Abstract
Staphylococcus aureus bloodstream infection (bacteremia) is a major cause of morbidity and mortality and places substantial cost burdens on health care systems. The role of peripheral blood dendritic cells (PBDCs) in the immune responses against S. aureus infection has not been well characterized. In this study, we demonstrated that BDCA1+ myeloid DCs (mDCs) represent a unique PBDC subset that can induce immune responses against S. aureus infection. BDCA1+ mDCs could engulf S. aureus and strongly upregulated the expression of costimulatory molecules and production of proinflammatory cytokines. Furthermore, BDCA1+ mDCs expressed high levels of major histocompatibility complex (MHC) class I and II molecules in response to S. aureus and greatly promoted proliferation and gamma interferon (IFN-γ) production in CD4 and CD8 T cells. Moreover, BDCA1+ mDCs expressed higher levels of Toll-like receptor 2 (TLR-2) and scavenger receptor A (SR-A) than those on CD16+ and BDCA3+ mDCs, and these two receptors were both required for the recognition of S. aureus and the subsequent activation of BDCA1+ mDCs. Finally, BDCA1+ mDC-mediated immune responses against S. aureus were dependent on MyD88 signaling pathways. These results demonstrate that human BDCA1+ mDCs represent a unique subset of mDCs that can respond to S. aureus to undergo maturation and activation and to induce Th1 and Tc1 immune responses.
INTRODUCTION
Staphylococcus aureus is one of the common causes of nosocomial and community-acquired bloodstream infections in the world (1). After entering the bloodstream, S. aureus establishes infection and disseminates to almost all organs. Consequently, S. aureus bacteremia, which is the presence of bacteria in the blood, is often associated with serious metastatic complications, including endocarditis, osteomyelitis, and sepsis, with a mortality rate of 20 to 30% (2, 3). The infection is extremely hard to treat, requiring prompt source control and, often, prolonged antimicrobial therapy (4). Furthermore, the growing prevalence of antibiotic-resistant strains, together with the increase in the number of patients with a compromised immune status because of immune suppression after transplantation, cancer chemotherapy, or HIV infection, has led to a significant increase in the incidence of S. aureus bacteremia (5, 6).
A clinically significant bacteremia is generally defined as the isolation of bacteria from one or more peripheral venous blood culture samples collected from a patient with associated relevant clinical symptoms of systemic infection (7). The rampant dissemination of S. aureus in almost any organ increases the difficulty of antimicrobial therapies, as inappropriate therapies can lead to failures of treatment and to greater mortality (8). For these reasons, rapid diagnosis and new effective therapeutic strategies are required to better control S. aureus bloodstream infections. Therapeutic approaches aiming at enhancing the efficiency of the immune activation specific to S. aureus may represent the best options. To this end, it is pivotal to understand the immune responses involved in host defense against S. aureus infection.
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) and key modulators of T and B cell immunities, mainly owing to their superior abilities to take up and present antigens (Ags) (9, 10). The nature of the immune response to a given pathogen is tightly regulated by the DC network, which consists of multiple subsets that are equipped with unique pattern recognition receptors (PRRs) and are endowed with specialized functions (9). Human peripheral blood DCs (PBDCs) account for >1% of circulating peripheral blood mononuclear cells (PBMCs) and are classically defined as Ag-presenting leukocytes that lack other leukocyte lineage markers and express high levels of major histocompatibility complex (MHC) class II molecules (11). The PBDCs are categorized into two main groups: CD11c− CD123+ plasmacytoid DCs (pDCs) and CD11c+ CD123inter myeloid DCs (mDCs). More recently, mDCs were further divided into three phenotypically distinct subsets, defined by their expression of CD1c (BDCA1), CD16, and CD141 (BDCA3) (12). Promising DC-based therapeutic trials have been reported to treat malignancies and infections (13, 14), but the majority of these trials use in vitro-generated, monocyte-derived DCs (MDDCs), and the physiological relevance of this DC subtype is currently unclear.
A recent report showed that DCs are crucial players in the host immune response to S. aureus bloodstream infection in a mouse model (15). However, there is limited knowledge about how human blood DCs respond to S. aureus. Moreover, the infection route of S. aureus in bacteremia is the bloodstream, but the role of PBDC subsets has not been investigated. Therefore, understanding the emerging complexities of human DC subsets and their respective functions is essential for the development of new therapeutics to treat S. aureus bacteremia by targeting DCs. In this study, we investigated the responses of highly purified BDCA1+, CD16+, and BDCA3+ human blood mDC subsets to S. aureus and revealed marked differences in these responses.
MATERIALS AND METHODS
Ethics statement.
This study was conducted according to the principles expressed in the Declaration of Helsinki. Peripheral blood was obtained from healthy donor volunteers (39 males and 14 females of 20 to 35 years of age, with no clinical signs of inflammation) at the Shanghai Public Health Clinical Center. The Institutional Review Board of the Shanghai Public Health Clinical Center approved this study (IRB number 2012ZX09303013). Written informed consent was obtained from all volunteers.
Chemicals and antibodies.
Fluorescence-conjugated antibodies (Abs) with the following specificities were used for staining: isotype control Abs (IgG1, IgG2a, and IgG2b), anti-BDCA1–allophycocyanin (APC)–Cy7 (IgG1; L161), anti-CD11c–APC or –phycoerythrin (PE)–Cy7 (IgG1; 3.9), anti-CD16–PE (IgG1; 3G8), anti-BDCA3–peridinin chlorophyll protein (PerCP)–Cy5.5 (IgG1; M80), anti-CD123–PE–Cy7 (IgG1; 6H6), anti-CD83–fluorescein isothiocyanate (FITC) (IgG1; HB15e), anti-CD86–APC (IgG2b; IT2.2), anti-CD4–Pacific Blue (IgG2b; OKT4), anti-CD8–APC (IgG1; HIT8a), anti-HLA-A,B,C–FITC (IgG2a; W6/32), and anti-gamma interferon (anti-IFN-γ)–Alexa488 (IgG1; 4S.B3) were obtained from Biolegend, and anti-HLA-DR,-DP,-DQ–FITC Ab (IgG1; Tu39) was purchased from BD Biosciences (San Diego, CA). The following neutralizing antibodies were used to block cytokine activity. Abs against human CD11b (IgG1; ICRF44), Toll-like receptor 2 (TLR-2) (IgG2a; TL2.1), and TLR-4 (IgG2a; HTA125) were purchased from Biolegend. An Ab against scavenger receptor A (SR-A) (IgG1; E-10) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). PKH26 red and PKH67 green fluorescent cell linkers were purchased from Sigma-Aldrich. An MyD88 inhibition peptide and control peptide were obtained from InvivoGen (San Diego, CA).
Bacteria.
S. aureus strain SH1000 wild-type cells were cultured in brain heart infusion broth (Sigma-Aldrich, St. Louis, MO) for 16 h at 37°C in air with shaking (180 rpm). Bacteria were pelleted by centrifugation, washed with phosphate-buffered saline (PBS), and centrifuged again before a final resuspension in PBS to an optical density at 600 nm (OD600) of 1.0. The number of viable bacteria was determined after serial diluting and plating onto agar containing 5% sheep blood (InvitroGen, San Diego, CA).
Isolation of PBDCs.
PBDCs were isolated from PBMCs by depletion of lineage-positive mononuclear cells followed by magnetic bead labeling as previously described (16), with minor modifications. Briefly, PBMCs were incubated with a cocktail of mouse anti-human monoclonal Abs (MAbs) containing Abs to CD3, CD14, CD19, CD20, CD56, CD34, and CD235a in cold PBS. After washing, cells were incubated with anti-mouse IgG microbeads to deplete the Ab-labeled cells. BDCA1+ mDCs were isolated from these PBMCs by positive selection using BDCA1+ DC isolation kits (Miltenyi Biotec, Auburn, CA). CD16+ and BDCA3+ mDC subsets were further isolated from PBDCs by positive selection or electronic sorting on a FACSAria II flow cytometer (Becton Dickinson, Franklin Lakes, NJ). These procedures routinely yielded blood DC preparations with a purity of >95%.
Bacterial phagocytosis.
S. aureus was labeled with PKH26, a cell-tracking and -labeling red fluorescence dye. Isolated PBDCs were preincubated with Fc receptor blocking solution for 30 min (Biolegend), and then cells were cultured at 4 or 37°C with PKH26-labeled S. aureus (ratio of 1:50) in culture medium (RPMI 1640 medium with 5% autologous serum) for 1 h, washed three times in ice-cold PBS–0.5% autologous serum, and then analyzed by FACSAria II flow cytometry. For confocal analysis, BDCA1+ mDCs that were labeled with PKH67, a cell-tracking and -labeling green fluorescence dye, were cultured with PKH26-labeled S. aureus for 1 h and then spun down on a glass slide by cytospin centrifugation (Shandon, Pittsburgh, PA). The samples were then mounted using glycerol and analyzed by confocal microscopy (Carl Zeiss LSM 510; Carl Zeiss, Thornwood, NY).
Flow cytometry analysis.
Cells were washed with PBS containing 0.5% autologous serum, preincubated for 30 min with unlabeled isotype control and Fc blocking Abs, and then incubated with fluorescence-conjugated Abs at 4°C for 30 min, followed by washing with PBS. The cells were analyzed on a FACSAria II flow cytometer (Becton Dickinson, Franklin Lakes, NJ) with FlowJo 8.6 software (Tree Star). Cellular debris was excluded from the analysis by gating on forward and side scatter, and dead cells were further excluded by gating on 7-aminoactinomycin D (7AAD)-negative cells. As a control for nonspecific staining, isotype-matched irrelevant MAbs were used.
Real-time RT-PCR.
Total RNA was reverse transcribed into cDNA by use of oligo(dT) and Superscript III (Invitrogen, Carlsbad, CA) or Moloney murine leukemia virus (M-MLV) reverse transcriptase (RT) (Promega, Madison, WI). The cDNA was subjected to real-time PCR amplification (Qiagen, Limburg, Netherlands) for 45 cycles, with an annealing and extension temperature of 60°C, on a Light Cycler 480 real-time PCR system (Roche, Switzerland). Primer sequences were as follows: for interleukin-1β (IL-1β), forward (F) primer 5′-CTCAGGTGTCCTCGAAGAAATCAA-3′ and reverse (R) primer 5′-GCTTTTTTTGCTGTGAGTCCCG-3′; for IL-6, F primer 5′-TTCTCCACAAGCGCCTTCGGTCCA-3′ and R primer 5′-AGGGCTGAGATGCCGTCGAGGATGTA-3′; for IL-12p40, F primer 5′-GTCAGAGGGGACAACAAGGA-3′ and R primer 5′-TGATGAAGAAGCTGCTGGTG-3′; for tumor necrosis factor alpha (TNF-α), F primer 5′-CGAGTGACAAGCCTGTAGC-3′ and R primer 5′-GGTGTGGGTGAGGAGCACAT-3′, and for IFN-γ, F primer 5′-TGGGTTCTCTTGGCTGTTACTG-3′ and R primer 5′-GAGTTCCATTATCCGCTACATCTG-3′.
Measurement of T cell responses.
mDC subsets and syngeneic CD4 and CD8 T cells from the same patients were purified from PBMCs. CD4 and CD8 T cells were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA). S. aureus-stimulated mDC subsets were treated with 50 μg/ml of mitomycin C for 1 h, after which they were cocultured with CFSE-labeled syngeneic CD4 or CD8 T cells for 4 days at 37°C in RPMI 1640 medium under 5% CO2. The cells were stimulated with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 μM) (both from Calbiochem, La Jolla, CA) for 4 h, with the addition of monensin solution (Biolegend, San Diego, CA) during the final 2 h. Cells were then stained for surface molecules first, fixed, permeabilized with Cytofix/Cytoperm buffer (eBioscience, San Diego, CA), and subsequently incubated with the indicated anti-cytokine antibodies in Perm/Wash buffer (eBioscience, San Diego, CA) for 30 min. The cells were analyzed for proliferation status by CFSE dilution on a FACSAria II flow cytometer.
ELISA.
The IL-1β, IL-6, IL-10, IL-12p70, and TNF-α concentrations in the culture supernatants were measured in triplicate by use of standard enzyme-linked immunosorbent assay (ELISA) kits (Biolegend, San Diego, CA), with standard cytokine preparations used as internal controls.
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEM). The statistical significance of differences between experimental groups was calculated using analysis of variance with a Bonferroni posttest or unpaired Student's t test. All P values of <0.05 were considered significant.
RESULTS
BDCA1+ mDCs are a unique phagocytic DC subset responsive to S. aureus.
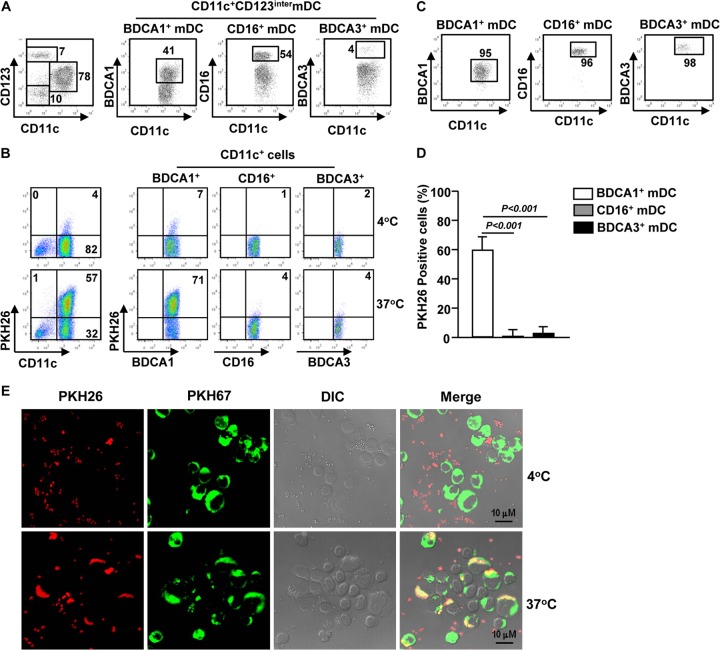
A recent report showed that DCs are crucial players in the host immune response to S. aureus bloodstream infection in a mouse model. However, the responses of human blood DCs, especially individual DC subsets, to S. aureus infection have not been investigated. To address this question, we first compared the abilities of human DC subsets to phagocytose S. aureus. We isolated PBDCs ex vivo from blood of healthy donors by depletion of lineage-positive mononuclear cells as described in Materials and Methods (Fig. 1A). PBDCs were cocultured with PKH26-labeled S. aureus to allow detection of cells that had phagocytosed bacteria. As shown by multicolor flow cytometry, more than 60% of BDCA1+ mDCs were positive for PKH26 red fluorescence, which indicates the presence of S. aureus in cells or on the cell surface. For comparison, no more than 5% of CD16+ or BDCA3+ mDCs were positive for PKH26 (Fig. 1B). To further confirm this observation, we purified BDCA1+, CD16+, and BDCA3+ mDC subsets (Fig. 1C) and cultured them with PKH26-labeled S. aureus for 1 h. As shown in Fig. 1D, a large proportion of BDCA1+ mDCs were positive for PKH26, whereas only a few CD16+ or BDCA3+ mDCs were positive for PKH26. To examine the cellular location of PKH26-labeled S. aureus, we performed confocal microscopy and found that PKH26-labeled S. aureus was mostly located in the cytoplasm of the BDCA1+ mDCs (Fig. 1E). Moreover, the BDCA1+ mDCs became PKH26 positive only after coculture with PKH26-labeled S. aureus at 37°C, not 4°C. These results indicate that BDCA1+ mDCs indeed engulfed S. aureus bacteria and demonstrate that BDCA1+ mDCs are a unique phagocytic DC subset in blood mDCs that can respond to S. aureus.
FIG 1.
BDCA1+ mDCs are a unique phagocytic DC subset responsive to S. aureus. (A) BDCA1+, CD16+, and BDCA3+ mDCs were enriched from PBDCs by an initial immunomagnetic depletion of lineage-positive cells by use of anti-human-lineage Abs (against CD3, CD19, CD20, CD34, CD56, and CD235a). The resulting cells were stained for a panel of surface markers and analyzed by flow cytometry. The BDCA1+, CD16+, and BDCA3+ mDC subsets among the CD11c+ CD123inter mDCs are shown. (B) Isolated PBDCs (1 × 106) were incubated with PKH26-labeled S. aureus for 1 h at 4 or 37°C. PKH26 fluorescence signals in the gated mDC subsets, as analyzed by flow cytometry, are shown. Data are representative of analyses of 3 samples. (C) BDCA1+, CD16+, and BDCA3+ mDCs were purified from PBDCs, and their purity was at least 95%, as shown in each graph. (D) The purified PBDC subsets described for panel C were incubated with PKH26-labeled S. aureus for 1 h and then analyzed for the PKH26 fluorescence signal. Data are averages from analyses of 4 samples from 4 donors. (E) PKH67-labeled BDCA1+ mDCs were cultured with PKH26-labeled S. aureus for 1 h and then analyzed for the PKH26 fluorescence signal and its cellular location by confocal microscopy. Images for one representative donor of four are shown.
BDCA1+ mDCs upregulate the expression of costimulatory molecules and the production of proinflammatory cytokines in response to S. aureus.
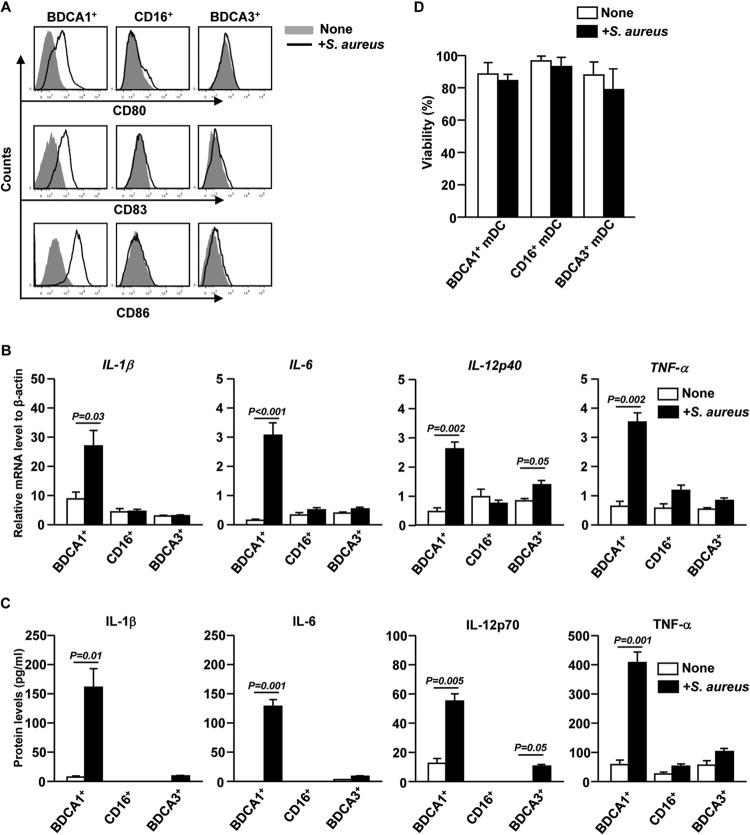
We next compared the activation and maturation of BDCA1+, CD16+, and BDCA3+ mDC subsets in response to S. aureus. Isolated BDCA1+, CD16+, and BDCA3+ mDCs were incubated with S. aureus for 12 h. Expression of the activation markers CD80, CD83, and CD86 on BDCA1+ mDCs was markedly upregulated by incubation with S. aureus, whereas that on CD16+ or BDCA3+ mDCs was not increased (Fig. 2A).
FIG 2.
S. aureus induces activation of BDCA1+ mDCs. (A) Purified BDCA1+, CD16+, and BDCA3+ mDCs were cocultured with S. aureusfor 12 h. CD80, CD83, and CD86 expression was measured by flow cytometry. The results are representative of 4 independent experiments with cells from 4 donors. (B) IL-1β, IL-6, IL-12p40, and TNF-α mRNA levels in S. aureus-stimulated PBDC subsets, presented relative to the β-actin mRNA level. Data are averages from analyses of 4 samples from 4 donors. (C) Concentrations of IL-1β, IL-6, IL-12p70, and TNF-α in culture media of S. aureus-activated BDCA1+, CD16+, and BDCA3+ mDCs, as measured by ELISA. Data are averages from analyses of 4 samples from 4 donors for each group. (D) Cell viability of S. aureus-treated DC subsets was measured by annexin V-FITC and propidium iodide staining. Data are averages from analyses of 3 samples from 3 donors.
We next examined whether mDC subsets can produce proinflammatory cytokines in response to S. aureus. Isolated mDC subsets were stimulated with S. aureus for 2 h, and cytokine mRNA levels were measured. S. aureus stimulation of BDCA1+ mDCs led to a marked increase in IL-1β, IL-6, IL-12p40, and TNF-α mRNA levels (Fig. 2B). Consistent with the mRNA levels, the protein concentrations of IL-1β, IL-6, IL-12p70, and TNF-α were dramatically increased in the supernatants of BDCA1+ mDCs cocultured with S. aureus for 12 h (Fig. 2C). In addition, BDCA3+ mDCs also upregulated IL-12p40 mRNA and IL-12p70 protein levels when stimulated by S. aureus, although both these levels were lower than those in BDCA1+ mDCs (Fig. 2B and C). In comparison, CD16+ mDCs did not upregulate the expression of any of these cytokines in response to S. aureus (Fig. 2B and C). Moreover, S. aureus did not markedly alter the viability of mDC subsets (Fig. 2D), suggesting that cell death was not a major contributing factor to the S. aureus-induced inflammatory response in BDCA1+ mDCs. These results suggest that S. aureus treatment induces upregulation of costimulatory molecules and proinflammatory cytokines in BDCA1+ mDCs, which exhibit phenotypic and functional characteristics of mature DCs.
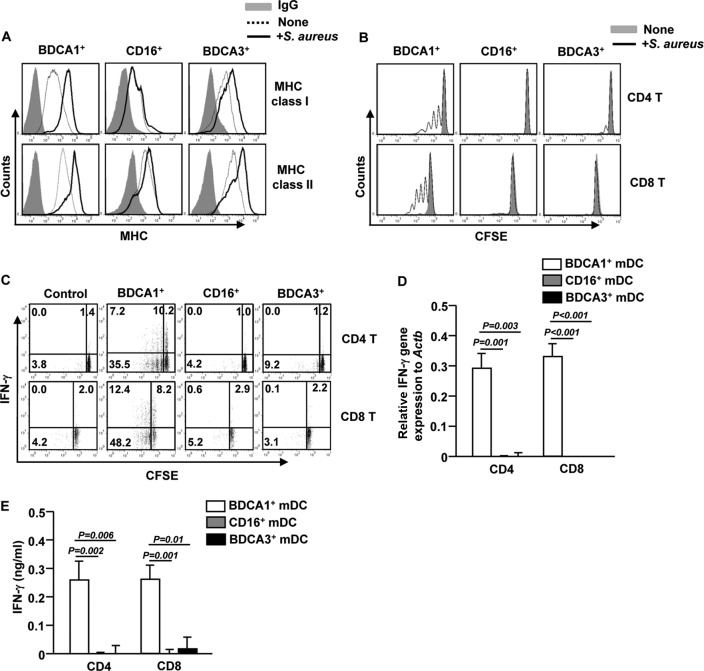
S. aureus-activated BDCA1+ mDCs promote expansion of IFN-γ-producing CD4 and CD8 T cells.
Recent studies have shown that BDCA3+ mDCs are specialized for facilitating CD8 T cell activation and proliferation (17–19). However, as we observed, the failure of BDCA3+ mDCs to ingest S. aureus suggests that they are not responsible for processing and presentation of bacterial Ags to T cells. Therefore, we compared the abilities of S. aureus-activated BDCA1+, CD16+, and BDCA3+ mDCs to induce proliferation and cytokine production of syngeneic T cells. S. aureus stimulation for 12 h led to substantial upregulation of MHC class I and II expression on BDCA1+ and BDCA3+ DCs (Fig. 3A). Next, we cocultured S. aureus-stimulated mDC subsets with syngeneic CD4 or CD8 T cells for 4 days. Proliferation of CD4 or CD8 T cells was markedly increased by S. aureus-activated BDCA1+ mDCs, as indicated by the CFSE labeling assay (Fig. 3B). Moreover, S. aureus-activated BDCA1+ mDCs induced a marked increase in the proportion of CD4 and CD8 T cells that produced IFN-γ, the signature cytokine for Th1 and Tc1 cells (Fig. 3C). In contrast, S. aureus-activated BDCA3+ mDCs were not able to induce proliferation and IFN-γ production in CD4 or CD8 T cells (Fig. 3B and C). To further confirm this observation, we examined IFN-γ mRNA and protein levels from syngeneic CD4 and CD8 T cells that were cocultured with S. aureus-stimulated mDC subsets. Consistent with intracellular IFN-γ production levels, CD4 and CD8 T cells cocultured with S. aureus-activated BDCA1+ mDCs had substantially higher expression levels of IFN-γ mRNA and protein than those cocultured with S. aureus-activated CD16+ or BDCA3+ mDCs (Fig. 3D and E). Collectively, these data demonstrate that BDCA1+ mDCs are the major human DC subset that is responsive to S. aureus with regard to phagocytosis, inflammatory cytokine secretion, and CD4 and CD8 T cell activation.
FIG 3.
S. aureus-activated BDCA1+ mDCs promote CD4 and CD8 T cell activation. (A) Expression levels of MHC class I and II in S. aureus-stimulated BDCA1+, CD16+, and BDCA3+ mDCs. Data for one representative donor of three are shown. The S. aureus-stimulated or unstimulated mDC subsets were cultured together with syngeneic naive CD4 or CD8 T cells for 4 days. (B) CFSE dilution in T cells was analyzed by flow cytometry. (C) Intracellular IFN-γ production was analyzed by flow cytometry of T cells cocultured with unstimulated BDCA1+ mDCs (left panel) or S. aureus-stimulated DC subsets (right three panels). (D) Relative IFN-γ mRNA levels in T cells cocultured with S. aureus-stimulated DC subsets were measured by real-time quantitative PCR (qPCR). (E) IFN-γ concentrations in the culture supernatants were measured by ELISA. The results shown represent the means and SEM for 4 independent experiments with samples from 4 donors.
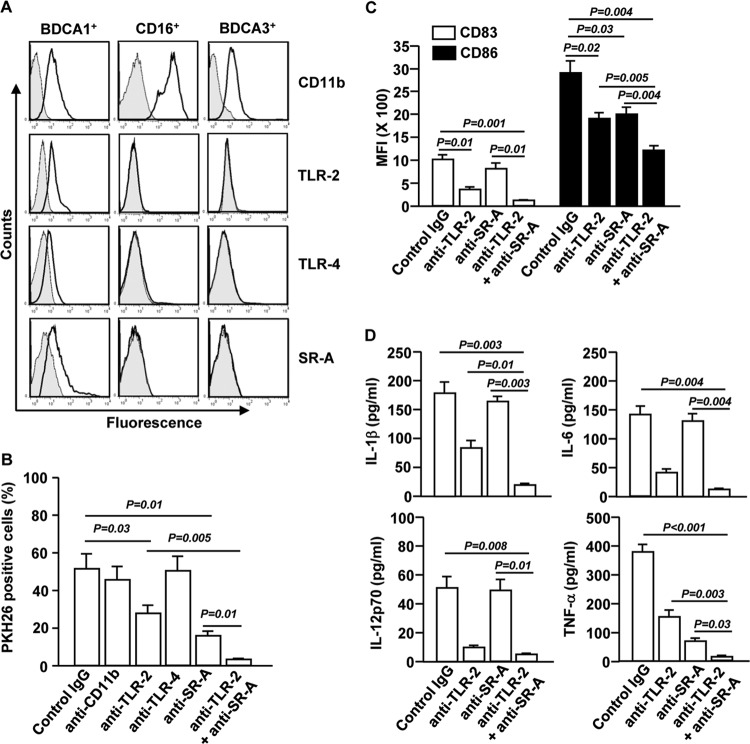
TLR-2 and SR-A contribute to S. aureus-induced BDCA1+ mDC maturation.
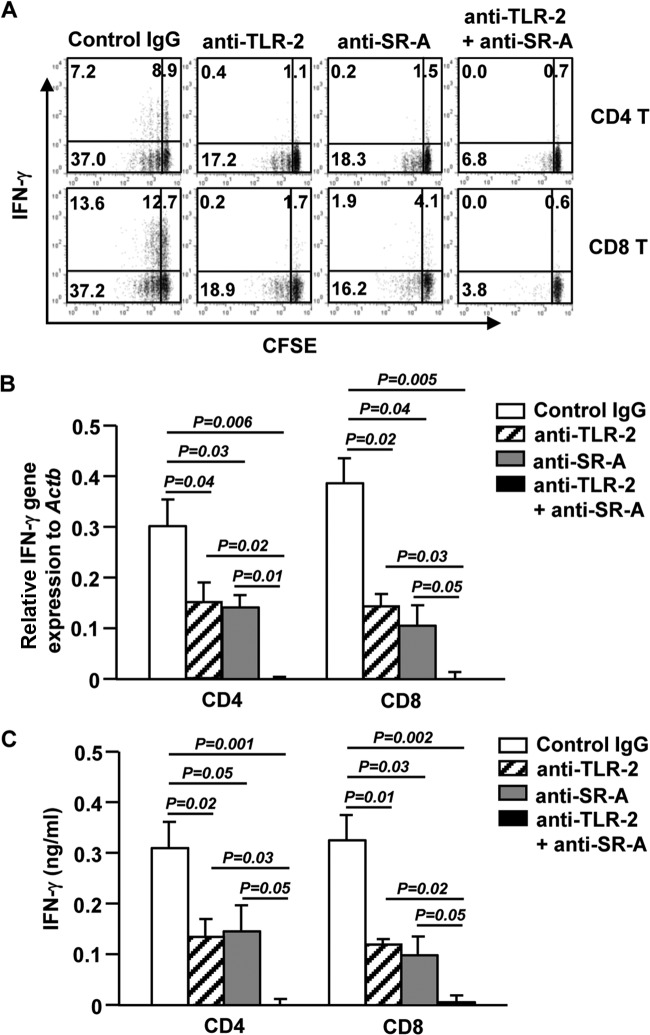
We next asked which phagocytosis receptors mediate S. aureus uptake by BDCA1+ mDCs. First, we compared the expression levels of CD11b, TLR-2, TLR-4, and SR-A in the mDC subsets. BDCA1+ mDCs showed higher levels of TLR-2 and TLR-4 than those in CD16+ and BDCA3+ mDCs. Moreover, consistent with our previous finding (20), BDCA1+ mDCs showed detectable levels of SR-A, whereas the other two subsets did not express this receptor (Fig. 4A). These observations prompted us to evaluate whether neutralization of these receptors can affect phagocytosis of S. aureus by BDCA1+ mDCs. Isolated BDCA1+ mDCs were pretreated with blocking Abs against CD11b, TLR-2, TLR-4, SR-A, or combination of TLR-2 and SR-A for 1 h and then incubated with S. aureus. One hour after incubation with S. aureus, we noticed that blockade of TLR-2 or SR-A partially prevented the phagocytosis of S. aureus by BDCA1+ mDCs (Fig. 4B). Additionally, a combination of anti-TLR-2 and anti-SR-A completely abolished the phagocytosis of S. aureus by BDCA1+ mDCs (Fig. 4B). Moreover, 12 h after stimulation by S. aureus, the induction of costimulatory molecules CD83 and CD86 in BDCA1+ mDCs was significantly decreased by blockade of TLR-2 or SR-A, although blockade of TLR-2 appeared to have a more potent effect than blockade of SR-A on CD83 expression. Furthermore, the combination of anti-TLR-2 and anti-SR-A decreased S. aureus-induced CD83 and CD86 expression more potently than the case with each Ab alone (Fig. 4C). Interestingly, the induction of IL-1β, IL-6, and IL-12p70 expression in S. aureus-stimulated BDCA1+ mDCs was significantly abrogated by anti-TLR-2 but not anti-SR-A (Fig. 4D). However, the inhibition of TNF-α induction by blockade of SR-A was more efficient than that of TLR-2 (Fig. 4D). The combination of anti-TLR-2 and anti-SR-A almost completely abrogated S. aureus-induced production of proinflammatory cytokines by BDCA1+ mDCs, with a greater efficiency than that with each Ab alone (Fig. 4D). These data indicate that S. aureus-induced production of proinflammatory cytokines by BDCA1+ mDCs is crucially dependent on TLR-2, with that of TNF-α also critically dependent on SR-A. Taken together, these results indicate that TLR-2 and SR-A expressed by BDCA1+ mDCs are critical mediators of these cells' phagocytic function and maturation in response to S. aureus and that the presence of both of them achieves the optimal effect.
FIG 4.
Activation of BDCA1+ mDCs in response to S. aureus is dependent on TLR-2 and SR-A. (A) CD11b, TLR-2, TLR-4, and SR-A expression levels on purified BDCA1+, CD16+, and BDCA3+ mDCs. Data for one representative donor of three are shown. (B) BDCA1+ mDCs were pretreated with the indicated neutralizing antibodies and then incubated with PKH26-labeled S. aureus for 1 or 12 h. The percentages of BDCA1+ mDCs that were PKH26 positive are shown. The results shown represent the means and SEM for 3 independent experiments with samples from 3 donors. (C) Mean fluorescence intensities (MFI) of CD83 and CD86 in BDCA1+ mDCs. (D) Concentrations of IL-1β, IL-6, IL-12p70, and TNF-α in culture medium of S. aureus-activated BDCA1+ mDCs, as measured by ELISA. Data are averages from analyses of 3 samples from 3 donors.
The ability of S. aureus-activated BDCA1+ mDCs to promote T cell responses is dependent on TLR-2 and SR-A.
To determine whether TLR-2 and SR-A are required for the ability of S. aureus-activated BDCA1+ mDCs to enhance T cell responses, we stimulated BDCA1+ mDCs with S. aureus in the presence or absence of anti-TLR-2, anti-SR-A, or a combination of both and then cocultured these mDCs with syngeneic CD4 or CD8 T cells for 4 days. The proliferation of CD4 or CD8 T cells was partially inhibited by either anti-TLR-2 or anti-SR-A and was almost completely inhibited by the addition of both Abs together (Fig. 5A). Moreover, the percentage of IFN-γ-producing CD4 and CD8 T cells was reduced by either anti-TLR-2 or anti-SR-A and was more dramatically reduced by the combination of both Abs (Fig. 5A). Similarly, IFN-γ mRNA and protein derived from CD4 and CD8 T cells were markedly decreased by anti-TLR-2 or anti-SR-A and were more potently reduced by the combination of the two Abs (Fig. 5B and C). These results further confirm that TLR-2 and SR-A are essential for the full activation of BDCA1+ mDCs in response to S. aureus, which enables them to induce innate and adaptive immune responses against S. aureus infection.
FIG 5.
The ability of S. aureus-activated BDCA1+ mDCs to promote T cell responses is dependent on TLR-2 and SR-A. BDCA1+ mDCs were stimulated with S. aureus in the presence or absence of anti-TLR-2, anti-SR-A, or anti-TLR-2 plus anti-SR-A antibodies for 12 h and then cocultured with CFSE-labeled syngeneic naive CD4 or CD8 T cells for 4 days. (A) CFSE dilution and intracellular IFN-γ production in CD4 or CD8 T cells were analyzed by flow cytometry. (B) Relative IFN-γ mRNA levels were measured by real-time qPCR. (C) Levels of IFN-γ in culture supernatants were measured by ELISA. Data are averages from analyses of 3 samples from 3 donors.
S. aureus-induced BDCA1+ mDC activation is mediated by the MyD88 signaling pathway.
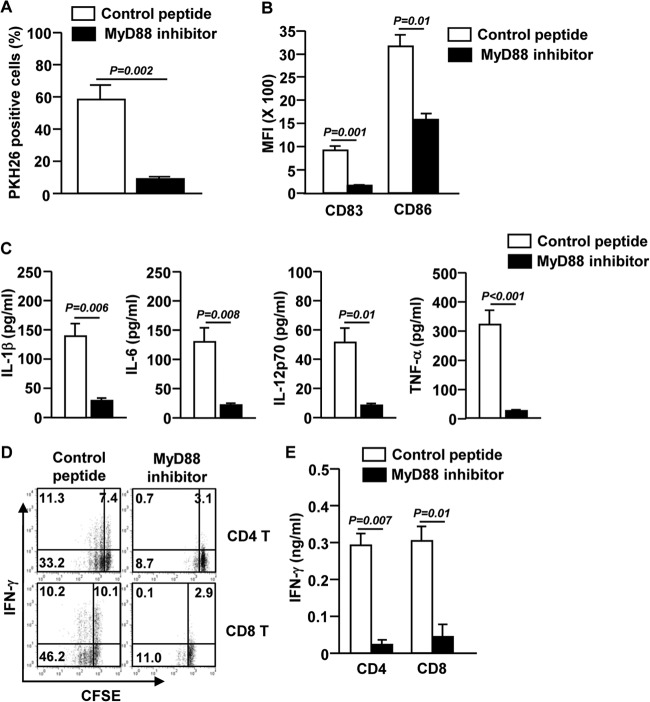
To further clarify the mechanism by which S. aureus promotes maturation of DCs, we examined the role of the MyD88 signaling pathway, a critical pathway activated by TLR stimulation in innate immune cells (21). It has also been reported that MyD88 is required for TLR signal transduction in mouse DCs in response to S. aureus (22, 23). Therefore, we assessed whether the activation of human blood BDCA1+ mDCs by S. aureus is dependent on the MyD88 signaling pathway. We treated BDCA1+ mDCs with a specific MyD88-inhibiting peptide or a control peptide before culturing the cells with S. aureus. BDCA1+ mDCs failed to take up S. aureus in the presence of the MyD88 inhibitor (Fig. 6A). In addition, S. aureus-induced upregulation of CD83 and CD86 was significantly abrogated by the MyD88 inhibitor (Fig. 6B). Moreover, proinflammatory cytokine production in BDCA1+ mDCs induced by S. aureus was also severely impaired by the MyD88 inhibitor (Fig. 6C). Consistent with the impaired BDCA1+ mDC activation, S. aureus-stimulated BDCA1+ mDCs cultured in the presence of the MyD88 inhibitor were unable to promote proliferation and IFN-γ production by syngeneic CD4 and CD8 T cells, as indicated by both intracellular and secreted protein levels (Fig. 6D and E). Thus, these results demonstrate that the MyD88 signaling pathway is essential for S. aureus-induced BDCA1+ mDC activation.
FIG 6.
The MyD88 signaling pathway is essential for S. aureus-induced activation of BDCA1+ mDCs. BDCA1+ mDCs were preincubated with the MyD88 inhibitor or a control peptide for 1 h and then cultured with S. aureus for 1 or 12 h. (A) Percentage of BDCA1+ mDCs that were PKH26+. The results shown represent the means and SEM for 3 independent experiments from 3 donors. (B) Mean fluorescence intensities of CD83 and CD86 in S. aureus-stimulated BDCA1+ mDCs. (C) Levels of IL-1β, IL-6, IL-12p70, and TNF-α in culture medium of S. aureus-stimulated BDCA1+ mDCs were measured by ELISA. Data are averages from analyses of 3 samples from 3 donors. (D) S. aureus-stimulated BDCA1+ mDCs were cocultured with syngeneic CD4 and CD8 T cells for 4 days, and CFSE dilution and intracellular IFN-γ production in T cells were analyzed by flow cytometry. (E) IFN-γ concentrations in culture supernatants were measured by ELISA. Data are representative of or averages from analyses of 3 samples from 3 donors.
DISCUSSION
In this study, we demonstrated for the first time that BDCA1+ mDCs represent a unique blood DC subset that can induce T cell responses against S. aureus infection. BDCA1+ mDCs, but not CD16+ or BDCA3+ mDCs, can engulf S. aureus and strongly upregulate the expression of costimulatory molecules and production of proinflammatory cytokines. A previous study has shown that DCs are central regulatory cells in the immune responses to S. aureus bloodstream infection in a mouse model (15). Consistent with our human PBDC results, infection with S. aureus in mice advanced the maturation status of DCs compared to that of DCs in uninfected mice (15). Moreover, depletion of DCs in the mouse model abolished S. aureus-induced IL-12 production and Th1 generation (15); in line with this, we found that S. aureus-stimulated BDCA1+ mDCs promote Th1 and Tc1 responses. Our finding greatly supports the previous finding in mice and suggests that human BDCA1+ mDCs are crucial coordinators of the host immune response to S. aureus infection. Although DCs have an important role in S. aureus bloodstream infection in mice, the responses and functions of individual mouse DC subsets to S. aureus need more study.
Different subsets of DCs have different abilities and modes of presenting Ags (24). Human BDCA1+ mDCs have the ability to effectively activate CD4 T cells via MHC class II-peptide complexes (25). In contrast, BDCA3+ mDCs can efficiently cross-present exogenous soluble and cell-bound antigens through MHC class I, inducing cytotoxic CD8 T cell activation (18). Although BDCA3+ mDCs present Ags for CD8 T cell activation, BDCA1+ mDCs are also able to cross-present soluble Ags and induce CD8 T cell activation (26, 27). Here we also found that S. aureus-stimulated human blood BDCA1+ mDCs have a stronger overall capacity to promote activation, not only of CD4 T cells but also of CD8 T cells, than the same mDCs not stimulated with S. aureus. We hypothesize that the enhanced T cell-stimulating capacity of BDCA1+ mDCs results from both the presentation of S. aureus antigens to T cells by these DCs and the increased maturation and cytokine production of these DCs. In addition, we hypothesize that BDCA1+ mDCs may have the ability to both cross-present and directly present particular types of Ags to elicit different responses (28). These will be important areas for future investigations.
We also highlighted that human BDCA3+ mDCs do not phagocytose and have a limited ability to promote CD4 and CD8 T cell activation in response to S. aureus. However, we cannot fully exclude a role of human BDCA3+ mDCs in the induction of immune activation against S. aureus, since BDCA3+ mDCs were able to upregulate MHC class I and II expression in response to S. aureus. Future studies will investigate how blood BDCA3+ mDCs upregulate MHC class I and II in response to S. aureus and whether the upregulation of MHC class I and II allows them to present self-Ags and induce certain autoimmune responses.
The crucial role of DCs in host immune responses against various types of bacterial infections, including S. aureus bloodstream infection, has been demonstrated in multiple in vivo mouse models (15, 29). However, there are limited reports about the specific roles of individual DC subsets in bacterial infections. Here we demonstrate that BDCA1+ mDCs play an important role in initiating inflammatory responses during S. aureus infection in in vitro culture systems. Indeed, it has been reported that BDCA1+ mDCs are expanded in the peripheral blood of human subjects with bacterial infection-induced chronic periodontitis (30), suggesting that BDCA1+ mDCs may be the major human DC subset responsive to bacterial infection. We hypothesize that BDCA1+ mDCs play a critical role in the protective immune responses against S. aureus bloodstream infection in vivo, and our future studies will test this possibility by using mouse models of S. aureus infection.
DCs can directly sense pathogen components through PRRs, such as TLRs, C-type lectins, mannose receptors, SRs, and complement receptors (31). In line with previous reports, we also found that BDCA1+ mDCs, but not BDCA3+ mDCs, express TLR-2 and TLR-4 (18, 32). Moreover, several studies have shown that TLR-2-deficient mice have increased mortality compared to wild-type mice during S. aureus infection, which may be due to high bacterial tissue burdens and impaired proinflammatory cytokine production in TLR-2-deficient mice (33, 34). We also found that maturation of human blood BDCA1+ mDCs in response to S. aureus was critically dependent on TLR-2, yet phagocytosis of S. aureus by these mDCs was only partially dependent on TLR-2. Consistent with our data, previous reports also showed that TLR-2 in mouse macrophages is important for S. aureus Ag recognition but that phagocytosis of S. aureus is not mainly dependent on TLR-2 (35, 36). Interestingly, we also found that BDCA1+ mDCs expressed higher levels of surface SR-A than CD16+ or BDCA3+ mDCs. The function of SR-A in human and mouse DCs and macrophages in response to Gram-negative and Gram-positive bacteria has been investigated, showing that SR-A functionally contributes to phagocytosis of those bacteria (37, 38). Moreover, many studies showed that SR-A plays a significant role in the phagocytosis of S. aureus (39–41). We found that the presence of both SR-A and TLR-2 is required for the optimal phagocytosis of S. aureus by BDCA1+ mDCs. Consistent with our finding, SR-A was previously shown to cooperate with TLR-2 to promote phagocytosis of S. aureus in mouse bone marrow-derived DCs (37). It is possible that similar to upregulation of SR-A expression in a mouse macrophage cell line by TLR-4 signaling (42), TLR-2 signaling may upregulate SR-A expression in BDCA1+ mDCs, thereby enhancing the phagocytic activities and maturation of these cells in response to S. aureus. The molecular mechanisms by which S. aureus affects TLR-2 and SR-A expression in BDCA1+ mDCs require further investigation. Moreover, the expression and contribution of other receptors that have been implicated in the recognition of S. aureus, such as CD36, integrin βv, and TNF-α receptor 1 (43–45), will be characterized.
We also demonstrated that the phagocytosis and maturation of human blood BDCA1+ mDCs in response to S. aureus require the MyD88 signaling pathway. Although previous reports showed that the TLR-2–MyD88 signaling pathway has an important role in S. aureus phagocytosis (34, 35, 46), we found that neutralization of TLR-2 and inhibition of the MyD88 signaling pathway have different effects on phagocytosis of S. aureus by human blood BDCA1+ mDCs. The MyD88 signaling pathway was crucial for phagocytosis and maturation, but TLR-2 was partially involved in phagocytosis. Interestingly, both SR-A and TLR-2 are required for phagocytosis and maturation of BDCA1+ mDCs. These data suggest that the MyD88 signaling pathway may be downstream of SR-A and TLR-2 in this event. Moreover, recent studies have shown that the MyD88 signaling pathway is essential for SR-A ligand-induced antitumor effects in mouse NK cells (47) and for migration of human and mouse monocytes/macrophages (48). To address this hypothesis, we will characterize the downstream cascades of SR-A and TLR-2 signaling pathways in human BDCA1+ mDCs stimulated by S. aureus, including the MyD88 signaling pathway.
In conclusion, our data provide compelling evidence that human BDCA1+ mDCs represent a unique myeloid DC subset which induces Th1 and Tc1 immune responses to S. aureus infection in a TLR-2-, SR-A-, and MyD88-dependent fashion. These results have identified critical cellular players in host immune responses against S. aureus and suggest that enhancing the maturation and functional capacity of BDCA1+ mDCs may lead to better control of S. aureus bloodstream infection.
Footnotes
Published ahead of print 11 August 2014
REFERENCES
- 1.Laupland KB. 2013. Incidence of bloodstream infection: a review of population-based studies. Clin. Microbiol. Infect. 19:492–500. 10.1111/1469-0691.12144. [DOI] [PubMed] [Google Scholar]
- 2.Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 3.Kern WV. 2010. Management of Staphylococcus aureus bacteremia and endocarditis: progresses and challenges. Curr. Opin. Infect. Dis. 23:346–358. 10.1097/QCO.0b013e32833bcc8a. [DOI] [PubMed] [Google Scholar]
- 4.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Torok ME, Walker S, Wertheim HF, Wilson P, Llewelyn MJ, UK Clinical Infection Research Group 2011. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect. Dis. 11:208–222. 10.1016/S1473-3099(10)70285-1. [DOI] [PubMed] [Google Scholar]
- 5.Garzoni C, Vergidis P, AST Infectious Diseases Community of Practice 2013. Methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus infections in solid organ transplantation. Am. J. Transplant. 13(Suppl 4):50–58. 10.1111/ajt.12098. [DOI] [PubMed] [Google Scholar]
- 6.Furuno JP, Johnson JK, Schweizer ML, Uche A, Stine OC, Shurland SM, Forrest GN. 2011. Community-associated methicillin-resistant Staphylococcus aureus bacteremia and endocarditis among HIV patients: a cohort study. BMC Infect. Dis. 11:298. 10.1186/1471-2334-11-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31:1250–1256. 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen RV, Fowler VG, Jr, Skov R, Bruun NE. 2011. Future challenges and treatment of Staphylococcus aureus bacteremia with emphasis on MRSA. Future Microbiol. 6:43–56. 10.2217/fmb.10.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. 1998. Dendritic cells and the control of immunity. Nature 392:245–252. 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Palucka AK. 2005. Dendritic cells as therapeutic vaccines against cancer. Nat. Rev. Immunol. 5:296–306. 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 11.Hart DN. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245–3287. [PubMed] [Google Scholar]
- 12.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. 2002. Characterization of human blood dendritic cell subsets. Blood 100:4512–4520. 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 13.Vulink A, Radford KJ, Melief C, Hart DN. 2008. Dendritic cells in cancer immunotherapy. Adv. Cancer Res. 99:363–407. 10.1016/S0065-230X(07)99006-5. [DOI] [PubMed] [Google Scholar]
- 14.Garcia F, Climent N, Assoumou L, Gil C, Gonzalez N, Alcami J, Leon A, Romeu J, Dalmau J, Martinez-Picado J, Lifson J, Autran B, Costagliola D, Clotet B, Gatell JM, Plana M, Gallart T, DCV2/MANON07 AIDS Vaccine Research Objective Study Group 2011. A therapeutic dendritic cell-based vaccine for HIV-1 infection. J. Infect. Dis. 203:473–478. 10.1093/infdis/jiq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler D, Gutierrez MG, Beineke A, Rauter Y, Rohde M, Foster S, Goldmann O, Medina E. 2012. Dendritic cells are central coordinators of the host immune response to Staphylococcus aureus bloodstream infection. Am. J. Pathol. 181:1327–1337. 10.1016/j.ajpath.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 16.Kassianos AJ, Jongbloed SL, Hart DN, Radford KJ. 2010. Isolation of human blood DC subtypes. Methods Mol. Biol. 595:45–54. 10.1007/978-1-60761-421-0_3. [DOI] [PubMed] [Google Scholar]
- 17.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, Kroczek RA. 2010. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 207:1273–1281. 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJ, Hart DN, Radford KJ. 2010. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 207:1247–1260. 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, Le Moine A, Faure F, Donckier V, Sancho D, Cerundolo V, Bonnet D, Reis e Sousa C. 2010. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J. Exp. Med. 207:1261–1271. 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin JO, Park HY, Xu Q, Park JI, Zvyagintseva T, Stonik VA, Kwak JY. 2009. Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha. Blood 113:5839–5847. 10.1182/blood-2008-10-184796. [DOI] [PubMed] [Google Scholar]
- 21.Takeda K, Akira S. 2004. TLR signaling pathways. Semin. Immunol. 16:3–9. 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Schmaler M, Jann NJ, Ferracin F, Landmann R. 2011. T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88-dependent T cell activation. J. Immunol. 186:443–452. 10.4049/jimmunol.1001407. [DOI] [PubMed] [Google Scholar]
- 23.Ip WK, Sokolovska A, Charriere GM, Boyer L, Dejardin S, Cappillino MP, Yantosca LM, Takahashi K, Moore KJ, Lacy-Hulbert A, Stuart LM. 2010. Phagocytosis and phagosome acidification are required for pathogen processing and MyD88-dependent responses to Staphylococcus aureus. J. Immunol. 184:7071–7081. 10.4049/jimmunol.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. 2000. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 164:2978–2986. 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 25.Villadangos JA, Schnorrer P. 2007. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 7:543–555. 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 26.Nizzoli G, Krietsch J, Weick A, Steinfelder S, Facciotti F, Gruarin P, Bianco A, Steckel B, Moro M, Crosti M, Romagnani C, Stolzel K, Torretta S, Pignataro L, Scheibenbogen C, Neddermann P, De Francesco R, Abrignani S, Geginat J. 2013. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 122:932–942. 10.1182/blood-2013-04-495424. [DOI] [PubMed] [Google Scholar]
- 27.Segura E, Durand M, Amigorena S. 2013. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J. Exp. Med. 210:1035–1047. 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgdorf S, Kurts C. 2008. Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 20:89–95. 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Parker D, Prince A. 2012. Staphylococcus aureus induces type I IFN signaling in dendritic cells via TLR9. J. Immunol. 189:4040–4046. 10.4049/jimmunol.1201055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrion J, Scisci E, Miles B, Sabino GJ, Zeituni AE, Gu Y, Bear A, Genco CA, Brown DL, Cutler CW. 2012. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J. Immunol. 189:3178–3187. 10.4049/jimmunol.1201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon S. 2002. Pattern recognition receptors: doubling up for the innate immune response. Cell 111:927–930. 10.1016/S0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 32.Villadangos JA, Shortman K. 2010. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J. Exp. Med. 207:1131–1134. 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yimin Kohanawa M, Zhao S, Ozaki M, Haga S, Nan G, Kuge Y, Tamaki N. 2013. Contribution of Toll-like receptor 2 to the innate response against Staphylococcus aureus infection in mice. PLoS One 8:e74287. 10.1371/journal.pone.0074287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeuchi O, Hoshino K, Akira S. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392–5396. 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 35.Blander JM, Medzhitov R. 2004. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304:1014–1018. 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 36.Mullaly SC, Kubes P. 2006. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J. Immunol. 177:8154–8163. 10.4049/jimmunol.177.11.8154. [DOI] [PubMed] [Google Scholar]
- 37.Amiel E, Alonso A, Uematsu S, Akira S, Poynter ME, Berwin B. 2009. Pivotal advance: Toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. J. Leukoc. Biol. 85:595–605. 10.1189/jlb.1008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amiel E, Nicholson-Dykstra S, Walters JJ, Higgs H, Berwin B. 2007. Scavenger receptor-A functions in phagocytosis of E. coli by bone marrow dendritic cells. Exp. Cell Res. 313:1438–1448. 10.1016/j.yexcr.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanchet C, Jouvion G, Fitting C, Cavaillon JM, Adib-Conquy M. 2014. Protective or deleterious role of scavenger receptors SR-A and CD36 on host resistance to Staphylococcus aureus depends on the site of infection. PLoS One 9:e87927. 10.1371/journal.pone.0087927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peiser L, Gough PJ, Kodama T, Gordon S. 2000. Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect. Immun. 68:1953–1963. 10.1128/IAI.68.4.1953-1963.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sever-Chroneos Z, Krupa A, Davis J, Hasan M, Yang CH, Szeliga J, Herrmann M, Hussain M, Geisbrecht BV, Kobzik L, Chroneos ZC. 2011. Surfactant protein A (SP-A)-mediated clearance of Staphylococcus aureus involves binding of SP-A to the staphylococcal adhesin eap and the macrophage receptors SP-A receptor 210 and scavenger receptor class A. J. Biol. Chem. 286:4854–4870. 10.1074/jbc.M110.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu WY, Wang L, Wang HM, Wang YQ, Liang YF, Zhao TT, Wu YZ. 2007. TLR2 and TLR4 agonists synergistically up-regulate SR-A in RAW264.7 through p38. Mol. Immunol. 44:2315–2323. 10.1016/j.molimm.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. 2005. CD36 is a sensor of diacylglycerides. Nature 433:523–527. 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 44.Shiratsuchi A, Mori T, Sakurai K, Nagaosa K, Sekimizu K, Lee BL, Nakanishi Y. 2012. Independent recognition of Staphylococcus aureus by two receptors for phagocytosis in Drosophila. J. Biol. Chem. 287:21663–21672. 10.1074/jbc.M111.333807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10:842–848. 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 46.Fournier B, Philpott DJ. 2005. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 18:521–540. 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azuma K, Ishihara T, Nakamoto H, Amaha T, Osaki T, Tsuka T, Imagawa T, Minami S, Takashima O, Ifuku S, Morimoto M, Saimoto H, Kawamoto H, Okamoto Y. 2012. Effects of oral administration of fucoidan extracted from Cladosiphon okamuranus on tumor growth and survival time in a tumor-bearing mouse model. Mar. Drugs 10:2337–2348. 10.3390/md10102337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geiger-Maor A, Levi I, Even-Ram S, Smith Y, Bowdish DM, Nussbaum G, Rachmilewitz J. 2012. Cells exposed to sublethal oxidative stress selectively attract monocytes/macrophages via scavenger receptors and MyD88-mediated signaling. J. Immunol. 188:1234–1244. 10.4049/jimmunol.1101740. [DOI] [PubMed] [Google Scholar]