling than buffer (APBS) controls (*, P < 0.01; **, P < 0.001 [paired Student's t test; alpha set to 0.017 for multiple tests]). The data show the mean (± the SEM) increase in actual size (A) or percent increase (B) in foot size compared to each foot's measurement before injection from both feet of six frogs. (C) Representative photograph of an individual 24 h after injection of APBS in the right foot and of killed E. coli into the left foot (the picture shows the ventral side of the frog).
Redness is a classical symptom of acute inflammation (32). However, no reddening of the foot near the site of injection was visible for either the PHA or the killed E. coli injections. The only visible effect of injection with inflammatory inducers was an increase in the size of the foot (Fig. 2E and F; Fig. 3C). The foot swelling did not appear to cause discomfort and did not obstruct movement of the X. laevis.
In vivo inhibition of a local immune response by B. dendrobatidis.
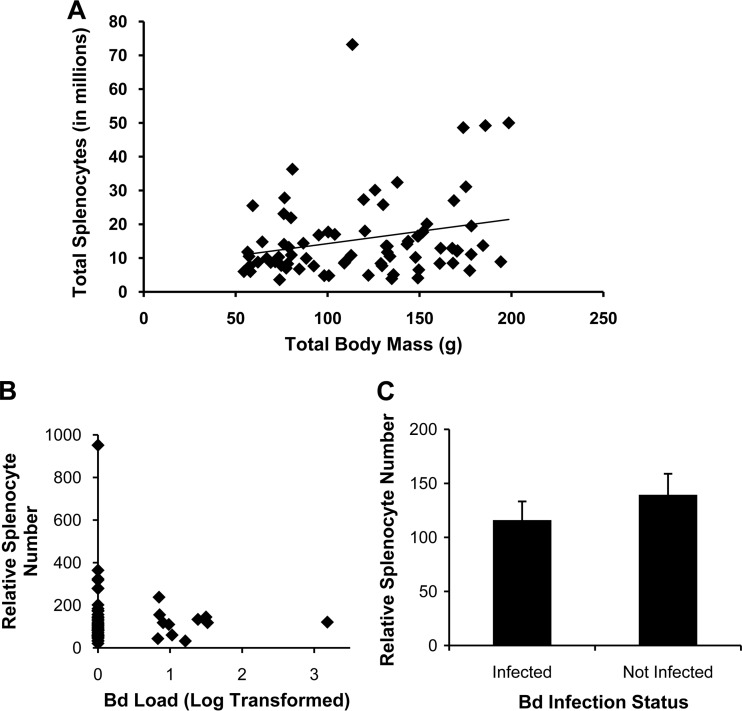
In our previous study demonstrating paralysis of lymphocytes by B. dendrobatidis factors, the B. dendrobatidis infection burdens of many X. laevis used to obtain splenocytes were assessed (19). Other frogs not screened for B. dendrobatidis infection were used to confirm that spleen size was significantly correlated with total mass in X. laevis (Fig. 4A). The data obtained from frogs with detectable B. dendrobatidis infections was used here to determine whether B. dendrobatidis infection in the skin would affect lymphocyte populations in the spleen (the only secondary lymphoid organ in frogs). There was no effect of intensity of infection (Fig. 4B) or infection status (Fig. 4C) on the number of splenocytes obtained from X. laevis. Because the X. laevis harbored very low infection burdens in these studies, B. dendrobatidis did not appear to significantly affect splenocyte populations. Therefore, the foot injection approach described here was taken to measure the effects of B. dendrobatidis soluble factors on a local induced immune response in the foot.
FIG 4.
B. dendrobatidis infection does not affect the number of leukocytes present in the spleen (splenocytes) of X. laevis. (A) Splenocyte number positively correlates with size in X. laevis. A significant correlation exists (P = 0.03) between the total body mass of individuals, and the total number of splenic leukocytes. n = 75, R = 0.25; the best fit line is shown. (B) No significant relationship exists between the infection load and relative number of splenocytes (relative to body mass) by regression analysis (all: P = 0.64; infected: P = 0.99). Bd Load, B. dendrobatidis load. (C) There is also no significant difference in the mean number of splenocytes (± the SEM) between individuals that were or were not infected with B. dendrobatidis by two-tailed Student's t test (P = 0.37). The number of splenocytes was normalized (B and C) to the size of each individual by dividing the total splenocytes by the mass in mg of the frog. The Bd load was determined by quantitative PCR using zoospore equivalent standards and log transformed. n = 50 for individuals that were not infected; n = 11 for infected individuals. The mean infection load (± the SEM) for infected individuals was 17.8 ± 0.9 zoospore equivalents.
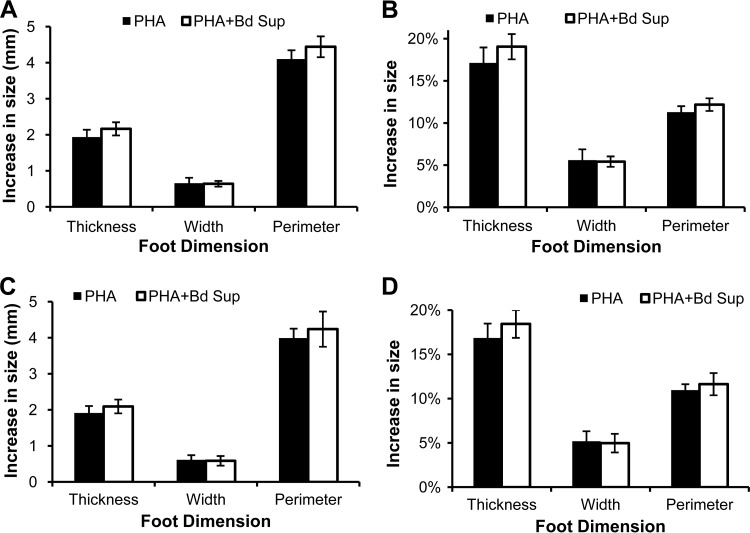
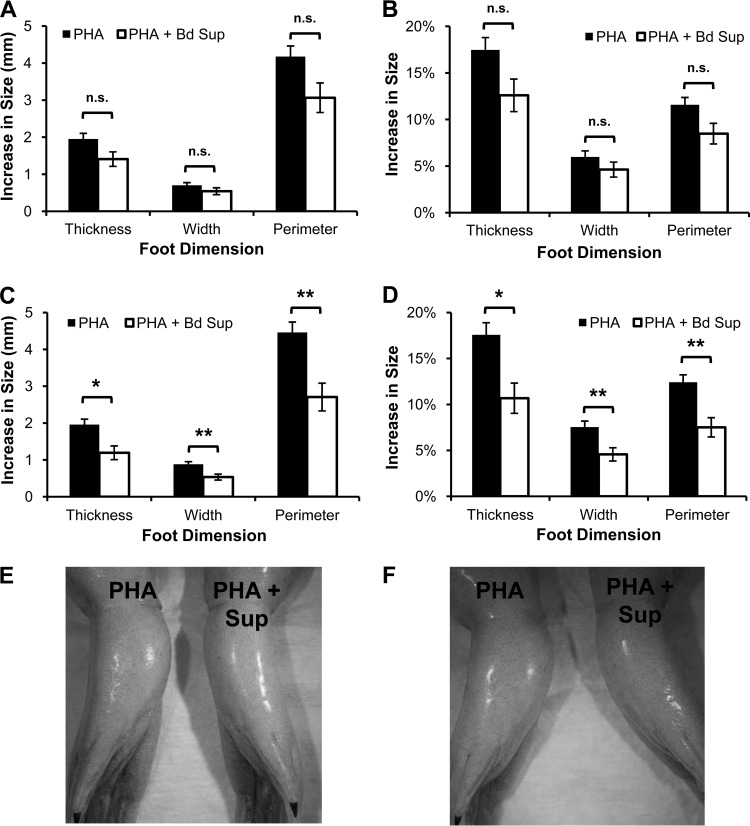
B. dendrobatidis supernatants were injected along with PHA to determine whether inhibitory factors decreased the swelling caused by innate and adaptive leukocytes activated by PHA. B. dendrobatidis supernatants had no significant impact on the increase of foot size following a single PHA injection (Fig. 5). Previous studies suggested that a single PHA injection typically promotes a more robust innate leukocyte response, and a second PHA injection promotes recruitment of phagocytes again but with greater activation and immigration of lymphocytes (27, 29). To investigate whether B. dendrobatidis factors impaired adaptive immune responses in vivo, X. laevis frogs were primed with a single intraperitoneal PHA injection before receiving foot injections of PHA alone or PHA with B. dendrobatidis supernatants. After PHA priming, B. dendrobatidis supernatants caused a significant reduction in foot swelling induced by PHA (Fig. 6). At 24 h after injection, the difference in swelling was substantial but not significant (Fig. 6A and B). However, by 48 h, the swelling of feet receiving B. dendrobatidis supernatants with PHA was significantly diminished compared to the feet receiving PHA alone (Fig. 6C and D). In some individuals, the decrease in swelling caused by B. dendrobatidis supernatants was visible without the need for measuring devices at both 24 and 48 h (Fig. 6E and F).
FIG 5.
B. dendrobatidis supernatant (Bd Sup) does not impair inflammatory swelling induced by a single PHA injection (frogs were not primed with PHA). X. laevis feet were injected with PHA alone or with PHA and Bd Sup. Feet were measured before injection and at 24 h (A and B) and 48 h (C and D) after injection. The data show the mean (± the SEM) increase in actual size (A, C) or percent increase (B, D) in foot size compared to each foot's measurement before injection from both feet of six frogs. Swelling was not significantly different between treatments (paired Student's t test).
FIG 6.
B. dendrobatidis supernatant (Bd Sup) reduces inflammatory swelling induced by a second PHA injection. X. laevis feet were injected with PHA alone or with PHA and Bd Sup 7 days after priming with intraperitoneal injection of PHA. Feet were measured before injection and at 24 h (A, B, and E) and 48 h (C, D, and F) after injection. The data show the mean (± the SEM) increase in actual size (A and C) or percent increase (B and D) in foot size compared to each foot's measurement before injection from both feet of 12 frogs. Swelling was only significantly different between treatments in feet 48 h after injection. n.s., P > 0.017; *, P < 0.01; **, P < 0.001 (paired Student's t test; alpha set to 0.017 for multiple tests). Representative photographs of an individual at 24 h (E) and 48 h (F) after the injection of PHA and Bd Sup in the left foot and PHA alone into the right foot (the pictures show the ventral side of the frog).
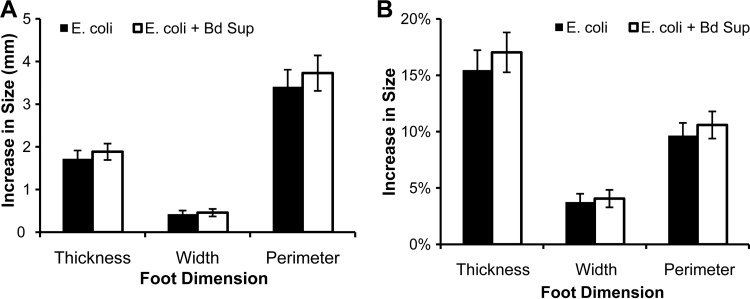
B. dendrobatidis supernatants were also injected along with heat-killed E. coli to determine whether factors present in the supernatants decreased inflammatory swelling caused by infiltrating phagocytes activated by dead bacteria. Injection with B. dendrobatidis supernatant had no significant effect on the increase in foot size caused by injection with killed E. coli, and foot dimensions were not different between the feet receiving E. coli alone and those receiving both E. coli and B. dendrobatidis supernatants (Fig. 7). The increase in foot size of this experiment was comparable to what was observed when only one foot received the killed E. coli injection.
FIG 7.
B. dendrobatidis supernatant (Bd Sup) does not impair inflammatory swelling induced by killed E. coli. X. laevis feet were injected with either killed E. coli alone or E. coli with Bd Sup. Feet were measured before injection and 24 h after injection. The data show the mean (± the SEM) increase in actual size (A) or percent increase (B) in foot size compared to each foot's measurement before injection from both feet of 12 frogs. Swelling was not significantly different between treatments (paired Student's t test).
DISCUSSION
Inhibition of local adaptive immune responses by B. dendrobatidis.
Early studies of chytridiomycosis noted minimal leukocyte infiltration into B. dendrobatidis-infected skin (9, 11, 33). Transcriptional studies of early infections also found little activation of immune gene expression during chytridiomycosis (34, 35). The absence of robust immune responses to B. dendrobatidis is not likely due to the incapacity of amphibian immunity. Adaptive immune defenses can be activated and promote resistance to chytridiomycosis in some species (15, 18, 36, 37). The ineffective clearance of B. dendrobatidis is most likely the result of immune evasion due to suppressive factors produced by the fungus (19, 37). B. dendrobatidis impairs lymphocytes in vitro (19), and very recent transcriptional expression studies in the highly susceptible frog, Atelopus zeteki, also seem to show a robust immune activation which is subsequently suppressed (37). Both observations may explain the lack of robust immunity in the most susceptible species.
The factors that impair and kill amphibian lymphocytes in vitro do not appear to inhibit amphibian phagocytes, suggesting that the targets of evasion are effector lymphocytes (19). The present study reproduces these in vitro observations in vivo using a modified protocol to induce inflammatory swelling in the feet of X. laevis. Primary injection with PHA or killed E. coli induces a primarily innate immune response mainly causing an infiltration of phagocytic leukocytes (19, 23, 27, 29). The inflammatory swelling induced by these injections was not significantly affected when B. dendrobatidis supernatants were simultaneously injected, suggesting no impairment of innate immune responses. Amphibian macrophages and neutrophils were not impaired in vitro by B. dendrobatidis supernatants in our previous study (19), and soluble B. dendrobatidis factors did not appear to kill or impair recruitment of innate leukocytes into the foot in the present study.
In a previous study, a single injection of PHA did induce a small amount of lymphocyte recruitment in an amphibian host, but this is likely to be a minor component of the inflammatory response causing swelling (27). A second PHA injection promoted a more robust lymphocyte response in birds (29) and induced greater swelling in amphibians (27). In our experimental design, X. laevis was primed with an intraperitoneal injection of PHA a week before feet were injected with either PHA alone or PHA with B. dendrobatidis supernatant. The priming injection of PHA allowed for a greater lymphocyte response during the second injection. Unlike the response induced with a single PHA injection, the inflammatory swelling induced by the second injection was significantly reduced by factors present in B. dendrobatidis supernatants, suggesting that lymphocytes were impaired by B. dendrobatidis factors in vivo as previously characterized in vitro. The full effect of B. dendrobatidis supernatants did not become significant until 48 h after injection. This correlates well with the observed peak of B. dendrobatidis-induced amphibian lymphocyte apoptosis (19). The delayed effect of B. dendrobatidis supernatant also may be explained by the later recruitment of lymphocytes during PHA injection compared to the infiltration of innate leukocytes (27). Because B. dendrobatidis factors inhibit activation and induce apoptosis in lymphocytes (19), the mechanism of decreased swelling induced by a secondary PHA injection probably is mediated by the inhibition of lymphocyte proliferation and induction of lymphocyte apoptosis after recruitment. However, B. dendrobatidis supernatants may also inhibit lymphocyte infiltration by disrupting or damping chemokine signaling (37).
We assumed that injection of E. coli induces a rapid immigration of neutrophils and macrophages rather than a lymphocyte-specific response because these cells are recruited during a single intraperitoneal injection of E. coli into X. laevis (19, 23). However, we did not test whether intraperitoneal priming with E. coli would result in enhanced swelling of the foot, as was observed with priming and injection of PHA. If priming with E. coli does induce an E. coli-specific lymphocyte response, we would expect that the B. dendrobatidis supernatants would inhibit that response as well.
The in vivo injection of B. dendrobatidis demonstrated that soluble factors from B. dendrobatidis inhibited local adaptive immune responses at the sites of lymphocyte recruitment and activation in X. laevis. The decrease in inflammation caused by B. dendrobatidis factors only appeared to affect the site where supernatants were injected. The amount of inflammation in feet injected with PHA alone resembled the amount of inflammation caused by PHA when no B. dendrobatidis supernatant was injected into X. laevis. However, a systemic inhibition of immunity may be possible during an infection that lasts longer than the duration of the foot injection experiment. The lymphotoxic factors released by B. dendrobatidis in the skin may be released into the bloodstream at a high enough concentration to have an impact on lymphocytes further away from the sites of infection. When we investigated the effect of low level B. dendrobatidis infection on splenocytes, we saw no effect of low-intensity infection on X. laevis splenocyte number. However, studies of gene transcription in A. zeteki late in infection and with heavy lethal pathogen burdens support the hypothesis that fungal products from the skin infection can alter splenic lymphocyte numbers and functions (37). These results suggest that the factors released by B. dendrobatidis may, indeed, interact with the immune system outside the skin.
An improved method for eliciting a DTH response in amphibians.
PHA injection is a common method used to measure a delayed-type-hypersensitivity (DTH) immune response in nonmodel vertebrates. In birds a consensus protocol of injecting patagia has been developed (24), but no such site has previously been determined for anuran amphibians. In previous studies, many of the sites chosen for injecting adult frogs showed a very small amount of swelling induced by PHA (26, 27). Such small differences in size require very precise tools and a large number of replicates to obtain statistical significance. For our experimental design, in which factors from B. dendrobatidis were examined to determine whether they would decrease swelling, greater inflammatory swelling was necessary to determine significant inhibition of inflammatory processes. A modified protocol in which PHA was injected intramuscularly into the feet of X. laevis was developed. The amount of swelling observed was between 3 and 20 times greater than previously recorded with subcutaneous injection in frogs (25–28). The amount of inflammatory swelling induced by intramuscular injection was sufficient so that a noticeable difference was visible between PHA and buffer injections. This method was also suitable for measuring swelling induced by killed bacteria.
Priming with a prior injection of PHA appears to be necessary to induce a robust lymphocyte response in our studies. In birds, a second injection of PHA into the patagium induces a greater infiltration of lymphocytes than a single injection (29). In amphibians, a single injection of PHA does induce some lymphocyte recruitment, and a second injection of PHA induces significantly greater swelling (27). In our experimental design, we injected PHA intraperitoneally 7 days before the second injection of PHA into the feet. The peritoneum was chosen for PHA priming so both feet did not have to be injected and because the peritoneum has previously been used as the site to induce immune responses in X. laevis (18, 19, 23).
Taken together, our studies suggest that intramuscular foot injection of PHA or other inducers of inflammation may be a better method for investigating DTH immune responses in amphibians than previously published sites of injection. Deeper injection into the foot and the increased difference in swelling requires less precision in injection and measuring, thus decreasing experimental error and decreasing the need for many experimental replicates. The simplicity of the method may also make it preferable for future studies.
In summary, we used an improved immunological method in amphibians to provide in vivo evidence that B. dendrobatidis is capable of inhibiting local lymphocyte responses in its amphibian host to promote infection. This adaptation by B. dendrobatidis to evade adaptive immune responses may help to explain how it is such a successful pathogen known to infect more than 500 species of amphibians (38) and why amphibians lacking robust innate immune responses are so susceptible to chytridiomycosis (reviewed in reference 16).
ACKNOWLEDGMENTS
This research was supported by National Science Foundation grants IOS-0843207 and IOS-1121758 (to L.A.R.-S.). T.M.C. was supported by an REU supplement to National Science Foundation grant IOS-1121758.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 25 August 2014
REFERENCES
- 1.Houlahan J, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL. 2000. Quantitative evidence for global amphibian population declines. Nature 404:752–755. 10.1038/35008052. [DOI] [PubMed] [Google Scholar]
- 2.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. 2004. Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786. 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 3.Lips KR, Bren F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins JP. 2006. Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proc. Natl. Acad. Sci. U. S. A. 103:3165–3170. 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rachowicz LJ, Knapp RA, Morgan JAT, Stice MJ, Vredenburg VT, Parker JM, Briggs CJ. 2006. Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 87:1671–1683. 10.1890/0012-9658(2006)87[1671:EIDAAP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Voyles J, Vredenburg VT, Tunstall TS, Parker JM, Briggs CJ, Rosenblum EB. 2012. Pathophysiology in mountain yellow-legged frogs (Rana muscosa) during a chytridiomycosis outbreak. PLoS One 7:e35374. 10.1371/journal.pone.0035374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4:125–134. 10.1007/s10393-007-0093-5. [DOI] [Google Scholar]
- 7.Wake DB, Vredenburg VT. 2008. Colloquium paper: are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. U. S. A. 105:11466–11473. 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins JP. 2010. Amphibian decline and extinction: what we know and what we need to learn. Dis. Aquat. Organ. 92:93–99. 10.3354/dao02307. [DOI] [PubMed] [Google Scholar]
- 9.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. U. S. A. 95:9031–9036. 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 91:219–227. 10.2307/3761366. [DOI] [Google Scholar]
- 11.Pessier AP, Nichols DK, Longcore JE, Fuller MS. 1999. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White's tree frogs (Litoria caerulea). J. Vet. Diagn. Invest. 11:194–199. 10.1177/104063879901100219. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann M, Hilton-Taylor C, Angulo A, Böhm M, Brooks TM, Butchart SHM, Carpenter KE, Chanson J, Collen B, Cox NA, Darwell WRT, Dulvy NK, Harrison LR, Katariya V, Pollock CM, Quader S, Richman NI, Rodrigues ASL, Tognelli MF, Vié J-C, Aguiar JM, Allen DJ, Allen GR, Amori G, Ananjeva NB, Andreone F, Andrew P, Aquino Ortiz AL, Baillie JEM, Baldi R, Bell BD, Biju SD, Bird JP, Black-Decima P, Blanc JJ, Bolaños F, Bolivar-G W, Burfield IJ, Burton JA, Capper DR, Castro F, Catullo G, Cavanagh RD, Channing A, Chao NL, Chenery AM, Chiozza F, Clausnitzer V, Collar NJ, Collett LC, Collette BB, Cortez Fernandez CF, Craig MT, Crosby MJ, Cumberlidge N, Cuttelod A, Derocher AE, Diesmos AC, Donaldson JS, Duckworth JW, Dutson G, Dutta SK, Emslie RH, Farjon A, Fowler S, Freyhof J, et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330:1503–1509. 10.1126/science.1194442. [DOI] [PubMed] [Google Scholar]
- 13.Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith LA. 2007. Resistance to chytridiomycosis varies by amphibian species and is correlated with skin peptide defenses. Animal Conservation 10:409–417. 10.1111/j.1469-1795.2007.00130.x. [DOI] [Google Scholar]
- 14.Becker MH, Harris RN. 2010. Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PLoS One 5:e10957. 10.1371/journal.pone.0010957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage AE, Zamudio KR. 2011. MHC genotypes associate with resistance to a frog-killing fungus. Proc. Natl. Acad. Sci. U. S. A. 108:16705–16710. 10.1073/pnas.1106893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollins-Smith LA, Ramsey JP, Pask Reinert JD LK, Woodhams DC. 2011. Amphibian immune defenses against chytridiomycosis: impacts of changing environments. Integr. Comp. Biol. 51:552–562. 10.1093/icb/icr095. [DOI] [PubMed] [Google Scholar]
- 17.Wüthrich M, Deepe GS, Jr, Klein BS. 2012. Adaptive immunity to fungi. Annu. Rev. Immunol. 30:115–148. 10.1146/annurev-immunol-020711-074958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA. 2010. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 78:3981–3992. 10.1128/IAI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fites JS, Ramsey JP, Holden WM, Collier SP, Sutherland DM, Reinert LK, Gayek AS, Dermody TS, Aune TM, Oswald-Richter K, Rollins-Smith LA. 2013. The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science 342:366–369. 10.1126/science.1243316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert J, Cohen N. 2011. The genus Xenopus as a multispecies model for evolutionary and comparative immunobiology of the 21st century. Dev. Comparative Immunol. 35:916–923. 10.1016/j.dci.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rollins-Smith LA, Parsons SC, Cohen N. 1984. During frog ontogeny, PHA and Con A responsiveness of splenocytes precedes that of thymocytes. Immunology 52:491–500. [PMC free article] [PubMed] [Google Scholar]
- 22.Morales H, Muharemagic A, Gantress J, Cohen N, Robert J. 2003. Bacterial stimulation upregulates the surface expression of the stress protein gp96 on B cells in the frog Xenopus. Cell Stress Chaperones 8:265–271. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nedelkovska H, Cruz-Luna T, McPherson P, Robert J. 2010. Comparative in vivo study of gp96 adjuvanticity in the frog Xenopus laevis. J. Vis. Exp. 43. 10.3791/2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. 2006. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Functional Ecol. 20:290–299. 10.1111/j.1365-2435.2006.01094.x. [DOI] [Google Scholar]
- 25.Gilbertson M-K, Haffner GD, Drouillard KG, Albert A, Dixon B. 2003. Immunosuppression in the northern leopard frog (Rana pipiens) induced by pesticide exposure. Environ. Toxicol. Chem. 22:101–110. 10.1002/etc.5620220113. [DOI] [PubMed] [Google Scholar]
- 26.Gervasi SS, Foufopoulos J. 2008. Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Functional Ecol. 22:100–108. 10.1111/j.1365-2435.2007.01340.x. [DOI] [Google Scholar]
- 27.Brown GP, Shilton CM, Shine R. 2011. Measuring amphibian immunocompetence: validation of the phytohemagglutinin skin-swelling assay in the cane toad, Rhinella marina. Methods Ecol. Evol. 2:341–348. 10.1111/j.2041-210X.2011.00090.x. [DOI] [Google Scholar]
- 28.Venesky MD, Wilcoxen TE, Rensel MA, Rollins-Smith L, Kerby JL, Parris MJ. 2012. Dietary protein restriction impairs growth, immunity, and disease resistance in southern leopard frog tadpoles. Oecologia 169:23–31. 10.1007/s00442-011-2171-1. [DOI] [PubMed] [Google Scholar]
- 29.Tella JL, Lemus JA, Carrete M, Blanco G. 2008. The PHA test reflects acquired T-cell mediated immunocompetence in birds. PLoS One 3:e3295. 10.1371/journal.pone.0003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyle DG, Boyle DB, Olsen V, Morgan JA, Hyatt AD. 2004. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time TaqMan PCR assay. Dis. Aquat. Organ. 60:141–148. 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 31.Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, Dalton A, Kriger K, Heros M, Hines H, Phillott R, Campbell R, Marantelli G, Gleason F, Coiling A. 2007. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 73:175–192. 10.3354/dao073175. [DOI] [PubMed] [Google Scholar]
- 32.Hurley JV. 1972. Acute inflammation. Churchill Livingstone, Edinburgh, United Kingdom. [Google Scholar]
- 33.Berger L, Speare R, Skerratt LF. 2005. Distribution of Batrachochytrium dendrobatidis and pathology in the skin of green tree frogs Litoria caerulea with severe chytridiomycosis. Dis. Aquat. Organ. 68:65–70. 10.3354/dao068065. [DOI] [PubMed] [Google Scholar]
- 34.Rosenblum EB, Poorten TJ, Settles M, Murdoch GK, Robert J, Maddox N, Eisen MB. 2009. Genome-wide transcriptional response of Silurana (Xenopus) tropicalis to infection with the deadly chytrid fungus. PLoS One 4:e6494. 10.1371/journal.pone.0006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenblum EB, Poorten TJ, Settles M, Murdoch GK. 2012. Only skin deep: shared genetic response to the deadly chytrid fungus in susceptible frog species. Mol. Ecol. 21:3110–3120. 10.1111/j.1365-294X.2012.05481.x. [DOI] [PubMed] [Google Scholar]
- 36.McMahon TM, Sears BF, Venesky M, Bessler SM, Brown JM, Deutsch K, Halstead NT, Lentz G, Tenouri N, Young S, Civitello DJ, Ortega N, Fites JS, Reinert LK, Rollins-Smith LA, Raffel TR, Rohr JR. 2014. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511:224–227. 10.1038/nature13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellison AR, Savage AE, DiRenzo GV, Langhammer P, Lips KR, Zamudio KR. 2014. Fighting a losing battle: vigourous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki. G3 (Bethesda) 4:1275–1289. 10.1534/g3.114.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TW, Weaver G, Bd Mapping Group. Fisher MC. 2013. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS One 8:e56802. 10.1371/journal.pone.0056802. [DOI] [PMC free article] [PubMed] [Google Scholar]