Abstract
In nature, mixed Borrelia burgdorferi infections are common and possibly can be acquired by either superinfection or coinfection. Superinfection by heterologous B. burgdorferi strains has been established experimentally, although the ability of homologous B. burgdorferi clones to superinfect a host has not been studied in detail. Information regarding any potential immune barriers to secondary infection also currently is unavailable. In the present study, the ability to superinfect various mouse models by homologous wild-type clones was examined and compared to superinfection by heterologous strains. To assess the ability of homologous B. burgdorferi clones to successfully superinfect a mouse host, primary- and secondary-infecting spirochetes were recovered via in vitro cultivation of collected blood or tissue samples. This was accomplished by generating two different antibiotic-resistant versions of the wild-type B31-A3 clone in order to distinguish superinfecting B. burgdorferi from primary-infecting spirochetes. The data demonstrate an inability of homologous B. burgdorferi to superinfect immunocompetent mice as opposed to heterologous strains. Attempts to superinfect different types of immunodeficient mice with homologous B. burgdorferi indicate that the murine innate immune system represents a major barrier to intrastrain superinfection. Consequently, the possibility of innate immunity as a driving force for B. burgdorferi heterogeneity during the enzootic cycle is discussed.
INTRODUCTION
Borrelia burgdorferi is the bacterial causative agent of Lyme disease, the most prevalent vector-borne disease in North America (1–6). Lyme disease is a debilitating multisystemic disorder that most commonly presents itself as skin lesions (erythema migrans), and ultimately it is followed by arthritis, carditis, and nervous system manifestations (7–10). In nature, B. burgdorferi is maintained in an enzootic cycle involving a mammalian reservoir and tick vector. Peromyscus spp. of mice are important reservoirs of B. burgdorferi in the wild (6, 11, 12), whereas ixodid ticks are responsible for the seasonally restricted transmission of Lyme disease (6, 13).
Mixed infections with various B. burgdorferi genotypes have been reported in questing ticks (14–16), reservoir animals (17), and humans (18). In Peromyscus leucopus mice, infections by heterologous B. burgdorferi populations are fairly common and potentially could be acquired by either superinfection or coinfection (17). For the purpose of this study, superinfection is defined as the introduction of B. burgdorferi into a mouse host that already harbors an ongoing, active B. burgdorferi infection, whereas coinfection is a simultaneous introduction of more than one B. burgdorferi clone into a naive animal. Moreover, superinfection or coinfection is considered established when a superinfecting or coinfecting B. burgdorferi clone can be cultured from any murine tissue postchallenge.
Superinfection by heterologous subclones or strains of B. burgdorferi has been experimentally established (17, 19). Peromyscus mice initially infected with the rRNA spacer type 3 (RST3) or RST1 B. burgdorferi genotype were able to be superinfected by RST1 or RST3, respectively. However, the results obtained from control groups of mice that were sequentially (14 days apart) challenged by the same B. burgdorferi strain (RST1/RST1 or RST3/RST3) are questionable due to the lack of any selectable marker that would allow the primary and secondary infections to be differentiated (19). Thus, despite the evidence for successful host superinfection by heterologous B. burgdorferi strains, the ability of homologous clones to superinfect a host has not been thoroughly investigated. Additionally, any potential immune barriers to superinfection, as well as a requirement for B. burgdorferi proteins with known roles in immune evasion and persistence, have not been examined to date. Such knowledge might provide insight into selective pressures that could drive heterogeneity of B. burgdorferi during its enzootic life cycle.
In the present study, the ability of homologous wild-type and mutant B. burgdorferi clones to superinfect various mouse models was assessed and compared to superinfection by heterologous strains. The ability to distinguish between primary- and secondary-infecting B. burgdorferi was accomplished by generating two different antibiotic-resistant versions of the wild-type B31-A3 clone. Overall, the data demonstrated an inability of homologous clones to superinfect immunocompetent mice. In contrast, heterologous B. burgdorferi strains did exhibit the capacity to establish superinfection in immunocompetent mice, supporting findings from previous studies (17, 19). Experiments also showed that homologous B. burgdorferi clones were unable to superinfect different types of antibody-deficient mice, suggesting that the host-acquired immune response is not involved in preventing host superinfection by B. burgdorferi. Importantly, data from additional immunodeficient mice are suggestive that the murine innate immune system is the main barrier to intrastrain superinfection. Finally, experiments involving B. burgdorferi devoid of VlsE demonstrated that the presence of the vls locus is not required to establish superinfection but is necessary for persistent superinfection by heterologous B. burgdorferi strains.
MATERIALS AND METHODS
Ethics statement.
All experimental procedures involving inbred mice were carried out in accordance with the American Association for Accreditation of Laboratory Animal Care (AAALAC) protocol and the institutional guidelines set by the Office of Campus Veterinarian at Washington State University (Animal Welfare Assurance A3485-01 and USDA registration number 91-R-002). Washington State University AAALAC and institutional guidelines are in compliance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. All inbred mice were maintained at Washington State University in an AAALAC-accredited animal facility. The Washington State University Institutional Animal Care and Use Committee reviewed and approved the animal protocols associated with the current studies.
Bacterial strains and culture conditions.
Borrelia burgdorferi strain B31-A3 (wtB31) was kindly provided by Patti Rosa (20). The B31-A3Δvls (ΔvlsE) clone was generated and characterized in previous studies (21, 22). The infectious B. burgdorferi 297 strain was a kind gift from Scott Samuels by way of Michael Norgard. The 5A4ΔD16-D25 clone was previously generated and characterized in our laboratory (23). All B. burgdorferi clones were cultivated in liquid Barbour-Stoenner-Kelly II media (BSK-II) supplemented with 6% rabbit serum (Cedarlane Laboratories, Burlington, NC) and incubated at 35°C under 2.5% CO2.
Plasmid construction.
In order to generate B31-A3 lp25::kan (wtB31-kanr) and B31-A3 lp25::gent (wtB31-gentr) clones, pAR14 and pAR15 were constructed to disrupt bbe02 located on plasmid lp25 (coordinates 323 to 4,156; NCBI reference sequence NC_001850.1). The 3,770-bp target of bbe02 (coordinates 361 to 4,130) was PCR amplified using P222 (5′-ATG AAA ACT AAT GAT ATC GTA AAA ACA-3′) and P223 (5′-TTA TTT ATG ATA AAA AAT TTT ATT ATT TAG TAA ATA ATT-3′) primers. The amplicon was cloned into the pJET1.2 vector by utilizing the CloneJET PCR cloning kit (Fermentas, USA). The flgBp-driven kanamycin and gentamicin resistance genes (flgBp-kan and flgBp-gent, respectively) carried on pTB44 and pGCL47-4G (21), respectively, were amplified with corresponding primer sets P509 (5′-TGA GCT AGC TAC CCG AGC TTC AAG GAA GA-3′) and P510 (5′-GCA TTA ATT AAT GCC AGT GTT ACA ACC AAT T-3′) as well as P507 (5′-TGA GCT AGC CAG TTG CGC AGC CTG AAT GG-3′) and P508 (5′-GCA TTA ATT AAA GGT GGC GGT ACT TGG GTC G-3′), which include NheI and PacI restriction sites (underlined). The flgBp-kan and flgBp-gent amplicons were individually cloned into pJET1.2, recovered by digestion with NheI and PacI, and then cloned into pJET1.2::bbe02.
Bacterial transformation.
B. burgdorferi cells were electroporated using previously described conditions (21). After electroporation with a total of 25 μg of DNA, spirochetes were resuspended in 10 ml of prewarmed BSK-II media. Cells were recovered at 35°C for 18 h and then diluted in 100 ml of prewarmed BSK-II supplemented with 200 μg ml−1 kanamycin or 100 μg ml−1 gentamicin. The transformed cell suspension was aliquoted into 96-well plates and incubated at 35°C for 21 to 28 days. Genomic DNA from positive wells was extracted using a DNeasy blood and tissue kit (Qiagen, Germantown, MD), and the presence of flgBp-kan and flgBp-gent cassettes was confirmed by PCR analysis using P54/P55 (5′-CAT ATG AGC CAT ATT CAA CGG GAA ACG-3′ and 5′-AAA GCC GTT TCT GTA ATG AAG GAG-3′) and P91/P92 (5′-CGC AGC AGC AAC GAT GTT AC-3′ and 5′-CTT GCA CGT AGA TCA CAT AAG C-3′) primer sets, respectively. Plasmid content for each verified transformant was determined by PCR using plasmid-specific primers as previously described (24).
Murine infection.
Male C3H/HeNHsd (C3H) and Hsd:Athymic Nude-Foxn1nu (nude) mice of 4 to 6 weeks of age were obtained from Harlan (Indianapolis, IN). Male C3SnSmn.CB17-Prkdcscid/J (SCID) and NOD.CB17-Prkdcscid/J (NOD) mice of 4 to 5 weeks of age were purchased from Jackson Laboratories (Bar Harbor, ME). The initial infection was performed on animals 5 to 7 weeks of age. Attempts at superinfection of mice occurred subcutaneously through either needle inoculation with 1.1 × 104 total spirochetes in the scapular region or by transplantation of ear tissue in the lumbar area. Inoculum of each B. burgdorferi clone containing a recombinant plasmid was cultured in BSK-II medium supplemented with the appropriate antibiotic and was diluted (approximately 1:1,000) with antibiotic-free BSK prior to murine infection. B. burgdorferi clones from frozen glycerol stock were passaged no more than two times in vitro prior to use in mouse infection assays. Ear tissues were derived from infected SCID mice at day 28 postinfection as previously described (22, 25) and stored at −80°C until use. Immunodeficient SCID mice were used to generate host-adapted B. burgdorferi due to the lack of their ability to clear infection by the vls mutant clones (21, 22). For tissue transplantation, ear pinnae were excised into small, circular pieces (3 mm in diameter) by a sterile ear punch and subcutaneously inserted through a skin incision in the lumbar region (two pieces per mouse). The infectivity of each in vitro- or tissue-derived clone was tested on naive mice (see Tables S1 and S2 in the supplemental material).
Infection was verified by culturing approximately 50 μl of blood aseptically drawn from a mouse via maxillary bleed in 3 ml of BSK-II containing Borrelia antibiotic cocktail (0.02 mg ml−1 phosphomycin, 0.05 mg ml−1 rifampin, and 2.5 mg ml−1 amphotericin B). To monitor the progress of infection, ear, heart, bladder, and tibiotarsal joint tissues were aseptically harvested at various time points postinfection and cultured in BSK-II supplemented with the antibiotic cocktail. Eight-ml polystyrene tubes (Becton Dickinson Labware, USA) were used for culturing blood (50 μl of blood in 3 ml of BSK medium) and heart tissue (approximately half of a heart in 3.0 ml of BSK medium), and 2.0-ml polypropylene microcentrifuge tubes (Fisherbrand, USA) were utilized for the other tissues (approximately half of a bladder, a tibiotarsal joint, and ear tissue [3 mm in diameter] in 1.0 ml of BSK medium). Murine tissues were incubated at 35°C under 2.5% CO2. The presence of viable spirochetes from each cultured tissue was confirmed by dark-field microscopy.
Statistical analysis.
A one-tailed Fisher's exact test was used. A P value of <0.05 was considered significantly different.
RESULTS
Generation and characterization of antibiotic-resistant wild-type B. burgdorferi clones.
To assess the ability of homologous B. burgdorferi clones to successfully superinfect a mouse host, primary- and secondary-infecting spirochetes were recovered via in vitro cultivation of collected blood or tissue samples. This was accomplished by generating two different antibiotic-resistant versions of the wild-type B. burgdorferi B31-A3 clone in order to distinguish superinfecting B. burgdorferi from primary-infecting spirochetes (Table 1). These modified wild-type clones obtained an insertion of either a gentamicin (gent) or kanamycin (kan) antibiotic resistance cassette in the bbe02 locus of the lp25 plasmid via allelic exchange. Inactivation of bbe02, a putative restriction-modification gene, does not result in loss of infectivity in mice (26–28). Ten transformants of each clone type were chosen for initial PCR analysis to screen for the presence of the gent or kan gene. Several clones were selected for further PCR analysis in order to ensure retention of all parental B. burgdorferi plasmids. One clone each of B31-A3 lp25::gent (wtB31-gentr) and B31-A3 lp25::kan (wtB31-kanr) that retained the full parent plasmid profile were chosen for use in the planned superinfection experiments (Fig. 1).
TABLE 1.
Borrelia burgdorferi clones used in the study
| B. burgdorferi clone | Presence of: |
Reference or source | |
|---|---|---|---|
| vls2-16a | vlsE | ||
| B31-A3 (wtB31) | + | + | 20 |
| B31-A3 lp25::gent (wtB31-gentr) | + | + | This study |
| B31-A3 lp25::kan (wtB31-kanr) | + | + | This study |
| B31-A3 lp28–1Δvls (ΔvlsE) | − | − | 21, 22 |
| 297 | NDb | + | 85 |
vls2-16 denotes silent cassettes of the vls locus.
The silent cassette region in this strain has not been determined to date.
FIG 1.
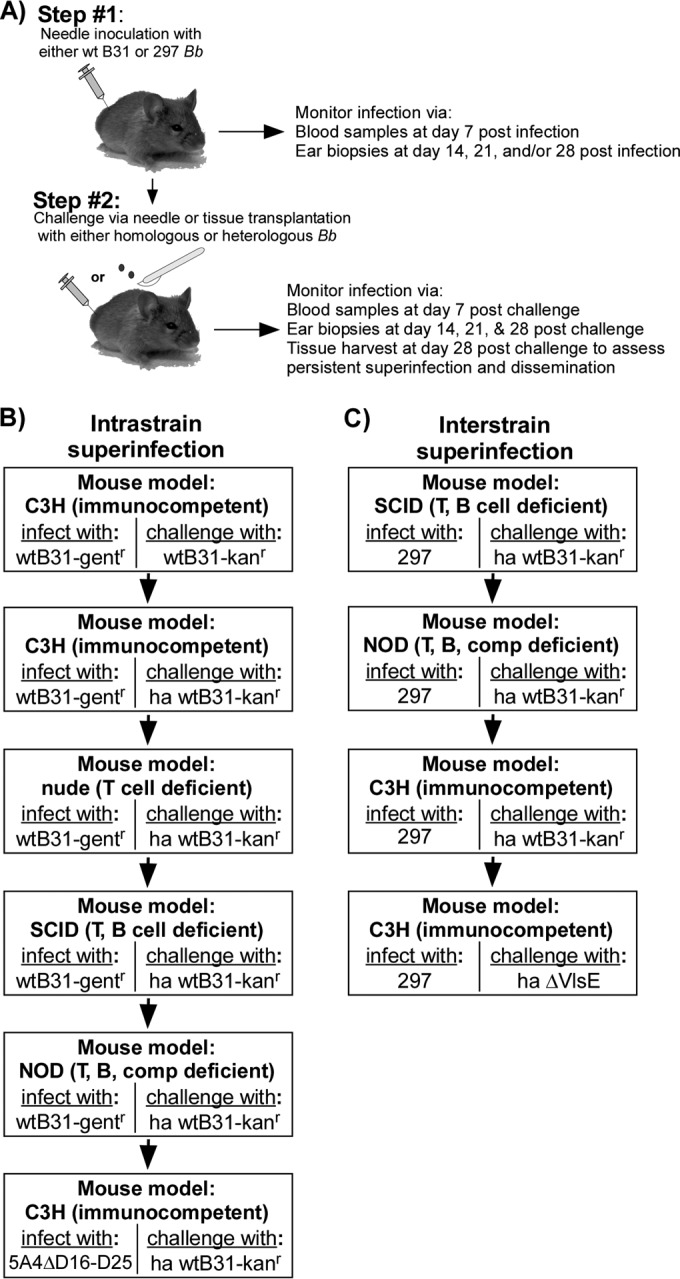
Experimental approach to assay host superinfection by homologous and heterologous B. burgdorferi. (A) Schematic for the general two-step approach to assay for host superinfection by B. burgdorferi. First, groups of mice were needle inoculated with either the B31 or 297 wild-type strain. The establishment of infection was confirmed by culturing blood taken at day 7 postinfection and ear skin tissues sampled weekly afterwards. At day 6, 13, 21, or 28 postinfection, mice were superinfected with either homologous or heterologous B. burgdorferi via needle inoculation or tissue transplantation. Blood, ear, heart, bladder, and tibiotarsal joint tissues were collected at the indicated time points and cultured in the presence of the appropriate antibiotic to assess the outcome of superinfection. (B and C) Flow chart of superinfection experiments involving homologous (B) or heterologous (C) B. burgdorferi. T, T cell; B, B cell; comp, host complement; ha, host adapted.
Inoculation of mice demonstrated that both wtB31-gentr and wtB31-kanr were infectious in C3SnSmn.CB17-Prkdcscid/J (SCID) and C3H/HeNHsd (C3H) mice, as determined by culture-detectable spirochetes in blood at day 7 postinfection (see Table S1 in the supplemental material). Similar to the parental wild-type B. burgdorferi, wtB31-gentr and wtB31-kanr clones were able to disseminate and establish persistent infection, as shown by positive cultures of ear biopsy specimens sampled at days 14, 21, and 28 postinfection. Heart, joint, and bladder tissues harvested at day 28 postinfection were consistently culture positive, demonstrating the ability of the clones to colonize these sites. While the in vitro-grown wtB31-gentr and wtB31-kanr clones exhibited full infectivity in C3H mice, it was also determined whether host-adapted forms of these B. burgdorferi clones were infectious. To obtain host-adapted versions of the wtB31-gentr and wtB31-kanr clones, aural skin tissues of SCID mice infected with either clone were utilized for tissue transplantation into naive mice. Both host-adapted B. burgdorferi clones exhibited full infectivity in C3H and Hsd:Athymic Nude-Foxn1nu (nude) mice (see Table S2). Overall, the newly generated clones did not show any impairment in their ability to infect either immunodeficient or immunocompetent mouse models compared to the parental wild-type B. burgdorferi.
Superinfection by homologous B. burgdorferi is limited to severely immunodeficient mice.
In order to examine if mice could become superinfected with homologous B. burgdorferi, initial infection of 5 C3H mice with in vitro-grown wtB31-gentr B. burgdorferi was followed by a secondary spirochete challenge with the homologous wtB31-kanr clone (Fig. 1A). Blood and tissue samples were cultured in the presence of either gentamicin or kanamycin in order to differentiate between the initial-infecting or superinfecting B. burgdorferi, respectively. All primary-infected mice displayed spirochetemia at day 7 postinfection, and ear biopsy specimens were culture positive at days 21 and 28. At day 28 postinfection, persistently infected mice (5 animals per group) were needle inoculated with in vitro-grown wtB31-kanr (Fig. 1B). The outcome of superinfection was monitored weekly by taking blood samples at day 7 and ear biopsy specimens at days 14, 21, and 28 postchallenge. Secondary-infecting B. burgdorferi was not detected by culture of blood or aural skin tissues, and collected heart, bladder, and tibiotarsal joint tissues were consistently culture negative (see Table S3 in the supplemental material). Conversely, control C3H mice were successfully infected by in vitro-grown wtB31-kanr, demonstrating that the clone (the same inoculum) was capable of primary infection (P < 0.05) (see Table S3). Together, the data suggest that in vitro-grown homologous B. burgdorferi is not able to superinfect C3H mice.
The inability to establish host superinfection by homologous B. burgdorferi might be partially accounted for by low expression levels of factors necessary for infectivity at the time of inoculation. Previous data have shown that wild-type B. burgdorferi can reinfect mice only when it has been host adapted or tick derived (22, 29). For this reason, superinfection was performed using host-adapted B. burgdorferi (Fig. 1A and B). At day 28 postinfection, persistently wtB31-gentr-infected C3H mice (5 animals per group) received host-adapted wtB31-kanr via tissue transplantation of ear tissues from infected SCID mice. The outcome of superinfection was assessed by culturing blood samples at day 7, ear biopsy specimens at days 14, 21, and 28, and heart, bladder, and tibiotarsal joint tissues harvested at day 28 postchallenge. Similar to results from the previous superinfection experiment, mice displayed neither spirochetemia nor dissemination as determined by negative cultures of murine tissues (Table 2). Again, control C3H mice all were infected by host-adapted wtB31-kanr, indicating that the clones were capable of primary infection in mice (P < 0.05) (Table 2). Thus, although host adaptation provides homologous wild-type B. burgdorferi clones with the capacity to establish reinfection (22), these results suggest that this is not the case with superinfection.
TABLE 2.
Assessment of superinfection by homologous B. burgdorferi in C3H and NOD mice
| Tissue collected (day postchallenge) | No. of samples positive for superinfection froma: |
No. of samples from naïve C3H mice (control) positive for infection with ha wtB31-kanr | ||
|---|---|---|---|---|
| wtB31-gentr- infected C3H mice | wtB31-gentr- infected NOD mice | wtB31-gentr- infected NOD mice treated with SCID mouse-derived sera | ||
| Blood (7) | 0/5b | 7/9 | 1/4 | 5/5 |
| Ear (14) | 0/5 | 0/9 | 0/4 | 5/5 |
| Ear (21) | 0/5 | 0/9 | 0/4 | 5/5 |
| Tissues (28) | ||||
| Ear | 0/5 | 0/9 | 0/4 | 5/5 |
| Heart | 0/5 | 1/9 | 0/4 | 5/5 |
| Bladder | 0/5 | 0/9 | 0/4 | 3/5 |
| Joint | 0/5 | 0/9 | 0/4 | 5/5 |
| Total no. of infected micec (28) | 0/5 | 1/9 | 0/4 | 5/5 |
Superinfection was performed via tissue transplantation with the host-adapted (ha) wtB31-kanr clone.
Values listed correspond to numbers of cultures positive/numbers tested.
Based on combined results obtained from culturing ear, heart, bladder, and tibiotarsal joint.
The inability of homologous B. burgdorferi to superinfect C3H mice may be due to an acquired antibody response that is constantly directed to a variety of B. burgdorferi surface antigens (30–34). To test if the extent of the host adaptive antibody response has any effect on the outcome of superinfection, we challenged wtB31-gentr-infected C3H mice with host-adapted wtB31-kanr B. burgdorferi at days 6 and 13 postsuperinfection (initial infection was confirmed by positive cultures of blood sampled immediately prior to secondary infection). At these time points, an adaptive humoral response is predicted to be either at a very low level (day 6) or just beginning to develop (day 13) in immunocompetent C3H mice (35). However, murine tissue samples cultured at day 4, 7, 14, 21, or 28 postsecondary challenge showed once again the presence of the primary-infecting B. burgdorferi clone and a lack of superinfecting spirochetes (see Table S4 in the supplemental material), suggesting that the acquired antibody response is not a factor in preventing B. burgdorferi host superinfection.
To further determine if acquired (T-cell-dependent) humoral immunity represents a barrier to superinfection, nude mice were utilized in the experimental design (Fig. 1B). These mice lack functional CD4+ and CD8+ T cells; thus, they are able to generate only T-cell-independent (TI) immunoglobulins. Five nude mice originally infected with host-adapted wtB31-gentr (see Table S2 in the supplemental material) were challenged with host-adapted wtB31-kanr at day 28 postinfection. Consistent with the previous results, blood, ear, joint, bladder, and heart tissues were negative by culture for any superinfecting clone (see Table S5 in the supplemental material). The absence of culture-detectable spirochetes in this assay further indicates that acquired humoral immunity does not prevent host superinfection by B. burgdorferi.
The above-described finding also suggests that TI antibodies are able to prevent superinfection by B. burgdorferi in mice. In fact, earlier studies have shown that immune sera from Borrelia-infected T-cell-deficient mice are able to protect naive animals against challenge with in vitro-grown wild-type B. burgdorferi (22, 36). In order to examine if TI immunoglobulins play any role in the prevention of superinfection, SCID mice lacking both T-cell-dependent (TD) and TI antibodies were utilized for the infection assay. Five SCID mice persistently infected with wtB31-gentr were challenged with wtB31-kanr at day 21 postinfection. Secondary challenge was performed by using host-adapted or in vitro-grown wtB31-kanr (at 1.1 × 105 cells per mouse). All SCID mice displayed a sustained primary wtB31-gentr-induced spirochetemia for at least 21 days postinfection, as determined by positive blood cultures (see Table S6 in the supplemental material). After secondary challenge, the results also showed that none of the mice challenged with host-adapted or in vitro-grown wtB31-kanr had superinfecting spirochetes, as determined by negative culture of murine tissues (see Table S5). The lack of culture-detectable superinfection in SCID mice suggests that neither TD nor TI antibodies are responsible for preventing host superinfection by homologous B. burgdorferi.
The absence of superinfecting spirochetes in mice deficient in antibody-mediated immunity suggests that innate immunity acts as a barrier to B. burgdorferi superinfection (13, 37, 38). A recent study involving mixed populations of B. burgdorferi found a significant role for the host innate response in controlling the early stage of B. burgdorferi infection (39). Additionally, the alternative pathway of the complement system has been shown to have a role in mediating B. burgdorferi host specificity (13, 38, 40). In order to further examine if host innate immunity, particularly host complement, could be involved in preventing superinfection by B. burgdorferi, another strain of T- and B-cell-deficient mice, NOD.CB17-Prkdcscid/J (NOD), was used. As opposed to the elevated complement activity observed in SCID mice, NOD mice are deficient in functional C5 complement (41) that precludes formation of any C5b-9 membrane attack complex (42) regardless of the pathway of complement activation. At day 21 postinfection, nine wtB31-gentr-infected NOD mice were challenged with host-adapted wtB31-kanr B. burgdorferi. Interestingly, seven out of nine NOD animals displayed spirochetemia at day 7 postsuperinfection, suggesting that homologous B. burgdorferi is capable of establishing superinfection in these severely immunodeficient mice (P < 0.05) (Table 2). However, both blood and murine tissues sampled at later time points were culture negative for superinfecting spirochetes, with the exception of one mouse that showed culture-positive cardiac tissue. In addition to a lack of dissemination, these results suggest a failure of homologous B. burgdorferi to establish persistent secondary infection. Collectively, the above-described experiments utilizing both immunocompetent and -deficient mice suggest that homologous B. burgdorferi is unable to establish persistent superinfection of a mouse host, and that some component(s) of the murine innate system represents a barrier to intrastrain superinfection by B. burgdorferi.
In order to test if blood-borne factors of the innate system are involved in preventing intrastrain superinfection, a passive transfer approach was undertaken. Specifically, 4 naive NOD mice were infected initially with in vitro-grown wtB31-gentr. All mice became infected with B. burgdorferi, as determined by positive cultures of blood and ear tissues sampled at days 7 and 20 postinfection (data not shown). At day 20 postinfection, these NOD mice were subcutaneously injected with filter-sterilized sera (150 μl) collected from wtB31-infected SCID mice at day 20 postinfection. The NOD mice were superinfected 24 h later with host-adapted wtB31-kanr. The culture results showed that 3 out of 4 mice were negative for superinfecting spirochetes in blood drawn at day 7 postsuperinfection (Table 2). In contrast, when left untreated with any sera, 7 out of 9 NOD mice were shown earlier to be successfully superinfected by homologous B. burgdorferi clones (Table 2). Although not quite statistically significant (1/4 versus 7/9; P = 0.1189), these results are somewhat suggestive that host innate factors passed along with sera from animals that are devoid of acquired immunity are responsible for preventing intrastrain B. burgdorferi superinfection.
Superinfection of mouse models by heterologous B. burgdorferi.
Superinfection by heterologous B. burgdorferi strains has been demonstrated previously (19). To test if long-term (>7 days) superinfection by heterologous B. burgdorferi is demonstrable under the experimental conditions applied in this study, the fully infectious B. burgdorferi 297 strain was used for initial inoculation of mice. The same experimental design was followed, with both SCID and NOD mice utilized as host mouse models (Fig. 1C). To assay for superinfection by heterologous B. burgdorferi in SCID mice, five animals were infected initially with in vitro-grown B. burgdorferi 297. All five mice exhibited spirochetemia in blood at day 7 and had culture-positive ear tissues sampled at days 14 and 21 postinfection (data not shown). At day 21, infected SCID mice were challenged with host-adapted wtB31-kanr B. burgdorferi, and the outcome of superinfection was monitored weekly via tissue harvest for detection by culture.
The results show that none of the SCID mice had culture-positive blood for wtB31-kanr B. burgdorferi at day 7 postsuperinfection (Table 3), and ear biopsy specimens taken at day 14 postchallenge were consistently negative for superinfecting spirochetes for all five mice. However, ear, heart, bladder, and tibiotarsal joint tissues harvested from four mice at day 28 were culture positive for the wtB31-kanr clone, indicating that the spirochetes eventually were able to establish detectable secondary infection in these immunodeficient mice (Table 3).
TABLE 3.
Assessment of superinfection by a heterologous strain of B. burgdorferi in SCID, NOD, and C3H mice
| Tissue collected (day postchallenge) | No. positive/total no. of samples superinfected fromb: |
||
|---|---|---|---|
| 297-infected SCID mice | 297-infected NOD mice | 297-infected C3H mice | |
| Blood (7) | 0/5a | 4/5 | 0/5 |
| Ear (14) | 0/5 | 0/5 | 0/5 |
| Ear (21) | NAc | 0/5 | 0/5 |
| Tissues (28) | |||
| Ear | 0/5 | 1/5 | 2/5 |
| Heart | 3/5 | 2/5 | 4/5 |
| Bladder | 1/5 | 1/5 | 3/5 |
| Joint | 3/5 | 1/5 | 3/5 |
| Total no. of infected miced (28) | 4/5 | 2/5 | 4/5 |
Values listed correspond to numbers of cultures positive/numbers tested.
Samples were superinfected via tissue transplantation with host-adapted (ha) wtB31-kanr.
NA, not assessed.
Based on combined results obtained from culturing ear, heart, bladder, and tibiotarsal joint.
When NOD mice were used, animals initially infected with the 297 strain became successfully superinfected by host-adapted wtB31-kanr (Table 3), with 4 out of 5 animals exhibiting spirochetemia at day 7 postchallenge. Curiously, murine tissues cultured at later time points were consistently culture negative for superinfecting spirochetes, suggesting a possible absence of dissemination. This is similar to the results obtained from intrastrain superinfection of NOD mice and indicates the presence of a general immune restriction to dissemination and tissue colonization by secondary challenging spirochetes in these mice (see Discussion). Together, the results demonstrate that persistent superinfection by B. burgdorferi is possible under the experimental conditions used here when heterologous strains are utilized on immunodeficient mouse models.
In order to test if immunocompetent mice could be superinfected by heterologous B. burgdorferi, five B. burgdorferi 297-infected C3H mice were challenged with host-adapted wtB31-kanr spirochetes. Mice showed no culture-detectable spirochetemia at day 7 postchallenge. However, in contrast to the failure to superinfect C3H mice with homologous B. burgdorferi strains, 4 out of 5 mice became superinfected with wtB31-kanr as determined by culture-positive tissues harvested at day 28 (P < 0.05) (Table 3). Thus, these results demonstrate that heterologous B. burgdorferi is indeed capable of long-term superinfection in immunocompetent mice.
Homologous B. burgdorferi is unable to populate an uncolonized niche in a persistently infected mouse.
A possible explanation for the observed inability of homologous B. burgdorferi clones to carry out superinfection is that available tissue niches already are occupied by primary-infecting homologous spirochetes. In such a case, it is possible that superinfecting B. burgdorferi either cannot establish colonization of these sites or that titers of superinfecting spirochetes would simply be below the limit of detection via culture. Urinary bladder tissue has been shown to be a consistent source of culture-detectable spirochetes from B. burgdorferi-infected rodents (43–48). However, a recent study reported that an infectious B. burgdorferi mutant (B31 5A4ΔD16-D25) lacking lp17-resident genes bbd16 to bbd25 exhibited an impaired ability to colonize bladder tissue in C3H mice (23). To test the hypothesis that an uncolonized murine bladder could serve as an available niche for a superinfecting homologous clone of B. burgdorferi, the bladder-colonizing-defective mutant was utilized for the superinfection assay (Fig. 1B).
For this experiment, 10 C3H mice initially were infected with in vitro-grown 5A4ΔD16-D25 spirochetes. All animals became persistently infected with the mutant B. burgdorferi clone, as determined by culture-positive ear biopsy specimens sampled at days 21 and 28 postinfection (data not shown). At day 28, five of these mice were challenged with host-adapted wtB31-gentr, while the remaining five mice served as a nonsuperinfected control for the lack of bladder colonization by the infecting 5A4ΔD16-D25 B. burgdorferi clone. As shown in Table 4, all blood samples were culture negative for superinfecting spirochetes. At day 28 postsuperinfection, all mice were sacrificed and bladder tissues harvested to test for the presence of spirochetes. The results show that all five 5A4ΔD16-D25-infected mice that had been challenged with host-adapted wtB31-gentr were consistently culture negative for superinfecting B. burgdorferi. Importantly, only bladder tissues of control animals were culture negative for viable spirochetes of 5A4ΔD16-D25, which is in agreement with previously published data (23 and data not shown). In contrast to the above-described findings involving B. burgdorferi intrastrain superinfection, 7 out of 8 297-infected mice challenged with B31 wtB31-kanr spirochetes provided culture-positive bladders for each heterologous strain at day 28 postsuperinfection, demonstrating that heterologous B. burgdorferi strains have the capacity to cocolonize this tissue site (P < 0.05) (Table 4). Collectively, these data suggest that the occupancy capacity of murine tissues does not prevent host superinfection by homologous B. burgdorferi strains.
TABLE 4.
Assessment of uninfected bladder tissue as an available niche for superinfecting B. burgdorferi in C3H mice
| Tissue collected (day after rechallenge) | No. of samples positive for superinfection with hawtB31-gentr froma: |
|
|---|---|---|
| 5A4ΔD16-D25-infected mice | 297-infected mice | |
| Blood (7) | 0/5b | 0/8 |
| Tissues (28) | ||
| Ear | 0/5 | 3/8 |
| Heart | 0/5 | 8/8 |
| Bladder | 0/5 | 7/8 |
| Joint | 0/5 | 7/8 |
| Total no. of infected micec (28) | 0/5 | 8/8 |
ha, host-adapted clone.
Values listed correspond to numbers of cultures positive/numbers tested.
Based on combined results obtained from culturing ear, heart, bladder, and tibiotarsal joint.
Lack of murine coinfection by mixed homologous B. burgdorferi clones.
An inherent inability of wtB31-kanr to outcompete wtB31-gentr also could account for the failure of this homologous clone to superinfect an immunocompetent murine host. In order to test this possibility, five C3H mice were simultaneously infected with wtB31-kanr and wtB31-gentr. In order to ensure equal numbers of each B. burgdorferi clone at the site of infection, coinfection was performed by subcutaneous inoculation with a total of 1.1 × 104 spirochetes for each clone per animal. Blood sampled at day 7 and other tissues collected at later time points were consistently culture positive for either wtB31-gentr or wtB31-kanr, but not both, in 4 out of 5 mice (Table 5). Ear biopsy specimen and other tissue samples taken at days 14 and 28 postinfection showed that the same individual B. burgdorferi clone detected from blood samples continued to be the only clone recovered from these four mice. One of the five C3H mice produced a positive blood culture for both B. burgdorferi clones, although only one clone type was recovered from all tissue sites sampled at later time points. These results indicate that either clone is capable of outcompeting the other, although wtB31-kanr was found to outcompete in 4 out of 5 mice in this assay. Moreover, the two mixed B. burgdorferi clones were coculture detectable in only one blood sample out of 30 tissues tested during the course of infection, suggesting a bottleneck either at the time of subcutaneous inoculation or prior to extravascular dissemination. Together, the findings are consistent with the above-described superinfection data and indicate that the two homologous B31 clones are unable to establish culture-detectable cocolonization in immunocompetent mice under the current experimental conditions. These data also highlight the ability of heterologous B. burgdorferi to successfully superinfect a host despite the likelihood that the primary-infecting strain greatly outnumbers the secondary-infecting population.
TABLE 5.
Assessment of coinfection by homologous B. burgdorferi in C3H mice
| Tissue collected (day postinfection) | Presence of virus in C3H mice coinfected with in vitro-grown wtB31-kanr and wtB31-gentra |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| kanr | gentr | kanr | gentr | kanr | gentr | kanr | gentr | kanr | gentr | |
| Blood (7) | − | + | + | − | + | − | + | − | + | + |
| Ear (21) | − | + | + | − | + | − | + | − | + | − |
| Ear (28) | − | + | + | − | + | − | + | − | + | − |
| Heart (28) | − | + | + | − | + | − | + | − | + | − |
| Bladder (28) | − | − | − | − | − | − | − | − | + | − |
| Joint (28) | − | + | + | − | + | − | + | − | + | − |
Positive or negative for the presence of wtB31-kanr (kanr) and/or wtB31-gentr (gentr) in culture from all 5 C3H mice.
A role for VlsE in persistent superinfection by heterologous B. burgdorferi.
B. burgdorferi persistence, a hallmark of mammalian Lyme infection, is provided by gene conversion at the vls locus that results in antigenic variation of the VlsE lipoprotein (49–51). The biological role of VlsE is unknown (21, 22, 24, 52–56), although recent evidence suggests that it functions to protect other B. burgdorferi surface antigens from host antibody recognition (22). Challenge experiments have demonstrated that variable VlsE is critical for establishing murine reinfection by B. burgdorferi, possibly through escape of non-VlsE surface antigens from humoral immune surveillance. Despite its well-known importance for persistent infection and the recent data suggesting a VlsE requirement for murine reinfection (22), a role for VlsE in host superinfection has not been investigated to date. Thus, in order to examine the significance of VlsE in host superinfection, a previously characterized B. burgdorferi clone lacking the vls locus (ΔvlsE) was utilized for superinfection assays by heterologous B. burgdorferi strains (Fig. 1C) (21, 22).
Five 297-infected C3H mice were challenged with wtB31-kanr or the B. burgdorferi ΔvlsE mutant at day 28 postinfection. Similar to experiments described above, there was no culture-detectable spirochetemia in mice at day 7 postchallenge with wtB31-kanr, although this B. burgdorferi clone was capable of superinfecting C3H mice as determined by culture-positive tissues harvested at day 28 (Table 6). Despite the inability of the wtB31-kanr clone to provide detectable spirochetemia in all 297-infected C3H mice by day 7, blood samples from 3 out of 5 mice superinfected with the ΔvlsE mutant provided positive cultures (P < 0.05) (Table 6). As expected, the ΔvlsE clone was not detected by culture at days 14, 21, and 28 in ear biopsy specimens and other tissues (heart, bladder, and joint) harvested at day 28 postsuperinfection, indicating that the mice had cleared infection by the VlsE-deficient clone. Taken together, the data demonstrate that superinfection of immunocompetent mice by a heterologous B. burgdorferi strain does not require the presence of VlsE, while persistent secondary infection is absolutely dependent on antigenically variable VlsE.
TABLE 6.
Assessment of superinfection by B. burgdorferi ΔvlsE mutant in C3H mice
| Tissue collected (day postchallenge) | 297-infected C3H mice superinfected via tissue transplantation witha: |
|
|---|---|---|
| hab wtB31-kanr | ha ΔvlsE mutant | |
| Blood (7) | 0/5 | 3/5 |
| Ear (14) | 0/5 | 0/5 |
| Ear (21) | 0/5 | 0/5 |
| Tissues (28) | ||
| Ear | 1/5 | 0/5 |
| Heart | 3/5 | 0/5 |
| Bladder | 4/5 | 0/5 |
| Joint | 4/5 | 0/5 |
| Total no. of infected micec (28) | 4/5 | 0/5 |
Values listed correspond to numbers of cultures positive/numbers tested.
ha, host-adapted clone.
Based on combined results obtained from culturing ear, heart, bladder and tibiotarsal joint.
DISCUSSION
The present study has attempted to examine the capacity of homologous B. burgdorferi to superinfect a murine host and the immune barriers that act to prevent superinfection. The findings reported here demonstrate that homologous B. burgdorferi is unable to effectively coinfect or superinfect an immunocompetent mouse host. The inability of in vitro-grown B31-A3 clones to establish culture-detectable coinfection may pose a limitation to competitive infection experiments used to assess colonization defects of isogenic B. burgdorferi clones after coinoculation (57). The results also show that host adaptation of spirochetes does not enable homologous B. burgdorferi clones to superinfect C3H mice. This is in contrast to the previous finding that host adaptation allows isogenic B. burgdorferi to successfully reinfect C3H mice (22). Such a discrepancy could be attributed to the difference in the overall strength of host immune responses. In the case of reinfection, the primary-infecting B. burgdorferi ΔvlsE clone was cleared by host antibodies by day 21 postinfection (22); therefore, the overall host immune response most likely would be weaker than that found during persistent infection (58). The findings from the superinfection assays involving various immunodeficient mouse strains suggest that anti-B. burgdorferi host antibodies, both T-cell dependent and independent, are not a restrictive barrier to host superinfection by homologous B. burgdorferi clones.
The presence of immune barriers to superinfection in severely immunodeficient animals also indicates that antibodies developed to OspC, an outer surface protein required for spirochetes to establish infection in a mammal (59–62), are unlikely to represent a limiting factor. Moreover, it has been demonstrated that OspC production is not necessary for establishing murine infection by tissue-transplanted B. burgdorferi (63). Thus, the finding reported here that host-adapted homologous B. burgdorferi could not successfully superinfect mice also casts doubt on the idea that anti-OspC antibodies present a barrier. Overall, the inability of homologous B. burgdorferi to superinfect animals that lack antibody suggests that components of the host innate immune system serve as obstacles to superinfecting B. burgdorferi. Thus, it is feasible that other immune responses to OspC are responsible for preventing intrastrain superinfection, and experiments are under way in an attempt to address this possibility.
Murine innate immunity as a potential barrier against intrastrain superinfection.
NOD mice that are lacking in both antibody and complement were utilized as a model in order to examine if the host innate system constitutes a barrier to superinfection by homologous B. burgdorferi. Results from superinfection assays show that these NOD mice indeed can be superinfected by homologous clones of wild-type B. burgdorferi. Inefficient clearance by innate immune cells in liver and spleen (e.g., resident macrophages) due to the absence of C5-mediated opsonization (64) may explain transient detection of intrastrain superinfection in the murine host. However, the data also demonstrate that superinfecting spirochetes are not able to colonize tissues (ear, heart, bladder, and joint) in 6 out of 7 bacteremic mice, suggesting a lack of tissue dissemination by the superinfecting B. burgdorferi. Superinfecting heterologous B. burgdorferi also displayed an inability to disseminate and colonize these same tissue sites in NOD mice. This is in contrast to results from superinfection of SCID mice by the same heterologous B. burgdorferi strains that showed successful dissemination and tissue colonization by secondary-infecting spirochetes. These contradictory findings suggest that some immune component(s) specific to NOD mice is responsible for preventing tissue colonization by superinfecting B. burgdorferi. NOD mice have been shown to have elevated macrophage and granulocytes levels (41), which implicates one or both as a potential preventive agent. In addition, the fact that intrastrain superinfection was prevented in 3 out of 4 NOD mice by SCID mouse-derived immune sera indicates that relevant factors in these sera likely are nonantibody components of the humoral innate immune response (65). The failure of this passive transfer to block superinfection by homologous B. burgdorferi in all four mice could be attributed to an inadequate (suboptimal) amount of immune sera being used. Further experiments delineating a role of the innate system in the prevention of superinfection by homologous B. burgdorferi are warranted and are under way.
It is also possible that the inability of wild-type B. burgdorferi to be cultured from the tissues of NOD mice is an issue of detection. Severely immunodeficient NOD mice may develop a very high burden of B. burgdorferi in their tissues upon initial infection, and this could either block colonization by a superinfecting clone or reduce superinfecting spirochete numbers to below the detection limit for the chosen culture-based method. Even for interstrain superinfection, B. burgdorferi persistence is observed in only 2 out of 4 superinfected NOD mice (Table 3), whereas superinfecting B. burgdorferi was able to establish persistence in 8 out of 10 C3H mice (Tables 3 and 6) and 4 out of 5 SCID mice (Table 3). However, the data also show that uncolonized bladders in C3H mice inoculated with bladder-colonizing-defective B. burgdorferi are unable to be occupied by homologous wild-type B. burgdorferi. The inability to populate bladder tissue by superinfecting homologous B. burgdorferi suggests that any lack of colonizable niches is not a restrictive factor for superinfection by homologous spirochetes. Additionally, the results from the coinfection assay showed that homologous B31 clones generally are unable to establish culture-detectable cocolonization in C3H mice. This further indicates that competition for available host tissue due to the original infecting population outnumbering the secondary spirochete population does not explain the failure of isogenic B. burgdorferi clones to establish host superinfection. Regardless, the present findings suggest that the host innate immune system represents a barrier to superinfection by homologous B. burgdorferi. The possibility that innate immunity can prevent homologous, but not heterologous, B. burgdorferi infection is surprising considering that the innate system is not thought to be able to differentiate between variations that exist among heterologous bacterial strains. However, as an example, various B. burgdorferi strains may simply differ in their ability to bind host complement negative regulators (e.g., factor H and factor H-like proteins) (66–68) and/or express their own functional complement inhibitors (e.g., CspA) (69). Thus, a disparate ability of a B. burgdorferi strain to inhibit overall activation of the innate system may ultimately predetermine the partial success of host superinfection.
Consistent with previous studies (17, 19), data presented here have demonstrated that B. burgdorferi B31 is able to superinfect both immunodeficient and immunocompetent mice originally infected with the heterologous 297 B. burgdorferi strain. Interestingly, the tissues from SCID and C3H mice were culture positive for superinfecting spirochetes only at later time points (day 28), suggesting that superinfecting spirochetes were below the detectable level during the early phases of superinfection. In contrast to SCID or C3H mice, NOD mice exhibited culture-detectable spirochetemia at day 7 postsuperinfection, further indicating that innate immunity is responsible for the low or absent numbers of superinfecting B. burgdorferi in the blood of SCID and C3H mice.
Importance of VlsE variability for persistent B. burgdorferi interstrain superinfection.
Previous data demonstrated the absolute requirement of variable VlsE for reinfection of mice by B. burgdorferi (22). However, reinfection assays in that study involved only prior exposure to VlsE or other Borrelia surface antigens. Variant VlsE-reactive antibody responses are consistently detected in human and animal patients with active Lyme disease (70–73). Thus, the current study takes into account the ongoing anti-B. burgdorferi antibody response that is expected to be stronger than that found after infection resolution (58).
The data presented here suggest that VlsE is also a specific target of the host antibody response during superinfection. Unlike wtB31-kanr, the ΔvlsE clone exhibited the capacity to establish spirochetemia in immunocompetent C3H mice persistently infected with 297. Thus, the ability of the ΔvlsE clone to establish spirochetemia in an infected immunocompetent host indicates that the antibody response against primary-infecting B. burgdorferi is directed mainly to VlsE, with antibodies against non-VlsE antigens potentially generated at subdominant levels. Despite the absence of spirochetes in blood, superinfecting wtB31-kanr B. burgdorferi was detectable from other tissue sites, indicating an ability of heterologous wild-type B. burgdorferi to disseminate and colonize occupied niches of the infected murine host. Given that spirochetes presumably are required to enter the bloodstream in order to travel to distal tissue sites, this result suggests that superinfecting wtB31-kanr is not completely prevented from transiently occupying blood. Rather, the titer of blood-residing spirochetes might simply be maintained at undetectable levels. Thus, during interstrain superinfection, host anti-B. burgdorferi antibodies seem to be responsible for primarily targeting B. burgdorferi that specifically expresses variable VlsE, inevitably selecting for spirochetes with novel VlsE variants that lead to persistent superinfection.
The absence of culture-detectable wtB31-kanr in blood from 297-infected C3H mice, as opposed to mutant ΔvlsE-induced spirochetemia, also suggests that any anti-297 VlsE antibodies are cross-reactive with B31 VlsE. Cross-reactivity of anti-VlsE antibodies has been noted for human sera from patients infected with different B. burgdorferi species (74). However, a recent study (75) showed that immune sera from Peromyscus leucopus infected with various B. burgdorferi strains contained no cross-reactive anti-VlsE antibodies compared to the more broadly cross-reactive immunoglobulins observed in laboratory mice and other animals (71, 76, 77), suggesting that the repertoire of anti-VlsE antibodies is more limited during infection of the natural reservoir host. Additionally, repertoire differences can result from diversity among the vls silent cassette sequences of heterologous B. burgdorferi strains due to the recently reported evolvability of the B. burgdorferi vls system (78). Experiments utilizing Peromyscus mice challenged by tick-derived secondary B. burgdorferi clones are ongoing to address whether the limited anti-VlsE repertoire affects the outcome of host superinfection.
Innate immunity as a selective driving force for B. burgdorferi heterogeneity.
In nature, B. burgdorferi is propagated in a life cycle that involves an arthropod vector and mammalian reservoir host (6, 11, 12). The capacity to superinfect may provide heterologous B. burgdorferi with an advantage for being maintained in the enzootic cycle, especially in ecological situations when naive Peromyscus mice populations are of limited availability. The data presented here suggest that murine innate immunity is a barrier to superinfection by homologous, but not heterologous, B. burgdorferi. The host innate system, specifically complement, has been shown to be able to affect the outcome of infection by different B. burgdorferi strains (13, 38, 40, 79). Thus, it is plausible that during superinfection, innate immunity in a reservoir host could act as a selective driving force for B. burgdorferi heterogeneity.
The simplified schematic shown in Fig. 2 illustrates this selection process. In this model, the innate immune response of a persistently infected mouse impedes secondary infection by spirochetes that are homologous to primary-infecting B. burgdorferi. In contrast, heterogeneous B. burgdorferi has the capacity to overcome this innate barrier, as demonstrated by findings from the present and previous work (17, 19), and eventually establish superinfection. While the data presented here demonstrate that both wild-type B31 and the ΔvlsE clone have the capacity to superinfect 297-infected C3H mice, only the wild-type clone is able to persist. In nature, this is likely facilitated through the limited vlsE repertoire of different B. burgdorferi strains that has been shown to lead to non-cross-reactive anti-VlsE antibodies during infection of Peromyscus mice (75). Thus, once superinfection has been initially established, VlsE variation is required for superinfecting B. burgdorferi to maintain persistence. Ultimately, only genetically diversified B. burgdorferi strains that have the capacity to overcome both innate and acquired immune responses will be the most likely candidates for the continuation of the B. burgdorferi life cycle.
FIG 2.
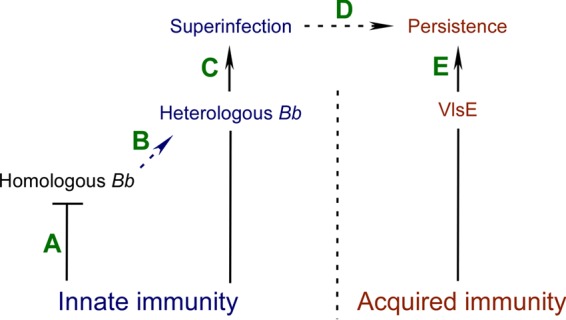
Innate immunity as a driving force for B. burgdorferi heterogeneity during superinfection. (A) Innate immunity of a persistently infected mouse host (represented by the “T” end of the line) blocks secondary-infecting spirochetes that are homologous to initial-infecting B. burgdorferi. (B) Immune pressure mediated by the host innate response acts as a driving force for selection of heterologous B. burgdorferi. (C) In turn, only heterologous B. burgdorferi has the capacity to overcome this innate barrier (represented by the arrow end of the line) and establish superinfection. (D and E) Initially, VlsE is not involved in this selection process. However, variable VlsE is required for the evasion of a host-acquired immune response that leads to persistent B. burgdorferi superinfection. The established persistence of secondary-infecting B. burgdorferi in the reservoir host increases the likelihood of transmitting a selected B. burgdorferi heterogeneity to seasonally available questing ticks.
Superinfection as a driver for genomic diversification has been proposed for another antigenically variant pathogen, Anaplasma marginale (80, 81). Strain superinfection by A. marginale is shown to be associated with a substantial increase in variant diversity of major surface protein 2 (MSP2) (81). A unique MSP2 allelic repertoire of A. marginale has been shown to predetermine the success of superinfection (56, 80, 81). However, in contrast to antibody-mediated selection for A. marginale, the primary driving force for B. burgdorferi heterogeneity during superinfection seems to be host innate immunity. In either case, superinfection could be an important selective process for diversification of bacterial pathogens, during which multiple bacterial strains with different degrees of fitness might be retained (56). This selective pressure may also partially explain the high levels of recombinational rearrangements exhibited by Lyme Borrelia that has led to the described flux state of the B. burgdorferi genome (82–84).
In conclusion, the present study demonstrates for the first time that spirochete heterogeneity is required for the ability of B. burgdorferi to superinfect a host. Moreover, extensive use of various murine models and B. burgdorferi mutants indicates that some component(s) of the murine innate system is partially accountable for preventing superinfection and suggests a role for innate immunity as a driving force for genomic diversification of B. burgdorferi. Although the relevance of superinfection to the life cycle of the Lyme pathogen is not yet known, future studies of the mechanism behind the immune barriers to secondary infection will allow for a better understanding of immune evasion by Borrelia species and may provide insight into the importance of superinfection for the evolution of this important pathogen in nature.
Supplementary Material
ACKNOWLEDGMENTS
We thank Tim Casselli, Allison James, Petronella Hove, and Yvonne Tourand for critically reviewing the manuscript.
This work was supported by an intramural grant from the College of Veterinary Medicine at Washington State University.
Footnotes
Published ahead of print 11 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01817-14.
REFERENCES
- 1.Ogden NH, Lindsay LR, Morshed M, Sockett PN, Artsob H. 2009. The emergence of Lyme disease in Canada. CMAJ 180:1221–1224. 10.1503/cmaj.080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steere AC, Coburn J, Glickstein L. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093–1101. 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi T, Kilpatrick AM, Mangel M, Wilmers CC. 2012. Deer, predators, and the emergence of Lyme disease. Proc. Natl. Acad. Sci. U. S. A. 109:10942–10947. 10.1073/pnas.1204536109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connell S. 2010. Lyme borreliosis: current issues in diagnosis and management. Curr. Opin. Infect. Dis. 23:231–235. 10.1097/QCO.0b013e32833890e2. [DOI] [PubMed] [Google Scholar]
- 5.Heymann WR, Ellis DL. 2012. Borrelia burgdorferi infections in the United States. J. Clin. Aesthet. Dermatol. 5:18–28. [PMC free article] [PubMed] [Google Scholar]
- 6.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10:87–99. 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray TS, Shapiro ED. 2010. Lyme disease. Clin. Lab. Med. 30:311–328. 10.1016/j.cll.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tibbles CD, Edlow JA. 2007. Does this patient have erythema migrans? JAMA 297:2617–2627. 10.1001/jama.297.23.2617. [DOI] [PubMed] [Google Scholar]
- 9.Marques AR. 2010. Lyme disease: a review. Curr. Allergy Asthma Rep. 10:13–20. 10.1007/s11882-009-0077-3. [DOI] [PubMed] [Google Scholar]
- 10.Marques A. 2008. Chronic Lyme disease: a review. Infect. Dis. Clin. North Am. 22:341–360. 10.1016/j.idc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rand PW, Lacombe EH, Smith RP, Jr, Rich SM, Kilpatrick CW, Dragoni CA, Caporale D. 1993. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetares: Spirochaetaceae) in the wild. J. Med. Entomol. 30:614–618. [DOI] [PubMed] [Google Scholar]
- 12.Peavey CA, Lane RS. 1995. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J. Parasitol. 81:175–178. [PubMed] [Google Scholar]
- 13.Kurtenbach K, Hanincova K, Tsao JI, Margos G, Fish D, Ogden NH. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 4:660–669. 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 14.Guttman DS, Wang PW, Wang IN, Bosler EM, Luft BJ, Dykhuizen DE. 1996. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by single-strand conformation polymorphism analysis. J. Clin. Microbiol. 34:652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Liveris D, Brei B, Wu H, Falco RC, Fish D, Schwartz I. 2003. Real-time PCR for simultaneous detection and quantification of Borrelia burgdorferi in field-collected Ixodes scapularis ticks from the northeastern United States. Appl. Environ. Microbiol. 69:4561–4565. 10.1128/AEM.69.8.4561-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmeister EK, Glass GE, Childs JE, Persing DH. 1999. Population dynamics of a naturally occurring heterogeneous mixture of Borrelia burgdorferi clones. Infect. Immun. 67:5709–5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, McKenna D, Nowakowski J, Nadelman RB, Wormser GP, Schwartz I. 1999. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J. Clin. Microbiol. 37:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derdakova M, Dudioak V, Brei B, Brownstein JS, Schwartz I, Fish D. 2004. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl. Environ. Microbiol. 70:6783–6788. 10.1128/AEM.70.11.6783-6788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139–2150. 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bankhead T, Chaconas G. 2007. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol. Microbiol. 65:1547–1558. 10.1111/j.1365-2958.2007.05895.x. [DOI] [PubMed] [Google Scholar]
- 22.Rogovskyy AS, Bankhead T. 2013. Variable VlsE is critical for host reinfection by the Lyme disease spirochete. PLoS One 8:e61226. 10.1371/journal.pone.0061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casselli T, Tourand Y, Bankhead T. 2012. Altered murine tissue colonization by Borrelia burgdorferi following targeted deletion of linear plasmid 17-carried genes. Infect. Immun. 80:1773–1782. 10.1128/IAI.05984-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 97:13865–13870. 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barthold SW. 1993. Antigenic stability of Borrelia burgdorferi during chronic infections of immunocompetent mice. Infect. Immun. 61:4955–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawabata H, Norris SJ, Watanabe H. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 72:7147–7154. 10.1128/IAI.72.12.7147-7154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrenz MB, Kawabata H, Purser JE, Norris SJ. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 70:4798–4804. 10.1128/IAI.70.9.4798-4804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs MB, Norris SJ, Phillippi-Falkenstein KM, Philipp MT. 2006. Infectivity of the highly transformable BBE02− lp56− mutant of Borrelia burgdorferi, the Lyme disease spirochete, via ticks. Infect. Immun. 74:3678–3681. 10.1128/IAI.00043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piesman J, Dolan MC, Happ CM, Luft BJ, Rooney SE, Mather TN, Golde WT. 1997. Duration of immunity to reinfection with tick-transmitted Borrelia burgdorferi in naturally infected mice. Infect. Immun. 65:4043–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Embers ME, Hasenkampf NR, Jacobs MB, Philipp MT. 2012. Dynamic longitudinal antibody responses during Borrelia burgdorferi infection and antibiotic treatment of rhesus macaques. Clin. Vaccine Immunol. 19:1218–1226. 10.1128/CVI.00228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fikrig E, Barthold SW, Kantor FS, Flavell RA. 1990. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250:553–556. 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 32.Fikrig E, Barthold SW, Marcantonio N, Deponte K, Kantor FS, Flavell RA. 1992. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect. Immun. 60:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fikrig E, Barthold SW, Sun W, Feng W, Telford SR, III, Flavell RA. 1997. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531–539. 10.1016/S1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 34.Hanson JM, Goebel JA. 1998. Comparison of manual whole-body and passive and active head-on-body rotational testing with conventional rotary chair testing. J. Vestib. Res. 8:273–282. 10.1016/S0957-4271(97)00071-2. [DOI] [PubMed] [Google Scholar]
- 35.Barthold SW, Bockenstedt LK. 1993. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect. Immun. 61:4696–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKisic MD, Barthold SW. 2000. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect. Immun. 68:5190–5197. 10.1128/IAI.68.9.5190-5197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alitalo A, Meri T, Lankinen H, Seppala I, Lahdenne P, Hefty PS, Akins D, Meri S. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847–3853. 10.4049/jimmunol.169.7.3847. [DOI] [PubMed] [Google Scholar]
- 38.Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, Kraiczy P. 2002. Host association of Borrelia burgdorferi sensu lato–the key role of host complement. Trends Microbiol. 10:74–79. 10.1016/S0966-842X(01)02298-3. [DOI] [PubMed] [Google Scholar]
- 39.Troy EB, Lin T, Gao L, Lazinski DW, Camilli A, Norris SJ, Hu LT. 2013. Understanding barriers to Borrelia burgdorferi dissemination during infection using massively parallel sequencing. Infect. Immun. 81:2347–2357. 10.1128/IAI.00266-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurtenbach K, Sewell HS, Ogden NH, Randolph SE, Nuttall PA. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL. 1995. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154:180–191. [PubMed] [Google Scholar]
- 42.Muller-Eberhard HJ. 1986. The membrane attack complex of complement. Annu. Rev. Immunol. 4:503–528. [DOI] [PubMed] [Google Scholar]
- 43.Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 44.Barthold SW. 1995. Animal models for Lyme disease. Lab. Investig. 72:127–130. [PubMed] [Google Scholar]
- 45.Czub S, Duray PH, Thomas RE, Schwan TG. 1992. Cystitis induced by infection with the Lyme disease spirochete, Borrelia burgdorferi, in mice. Am. J. Pathol. 141:1173–1179. [PMC free article] [PubMed] [Google Scholar]
- 46.Goodman JL, Jurkovich P, Kodner C, Johnson RC. 1991. Persistent cardiac and urinary tract infections with Borrelia burgdorferi in experimentally infected Syrian hamsters. J. Clin. Microbiol. 29:894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petney TN, Hassler D, Bruckner M, Maiwald M. 1996. Comparison of urinary bladder and ear biopsy samples for determining prevalence of Borrelia burgdorferi in rodents in central Europe. J. Clin. Microbiol. 34:1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwan TG, Burgdorfer W, Schrumpf ME, Karstens RH. 1988. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J. Clin. Microbiol. 26:893–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norris SJ. 2006. Antigenic variation with a twist–the Borrelia story. Mol. Microbiol. 60:1319–1322. 10.1111/j.1365-2958.2006.05204.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang JR, Hardham JM, Barbour AG, Norris SJ. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275–285. 10.1016/S0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JR, Norris SJ. 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect. Immun. 66:3698–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawrenz MB, Wooten RM, Norris SJ. 2004. Effects of vlsE complementation on the infectivity of Borrelia burgdorferi lacking the linear plasmid lp28-1. Infect. Immun. 72:6577–6585. 10.1128/IAI.72.11.6577-6585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labandeira-Rey M, Skare JT. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446–455. 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48:753–764. 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 55.Labandeira-Rey M, Seshu J, Skare JT. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608–4613. 10.1128/IAI.71.8.4608-4613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmer GH, Bankhead T, Lukehart SA. 2009. “Nothing is permanent but change”–antigenic variation in persistent bacterial pathogens. Cell Microbiol. 11:1697–1705. 10.1111/j.1462-5822.2009.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bestor A, Rego RO, Tilly K, Rosa PA. 2012. Competitive advantage of Borrelia burgdorferi with outer surface protein BBA03 during tick-mediated infection of the mammalian host. Infect. Immun. 80:3501–3511. 10.1128/IAI.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kannian P, McHugh G, Johnson BJ, Bacon RM, Glickstein LJ, Steere AC. 2007. Antibody responses to Borrelia burgdorferi in patients with antibiotic-refractory, antibiotic-responsive, or non-antibiotic-treated Lyme arthritis. Arthritis Rheum. 56:4216–4225. 10.1002/art.23135. [DOI] [PubMed] [Google Scholar]
- 59.Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. U. S. A. 101:3142–3147. 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart PE, Wang X, Bueschel DM, Clifton DR, Grimm D, Tilly K, Carroll JA, Weis JJ, Rosa PA. 2006. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect. Immun. 74:3547–3553. 10.1128/IAI.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tilly K, Bestor A, Jewett MW, Rosa P. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 75:1517–1519. 10.1128/IAI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554–3564. 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tilly K, Bestor A, Dulebohn DP, Rosa PA. 2009. OspC-independent infection and dissemination by host-adapted Borrelia burgdorferi. Infect. Immun. 77:2672–2682. 10.1128/IAI.01193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ricklin D, Hajishengallis G, Yang K, Lambris JD. 2010. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11:785–797. 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shishido SN, Varahan S, Yuan K, Li X, Fleming SD. 2012. Humoral innate immune response and disease. Clin. Immunol. 144:142–158. 10.1016/j.clim.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haupt K, Kraiczy P, Wallich R, Brade V, Skerka C, Zipfel PF. 2007. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J. Infect. Dis. 196:124–133. 10.1086/518509. [DOI] [PubMed] [Google Scholar]
- 67.Kraiczy P, Skerka C, Brade V, Zipfel PF. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800–7809. 10.1128/IAI.69.12.7800-7809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siegel C, Hallstrom T, Skerka C, Eberhardt H, Uzonyi B, Beckhaus T, Karas M, Wallich R, Stevenson B, Zipfel PF, Kraiczy P. 2010. Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS One 5:e13519. 10.1371/journal.pone.0013519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hallstrom T, Siegel C, Morgelin M, Kraiczy P, Skerka C, Zipfel PF. 2013. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. mBio 4:e00481–13. 10.1128/mBio.00481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lawrenz MB, Hardham JM, Owens RT, Nowakowski J, Steere AC, Wormser GP, Norris SJ. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 37:3997–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang FT, Jacobson RH, Straubinger RK, Grooters A, Philipp MT. 2000. Characterization of a Borrelia burgdorferi VlsE invariable region useful in canine Lyme disease serodiagnosis by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:4160–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner B, Goodman LB, Rollins A, Freer HS. 2013. Antibodies to OspC, OspF and C6 antigens as indicators for infection with Borrelia burgdorferi in horses. Equine Vet. J. 45:533–537. 10.1111/evj.12033. [DOI] [PubMed] [Google Scholar]
- 73.Wagner B, Freer H, Rollins A, Garcia-Tapia D, Erb HN, Earnhart C, Marconi R, Meeus P. 2012. Antibodies to Borrelia burgdorferi OspA, OspC, OspF, and C6 antigens as markers for early and late infection in dogs. Clin. Vaccine Immunol. 19:527–535. 10.1128/CVI.05653-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goettner G, Schulte-Spechtel U, Hillermann R, Liegl G, Wilske B, Fingerle V. 2005. Improvement of Lyme borreliosis serodiagnosis by a newly developed recombinant immunoglobulin G (IgG) and IgM line immunoblot assay and addition of VlsE and DbpA homologues. J. Clin. Microbiol. 43:3602–3609. 10.1128/JCM.43.8.3602-3609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baum E, Hue F, Barbour AG. 2012. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. mBio 3:e00434–00412. 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bacon RM, Biggerstaff BJ, Schriefer ME, Gilmore RD, Jr, Philipp MT, Steere AC, Wormser GP, Marques AR, Johnson BJ. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187–1199. 10.1086/374395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, Philipp MT. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J. Clin. Microbiol. 37:3990–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graves CJ, Ros VID, Stevenson B, Sniegowski PD, Brisson D. 2013. Natural selection promotes antigenic evolvability. PLoS Pathog. 9:e1003766. 10.1371/journal.ppat.1003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491–497. 10.1128/IAI.70.2.491-497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Futse JE, Brayton KA, Dark MJ, Knowles DP, Jr, Palmer GH. 2008. Superinfection as a driver of genomic diversification in antigenically variant pathogens. Proc. Natl. Acad. Sci. U. S. A. 105:2123–2127. 10.1073/pnas.0710333105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ueti MW, Tan Y, Broschat SL, Castaneda Ortiz EJ, Camacho-Nuez M, Mosqueda JJ, Scoles GA, Grimes M, Brayton KA, Palmer GH. 2012. Expansion of variant diversity associated with a high prevalence of pathogen strain superinfection under conditions of natural transmission. Infect. Immun. 80:2354–2360. 10.1128/IAI.00341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Casjens S, Palmer N, Van Vugt R, Huang WH, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516. 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 83.Chaconas G, Kobryn K. 2010. Structure, function, and evolution of linear replicons in Borrelia. Annu. Rev. Microbiol. 64:185–202. 10.1146/annurev.micro.112408.134037. [DOI] [PubMed] [Google Scholar]
- 84.Kobryn K, Chaconas G. 2005. Fusion of hairpin telomeres by the B. burgdorferi telomere resolvase ResT: implications for shaping a genome in flux. Mol. Cell 17:783–791. 10.1016/j.molcel.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 85.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733–740. 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


