Abstract
Blastocystis is a common enteric protistan parasite that can cause acute, as well as chronic, infection and is associated with irritable bowel syndrome (IBS). However, the pathogenic status of Blastocystis infection remains unclear. In this study, we found that Blastocystis antigens induced abundant expression of proinflammatory cytokines, including interleukin 1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α), in mouse intestinal explants, in mouse colitis colon, and in macrophages. Further investigation utilizing RAW264.7 murine macrophages showed that Blastocystis treatment in RAW264.7 macrophages induced the activation of ERK, JNK, and p38, the three major groups of mammalian mitogen-activated protein (MAP) kinases that play essential roles in the expression of proinflammatory cytokines. ERK inhibition in macrophages significantly suppressed both mRNA and protein expression of IL-6 and TNF-α and mRNA expression of IL-1β. On the other hand, JNK inhibition resulted in reductions in both c-Jun and ERK activation and significant suppression of all three proinflammatory cytokines at both the mRNA and protein levels. Inhibition of p38 suppressed only IL-6 protein expression with no effect on the expression of IL-1β and TNF-α. Furthermore, we found that serine proteases produced by Blastocystis play an important role in the induction of ERK activation and proinflammatory cytokine expression by macrophages. Our study thus demonstrated for the first time that Blastocystis could induce the expression of various proinflammatory cytokines via the activation of MAP kinases and that infection with Blastocystis may contribute to the pathogenesis of inflammatory intestinal diseases through the activation of inflammatory pathways in host immune cells, such as macrophages.
INTRODUCTION
Activation of the immune system in the gut by microbial infection or loss of tolerance for nonpathogenic commensal microbes leads to inflammation and contributes to the development of intestinal disorders, such as inflammatory bowel diseases (1, 2). Blastocystis is a common enteric protistan parasite and an emerging pathogen found in humans and many animals (3). It is the most frequently isolated protist reported in human fecal samples and has prevalences of up to 10% in developed countries and as much as 50 to 60% in developing countries (4, 5). At least 17 subtypes (STs) of Blastocystis have been identified, with 9 of them (subtype 1 [ST-1] to ST-9) detected in humans (6). Transmission occurs via the fecal-oral route in cyst form via contaminated food or water (4, 7). Higher incidences of Blastocystis infections are reported in immunocompromised patients, such as HIV-infected and cancer patients (8–10). Common clinical symptoms of human Blastocystis infection, blastocystosis, are abdominal pain, diarrhea, vomiting, and bloating (4). Blastocystis infections are frequently associated with irritable bowel syndrome (IBS), as well (11–15).
While the mechanisms of mucosal injury induced by Blastocystis infection in the human gastrointestinal tract remain unclear (16), it has been shown that Blastocystis is able to disrupt barrier function and increase permeability in intestinal cells via rearrangement of F actin and ZO-1 distribution (17). Furthermore, Blastocystis could induce apoptosis of intestinal cells in a contact-independent manner by involving caspase 3, suggesting that the parasite may secrete virulence factors that initiate the apoptotic pathway (17). Blastocystis was able to induce the production of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 8 (IL-8) in HT-29 and T-84 human colonic epithelial cells (18). The induction of IL-8 in T-84 cells is dependent on NF-κB with the involvement of cysteine proteases (19). Upregulation of proinflammatory gene expression and intense infiltration of proinflammatory cells in the colon have been shown in rat and mouse models of Blastocystis infection (20, 21). A recent study also showed significantly higher levels of IL-17 and IL-23 in mice infected with Blastocystis (22). These studies demonstrate the pathogenic potential of Blastocystis despite a longstanding controversy as to whether it is an intestinal pathogen.
The uncertainty of Blastocystis pathogenesis and observations of differences in virulence have been attributed to different subtypes of the intestinal parasite. This is supported by studies revealing variation of cysteine protease activity between subtypes (23). Additionally, while ST-7 (B) was shown to be more sensitive to nitrosative stress than ST-4 (WR-1), it appeared to possess unique evasion strategies, with its ability to inhibit epithelial nitric oxide (NO) production by downregulating epithelial inducible nitric oxide synthase (iNOS) expression (24). These studies suggesting subtype-dependent variation show the need to further investigate differences in pathogenic potential across different subtypes of Blastocystis. In addition, host genetic polymorphism could influence the expression of symptoms in response to Blastocystis infection. For instance, it has been reported that some IL-8 and IL-10 single-nucleotide polymorphisms (SNPs) could change individual susceptibility, increasing the relative risk of development of IBS in Blastocystis carriers (25).
The mitogen-activated protein kinase (MAPK) signaling pathways are evolutionarily conserved and important mediators involved in transducing extracellular signals, such as stress, growth factors, and cytokines, to intracellular responses in order to generate an appropriate physiological response to the stimuli (26, 27). Three major groups of MAPKs exist in mammalian species, namely, the extracellular signal-regulated protein kinases (ERKs), the p38 MAP kinases, and the c-Jun NH2-terminal kinases (JNKs). MAPKs are activated through a core triple-kinase cascade whereby each MAPK is activated by its specific upstream MAPK kinase (MKK) via phosphorylation of both threonine and tyrosine residues in its activation loop. MKKs are activated after phosphorylation of their serine/threonine residues by MAPK kinase kinases (MKKKs) (28). Activated MAPKs phosphorylate a wide range of substrates, including transcription factors and cytoskeletal proteins (29).
MAPKs play crucial roles in innate immunity by triggering cellular responses and production of cytokines during pathogenic infections (see Fig. S1 in the supplemental material) (30). MAPK activation in macrophages in response to protozoan parasites has been reported extensively. Studies on Trypanosoma cruzi have revealed its ability to activate ERK and p38 with kinetics similar to those of lipopolysaccharide (LPS)-induced MAPK activation, leading to the production of IL-12 and tumor necrosis factor alpha (TNF-α) by macrophages (31). Infection with Toxoplasma gondii activates ERK, JNK, and p38 during invasion and proliferation of its tachyzoites (32). MAPK signaling pathways appeared to be involved in increased secretion and expression of monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), cyclooxygenase 2 (COX-2), and prostaglandin E2 (PGE2) in RAW264.7 macrophages in response to T. gondii (32). Parallel studies with Entamoeba histolytica showed MAPK activation in RAW264.7 macrophages by its DNA and Gal/GalNAc adherence lectin (33, 34). Apart from cytokine production, p38 and JNK appeared to be involved in macrophage apoptosis induced by Trichomonas vaginalis (27). However, whether Blastocystis infection could induce MAPK activation in host cells is unknown.
In this study, we investigated host-pathogen interactions using the RAW264.7 murine macrophage line model. Using two Blastocystis subtypes, ST-4 (WR-1) and ST-7 (B), we demonstrated for the first time that Blastocystis can activate MAPK pathways and induce proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, in a subtype-dependent manner. In addition, we investigated the role of each MAPK signaling pathway in proinflammatory cytokine expression in murine macrophages in response to Blastocystis ST-7 (B) and found for the first time that ST-7 (B) serine proteases play a major role in the activation of MAPKs and the expression of proinflammatory cytokines in macrophages.
MATERIALS AND METHODS
Parasite culture and preparation of lysate.
For this study, Blastocystis ST-4 (WR-1) and ST-7 (B) were used. ST-4 (WR-1) was isolated from a healthy rat during an animal survey (35). ST-7 (B) was recovered from a patient suffering from gastrointestinal symptoms at the Singapore General Hospital (23). All isolates were categorized according to a recent Blastocystis sp. classification system (36). The parasites were cultured axenically, as described previously (23, 24). In short, cultures of each isolate were maintained in 10 ml of prereduced Iscove's modified Dulbecco's medium (IMDM) containing 10% inactivated horse serum (Gibco) and incubated at 37°C in an anaerobic jar (Oxoid, United Kingdom) with an AnaeroGen gas pack (Oxoid, United Kingdom). Under these conditions, the morphology of the parasites is in the vacuolar state. Subculturing of parasites was performed at intervals of 3 or 4 days. Cultures harvested from log-phase in vitro cultivation at 24 hours postincubation were washed thrice in phosphate-buffered saline (PBS) at 500 × g for 15 min at room temperature (23). The pellet was resuspended in PBS, and cells were counted with a hemocytometer. Parasitic lysates were then prepared by three freeze-thaw cycles in liquid nitrogen and a 37°C water bath. Saccharomyces boulardii was obtained from the ATCC (MYA-796), and cells were grown in 10 ml YPDA (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] dextrose, 0.04% [wt/vol] adenine) liquid medium inside an incubator shaker at 28°C and 200 rpm. Cells were harvested at log phase by centrifugation at 800 × g for 5 min and washed with 1 ml sterile water. The pellet was resuspended in 1 ml yeast lysis buffer (100 mM Tris-HCl, pH 7.4, 50 mM KCl, 1 mM EDTA, 0.1% [wt/vol] NP-40). Glass beads (100 mg, acid washed, 425 to 600 μm; Sigma; G8772) were added, and the lysate was prepared with a bead beater (Precellys 24; five times at 6,000 rpm for 30 s with 1 min cooling on ice during the intervals).
Experimental in vivo inoculations.
C57BL/6 mice were purchased from the National University of Singapore (CARE) unit. All animals were housed in an animal biosafety level 2 (ABSL-2) conventional clean animal facility. The animal experiments were performed in accordance with the Singapore National Advisory Committee for Laboratory Animal Research guidelines. The protocol (R13-5890) was reviewed and approved by the National University of Singapore Institutional Animal Care and Use Committee.
Eight-week-old C57BL/6 male mice were first given 2% dextran sulfate sodium salt (DSS) in drinking water for 5 days (day 1 to day 5). On day 5, the mice were divided into two groups. One group (the inoculated mice) were inoculated with 3.75 × 107 ST-7 (B) lysates by oral gavage for 3 days. The other group, the control mice, were given PBS. Both infected mice and control mice were anesthetized and killed for examination. The small intestine, cecum, proximal colon, and distal colon were harvested to investigate the mRNA expression of the proinflammatory cytokines using real-time reverse transcription (RT)-PCR.
Culture of the RAW264.7 mouse macrophage line.
The murine macrophage line RAW264.7 was purchased from the American Type Culture Collection (ATCC TIB-71). RAW264.7 stock cultures were maintained in T-75 flasks (Fisher Scientific) in a humidified 37°C incubator with 5% CO2, and cultures within 5 to 20 passages were used for this study. RAW264.7 cells were cultured in RPMI 1640 medium with l-glutamine (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen) and 1% penicillin-streptomycin mixture (Invitrogen) (RPMI 1640 complete medium). Cell viability was assessed by trypan blue assay, and cell cultures with more than 95% viability were used for all experiments. Cells were trypsinized with 0.25% trypsin-EDTA (Invitrogen), seeded in either 6-well plates (Nunc) for Western blot experiments or 12-well plates (Nunc) for real-time PCR and enzyme-linked immunosorbent assays (ELISAs), and grown to 80 to 90% confluence.
For MAPK inhibition experiments, RAW264.7 cells were pretreated in RPMI 1640 complete medium containing one of the following inhibitors: PD98059 (Sigma) (100 μM), SB203580 (Biorbyt) (20 μM), or SP600125 (Calbiochem) (20 μM). After 1 h pretreatment, the RAW264.7 cells were stimulated with ST-7 (B) lysates for the indicated times.
Western blot analysis.
After the desired period of coincubation at 37°C in 5% CO2, the stimulation reaction was ended by removing the RPMI 1640 complete medium, and the monolayers were washed twice with PBS. The cells were then harvested with a cell scraper and spun down at 4°C for 3 min. The supernatant was discarded, while the remaining cell pellet was lysed by adding 150 to 250 pg/ml of cell lysis buffer (50 mM Tris-HCl [pH 7.8], 150 mM NaCl, 1% NP-40) adjusted to the pellet size. The protein concentration of whole-cell lysates was quantitated by spectrophotometric analysis using Bradford reagent (Bio-Rad), according to the manufacturer's instructions. Equal amounts of proteins (30 to 60 μg) were then loaded on 10% polyacrylamide gels for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using the Mini-Protean 3 Electrophoresis System (Bio-Rad Laboratories) and transferred to a 0.2-μm polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories). After being blocked with 5% nonfat milk in 20 mM Tris-HCl, pH 8.0, 154 M NaCl, 0.05% Tween, the membrane was incubated in primary MAPK antibody at 4°C overnight, followed by incubation with Amersham ECL anti-rabbit IgG and horseradish peroxidase-linked species-specific whole antibody (from donkey; GE Healthcare) for 30 min. The primary MAPK antibodies used were phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit monoclonal antibody (Cell Signaling Technology), phospho-SAPK/JNK (Thr183/Tyr185) rabbit monoclonal antibody (Cell Signaling Technology), and phospho-p38 MAPK (Thr180/Tyr182) rabbit monoclonal antibody (Cell Signaling Technology). These antibodies were raised against human MAP kinases using epitopes conserved in their murine orthologs. The blots were treated for chemiluminescence detection using Supersignal West Dura Extended Duration Substrate (Pierce). Luminescence was detected using Amersham hyperfilm MP enhanced chemiluminescence (ECL) autoradiography film (GE Healthcare) and processed using an X-ray film processor (Kodak).
ELISA and quantitative real-time PCR (qRT-PCR) for cytokines.
An ELISA kit (BD Pharmingen) was used to detect the levels of proinflammatory cytokines, IL-6, IL-1β, and TNF-α, in supernatants of RAW264.7 cultures coincubated with isolate ST-4 (WR-1) or ST-7 (B) parasite lysates. For live-parasite infection, ST-4 (WR-1) and ST-7 (B) log-phase cultures were harvested and washed twice with PBS at 500 × g for 15 min at room temperature. The pellet was resuspended in PBS and counted with a hemocytometer. Multiplicities of infection (MOIs) of 20 for ST-4 (WR-1) and 10 for ST-7 (B) were used for infection. For real-time PCR, total cellular RNA was isolated from cells using TRIzol reagent (Invitrogen). First-strand cDNA was prepared using the ImProm-II Reverse Transcription System (Promega) following the manufacturer's protocol. Quantitative real-time PCR was performed using Fast-SYBR green master mix (ABI Applied Biosystems), cDNA, and appropriate primers (see below). Real-time PCR was then run in a 7900HT Fast real-time PCR system at 95°C for 30 s, followed by 40 cycles of 5 s at 95°C and 20 s at 60°C. Melting-curve analysis was conducted after each reaction and examined for a single peak to ensure specificity of the amplification reactions. Relative quantitation of mRNA levels was calculated by the ΔΔCT method, and the amount of the target relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was expressed as 2−ΔΔCT. The primers used were IL-6 sense (5′ GAT GCT ACC AAA CTG GAT ATA ATC 3′) and antisense (5′ GGT CCT TAG CCA CTC CTT CTG TG 3′), IL-1β sense (5′ CCT TCA TCT TTG AAG AAG A 3′) and antisense (5′ GAG GTG CTG ATG TAC CAG TTG 3′), TNF-α sense (5′ TCC CAG GTT CTC TTC AAG GGA 3′) and antisense (5′ GGT GAG GAG CAC GTA GTC GG 3′), and GAPDH sense (5′ GAG AAC TTT GGC ATT GTG G 3′) and antisense (5′ ATG CAG GGA TGA TGT TCT G 3′).
RESULTS
Blastocystis induces proinflammatory cytokine expression in the intestine.
The intestinal epithelium provides nonspecific defenses against potential pathogens, and enterocytes secrete cytokines, such as IL-1β, to regulate inflammation (37). To investigate the possible role of Blastocystis infection in intestinal inflammation, we treated intestinal explants isolated from C57/BL6 mice with Blastocystis ST-7 (B) lysate to test its proinflammatory potential. We found that treatment with ST-7 (B) resulted in secretion of IL-1β and TNF-α from the explants (Fig. 1A). To confirm that the inflammatory cytokine expression in intestinal explants was indeed due to Blastocystis, we treated the explants with live ST-7 (B) and found that live ST-7 (B) treatment induced the secretion of IL-1β and IL-6 (see Fig. S2 in the supplemental material). Together, these results suggested that infection with Blastocystis could contribute to acute and chronic inflammatory intestinal diseases.
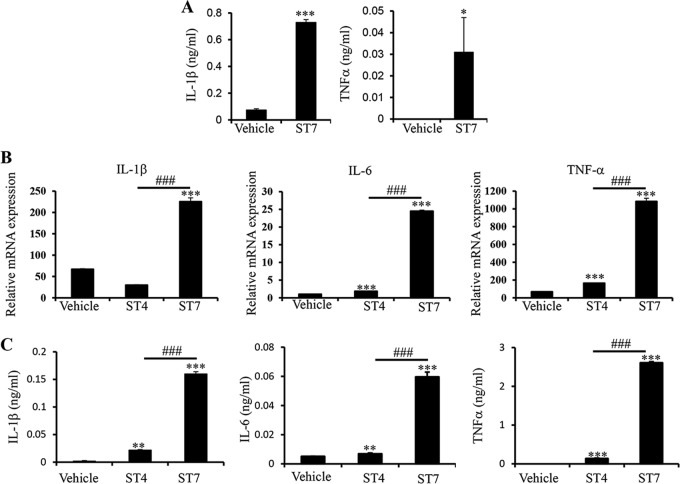
FIG 1.
Blastocystis treatment upregulates proinflammatory cytokines in mouse intestinal explants and BMDMs. (A) Murine intestinal explants were isolated from C57/BL6 mice and treated with Blastocystis ST-7 (B) lysate. The culture supernatants were tested using ELISA for protein concentrations of proinflammatory cytokines, IL-1β and TNF-α. (B) Mouse BMDMs were generated and treated with either Blastocystis ST-4 (WR-1) or ST-7 (B) at MOIs of 20 and 10, respectively. The mRNA levels of TNF-α, IL-1β, IL-6, and GAPDH were also measured by qRT-PCR. (C) The concentrations of IL-6, TNF-α, and IL-1β in the culture supernatants of BMDMs were measured by ELISA. Each value represents the mean and standard deviation from at least 3 individual experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005 (representing a statistically significant difference compared to the negative control). ###, P < 0.005 (representing a statistically significant difference between Blastocystis ST-4 and ST-7).
To examine the possible contribution of Blastocystis to intestinal inflammation, we treated C57/BL6 mice for 5 days with 2% DSS, a heparin-like polysaccharide that induces intestinal mucosal damage, exposing the underlying epithelium to the luminal contents, including microbes, and that therefore is commonly used to induce mouse colitis (1), followed or not by treatment with ST-7 (B) lysate. The expression of inflammatory cytokines, including IL-1β, IL-6, and TNF-α, in the colon was examined. We observed an increase in the average expression of IL-1β, IL-6, and TNF-α in response to ST-7 (B) treatment in each of the three experiments we performed (see Fig. S3 in the supplemental material). However, the differences between treated and nontreated groups were not significant. It is possible that the effect of Blastocystis treatment was overshadowed by the existing high expression of these cytokines caused by DSS, indicating a suitable mouse model for Blastocystis infection is needed.
Blastocystis induces MAP kinase activation in macrophages.
It has been shown that infection of mice by Blastocystis ST-7 (B) resulted in intensive proinflammatory cell infiltration and inflammation in the colon (20). Macrophages, one of the major components of innate immunity, are the first cells that encounter pathogens that cross the intestinal epithelium (38). To examine macrophage responses to Blastocystis infection, we generated mouse bone marrow-derived macrophages (BMDMs) and treated them with Blastocystis ST-4 (WR-1) or ST-7 (B) lysates. We found that ST-7 (B) treatment induced strong expression of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, at both the mRNA and protein levels in macrophages compared with cells treated with ST-4 (WR-1) (Fig. 1B and C).
To facilitate the study of the role of Blastocystis infection in intestinal inflammation, particularly in proinflammatory cytokine expression by macrophages, we utilized the murine macrophage line most commonly used in medical research, RAW264.7. We treated RAW264.7 macrophages with ST-4 (WR-1) or ST-7 (B) lysate to examine proinflammatory cytokine expression. As shown in Fig. 2A and B, both ST-4 (WR-1) and ST-7 (B) induced the expression of IL-1β, IL-6, and TNF-α. However, ST-7 (B) induced significantly higher expression of these cytokines in macrophages than ST-4 (WR-1), which is consistent with our findings in BMDMs (Fig. 1B and C). Similar results were observed when RAW264.7 cells were treated with live ST-4 (WR-1) and ST-7 (B) parasites (see Fig. S4 in the supplemental material). Of note, the lysate of S. boulardii, a strain of yeast, does not induce significant expression of IL-1β, IL-6, and TNF-α from macrophages (Fig. 2C). This result demonstrates that the inflammatory cytokine expression by macrophages in response to ST7 (B) lysate is indeed caused by factors from Blastocystis ST-7 (B).
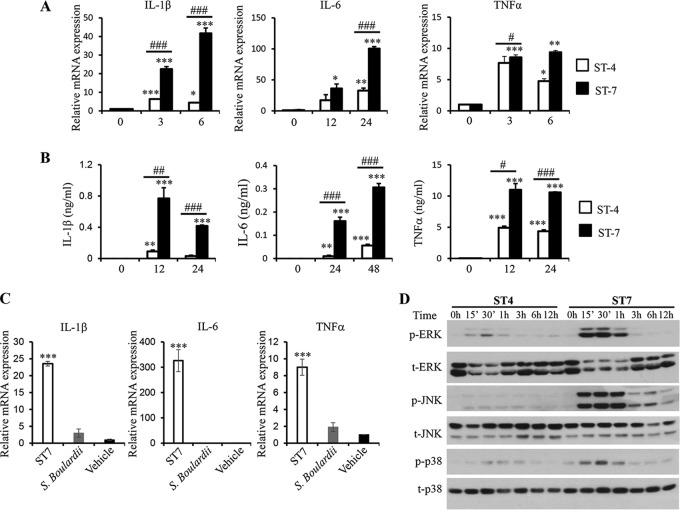
FIG 2.
Blastocystis ST-7 (B) induces stronger proinflammatory cytokine expression and MAPK activation in murine macrophages than ST-4 (WR1). RAW264.7 cells were incubated with lysates of either Blastocystis ST-4 (WR1) or ST-7 (B) at MOIs of 20 and 10, respectively. (A) mRNA levels of TNF-α, IL-1β, IL-6, and GAPDH in macrophages were measured by qRT-PCR. The results are normalized to GAPDH and expressed as the mean fold change relative to the 0-h time point, which is set at 1. (B) The concentrations of IL-6, TNF-α, and IL-1β in the culture supernatants of RAW264.7 cells were measured by ELISA. Each value represents the mean and standard deviation from at least 3 individual experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005 (representing a statistically significant difference compared to the negative control). #, P < 0.05; ##, P < 0.01; ###, P < 0.005 (representing a statistically significant difference between Blastocystis ST-4 and ST-7). (C) S. boulardii lysate does not induce inflammatory cytokine expression in macrophages. RAW264.7 cells were incubated with lysate of either Blastocystis ST-7 (B) (MOI, 10) or S. boulardii (MOI, 20). The mRNA levels of TNF-α, IL-1β, IL-6, and GAPDH in macrophages were measured by qRT-PCR. (D) Representative Western blots of MAPK activation in RAW264.7 cells in response to ST-4 (WR1) or ST-7 (B) lysate stimulation. Cells were lysed and were processed by SDS-PAGE. Membranes were then blotted with phospho-ERK (p-ERK), total ERK (t-ERK), phospho-JNK, total JNK, phospho-p38, and total p38 antibodies. Each blot is representative of three separate experiments.
Signaling pathways such as the MAP kinases (MAPKs) play a critical role in regulating the expression of proinflammatory cytokines like IL-1β, IL-6, and TNF-α (39). To investigate the potential role of MAPKs in the expression of proinflammatory cytokines by macrophages in response to Blastocystis, RAW264.7 cells were treated with ST-4 (WR-1) or ST-7 (B) to assess the activation of MAPKs. As shown in Fig. 2D, ST-4 (WR-1) induced ERK activation at 15 min posttreatment, and activation peaked at 30 min. ST-7 (B) induced stronger ERK activation at 15 and 30 min posttreatment, which then decreased at 3 h. Also, ST-7 (B) induced much stronger JNK activation in RAW264.7 that peaked within the first hour and was sustained until 6 h after treatment. Peak phosphorylation of p38 was seen in cells at 30 min when treated with ST-4 (WR-1) or ST-7 (B). Evidently, ST-7 (B) induced much stronger activation of all three MAPKs than ST-4 (WR-1). These results demonstrated the different abilities of these two subtypes to activate the three MAPK pathways, which is correlated with their abilities to induce proinflammatory cytokine expression in macrophages. In the studies described below, we focused on investigating the regulatory function of MAPKs in macrophage proinflammatory cytokine expression induced by ST-7 (B).
Blastocystis-induced proinflammatory cytokine expression is dependent on ERK and JNK.
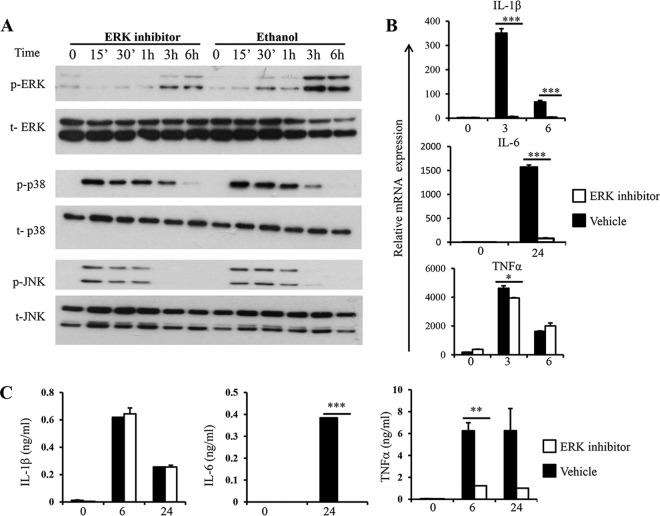
Next, we examined the roles of the three major groups of MAPKs in the expression of proinflammatory cytokines in macrophages in response to Blastocystis, using their specific inhibitors. To elucidate the role of ERK in macrophage cytokine expression in response to Blastocystis, we treated RAW264.7 cells with PD98059, an MEK1-specific inhibitor. Pretreatment of macrophages with PD98059 for an hour reduced ERK activation upon ST-7 (B) stimulation (Fig. 3A). Treatment with PD98059 did not affect the activation of p38 and JNK in macrophages in response to ST-7 (B) stimulation (Fig. 3A).
FIG 3.
Inhibition of ERK leads to reduced cytokine expression in response to Blastocystis. RAW264.7 cells were pretreated with either 100 μM PD98059 or ethanol vehicle dissolved in RPMI complete medium for 1 hour prior to stimulation with Blastocysis ST-7 at an MOI of 10 for the indicated times. (A) Representative Western blots for MAPK activation in RAW264.7 cells. The cells were lysed and processed by SDS-PAGE. Membranes were then blotted with phospho-ERK, ERK, phospho-JNK, JNK, phospho-p38, and p38 antibodies. Each blot is representative of three separate experiments. (B) mRNA levels of TNF-α, IL-1β, IL-6, and GAPDH were measured by qRT-PCR. The values are expressed as mean relative transcript levels normalized to GAPDH and are expressed relative to the 0-h time point, which is set at 1. (C) Concentrations of IL-6, TNF-α, and IL-1β in the culture supernatants were measured by ELISA. Each value represents the mean and standard deviation from at least 3 individual experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005 (representing a statistically significant difference between inhibitor and vehicle treatments).
Next, we examined the effect of ERK inhibition on the expression of IL-1β, IL-6, and TNF-α in macrophages in response to Blastocystis by qRT-PCR and ELISA. As shown in Fig. 3B, inhibition of ERK significantly impaired the expression of IL-1β, IL-6, and TNF-α at the mRNA level. Notably, inhibition of ERK more profoundly suppressed the protein production of IL-6 and TNF-α than that of IL-1β (Fig. 3C). Together, these data indicated that ERK plays a major role in regulating the expression of IL-6 and TNF-α by macrophages in response to Blastocystis infection.
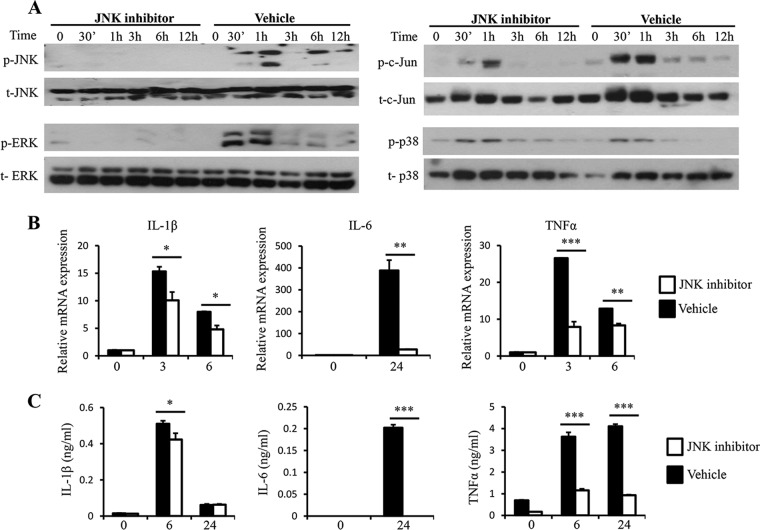
To understand the contribution of JNK to the expression of proinflammatory cytokine expression upon Blastocystis treatment, RAW264.7 macrophages were pretreated with SP100625, a JNK-specific inhibitor. As shown in Fig. 4A, inhibition of JNK activation in macrophages led to a decrease in activation of both JNK and its downstream target, c-Jun. At the same time, ERK activation was also completely abrogated in response to SP100625 treatment, whereas p38 activation was not affected. A significant reduction of IL-1β, IL-6, and TNF-α in response to Blastocystis stimulation was observed at both the mRNA and protein levels (Fig. 4B and C). Interestingly, SP100625 treatment resulted in reduced expression of IL-1β at both the mRNA and protein levels. These results showed the important regulatory role of JNK and ERK pathways for these proinflammatory cytokines in response to Blastocystis infection.
FIG 4.
JNK inhibition leads to reduced c-Jun and ERK activation and expression of proinflammatory cytokines upon Blastocystis treatment. RAW264.7 cells were pretreated with either 20 μM SP100625 or DMSO (dimethyl sulfoxide) vehicle dissolved in RPMI complete medium for 1 hour prior to stimulation with Blastocystis ST-7 (B) at an MOI of 10. (A) Representative Western blots for activation of c-Jun and MAPKs in RAW264.7 cells. The cells were lysed and processed by SDS-PAGE. Membranes were then blotted with phospho-ERK, ERK, phospho-JNK, JNK, phospho-cJun, c-Jun, phospho-p38, and p38 antibodies. Each blot is representative of three separate experiments. (B) mRNA levels of TNF-α, IL-1β, IL-6, and GAPDH were measured by qRT-PCR. The values are expressed as mean relative transcript levels normalized to GAPDH and are expressed relative to the 0-h time point, which is set at 1. (C) Concentrations of IL-6, TNF-α, and IL-1β in the supernatants of RAW264.7 cells were measured by ELISA. Each value represents the mean and standard deviation from at least 3 individual experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005 (representing a statistically significant difference between inhibitor and vehicle treatments).
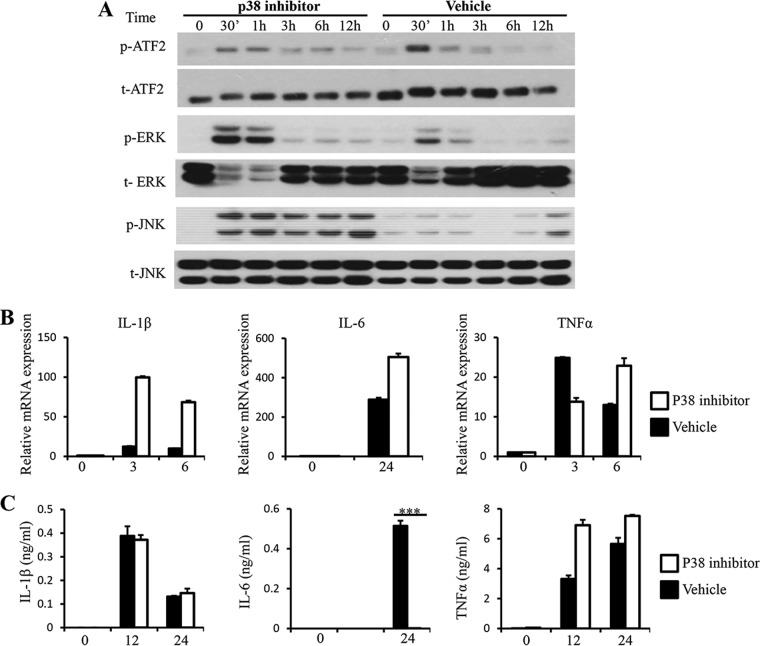
p38 MAP kinase has been shown to be important for proinflammatory cytokine expression in response to microbial infection (39). To address whether p38 regulates proinflammatory cytokine expression by macrophages in response to Blastocystis, we treated RAW264.7 cells with the p38-specific inhibitor SB203580, which blocks p38 catalytic activity without inhibiting its phosphorylation prior to stimulation with ST-7 (B). As shown in Fig. 5A, treatment with SB203580 resulted in reduced phosphorylation of ATF2, the substrate of p38. Meanwhile, an increase in ERK and JNK phosphorylation was observed after the treatment, suggesting cross talk by p38 with ERK and JNK in macrophages. At the same time, inhibition of p38 did not suppress mRNA expression of IL-1β, IL-6, and TNF-α in response to Blastocystis stimulation or their protein production, except for IL-6 (Fig. 5B and C). This is consistent with the observation that ERK and JNK are required for mRNA expression of these cytokines in response to Blasotcystis (Fig. 4B and C). Together, these results demonstrated that ERK and possibly JNK activation triggered by Blastocystis infection play major roles in regulating the expression of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α.
FIG 5.
Inhibition of p38 leads to increased phosphorylation of ERK and JNK and reduced secretion of IL-6. RAW264.7 cells were pretreated with either 20 μM SB203580 or DMSO vehicle dissolved in RPMI complete medium for 1 hour prior to stimulation with Blastocystis ST-7 at an MOI of 10. (A) Representative Western blots for activation of ATF2, ERK, and JNK. Cells were lysed and processed by SDS-PAGE. Membranes were then blotted with phospho-ERK, ERK, phospho-JNK, JNK, phospho-ATF2, and ATF2 antibodies. Each blot is representative of three separate experiments. (B) mRNA levels of TNF-α, IL-1β, IL-6, and GAPDH were measured by qRT-PCR. The values are expressed as mean relative transcript levels normalized to GAPDH and are expressed relative to the 0-h time point, which is set at 1. (C) Concentrations of IL-6, TNF-α and IL-1β in the culture supernatants were measured by ELISA. Each value represents the mean and standard deviation from at least 3 individual experiments. ***, P < 0.005 (representing a statistically significant difference between inhibitor and vehicle treatments).
Blastocystis-derived serine proteases play a major role in inducing MAPK activation and proinflammatory cytokine expression in macrophages.
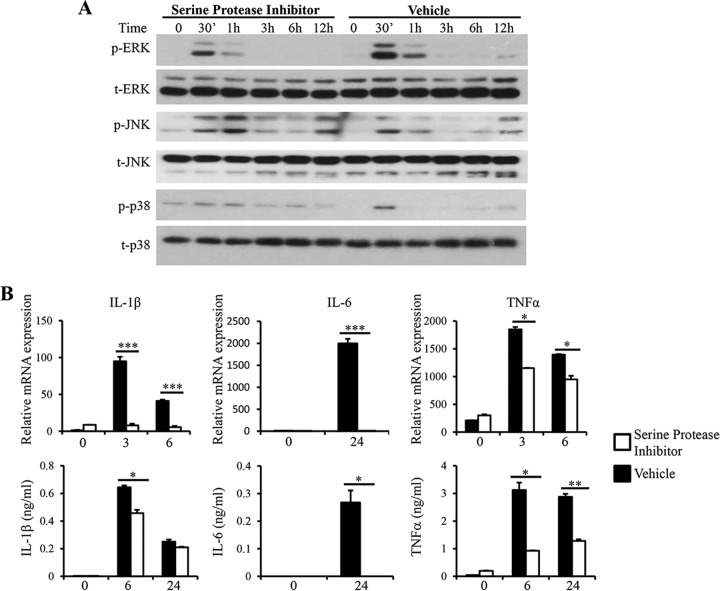
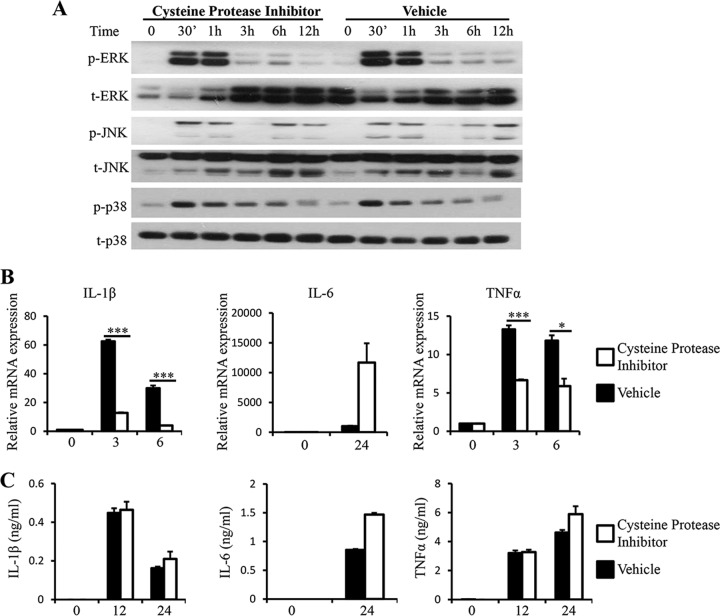
Proteases produced by parasitic protozoa contribute to their pathogenicity in many ways, ranging from invasion of the host to immune evasion and activation of inflammation (40). Proteases expressed by Blastocystis were able to degrade human secretory IgA and induce IL-8 expression in human colonic epithelial cells, indicating their roles in the pathogenesis of Blastocystis (19, 41). We examined the role of Blastocystis proteases in the regulation of proinflammatory cytokine expression in macrophages by incubating ST-7 (B) lysates with serine or cysteine protease inhibitors before macrophage stimulation. ST-7 (B) treated with the serine protease inhibitor 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF) induced weaker activation of ERK in RAW264.7 cells than ST-7 (B) treated with vehicle, whereas the activation of JNK and p38 was not reduced (Fig. 6A). In correlation with reduced ERK activation, RAW264.7 cells stimulated with AEBSF-treated ST-7 (B) produced significantly smaller amounts of IL-6, TNF-α, and IL-1β (Fig. 6B). In contrast, treatment of cysteine protease inhibitor with ST-7 (B) induced activation of MAPKs in RAW264.7 cells similar to that induced by the control (Fig. 7A). IL-6 mRNA and protein secretion levels were not affected by the cysteine protease inhibition. Interestingly, inhibiting cysteine proteases in ST-7 (B) resulted in significantly lower levels of IL-1β and TNF-α mRNA expression (Fig. 7B). However, protein expression of IL-1β and TNF-α was not reduced (Fig. 7B). Together, these results suggested that serine proteases produced by Blastocystis play a critical role in the induction of MAPK activation and proinflammatory cytokine expression in macrophages and that signaling triggered by cysteine proteases produced by Blastocystis plays a role in the mRNA expression of IL-1β and TNF-α that is independent of MAPKs.
FIG 6.
Serine protease inhibition leads to decreased ERK activation and proinflammatory cytokine expression. ST-7 (B) lysates were pretreated with 500 μM serine protease inhibitor, AEBSF, for 1 hour before coincubation with RAW264.7 cells at 37°C. (A) Representative Western blots for activation of MAPKs. Cells were lysed, and cell extracts were analyzed by Western blotting using phospho-ERK, total ERK, phospho-JNK, total JNK, phospho-p38, and total p38 antibodies. (B) mRNA levels of TNF-α, IL-1β, IL-6, and GAPDH were measured by qRT-PCR. The values are expressed as mean relative transcript levels normalized to GAPDH and are expressed relative to the 0-h time point, which is set at 1. (C) Concentrations of IL-6, TNF-α, and IL-1β in the supernatants were measured by ELISA. Each value represents the mean and standard deviation from at least 3 individual experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005 (representing a statistically significant difference between inhibitor and vehicle treatments).
FIG 7.
Cysteine protease inhibition results in a decrease in mRNA expression of TNF-α and IL-1β but does not have an effect on protein secretion levels for all three proinflammatory cytokines examined. ST-7 (B) lysates were pretreated with 100 μM cysteine protease inhibitor, E-64, for 1 hour before coincubation with RAW264.7 cells at 37°C. (A) Representative Western blots for activation of MAPKs. Cells were lysed, and cell extracts were analyzed by Western blotting using phospho-ERK, total ERK, phospho-JNK, total JNK, phospho-p38, and p38 antibodies. (B) mRNA levels of TNF-α, IL-1β, IL-6, and GAPDH were measured by qRT-PCR. The values are expressed as mean relative transcript levels normalized to GAPDH and are expressed relative to the 0-h time point, which is set at 1. (C) Concentrations of IL-6, TNF-α, and IL-1β in the supernatants were measured by ELISA. Each value represents the mean and standard deviation from at least 3 individual experiments. *, P < 0.05; ***, P < 0.005 (representing a statistically significant difference between inhibitor and vehicle treatments).
DISCUSSION
Blastocystis, the most common single-cell parasite in the human large intestine, is associated with various human intestinal disorders, such as acute and chronic diarrhea, irritable bowel syndrome, and possibly inflammatory bowel disease (3, 42, 43). However, the pathogenic potential of Blastocystis remains unclear. Previous studies showed that Blastocystis infection led to upregulation of proinflammatory gene expression and intense infiltration of proinflammatory cells into the colon in rats and mice (20, 21). In agreement with these findings, in this study, we found that ST-7 (B) lysate was able to induce inflammatory cytokine secretion in mouse colon explants (Fig. 1A). We further showed that in mice with colitis, treatment with ST-7 (B) lysate resulted in increased inflammatory cytokine gene expression, including the expression of IL-6, TNF-α, and IL-1β genes (Fig. 1B), suggesting the inflammatory potential of Blastocystis infection in the intestine.
Proinflammatory cytokines, such as IL-1, IL-8, gamma interferon (IFN-γ), and TNF-α, secreted by epithelial cells or immune cells, including macrophages, monocytes, neutrophils, and lymphocytes, contribute to mucosal intestinal inflammation and inflammatory disorders (44, 45). Extensive reports have shown that common protozoan pathogens, such as T. cruzi, E. histolytica, T. vaginalis, and Leishmania, cause upregulation of proinflammatory cytokines to induce immune responses (46–49). Macrophages, the first cells that encounter pathogens that cross the intestinal epithelium, may be resident in strategic locations, such as the lamina propria, or may be recruited to the inflamed gut by chemokine signals (38, 50). Blastocystis has been reported to disrupt intestinal barrier integrity (17, 51). Such tissue damage would presumably allow Blastocystis antigens to cross the epithelium into the lamina propria and contact cells of the immune system. Different types of macrophages with different features and functions exist in the gut, playing important roles in the regulation of the immune response to commensal microorganisms, as well as contributing to the pathogenesis of intestinal inflammatory disorders (50). For instance, the CD14+ inflammatory macrophages in the intestine can produce large amounts of proinflammatory cytokines, including IL-23, IL-6, and TNF-α, in response to commensal bacteria to contribute to the intestinal inflammation and pathogenesis of IBD (50, 52). We showed that macrophages treated with Blastocystis ST-4 (WR-1) or ST-7 (B) resulted in upregulation of the proinflammatory cytokines IL-6, IL-1β, and TNF-α (Fig. 1 and 2). In addition, ST-7 (B) evoked a stronger inflammatory response than ST-4 (WR-1), demonstrating the different pathogenic potentials of the two subtypes.
Interestingly, the different abilities of ST7 (B) and ST4 (WR-1) to induce the expression of proinflammatory cytokines in macrophages were correlated with their abilities to induce the activation of MAPKs (Fig. 2). MAPKs are known to play crucial roles in the regulation of cytokine expression in immune responses (28). While the kinetics of MAPK activation induced by the two subtypes were similar, the intensity of phosphorylation of each MAPK activated by ST-7 (B) was distinctly stronger than that by ST-4 (WR-1).
We further examined the contributions of individual MAPK pathways to the expression of these cytokines in macrophages in response to Blastocystis using specific inhibitors. The results suggested that the ERK and possibly JNK pathways play the main roles in regulating the expression of IL-6, IL-1β, and TNF-α. Interestingly, inhibition of p38 activation resulted in an increase in mRNA expression of IL-1β, IL-6, and TNF-α, which was correlated with increased JNK and ERK activation (Fig. 5A and B), suggesting cross talk among the three MAPK signaling pathways and supporting the major role of ERK and JNK in the expression of IL-1β, IL-6, and TNF-α in macrophages in response to Blastocystis. While mechanisms are in place to ensure the specificity of each MAPK pathway, studies have shown that cross talk does occur among different signaling pathways when inhibition of one MAPK pathway augments the phosphorylation of other MAPKs (53, 54).
The pathogenic status of Blastocystis remains controversial due to lack of knowledge about virulence factors and the existence of intrasubtype variation in pathogenic potential (55). Previous studies suggested that aspartic and cysteine proteases are candidates for Blastocystis virulence factors. Proteases derived from parasitic protozoa are major virulence factors that contribute to their pathogenicity by manipulating the immune response in a number of ways, such as tissue invasion, immune evasion, and activation of inflammation (40). Genome sequencing of ST-7 (B) has revealed the major classes of proteolytic enzymes to be serine, aspartic, and cysteine proteases and metalloproteases (56). It has been shown that aspartic and cysteine proteases from Blastocystis are able to degrade human secretory IgA, while cysteine proteases could reduce epithelial barrier function and induce IL-8 expression (19, 41, 57). In this study, we found that serine proteases, but not cysteine proteases, play a major role in induction of MAPK activation and expression of proinflammatory cytokines in macrophages (Fig. 6 and 7). Therefore, serine proteases are possible virulence factors of Blastocystis, as well. Serine proteases have been shown to play a role in the viability and pathogenicity of protozoan parasites, such as Plasmodium falciparum, Leishmania amazonensis, and T. cruzi, and have been implicated in gastrointestinal symptoms, such as abdominal aches, muscular contractions, and widespread pain (58–60). Several reports have established a link between Blastocystis and IBS after finding a higher prevalence of the intestinal parasite in IBS patients (14, 15, 61). Fecal supernatants collected from IBS patients were found to have significantly higher serine protease activity than those from healthy subjects (62). This elevated serine protease activity in fecal supernatants resulted in an increase in colonic paracellular permeability in vitro and evoked visceral hypersensitivity in mice after intracolonic infusion. Blastocystis has been reported to induce contact-independent apoptosis and to disrupt barrier function in rat intestinal epithelial IEC-6 cells (17). It would be interesting to investigate if Blastocystis serine protease activity is associated with increased intestinal epithelial permeability in future studies.
The link between Blastocystis serine protease, MAPK activation, and inflammation is currently unclear. Macrophages express a group of receptors known as protease-activated receptors (PARs) (63), a family of G-protein-coupled seven-transmembrane receptors (64), on the surface. The activation of PARs is initiated by cleavage of the N terminus of the receptor by a serine protease, resulting in the generation of a new tethered ligand that interacts with the receptor within extracellular loop 2, leading to downstream signaling. PAR2 is one of the four members in this family. PAR2 has been shown to be activated by proteases from enteric bacteria, such as Serratia marcescens (65), or proteases from the host in response to bacterial infection, such as infection by Citrobacter rodentium (66), playing a major role in the host inflammatory response to enteric bacterial infection (65, 66). PAR2 activation induces MAPK signaling, including ERK1/2 and to a lesser extent p38 and JNK (67). We found that ST-7 (B) lysate-induced ERK activation and inflammatory cytokine expression was reduced by treatment with serine protease inhibitor (Fig. 6). It is possible that serine protease from Blastocystis activates PAR2 in macrophages, which triggers MAPK activation and inflammatory cytokine expression. We will further validate this possibility in future investigations.
The different abilities of ST-7 (B) and ST-4 (WR-1) to trigger MAPK activation and proinflammatory cytokine expression could be due to their differential expression of serine proteases or different serine protease activities. Previous studies have shown such intrasubtype variation of virulence potential. For instance, cysteine protease activity from ST-7 (B) exhibited approximately twice the level of cysteine protease activity from ST-4 (WR-1) (23). In addition, ST-7 (B) was found to be resistant to metronidazole, a first-line therapy for Blastocystis infections, while ST-4 (WR-1) was susceptible to the drug (68). Despite being more sensitive to NO production by intestinal epithelial cells, ST-7 (B) also exhibited an ability to downregulate iNOS, indicating it possesses unique strategies to evade potential host defenses (24). Together, this evidence suggested that ST-7 (B) is more pathogenic than ST-4 (WR-1). Recent studies have implicated ST-7 (B) as a pathogenic subtype associated with intestinal symptoms, as it was found to be more prevalent in symptomatic patients than in healthy carriers (69). In addition, the prevalence of Blastocystis spp. is significant in IBS patients, and inflammation of the mucosa is well recognized in these patients (70). The significantly higher induction of proinflammatory cytokine production by ST-7 (B) found in this study may suggest a contribution by the parasite to the immunopathology of symptomatic patients seen in the clinical setting and supports the pathogenic nature of ST-7 (B) reported in clinical manifestations.
The pathogenic potential of ST-4 (WR-1) also should not be underestimated due to the increasing number of reports associating the subtype with symptomatic infections. Subtype analysis done on 51 clinical isolates has revealed a high prevalence of ST-4 (WR-1) among symptomatic patients from a health district of Valencia, Spain (7). ST-4 (WR-1) was revealed to be predominant in rural communities of Nepal and was also found to be common among Blastocystis-positive patients presenting with acute diarrhea in Denmark (71, 72). Oral inoculation of rats with ST-4 (WR-1) resulted in mild goblet cell hyperplasia and significant upregulation of proinflammatory cytokines (21). With these observations showing the pathogenic potential of both ST-4 (WR-1) and ST-7 (B), the results from our study suggest that while ST-4 (WR-1) could induce significant upregulation of proinflammatory cytokines, comparisons between the two subtypes have shown that ST-7 (B) could induce stronger upregulation of proinflammatory cytokines, which suggests that it could be more virulent. Future clinical studies can attempt to determine whether ST-7 (B) is associated with the marked immunopathology seen in symptomatic patients compared to ST-4 (WR-1).
In summary, Blastocystis ST-4 (WR-1) and ST-7 (B) exhibit subtype-dependent upregulation of proinflammatory cytokines and MAPK activation in macrophages. This is the first study that demonstrates differential regulation of proinflammatory cytokines by MAPKs in response to Blastocystis in macrophages. The present study also demonstrates for the first time that Blastocystis ST-7 (B) serine proteases play a major role in MAPK activation and the expression of proinflammatory cytokines, including IL-6, IL-1β, and TNF-α, supporting serine proteases as virulence factors of Blastocystis. While these findings shed new light on the pathogenesis of Blastocystis, future studies should focus on the development of suitable animal models to gain further understanding of Blastocystis-host interactions.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Office of the Deputy President, National University of Singapore, the Ministry of Education (MOE2010-T2-1-079), the National Medical Research Council (IRG10nov091 and CBRG11nov101), and the National Research Foundation (NRF-CRP7-2010-03) of Singapore to Y.Z. and a grant from the Ministry of Education (MOE2012-T2-101) of Singapore to N.L.
Footnotes
Published ahead of print 25 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02279-14.
REFERENCES
- 1.Strober W, Fuss IJ, Blumberg RS. 2002. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 20:495–549. 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 2.Kaser A, Zeissig S, Blumberg RS. 2010. Inflammatory bowel disease. Annu. Rev. Immunol. 28:573–621. 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan KS, Mirza H, Teo JD, Wu B, Macary PA. 2010. Current views on the clinical relevance of Blastocystis spp. Curr. Infect. Dis. Rep. 12:28–35. 10.1007/s11908-009-0073-8. [DOI] [PubMed] [Google Scholar]
- 4.Tan KS. 2008. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 21:639–665. 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark CG. 1997. Extensive genetic diversity in Blastocystis hominis. Mol. Biochem. Parasitol. 87:79–83. 10.1016/S0166-6851(97)00046-7. [DOI] [PubMed] [Google Scholar]
- 6.Alfellani MA, Taner-Mulla D, Jacob AS, Imeede CA, Yoshikawa H, Stensvold CR, Clark CG. 2013. Genetic diversity of blastocystis in livestock and zoo animals. Protist 164:497–509. 10.1016/j.protis.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez-Marquez MV, Guna R, Munoz C, Gomez-Munoz MT, Borras R. 2009. High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain). Parasitol. Res. 105:949–955. 10.1007/s00436-009-1485-y. [DOI] [PubMed] [Google Scholar]
- 8.Hailemariam G, Kassu A, Abebe G, Abate E, Damte D, Mekonnen E, Ota F. 2004. Intestinal parasitic infections in HIV/AIDS and HIV seronegative individuals in a teaching hospital, Ethiopia. Jpn. J. Infect. Dis. 57:41–43. [PubMed] [Google Scholar]
- 9.Cirioni O, Giacometti A, Drenaggi D, Ancarani F, Scalise G. 1999. Prevalence and clinical relevance of Blastocystis hominis in diverse patient cohorts. Eur. J. Epidemiol. 15:389–393. [DOI] [PubMed] [Google Scholar]
- 10.Tasova Y, Sahin B, Koltas S, Paydas S. 2000. Clinical significance and frequency of Blastocystis hominis in Turkish patients with hematological malignancy. Acta Med. Okayama 54:133–136. [DOI] [PubMed] [Google Scholar]
- 11.Yakoob J, Jafri W, Jafri N, Islam M, Asim Beg M. 2004. In vitro susceptibility of Blastocystis hominis isolated from patients with irritable bowel syndrome. Br. J. Biomed. Sci. 61:75–77. [DOI] [PubMed] [Google Scholar]
- 12.Stark D, van Hal S, Marriott D, Ellis J, Harkness J. 2007. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int. J. Parasitol. 37:11–20. 10.1016/j.ijpara.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Dogruman-Al F, Kustimur S, Yoshikawa H, Tuncer C, Simsek Z, Tanyuksel M, Araz E, Boorom K. 2009. Blastocystis subtypes in irritable bowel syndrome and inflammatory bowel disease in Ankara, Turkey. Mem. Inst. Oswaldo Cruz 104:724–727. 10.1590/S0074-02762009000500011. [DOI] [PubMed] [Google Scholar]
- 14.Dogruman-Al F, Simsek Z, Boorom K, Ekici E, Sahin M, Tuncer C, Kustimur S, Altinbas A. 2010. Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD patients in Ankara, Turkey. PLoS One 5:e15484. 10.1371/journal.pone.0015484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez-Gonzalez DE, Martinez-Flores WA, Reyes-Gordillo J, Ramirez-Miranda ME, Arroyo-Escalante S, Romero-Valdovinos M, Stark D, Souza-Saldivar V, Martinez-Hernandez F, Flisser A, Olivo-Diaz A, Maravilla P. 2012. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol. Res. 110:1269–1275. 10.1007/s00436-011-2626-7. [DOI] [PubMed] [Google Scholar]
- 16.Zuckerman MJ, Watts MT, Ho H, Meriano FV. 1994. Blastocystis hominis infection and intestinal injury. Am. J. Med. Sci. 308:96–101. 10.1097/00000441-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Puthia MK, Sio SW, Lu J, Tan KS. 2006. Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect. Immun. 74:4114–4123. 10.1128/IAI.00328-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long HY, Handschack A, Konig W, Ambrosch A. 2001. Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells. Parasitol. Res. 87:1029–1030. [DOI] [PubMed] [Google Scholar]
- 19.Puthia MK, Lu J, Tan KS. 2008. Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-kappaB-dependent manner. Eukaryot. Cell 7:435–443. 10.1128/EC.00371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moe KT, Singh M, Howe J, Ho LC, Tan SW, Chen XQ, Ng GC, Yap EH. 1997. Experimental Blastocystis hominis infection in laboratory mice. Parasitol. Res. 83:319–325. 10.1007/s004360050256. [DOI] [PubMed] [Google Scholar]
- 21.Iguchi A, Yoshikawa H, Yamada M, Kimata I, Arizono N. 2009. Expression of interferon gamma and proinflammatory cytokines in the cecal mucosa of rats experimentally infected with Blastocystis sp. strain RN94-9. Parasitol. Res. 105:135–140. 10.1007/s00436-009-1373-5. [DOI] [PubMed] [Google Scholar]
- 22.Wu LY, Fu RJ, Lu ZC, Tang LL, Zhang F, Liu DY. 2012. Expressions and significance of IL-17 and IL-23 in intestinal mucosa of mice infected with Blastocystis hominis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 24:676–680. [PubMed] [Google Scholar]
- 23.Mirza H, Tan KS. 2009. Blastocystis exhibits inter- and intra-subtype variation in cysteine protease activity. Parasitol. Res. 104:355–361. 10.1007/s00436-008-1203-1. [DOI] [PubMed] [Google Scholar]
- 24.Mirza H, Wu Z, Kidwai F, Tan KS. 2011. A metronidazole-resistant isolate of Blastocystis spp. is susceptible to nitric oxide and downregulates intestinal epithelial inducible nitric oxide synthase by a novel parasite survival mechanism. Infect. Immun. 79:5019–5026. 10.1128/IAI.05632-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivo-Diaz A, Romero-Valdovinos M, Gudino-Ramirez A, Reyes-Gordillo J, Jimenez-Gonzalez DE, Ramirez-Miranda ME, Martinez-Flores WA, Martinez-Hernandez F, Flisser A, Maravilla P. 2012. Findings related to IL-8 and IL-10 gene polymorphisms in a Mexican patient population with irritable bowel syndrome infected with Blastocystis. Parasitol. Res. 111:487–491. 10.1007/s00436-012-2830-0. [DOI] [PubMed] [Google Scholar]
- 26.Keyse SM. 2000. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol. 12:186–192. 10.1016/S0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 27.Ryang YS, Chang JH, Park JY. 2004. Involvement of MAP kinases in apoptosis of macrophage treated with Trichomonas vaginalis. Yonsei Med. J. 45:751–754. [DOI] [PubMed] [Google Scholar]
- 28.Zhang YL, Dong C. 2005. MAP kinases in immune responses. Cell Mol. Immunol. 2:20–27. [PubMed] [Google Scholar]
- 29.Imajo M, Tsuchiya Y, Nishida E. 2006. Regulatory mechanisms and functions of MAP kinase signaling pathways. IUBMB Life 58:312–317. 10.1080/15216540600746393. [DOI] [PubMed] [Google Scholar]
- 30.Lee HY, Hyung S, Lee NY, Yong TS, Han SH, Park SJ. 2012. Excretory-secretory products of Giardia lamblia induce interleukin-8 production in human colonic cells via activation of p38, ERK1/2, NF-kappaB and AP-1. Parasite Immunol. 34:183–198. 10.1111/j.1365-3024.2012.01354.x. [DOI] [PubMed] [Google Scholar]
- 31.Ropert C, Almeida IC, Closel M, Travassos LR, Ferguson MA, Cohen P, Gazzinelli RT. 2001. Requirement of mitogen-activated protein kinases and I kappa B phosphorylation for induction of proinflammatory cytokines synthesis by macrophages indicates functional similarity of receptors triggered by glycosylphosphatidylinositol anchors from parasitic protozoa and bacterial lipopolysaccharide. J. Immunol. 166:3423–3431. 10.4049/jimmunol.166.5.3423. [DOI] [PubMed] [Google Scholar]
- 32.Kim JY, Ahn MH, Song HO, Choi JH, Ryu JS, Min DY, Cho MH. 2006. Involvement of MAPK activation in chemokine or COX-2 productions by Toxoplasma gondii. Korean J. Parasitol. 44:197–207. 10.3347/kjp.2006.44.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawal S, Majumdar S, Vohra H. 2005. Activation of MAPK kinase pathway by Gal/GalNAc adherence lectin of E. histolytica: gateway to host response. Mol. Cell. Biochem. 268:93–101. 10.1007/s11010-005-3698-4. [DOI] [PubMed] [Google Scholar]
- 34.Ivory CP, Prystajecky M, Jobin C, Chadee K. 2008. Toll-like receptor 9-dependent macrophage activation by Entamoeba histolytica DNA. Infect. Immun. 76:289–297. 10.1128/IAI.01217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen XQ, Singh M, Ho LC, Moe KT, Tan SW, Yap EH. 1997. A survey of Blastocystis sp. in rodents. Lab. Anim. Sci. 47:91–94. [PubMed] [Google Scholar]
- 36.Stensvold CR, Suresh GK, Tan KS, Thompson RC, Traub RJ, Viscogliosi E, Yoshikawa H, Clark CG. 2007. Terminology for Blastocystis subtypes—a consensus. Trends Parasitol. 23:93–96. 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Stadnyk AW. 2002. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can. J. Gastroenterol. 16:241–246. [DOI] [PubMed] [Google Scholar]
- 38.Murphy K, Travers P, Walport M. 2008. Janeway's immunobiology. Garland Science, New York, NY. [Google Scholar]
- 39.Dong C, Davis RJ, Flavell RA. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55–72. 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 40.McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. 2006. Proteases in parasitic diseases. Annu. Rev. Pathol. 1:497–536. 10.1146/annurev.pathol.1.110304.100151. [DOI] [PubMed] [Google Scholar]
- 41.Puthia MK, Vaithilingam A, Lu J, Tan KS. 2005. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol. Res. 97:386–389. 10.1007/s00436-005-1461-0. [DOI] [PubMed] [Google Scholar]
- 42.Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li LH, Zhou XN, Ok UZ, Leelayoova S, Jones MS. 2008. Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit. Vectors 1:40. 10.1186/1756-3305-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scanlan PD. 2012. Blastocystis: past pitfalls and future perspectives. Trends Parasitol. 28:327–334. 10.1016/j.pt.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Kasper LH, Buzoni-Gatel D. 2001. Ups and downs of mucosal cellular immunity against protozoan parasites. Infect. Immun. 69:1–8. 10.1128/IAI.69.1.1-8.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermann GE, Rogers RC. 2008. TNFalpha: a trigger of autonomic dysfunction. Neuroscientist 14:53–67. 10.1177/1073858407305725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zwingenberger K, Harms G, Pedrosa C, Pessoa MC, Sandkamp B, Scheibenbogen C, Andreesen R. 1991. Generation of cytokines in human visceral leishmaniasis: dissociation of endogenous TNF-alpha and IL-1 beta production. Immunobiology 183:125–132. 10.1016/S0171-2985(11)80192-0. [DOI] [PubMed] [Google Scholar]
- 47.Camargo MM, Almeida IC, Pereira ME, Ferguson MA, Travassos LR, Gazzinelli RT. 1997. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J. Immunol. 158:5890–5901. [PubMed] [Google Scholar]
- 48.Sharma M, Vohra H, Bhasin D. 2005. Enhanced pro-inflammatory chemokine/cytokine response triggered by pathogenic Entamoeba histolytica: basis of invasive disease. Parasitology 131:783–796. 10.1017/S0031182005008541. [DOI] [PubMed] [Google Scholar]
- 49.Han IH, Goo SY, Park SJ, Hwang SJ, Kim YS, Yang MS, Ahn MH, Ryu JS. 2009. Proinflammatory cytokine and nitric oxide production by human macrophages stimulated with Trichomonas vaginalis. Korean J. Parasitol. 47:205–212. 10.3347/kjp.2009.47.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cader MZ, Kaser A. 2013. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut 62:1653–1664. 10.1136/gutjnl-2012-303955. [DOI] [PubMed] [Google Scholar]
- 51.Mirza H, Wu Z, Teo JD, Tan KS. 2012. Statin pleiotropy prevents rho kinase-mediated intestinal epithelial barrier compromise induced by Blastocystis cysteine proteases. Cell. Microbiol. 14:1474–1484. 10.1111/j.1462-5822.2012.01814.x. [DOI] [PubMed] [Google Scholar]
- 52.Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K, Akagawa KS, Hibi T. 2008. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 118:2269–2280. 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheth K, Friel J, Nolan B, Bankey P. 2001. Inhibition of p38 mitogen activated protein kinase increases lipopolysaccharide induced inhibition of apoptosis in neutrophils by activating extracellular signal-regulated kinase. Surgery 130:242–248. 10.1067/msy.2001.115902. [DOI] [PubMed] [Google Scholar]
- 54.Jijon HB, Panenka WJ, Madsen KL, Parsons HG. 2002. MAP kinases contribute to IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism. Am. J. Physiol. Cell Physiol. 283:C31–C41. 10.1152/ajpcell.00113.2001. [DOI] [PubMed] [Google Scholar]
- 55.Scanlan PD, Stensvold CR. 2013. Blastocystis: getting to grips with our guileful guest. Trends Parasitol. 29:523–529. 10.1016/j.pt.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Denoeud F, Roussel M, Noel B, Wawrzyniak I, Da Silva C, Diogon M, Viscogliosi E, Brochier-Armanet C, Couloux A, Poulain J, Segurens B, Anthouard V, Texier C, Blot N, Poirier P, Ng GC, Tan KS, Artiguenave F, Jaillon O, Aury JM, Delbac F, Wincker P, Vivares CP, El Alaoui H. 2011. Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biol. 12:R29. 10.1186/gb-2011-12-3-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sio SW, Puthia MK, Lee AS, Lu J, Tan KS. 2006. Protease activity of Blastocystis hominis. Parasitol. Res. 99:126–130. 10.1007/s00436-006-0131-1. [DOI] [PubMed] [Google Scholar]
- 58.Arastu-Kapur S, Ponder EL, Fonovic UP, Yeoh S, Yuan F, Fonovic M, Grainger M, Phillips CI, Powers JC, Bogyo M. 2008. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat. Chem. Biol. 4:203–213. 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- 59.de Matos Guedes HL, Pinheiro RO, Chaves SP, De-Simone SG, Rossi-Bergmann B. 2010. Serine proteases of Leishmania amazonensis as immunomodulatory and disease-aggravating components of the crude LaAg vaccine. Vaccine 28:5491–5496. 10.1016/j.vaccine.2010.04.109. [DOI] [PubMed] [Google Scholar]
- 60.de Almeida Nogueira NP, Morgado-Diaz JA, Menna-Barreto RF, Paes MC, da Silva-Lopez RE. 2013. Effects of a marine serine protease inhibitor on viability and morphology of Trypanosoma cruzi, the agent of Chagas disease. Acta Trop. 128:27–35. 10.1016/j.actatropica.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R. 2010. Irritable bowel syndrome: is it associated with genotypes of Blastocystis hominis. Parasitol. Res. 106:1033–1038. 10.1007/s00436-010-1761-x. [DOI] [PubMed] [Google Scholar]
- 62.Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztoczy A, Izbeki F, Fioramonti J, Wittmann T, Bueno L. 2008. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57:591–599. 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 63.Colognato R, Slupsky JR, Jendrach M, Burysek L, Syrovets T, Simmet T. 2003. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood 102:2645–2652. 10.1182/blood-2002-08-2497. [DOI] [PubMed] [Google Scholar]
- 64.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. 2001. Proteinase-activated receptors. Pharmacol. Rev. 53:245–282. [PubMed] [Google Scholar]
- 65.Kida Y, Inoue H, Shimizu T, Kuwano K. 2007. Serratia marcescens serralysin induces inflammatory responses through protease-activated receptor 2. Infect. Immun. 75:164–174. 10.1128/IAI.01239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen KK, Sherman PM, Cellars L, Andrade-Gordon P, Pan Z, Baruch A, Wallace JL, Hollenberg MD, Vergnolle N. 2005. A major role for proteolytic activity and proteinase-activated receptor-2 in the pathogenesis of infectious colitis. Proc. Natl. Acad. Sci. U. S. A. 102:8363–8368. 10.1073/pnas.0409535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothmeier AS, Ruf W. 2012. Protease-activated receptor 2 signaling in inflammation. Semin. Immunopathol. 34:133–149. 10.1007/s00281-011-0289-1. [DOI] [PubMed] [Google Scholar]
- 68.Mirza H, Teo JD, Upcroft J, Tan KS. 2011. A rapid, high-throughput viability assay for Blastocystis spp. reveals metronidazole resistance and extensive subtype-dependent variations in drug susceptibilities. Antimicrob. Agents Chemother. 55:637–648. 10.1128/AAC.00900-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stensvold CR, Nielsen HV, Molbak K, Smith HV. 2009. Pursuing the clinical significance of Blastocystis—diagnostic limitations. Trends Parasitol. 25:23–29. 10.1016/j.pt.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Poirier P, Wawrzyniak I, Vivares CP, Delbac F, El Alaoui H. 2012. New Insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLoS Pathog. 8:e1002545. 10.1371/journal.ppat.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stensvold CR, Christiansen DB, Olsen KE, Nielsen HV. 2011. Blastocystis sp. subtype 4 is common in Danish Blastocystis-positive patients presenting with acute diarrhea. Am. J. Trop. Med. Hyg. 84:883–885. 10.4269/ajtmh.2011.11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee IL, Tan TC, Tan PC, Nanthiney DR, Biraj MK, Surendra KM, Suresh KG. 2012. Predominance of Blastocystis sp. subtype 4 in rural communities, Nepal. Parasitol. Res. 110:1553–1562. 10.1007/s00436-011-2665-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.