Abstract
Listeria monocytogenes is a food-borne pathogen that can result in adverse pregnancy outcomes, such as stillbirth or premature delivery. The Mongolian gerbil was recently proposed as the most appropriate small-animal model of listeriosis due to its susceptibility to the same invasion pathways as humans. The objectives of this study were to investigate invasion and adverse pregnancy outcomes in gerbils orally exposed to L. monocytogenes, to compare the dose-response data to those of other animal models, and to investigate differences in the responses of pregnant versus nonpregnant gerbils. Gerbils were orally exposed to 0 (control), 103, 105, 107, or 109 CFU L. monocytogenes in whipping cream. L. monocytogenes was recovered in a dose-dependent manner from fecal samples, adult organs, and pregnancy-associated tissues. Dams exposed to 109 CFU had more invaded organs and higher concentrations of L. monocytogenes in almost all organs than nonpregnant animals, though no differences in fecal shedding were seen between the two groups. Adverse pregnancy outcomes occurred only in the dams treated with 109 CFU. A 50% infectivity dose (ID50) of 2.60 × 106 CFU for fetuses was calculated by fitting the data to a logistic model. Our results suggest that the 50% lethal dose (LD50) falls within the range of 5 × 106 to 5 × 108 CFU. This range includes the guinea pig and nonhuman primate LD50s, but the observation that L. monocytogenes-induced stillbirths can be seen in guinea pigs and primates exposed to lower doses than those at which stillbirths were seen in gerbils indicates that gerbils are not more sensitive to L. monocytogenes invasion.
INTRODUCTION
The food-borne pathogen Listeria monocytogenes is responsible for approximately 1,600 cases of listeriosis every year in the United States. While this number is small in comparison to food-borne illnesses caused by other agents, the 15.9% case fatality rate of listeriosis makes L. monocytogenes the third leading cause of death from a food-borne pathogen (1). Humans are usually exposed through the consumption of refrigerated ready-to-eat foods, delicatessen meats, and soft cheeses (2). At-risk individuals include the elderly, persons who are immunocompromised or immunosuppressed, and the fetuses of pregnant women, with one in six listeriosis cases (17%) occurring during pregnancy (3). The risk to a fetus of miscarriage, stillbirth, or premature delivery or of serious illness in a neonate (e.g., septicemia or meningitis) greatly increases if its mother has been exposed to L. monocytogenes (4), though she herself may experience only mild flu-like symptoms (5). One review of historical data (4) reported that 36 of 178 maternal listeriosis cases (20.2%) resulted in spontaneous abortion or stillbirth. Of the remaining 142 cases, 97 neonates (68.3%) were born with the infection. Data available on 94 of these neonates reported that 23 of the affected neonates (24.5%) died and another 12 (12.8%) experienced some form of serious long-term complication (4). Listeriosis is therefore of great concern to pregnant women and their unborn children.
L. monocytogenes possesses almost 50 virulence factors that help it adhere to, invade, replicate within, and spread among cells (6). Of the invasion virulence factors, InlA and InlB are generally considered to be the most important for entering the human body, initial invasion of tissues, and crossing the placental barrier (7). InlA binds to human E-cadherin, hijacking the mechanosensor's cytoskeleton reorganization capabilities and inducing membrane extensions that allow L. monocytogenes to enter the cell via endocytosis (8). InlB binds preferentially to the human Met receptor, initiating a cascade that culminates in actin rearrangement and internalization of the pathogen via clathrin-coated endocytosis (9, 10). The InlA–E-cadherin interaction is important to the efficient crossing of the intestinal barrier (11), while the InlB-Met interaction is thought to be important for the entry of L. monocytogenes into other mammalian cell types (12, 13) and could play a role in enhancing intestinal invasion (14). Some animal models of listeriosis differ from humans in either the E-cadherin or Met targets, offering the opportunity to study the importance of these in pregnancy-related listeriosis.
Similarity to human listeriosis is the chief consideration for the study of L. monocytogenes invasion in vivo. Ideally, the model should be (i) similar to humans in every aspect important for L. monocytogenes invasion and spread within the body and (ii) easily obtainable and capable of being timed bred in numbers large enough for studies involving pregnancy. Various animals have been used as models, but some of the most common are lacking in one or both of these areas. Listeriosis in nonhuman primates is perhaps most similar to listeriosis in humans (15). However, it can be difficult to acquire large numbers of primates for a single study. Additionally, the studies are expensive, and the primates are not usually sacrificed, which results in a loss of valuable information that addresses what is happening within tissues. Mice and rats possess an E-cadherin with a single amino acid difference from that of humans in the active site (16), and because L. monocytogenes is incapable of binding to the murine E-cadherin, many strains of mice and rats are highly resistant when exposed by the oral route (16). While these rodents can contract listeriosis if injected with the pathogen, injection removes the first barrier L. monocytogenes must cross (i.e., the intestine) and introduces the bacterium directly into the bloodstream, whereas it is normally an intracellular pathogen. Additionally, injection does not reflect human exposure, which is normally by the oral route. Although guinea pigs and rabbits are susceptible to listeriosis when exposed orally, they posses a polymorphism in their Met receptors that affects invasion of cells such as hepatocytes, potentially making guinea pigs and rabbits less sensitive than humans to listeriosis (17). However, despite the difference in the Met receptor, studies in guinea pigs demonstrate that they are susceptible to listeriosis at about the same concentrations as nonhuman primates and humans (18), suggesting that other pathways play a role in fetoplacental invasion in these animals (19). Likewise, other invasion pathways independent of InlA and InlB have been suggested for mice (20).
The Mongolian gerbil (Meriones unguiculatus) has recently been proposed as the small-animal model of choice because both InlA and InlB entry pathways are operative in gerbils. L. monocytogenes mutants lacking these two virulence factors are severely attenuated in crossing the gerbil placental barrier (21), similar to what is seen in human placental explants (22). This permissiveness theoretically makes the gerbil similar to humans in both exposure route and invasion susceptibility. However, the sensitivity of pregnant gerbils to listeriosis has not been previously determined. The objective of this research was therefore to provide dose-response data for fetal morbidity and mortality after oral exposure to L. monocytogenes in Mongolian gerbils, allowing comparisons to other animal models and humans. Furthermore, L. monocytogenes invasion in pregnant gerbils and that in their nonpregnant counterparts were compared.
MATERIALS AND METHODS
Animals.
All animal work was done in full compliance with federal regulations, including the Animal Welfare Act. All procedures were approved by the IACUC at the University of Georgia. The University of Georgia's Animal Care Program is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Thirty-eight female and 8 male Mongolian gerbils were obtained from Charles River Laboratories, International (Wilmington, MA). All gerbils were housed individually upon arrival at the animal facility, with males and females kept in separate rooms. Both rooms were set to a 14-h/10-h light-dark cycle conducive to breeding (23). The gerbils were provided PicoLab Rodent Diet 20 5053* (PMI Nutrition, St. Louis, MO) and water ad libitum.
The animals were bred in-house according to a method developed for breeding timed-pregnant gerbils described by Roulo et al. (24). Briefly, male gerbils aged >90 days and virgin female gerbils aged 80 to 95 days and averaging 71.0 g (±8.4 g) were acclimated in separate rooms for a minimum of 7 days before the female was placed into a divided cage with a male for a 3-day period meant to induce estrus. After 3 days, the pairs were allowed to breed overnight. The males were removed the following morning. On gestation day (GD) 15, the females were transferred from the breeding room to a separate treatment room, where they remained until sacrifice. A microisolator was placed on each cage to prevent cross-contamination between gerbils within the treatment room.
All females were weighed beginning on GD 7 and regularly thereafter as a means of monitoring pregnancy. After treatment, the females were also monitored three times daily for adverse effects, such as lethargy, preterm labor, and/or death.
Bacterial preparation and treatment.
Cells were prepared as previously described by Williams et al. (18), with some minor modifications. L. monocytogenes strain 12443 (serotype 1/2a), an isolate known to cause stillbirths in primates (25) and guinea pigs (26), was grown at 37°C for 24 h in 10 ml tryptic soy broth (TSB) (Becton, Dickinson and Company [BD], Sparks, MD) and activated by two subcultures at 24-h intervals. Cells were harvested by centrifugation (3,600 × g at 15°C for 10 min) and washed three times in 10 ml phosphate-buffered saline (PBS) (BD). The final pellet was resuspended in 1 ml PBS and then diluted with an additional 9 ml of either PBS or ultrapasteurized heavy whipping cream (Publix, Lakeland, FL). This mixture was serially diluted in either PBS or whipping cream to give concentrations ranging from 109 to 103 CFU/ml L. monocytogenes. The control treatment was prepared by diluting 1 ml PBS into 9 ml whipping cream. The exposure doses were confirmed by duplicate plating onto tryptic soy agar (TSA) (BD) and enumeration after incubation at 37°C for 48 h.
All female gerbils were orally exposed to 0.5 ml of the whipping cream containing either control, 103, 105, 107, or 109 CFU L. monocytogenes via an 18-gauge-by-1.25-in animal-feeding needle (Cadence Science, Cranston, RI) on GD 15. All media and the whipping cream vehicle were sterilized by autoclaving at 121°C for 20 min before use.
Tissue collection and analysis.
Fecal samples were collected every day, starting with a pretreatment sample collected immediately prior to exposure and ending the day before sacrifice. With the exception of one high-dose dam who died on GD 21, the gerbils were sacrificed by CO2 overdose on GD 22, 2 to 4 days short of full-term gestation (GD 24 to 26), allowing a total invasion period of just under 7 full days. All fetuses, placentas, and resorptions were collected from the dams. The fetuses were directly checked for viability (i.e., movement and breathing when removed from their amniotic sacs), as well as visually inspected for differences in coloration, size, and development compared to their littermates. Adverse effects were defined as fetal death (stillbirth or resorption) or underdevelopment compared to littermates (small size and earlier stage of development). The liver, spleen, brain, intestine, and gallbladder of each adult animal were harvested. Blood samples available from exsanguination were also saved for analysis in nonheparinized blood collection tubes (BD). Only portions of the liver and intestine were analyzed due to the large size of these organs; all other organs were analyzed in their entirety. All samples were weighed upon collection, placed into individual 24-oz Whirl-Pak bags with filters (Nasco, Fort Atkinson, WI), and transferred to ice. Processing of samples was completed within 24 h.
Individual fecal samples were macerated in a volume of UVM modified Listeria enrichment broth (UVM) (BD) equal to 10 times their weight; individual tissue samples were homogenized on ice using the same weight-to-UVM ratio. Each entire fetus, placenta, or resorption was processed individually. Blood samples were allowed to clot at room temperature for at least 1 h before centrifugation (1,000 × g at 4°C for 20 min). After discarding the serum, the remaining blood components were processed as described above. Samples and/or sample dilutions were plated in duplicate onto modified Oxford Listeria selective agar (Oxford) (BD) to obtain direct counts; these plates were incubated at 37°C for 48 h prior to enumeration. Of the remaining homogenized sample, 1 ml was diluted in 9 ml Fraser Listeria selective enrichment broth (FB) (BD); in cases where less than 1 ml was available, all remaining sample was placed into FB. The FB tubes were incubated at 37°C for 24 h and then plated in duplicate onto Oxford and incubated at 37°C for 48 h to determine the presence or absence of L. monocytogenes. Samples on Oxford plates taken from FB were considered positive if any L. monocytogenes colonies were present.
Direct counts were used to calculate the final numbers of L. monocytogenes bacteria in CFU/g. Any sample that did not have counts but was positive for FB enrichment was set at the detection limit for counts (50 CFU/g). Any sample that did not have counts and was negative for FB enrichment, including control samples that were presumably negative for L. monocytogenes, was set at the detection limit for FB (10 CFU/g). As a final L. monocytogenes confirmation, a random sample of colonies from both direct-count plates and FB plates was replated onto Rapid'L.Mono agar (Bio-Rad Laboratories, Hercules, CA) and incubated at 37°C for 24 h.
Statistical analysis.
After calculating the amount of L. monocytogenes bacteria present in each sample, all values were log transformed for statistical analysis. Fetal data were combined within litters before analysis to control for within-litter similarities; thus, the sample size was equal to the number of dams. Both positive and negative enrichment data and count data were analyzed using a Kruskal-Wallis test for multiple comparisons combined with a Sidak correction test (Stata, College Station, TX) to investigate potential differences between dose groups. Relationships between pregnant and nonpregnant animals within a single dose group were analyzed using two-sample t tests, while relationships between fetuses and their corresponding placentas were analyzed with paired t tests. All t tests were performed with Microsoft (Redmond, WA) Excel. The significance level was set at an α value of 0.05. The dose-response curve was created using a user-defined logistic fit model in PSI-Plot (Pearl River, NY) with the following equation: y(x) = 1/{1 + exp[−A × (x − B)]}, where parameter A is 0.9752, parameter B is −6.2557, and the goodness-of-fit correlation was 0.986.
RESULTS
The purposes of this study were to collect dose-response data on invasion and adverse fetal outcomes in Mongolian gerbils after a single oral exposure to L. monocytogenes, to compare the dose response of pregnant gerbils to that of nonpregnant gerbils and other animal models of listeriosis, and to investigate similarities to and differences from human listeriosis. The results from fecal shedding, adult organs, and pregnancy-associated tissues (fetuses, placentas, and resorptions) are reported below. Each was analyzed using two different methods: the amount of L. monocytogenes bacteria recovered directly from each sample and the presence/absence of L. monocytogenes through sample enrichment.
Most dams gained weight steadily throughout their pregnancies, though the dams exposed to 109 CFU L. monocytogenes experienced a significant net loss of weight indicative of maternal toxicity (Table 1). One dam treated with 109 CFU died on GD 21, and her fetuses averaged only 0.48 g. This was significantly different from the other two litters of this dose group (analyzed 1 day later), whose fetuses averaged 1.45 g and 0.99 g. Additionally, one litter of this highest-dose group was totally resorbed. Other summary characteristics of the dams and their litters are presented in Table 1.
TABLE 1.
Summary characteristics of gerbil pregnancies after oral challenge with L. monocytogenes
| Maternal dose (CFU) | Maternal wt gain (g ± SD) | No. of implantations/litter (±SD) | No. of fetuses/litter (±SD) | No. of litters with ≥1 resorption site/total (%) | No. of litters totally resorbed/total (%) |
|---|---|---|---|---|---|
| Control | 18.5 ± 3.7 | 9.5 ± 1.3 | 7.5 ± 1.7 | 4/4 (100) | 0/4 (0) |
| 103 | 18.2 ± 4.3 | 10.3 ± 1.5 | 9.8 ± 1.9 | 2/4 (50) | 0/4 (0) |
| 105 | 15.8 ± 7.6 | 5.8 ± 2.8 | 5.8 ± 2.8 | 0/4 (0) | 0/4 (0) |
| 107 | 16.1 ± 4.0 | 8.5 ± 0.6 | 8.5 ± 0.6 | 0/4 (0) | 0/4 (0) |
| 109a | −1.5 ± 10.7 | 9.3 ± 2.2 | 6.0 ± 4.1 | 2/4 (50) | 1/4 (25) |
This group includes one dam who died prematurely (GD 21).
Fecal shedding.
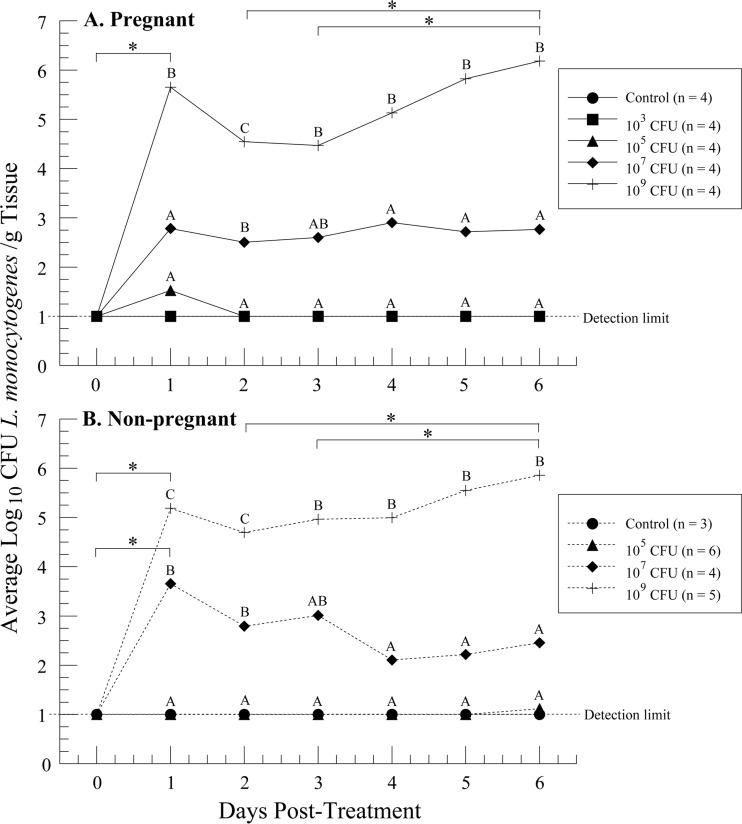
To determine whether the amount of L. monocytogenes bacteria ingested was correlated with either the number of CFU or the length of time L. monocytogenes was shed, fecal samples were analyzed daily. Looking at each day individually, the dams showed a trend toward dose-dependent increases in both the amount of L. monocytogenes bacteria shed (Fig. 1A) and the numbers of fecal samples positive for L. monocytogenes (P < 0.05) (see Table S1 in the supplemental material). In general, dams exposed to 109 CFU shed significantly more L. monocytogenes bacteria than all other groups (Fig. 1A). Dams exposed to 105 CFU shed only on the first day posttreatment (PTD 1), while at least 50% of the dams exposed to 107 CFU and all dams receiving 109 CFU shed every day throughout the collection period (see Table S1 in the supplemental material). None of the 38 animals shed L. monocytogenes prior to treatment, and the control animals remained negative for L. monocytogenes throughout the study. Likewise, the dams exposed to 103 CFU did not shed L. monocytogenes at any point during fecal collection (Fig. 1A; see Table S1 in the supplemental material). In nonpregnant animals, many of the relationships between when L. monocytogenes was shed and the amount of bacteria and number of days shed were the same as for pregnant animals (Fig. 1B; see Table S1 in the supplemental material).
FIG 1.
Fecal shedding of L. monocytogenes from before treatment (day 0) to the day prior to sacrifice (day 6). Fecal material from every dam (A) and nonpregnant animal (B) was gathered over seven 24-hour periods. Dose groups that are significantly different from one another (P < 0.05) on the same day are denoted by different letters. Control and 103- and 105-CFU-treated groups in panel A and control and 105-CFU-treated groups in panel B were given a single set of letters, as these groups were not different from each other on any posttreatment day. Days that differ significantly from one another within dose groups are denoted by bars with asterisks. Samples that had no countable colonies and were negative for enrichment were set at a log10(10 CFU/g) value of 1.00, the detection limit for the enrichment method.
Adult organs.
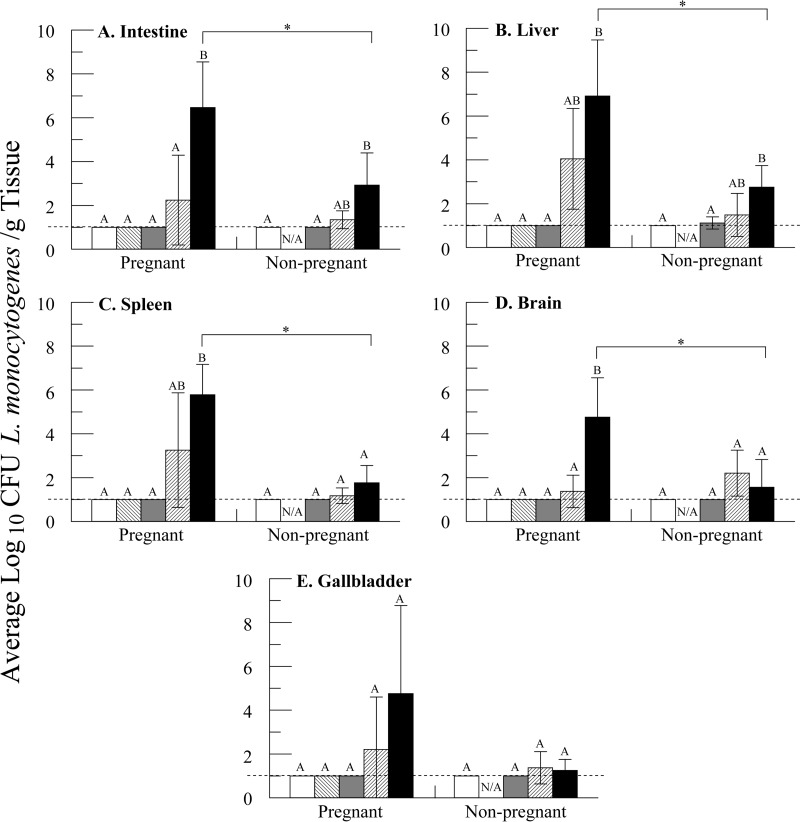
While fecal shedding is an indication of exposure, it may not indicate whether L. monocytogenes gained access to the body and invaded tissues. Isolating L. monocytogenes directly from adult tissues showed a trend toward a dose-dependent increase in both the amount of L. monocytogenes bacteria isolated from dams and the number of positive organs (Fig. 2; see Table S2 in the supplemental material). As expected, the dams receiving the highest dose (109 CFU) of L. monocytogenes bacteria had significantly more (P < 0.05) invasion in the intestine and brain tissues than any other dose group by both enumeration and enrichment (Fig. 2A and D; see Table S2 in the supplemental material). Likewise, liver and spleen samples from dams treated with 109 CFU were different from those of the two low-dose groups in both the total amount of bacteria recovered and the number of samples from which L. monocytogenes was isolated (Fig. 2B and C; see Table S2 in the supplemental material); however, the amounts of L. monocytogenes bacteria recovered from these organs were not different from those from the dams treated with 107 CFU. No differences were seen in the amounts recovered from gallbladders harvested from any group (Fig. 2E; see Table S2 in the supplemental material).
FIG 2.
Isolation of L. monocytogenes from adult organs after a single oral challenge. All organs were harvested and analyzed 7 days postchallenge, with the exception of one 109-CFU-treated dam, whose organs were harvested a day earlier due to her premature death. Dose groups that are significantly different from one another (P < 0.05) are denoted by different letters; pregnancy groups that are significantly different from one another are denoted by a bar with an asterisk. Samples that had no countable colonies and were negative for enrichment were set at a log10(10 CFU/g) value of 1.00, the detection limit for the enrichment method, denoted by the dotted lines. The error bars represent the standard deviations. n was ≥3 for each dose group comprised of pregnant animals; n was ≥3 for each dose group comprised of nonpregnant animals, except for control gallbladders, where n was equal to 2. There were no nonpregnant animals treated at 103 CFU. □, control; ▧, 103 CFU;  , 105 CFU; ▨, 107 CFU; ■, 109 CFU.
, 105 CFU; ▨, 107 CFU; ■, 109 CFU.
Dose-dependent increases in the amount of bacteria recovered or the number of organ samples in which L. monocytogenes was detected by enrichment were not as pronounced in nonpregnant animals as they were in pregnant animals. While the numbers of positive samples for the intestine, liver, and spleen all showed a trend toward dose-dependent increases, only the liver showed a significant difference between animals treated with 109 CFU and the other groups (see Table S2 in the supplemental material).
Within dose groups, no differences could be seen between the numbers of positive samples collected from pregnant and nonpregnant animals, with the exceptions of brain tissues (100% versus 20%, respectively) and overall tissues (95% versus 58%, respectively) in the groups treated with 109 CFU L. monocytogenes. In both of these cases, the dams had significantly more samples from which L. monocytogenes was isolated than the nonpregnant animals (see Table S2 in the supplemental material). More interesting, however, is the dramatic difference in the amounts of L. monocytogenes bacteria isolated from pregnant and nonpregnant animals in the 109-CFU-treated groups. In the intestine, liver, spleen, and brain tissues of animals treated with 109 CFU, L. monocytogenes was isolated in far greater numbers in pregnant animals than in nonpregnant animals (P < 0.05) (Fig. 2).
As with fecal shedding, L. monocytogenes was not isolated from any of the organs of the control animals. Additionally, it was not isolated from any organs of animals exposed to 103 or 105 CFU, with the exception of one liver of a nonpregnant animal treated with 105 CFU (Fig. 2; see Table S2 in the supplemental material).
In addition to the tissues shown in Fig. 2, the cellular fractions of blood samples from several control and treated animals were analyzed. L. monocytogenes was recovered from 50% of blood samples collected from 107-CFU-treated dams (n = 4) and 100% of blood samples from 109-CFU-treated dams (n = 3), but never from any blood samples collected from nonpregnant, treated animals (n = 10) (data not shown).
Pregnancy-associated tissues.
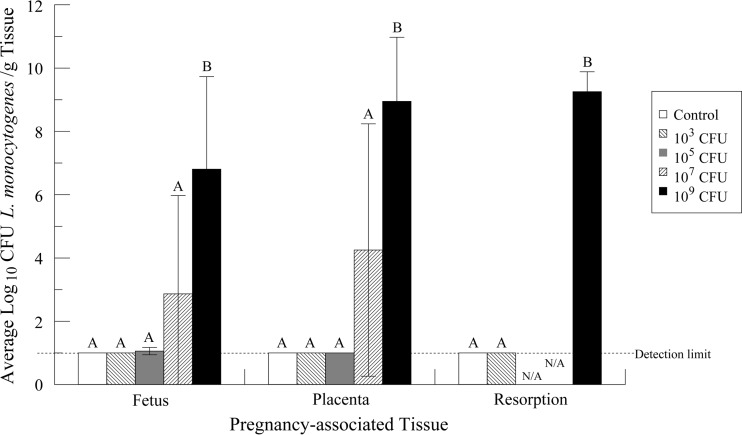
In dams exposed to 105, 107, and 109 CFU L. monocytogenes, 25, 50, and 100% of litters, respectively, contained at least one fetus from which L. monocytogenes was isolated (Table 2). In the two higher-dose groups, if L. monocytogenes was isolated from one fetus, it was isolated from all fetuses within that litter. Interestingly, in the group exposed to 105 CFU, L. monocytogenes was isolated from only one litter and within that litter from only one of three fetuses, indicating that infection of one fetus does not necessarily result in infection of all fetuses. Although resorptions were seen in the control and 103-CFU- and 109-CFU-treated groups, L. monocytogenes was recovered only from the resorptions of the highest-dose group, and in that group, all resorptions tested positive for L. monocytogenes (Table 2). L. monocytogenes was not isolated from any fetus, placenta, or resorption from dams in the control or lowest-dose group.
TABLE 2.
Isolation of L. monocytogenes from pregnancy-associated tissues after oral challenge of pregnant gerbils
| Maternal dose (CFU) | Fetus (%)a,b | Placenta (%)a | Resorption (%)a |
|---|---|---|---|
| Control | 0/4 (0)A | 0/4 (0)A | 0/4 (0)A |
| 103 | 0/4 (0)A | 0/4 (0)A | 0/2 (0)A |
| 105 | 1/4 (25)AB | 0/4c (0)A | NAd |
| 107 | 2/4 (50)AB | 2/4 (50)AB | NA |
| 109 | 4/4 (100)B | 4/4 (100)B | 2/2 (100)B |
Number of litters with ≥1 tissue positive through enrichment/total number of litters from which the tissue was collected (% positive). If any L. monocytogenes bacteria were isolated from an individual tissue, the litter was designated a positive litter for that specific tissue.
Dose groups followed by different letters are significantly different from one another (P < 0.05).
Two placentas, including the placenta paired with the one fetus of this group from which L. monocytogenes was recovered, could not be analyzed.
NA, not applicable; no resorptions were present in these groups.
Although a trend toward increased isolation of L. monocytogenes from fetuses, placentas, and resorptions could be seen with increasing doses, only the pregnancy-associated tissues from dams treated with 109 CFU were significantly different from those of the other dose groups (Fig. 3). When comparing individual placental and fetal units positive for L. monocytogenes from both 107- and 109-CFU-treated groups, we observed that the placentas were always more invaded than their corresponding fetuses, though this trend was not statistically significant when litters were compiled into their dose groups (Fig. 3). Resorptions were invaded to the same extent as placentas (Fig. 3).
FIG 3.
Invasion of pregnancy-associated tissues 7 days after maternal challenge with L. monocytogenes. Dose groups that are significantly different from one another (P < 0.05) are denoted by different letters. Samples that had no countable colonies and were negative for enrichment were set at a log10(10 CFU/g) value of 1.00, the detection limit for the enrichment method. The error bars represent the standard deviations. The sample size is equal to the number of dams. For fetal and placental data, n was equal to 4 for each dose group. For resorptions, n was equal to 4, 2, and 2 for control and 103- and 109-CFU groups, respectively.
Dose-response curve.
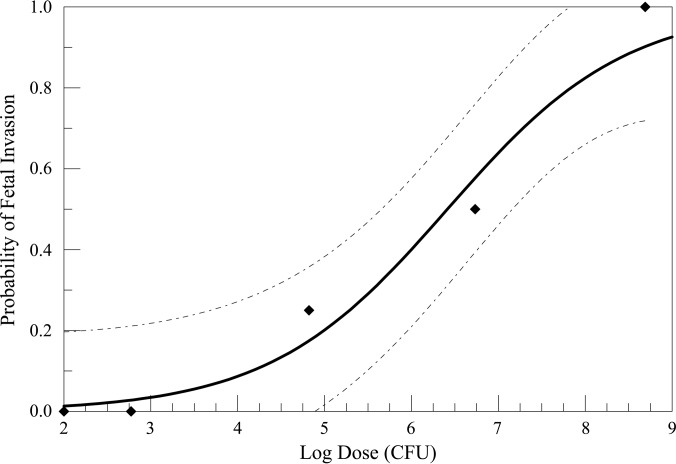
A dose-response curve based on fetal invasion data was created using a logistic fit model with the following equation (Fig. 4):
where p represents the invasion rate, x represents the log dose, A is 0.9752, and B is −6.2557. Using this formula, the log 50% infectivity dose for fetuses (log ID50) is estimated to be 6.415. The ID50 is therefore 2.599 × 106 CFU L. monocytogenes (95% confidence limits, 4.457 × 105 and 1.442 × 107 CFU). A dose-response curve for fetal mortality could not be calculated because fetal deaths occurred only in the highest-dose group.
FIG 4.
Dose response of L. monocytogenes invasion in gerbil fetuses. A logistic model was used to fit the data (solid line) on the basis of a dose resulting in fetal invasion. The calculated ID50 is 2.599 × 106 CFU L. monocytogenes. The diamonds represent the average invasion for each dose group. The dashed lines represent the 95% confidence limits.
DISCUSSION
The primary objectives of this research were to investigate maternal and fetal invasion of L. monocytogenes within the Mongolian gerbil, to construct its dose-response curve for fetal morbidity and/or mortality, and to evaluate its appropriateness for modeling human listeriosis. The FAO-WHO risk assessment for listeriosis during pregnancy estimates a 50% lethal dose (LD50) of 1.9 × 106 CFU for human fetuses (27). Previous studies using guinea pigs and nonhuman primates with fetal death as an endpoint have yielded LD50s of 2.00 × 107 CFU and 8.45 × 107 CFU, respectively (18, 25). The 95% confidence intervals of these three dose-response curves overlap, but the inability of L. monocytogenes to interact with the guinea pig Met receptor during invasion has led some to believe that guinea pig susceptibility to the pathogen may not be equivalent mechanistically to that of humans (17). The gerbil has therefore been proposed as the small-animal model of choice for the study of listeriosis, as its InlA–E-cadherin and InlB-Met receptor interaction pathways, the two invasion pathways considered most important to successful invasion of L. monocytogenes, are similar to those of humans (21) in ways that those of mice and guinea pigs are not (16, 17). This study adds to the knowledge of L. monocytogenes invasion within gerbils and allows a primary comparison with the guinea pig and nonhuman-primate models.
Because there were no fetal deaths or stillbirths at doses less than 109 CFU, the dose-response curve presented in Fig. 4 was based on data from the invasion of fetuses, with a calculated ID50 of 2.599 × 106 CFU L. monocytogenes. However, the presence of L. monocytogenes within a given sample does not necessarily mean there will be an adverse effect. Indeed, L. monocytogenes (up to 5.0 × 108 CFU/g) could be found in some of the viable fetuses of dams exposed to 107 CFU without any concurrent overt signs of illness or adverse developmental effects; however, the potential for long-term adverse effects due to L. monocytogenes invasion was beyond the scope of this study and should be investigated in the future. In adults, up to 6.3 × 103 CFU L. monocytogenes could be isolated from a single brain without any obvious change in the health or behavior of the animal. While the LD50 for gerbil fetuses cannot be calculated from our study due to the lack of any fetal deaths/resorptions at doses less than 109 CFU, an estimated range can be given. No adverse pregnancy outcomes were seen in any of the litters exposed to 103, 105, or 107 CFU, but 75% of 109-CFU litters contained resorptions and/or nonviable fetuses positive for L. monocytogenes. The LD50 can therefore be tentatively said to lie between 107 and 109 CFU (confirmed doses of 5.68 × 106 CFU and 5.08 × 108 CFU, respectively).
One of the more interesting findings of this study was the almost complete lack of L. monocytogenes invasion and adverse pregnancy outcomes within the two lower-dose groups (103 and 105 CFU) compared to guinea pigs and nonhuman primates. Given that gerbils are theoretically more susceptible to L. monocytogenes invasion than are guinea pigs (21), we expected to see more positive samples in the gerbils exposed to these lower doses. However, only a single gerbil fetus in these groups was positive for L. monocytogenes out of over 100 samples of maternal organs, placentas, and fetuses analyzed. In contrast, L. monocytogenes was recovered from 25% and 64% of livers collected from guinea pig dams exposed to 104 CFU and 105 CFU, respectively (18). Additionally, only 50% of litters from 107-CFU-treated gerbils were invaded, none of which showed L. monocytogenes-induced resorptions or stillbirths, whereas guinea pigs had fetal invasion in dams exposed to 105 CFU and stillbirths in all groups exposed to ≥106 CFU. These results can be further contrasted with those for nonhuman primates, a model that is also permissive to both InlA and InlB invasion pathways (15), where stillbirths occurred in animals exposed to as little as 103 CFU (25); if invasion in gerbils is indeed similar to invasion in primates and humans, we would have expected more stillbirths to occur at lower doses than 109 CFU in gerbils. Taken together, these results indicate that permissiveness to InlA- and InlB-dependent invasion pathways is insufficient to explain the susceptibility of various animals to L. monocytogenes, and they offer further evidence that other mechanisms or pathways may contribute to susceptibility in humans.
Another striking observation from our study is the variability in data collected from dams exposed to 107 CFU L. monocytogenes. In this group, some dams had no L. monocytogenes bacteria isolated from any sample while others had tens of thousands of CFU L. monocytogenes isolated from almost every sample. This variability may reflect variation within individuals in their responses to invasion by L. monocytogenes and presents an opportunity for future studies to determine why some individuals are susceptible at lower concentrations than others.
Finally, while no differences could be seen between pregnant and nonpregnant animals in the number of days L. monocytogenes was shed or the amount of L. monocytogenes bacteria per day that was shed in feces, L. monocytogenes was isolated in significantly higher numbers from pregnant organs than from nonpregnant organs in the highest-dose group. This high-dose group also contained one dam that died prematurely, and almost all of the highest counts of L. monocytogenes bacteria isolated from single tissue samples came from this animal. It has been hypothesized that the placenta may act as a reservoir for L. monocytogenes growth and spread within the maternal body, and this may be the reason for more pregnancy-related listeriosis cases than are seen in the general population (28, 29). Indeed, in 6 of the 7 dams in the two highest-dose groups from which L. monocytogenes was recovered, the single most invaded tissue type harvested from each animal was a placenta.
In conclusion, the food-borne pathogen L. monocytogenes can cause adverse outcomes in the pregnant Mongolian gerbil, though L. monocytogenes-induced fetal deaths were seen only in the highest-dose group (109 CFU). While a dose-response curve for fetal mortality could not be calculated, the LD50 falls somewhere between 5.68 × 106 and 5.08 × 108 CFU, where a threshold for lethality is seen. The ID50 is calculated to be 2.60 × 106 CFU L. monocytogenes. These results indicate that the gerbil is not more sensitive and is actually less sensitive to L. monocytogenes than the guinea pig and nonhuman-primate models of listeriosis for both invasion and adverse pregnancy outcomes. More research is therefore needed to elucidate which pathways are involved in fetoplacental invasion by L. monocytogenes, as InlA- and InlB-mediated pathways alone are insufficient to explain the differences in susceptibility among the various animal models.
Supplementary Material
ACKNOWLEDGMENTS
We thank Steve Rathbun for assistance with the statistical modeling and Leanne Alworth and Charles River Laboratories for their expertise in gerbil husbandry. Additionally, we thank all members of the Smith laboratory who assisted with the project: Malkah Glaser, Kwaku Agyekum, Rahat Wadhwa Desai, and Kendra Edwards.
Funding for this project was provided by the Food and Drug Administration (10223SOL00303). Partial salary support was provided by the Department of Environmental Health Science at the University of Georgia (R.M.R. and M.A.S.) and by the Office of the Vice President for Research at UGA (M.A.).
Footnotes
Published ahead of print 25 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01514-14.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15. 10.3201/eid1701.09-1101p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamont RF, Sobel J, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, Kim SK, Uldbjerg N, Romero R. 2011. Listeriosis in human pregnancy: a systematic review. J. Perinat. Med. 39:227–236. 10.1515/JPM.2011.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 4 December 2013. Listeriosis (Listeria infection). http://www.cdc.gov/listeria/.
- 4.Mylonakis E, Paliou M, Hohmann E, Calderwood S, Wing E. 2002. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine 81:260–269. 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Benshushan A, Tsafrir A, Arbel R, Rahav G, Ariel I, Rojansky N. 2002. Listeria infection during pregnancy: a 10 year experience. Isr. Med. Assoc. J. 4:776–780. [PubMed] [Google Scholar]
- 6.Camejo A, Carvalho F, Reis O, Leitao E, Sousa S, Cabanes D. 2011. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence 2:379–394. 10.4161/viru.2.5.17703. [DOI] [PubMed] [Google Scholar]
- 7.Lecuit M. 2005. Understanding how Listeria monocytogenes targets and crosses host barriers. Clin. Microbiol. Infect. 11:430–436. 10.1111/j.1469-0691.2005.01146.x. [DOI] [PubMed] [Google Scholar]
- 8.Lecuit M, Hurme R, Pizarro-Cerda J, Ohayon H, Geiger B, Cossart P. 2000. A role for alpha-and beta-catenins in bacterial uptake. Proc. Natl. Acad. Sci. U. S. A. 97:10008–10013. 10.1073/pnas.97.18.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizarro-Cerdá J, Bonazzi M, Cossart P. 2010. Clathrin-mediated endocytosis: what works for small, also works for big. Bioessays 32:496–504. 10.1002/bies.200900172. [DOI] [PubMed] [Google Scholar]
- 10.Veiga E, Cossart P. 2005. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat. Cell Biol. 7:894–900. 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 11.Lecuit M. 2007. Human listeriosis and animal models. Microbes Infect. 9:1216–1225. 10.1016/j.micinf.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Braun L, Ohayon H, Cossart P. 1998. The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077–1087. 10.1046/j.1365-2958.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Naujokas K, Park M, Ireton K. 2000. InlB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103:501–510. 10.1016/S0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 14.Pentecost M, Kumaran J, Ghosh P, Amieva MR. 2010. Listeria monocytogenes internalin B activates junctional endocytosis to accelerate intestinal invasion. PLoS Pathog. 6:e1000900. 10.1371/journal.ppat.1000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoelzer K, Pouillot R, Dennis S. 2012. Animal models of listeriosis: a comparative review of the current state of the art and lessons learned. Vet. Res. 43:18. 10.1186/1297-9716-43-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956–3963. 10.1093/emboj/18.14.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khelef N, Lecuit M, Bierne H, Cossart P. 2006. Species specificity of the Listeria monocytogenes InlB protein. Cell Microbiol. 8:457–470. 10.1111/j.1462-5822.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 18.Williams D, Irvin EA, Chmielewski RA, Frank JF, Smith MA. 2007. Dose-response of Listeria monocytogenes after oral exposure in pregnant guinea pigs. J. Food Prot. 70:1122–1128. [DOI] [PubMed] [Google Scholar]
- 19.Bakardjiev AI, Stacy BA, Fisher SJ, Portnoy DA. 2004. Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect. Immun. 72:489–497. 10.1128/IAI.72.1.489-497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Monnier A, Autret N, Join-Lambert OF, Jaubert F, Charbit A, Berche P, Kayal S. 2007. ActA is required for crossing of the fetoplacental barrier by Listeria monocytogenes. Infect. Immun. 75:950–957. 10.1128/IAI.01570-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Disson O, Grayo S, Huillet E, Nikitas G, Langa-Vives F, Dussurget O, Ragon M, Le Monnier A, Babinet C, Cossart P, Lecuit M. 2008. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature 455:1114–1118. 10.1038/nature07303. [DOI] [PubMed] [Google Scholar]
- 22.Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. 2010. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 6:e1000732. 10.1371/journal.ppat.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batchelder M, Keller LS, Sauer MB, West WL. 2012. Gerbils, p 1131–1155 In Suckow MA, Stevens KA, Wilson RP. (ed), The laboratory rabbit, guinea pig, hamster, and other rodents. Academic Press, Waltham, MA. [Google Scholar]
- 24.Roulo RM, Fishburn JD, Alworth L, Hoberman AM, Smith MA. 2013. Producing timed-pregnant Mongolian gerbils for developmental studies. Lab. Anim. 42:380–383. 10.1038/laban.297. [DOI] [PubMed] [Google Scholar]
- 25.Smith MA, Raybourne RB, Mytle N, Doyle MP, McClure HM, Takeuchi K, Anderson G, Ware GO. 2008. Dose-response model for Listeria monocytogenes-induced stillbirths in nonhuman primates. Infect. Immun. 76:726–731. 10.1128/IAI.01366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irvin EA, Williams D, Hamler SE, Smith MA. 2008. Immunological and pathological changes in the placenta during infection with Listeria monocytogenes in pregnant guinea pigs. Reprod. Toxicol. 26:151–155. 10.1016/j.reprotox.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Food and Agriculture Organization of United Nations and World Health Organization. 2004. Risk assessment of Listeria monocytogenes in ready-to-eat foods. Interpretive summary no. 4. World Health Organization; http://www.fao.org/fileadmin/templates/agns/pdf/jemra/mra4_en.pdf. [Google Scholar]
- 28.Bakardjiev AI, Stacy BA, Portnoy DA. 2005. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J. Infect. Dis. 191:1889–1897. 10.1086/430090. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan L, Pejcic-Karapetrovic B, Gurnani K, Zafer A, Sad S. 2010. Pregnancy does not deter the development of a potent maternal protective CD8+ T-cell acquired immune response against Listeria monocytogenes despite preferential placental colonization. Am. J. Reprod. Immunol. 63:54–65. 10.1111/j.1600-0897.2009.00766.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.