Abstract
Glycerol monolaurate (GML) is a natural surfactant with antimicrobial properties. At ∼0.3 mM, both GML and its component lauric acid were bactericidal for antibiotic-resistant Staphylococcus aureus biofilms. With the use of MICs of antibiotics obtained from planktonic cells, GML and lauric acid acted synergistically with gentamicin and streptomycin, but not ampicillin or vancomycin, to eliminate detectable viable biofilm bacteria. Images of GML-treated biofilms suggested that GML may facilitate antibiotic interaction with matrix-embedded bacteria.
TEXT
Glycerol monolaurate monoester (GML) is a mild surfactant formed by glycerol and lauric acid. It is used as a preservative and emulsifier in the food and cosmetic industries and is generally recognized as safe (GRAS) for oral use by the FDA (1). GML can be considered a natural surfactant in humans, where lauric acid is converted into GML found in breast milk. Although its clinical usefulness has not been firmly established, GML has antimicrobial activity against enveloped viruses (2) and a variety of bacteria, including some Gram-negative bacteria and some Gram-positive bacteria such as Streptococcus spp. and Staphylococcus aureus (3, 4).
In human infections, including those related to indwelling devices such as catheters, sutures, and stents, both the prevalence and the importance of bacterial biofilms are becoming increasingly recognized (5–7). Biofilms can be defined as surface-associated microbial communities that develop in liquid environments. Microbes within biofilms are often embedded in a hydrated matrix composed of an extracellular polymeric substance containing proteins, polysaccharides, lipids, and extracellular DNA (8, 9). Biofilms are typically resistant to therapeutic concentrations of antibiotics that are based in part on the MICs of planktonic cells (5, 6, 10). Thus, infected devices can be a therapeutic challenge and often require surgical removal.
The antibacterial effect of GML in biofilms has received little attention. Using an in vitro model of S. aureus suture-associated biofilms, we investigated the antibacterial effect of GML alone as well as GML's ability to act synergistically with specific antibiotics. S. aureus RN6390 is a wild-type strain known to produce biofilms (11–13). Antibacterial agents (Sigma-Aldrich, Inc., St. Louis, MO) included gentamicin sulfate (GEN), streptomycin sulfate, ampicillin, and vancomycin. With the use of CLSI guidelines (14), the MICs for this S. aureus strain were 1 μg/ml for GEN, 0.25 to 0.5 μg/ml for ampicillin, 2 μg/ml for vancomycin, and 32 μg/ml for streptomycin. For experiments, stock solutions of GML and lauric acid (Sigma-Aldrich) were dissolved in biofilm growth medium, namely, 66% tryptic soy broth supplemented with 0.2% glucose (12). Stock GML (182 mM in chloroform) was stored in the dark at room temperature, and stock lauric acid (500 and 100 mM in 100% ethanol) was stored at 4°C. In working dilutions, residual concentrations of solvents had no effect on S. aureus viability. Suture-associated biofilms were cultivated as described previously (13). Each well of a 24-well microtiter plate contained a 1-cm segment of black braided 3-0 silk suture (Ethicon, Inc., Somerville, NJ) suspended in 1 ml of growth medium and was inoculated with ∼105 S. aureus bacteria and then incubated for 24 h at 37°C with gentle rotation. The medium was then gently replaced with fresh medium supplemented with various concentrations of GML or lauric acid. After 16 h, suture biofilms were gently rinsed and assayed for viability, i.e., biofilm CFU were quantified from sonicated samples (13). Statistical differences between two groups were analyzed by an unpaired Student t test, and differences between more than two groups were analyzed by one-way analysis of variance followed by Fisher's post hoc test. P values of <0.05 were considered significant. For scanning electron microscopy (SEM), biofilms were prepared as described above, except 11-mm-diameter silicone elastomer coupons (Invotec International, Inc., Jacksonville, FL) were substituted for the silk suture to facilitate high-resolution imaging. To help preserve the biofilm matrix, samples were fixed in a solution that included the anionic dye alcian blue and then processed and viewed with a Hitachi S-4700 field emission scanning electron microscope operated at 2 to 3 kV (15).
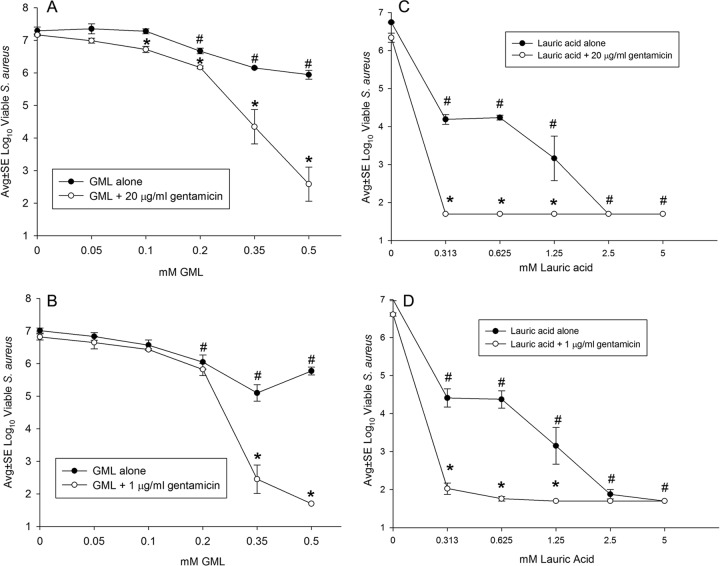
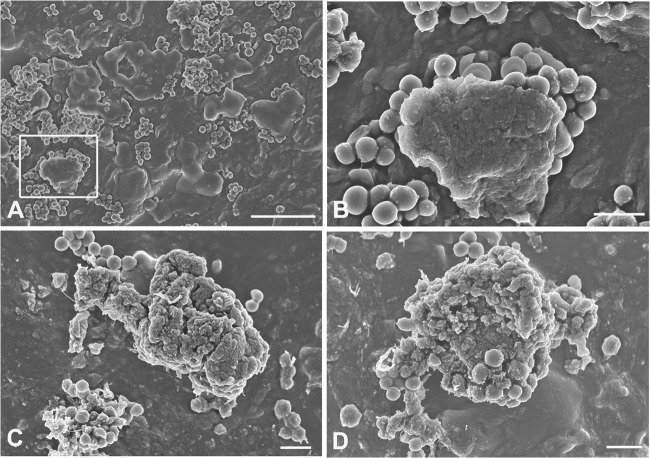
Initial experiments assessed the effect of GML and lauric acid on S. aureus biofilms incubated with or without 20 μg/ml or 1 μg/ml GEN. These GEN concentrations were chosen because 1 μg/ml is the MIC of planktonic cells and this biofilm model is resistant to 20 μg/ml GEN (16). Without GEN, both GML and lauric acid were bactericidal for S. aureus at ∼0.3 mM, indicating that the bactericidal activities of GML and lauric acid were comparable on a molar basis (Fig. 1). Additional testing showed antibacterial activity for lauric acid at 0.15 mM but not 0.78 mM (data not shown). To gain insight into the mechanism behind the antibacterial activity of GML, S. aureus biofilms were viewed by SEM (Fig. 2). Without GML, control S. aureus biofilms consisted of clumps of cocci, some covered by a relatively smooth homogenous matrix (Fig. 2A and B). Biofilms incubated with 0.35 mM GML had a similar appearance except the matrix appeared porous (Fig. 2C and D), indicating that GML might facilitate drug interaction with matrix-embedded bacteria. There is evidence that signal transduction plays a role in GML-mediated inhibition of S. aureus virulence factors, such as beta-lactamase, alpha-hemolysin, and toxic shock syndrome toxin 1 (TSST-1), likely through inhibition of activity at microbial membranes (17–19). Because we have visual evidence that lipid may be a component of the matrix of S. aureus biofilms (20), it is plausible that GML's role as a surfactant might facilitate matrix penetration, and there is evidence that rhamnolipids and plant biosurfactants have antibiofilm effects (21). In support of this hypothesis is the observation (Fig. 1A and B) that similar bacterial killing was noted following addition of either 20 μg/ml or 1 μg/ml gentamicin to GML. Although others have reported unrestricted antibiotic diffusion through biofilms (5, 22, 23), these studies did not have the resolution to determine the ability of antibiotics to contact individual cells within the biofilm, and the ability of an antibiotic to contact individual bacteria could greatly increase the killing of matrix-covered cells.
FIG 1.
(A to D) Antibacterial effect of GML (A, B) and lauric acid (C, D) on the viability of S. aureus biofilms cultivated with or without 20 μg/ml (A, C) or 1 μg/ml GEN (B, D). Compared to 0 mM GML or lauric acid, both GML and lauric acid decreased (#, P < 0.01) bacterial viability in the absence of GEN. In the presence of GEN, GML and lauric acid each acted synergistically to decrease (*, P ≤ 0.01) bacterial viability compared to the corresponding concentration of GML or lauric acid alone. Each data point represents four biofilms. The lower detection limit was 1.7 log10 CFU per biofilm. Avg, average; SE, standard error.
FIG 2.
SEM images of S. aureus biofilms cultivated on silicone coupons for 1 day plus 16 h in growth medium alone (A, B) or for 1 day in growth medium followed by 16 h in medium supplemented with 0.35 mM GML (C, D). (A) Low magnification of a control biofilm without GML containing areas of staphylococcal cells covered by relatively homogenous and smooth matrix material; (B) higher magnification of the area highlighted in panel A; (C, D) more irregular and porous appearance of the matrix associated with GML-treated biofilms. Scale bars, 10 μm (A) and 2 μm (B, C, D).
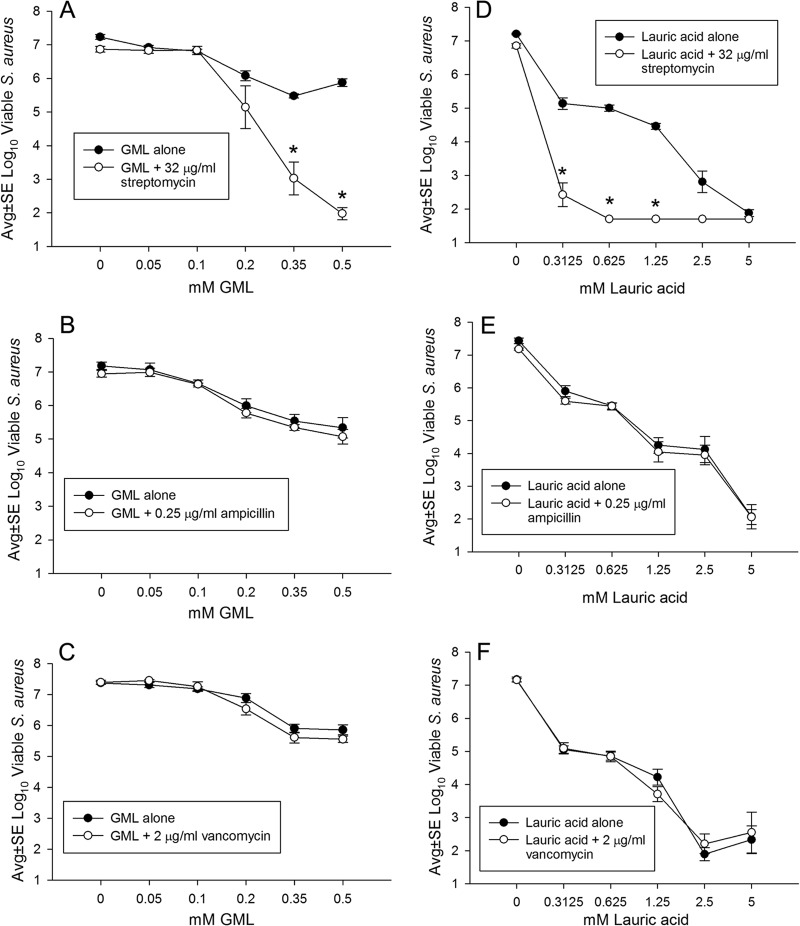
To investigate the specificity of GML's synergistic antibacterial effect with GEN, the experimental model was repeated using the MIC of streptomycin, another aminoglycoside that inhibits protein synthesis, as well as the MICs of ampicillin and vancomycin, two cell wall-active drugs. Figure 3 shows synergistic activity of GML with streptomycin but not ampicillin or vancomycin. Further experiments are necessary to dissect the mechanism behind this specificity. However, if GML facilitates antibiotic contact with matrix-embedded bacteria, these cells may not be actively dividing (24), rendering ampicillin and vancomycin relatively inactive. Additional experiments are needed to determine if GML and/or lauric acid facilitates the antibacterial effect of specific antibiotics in various types of biofilms.
FIG 3.
Antibacterial effect of GML (A to C) and lauric acid (D to F) on the viability of S. aureus biofilms cultivated with or without streptomycin (A, D), ampicillin (B, E), or vancomycin (C, F), showing a synergistic effect (*, P ≤ 0.01 for comparison to the corresponding concentration of GML or lauric acid alone) of streptomycin with GML (A) or lauric acid (D) but no synergistic effect with ampicillin (B, E) or vancomycin (C, F). Each data point represents four to six biofilms. The lower detection limit was 1.7 log10 CFU per biofilm.
Data from the present study indicated that the bactericidal activities of GML and lauric acid were comparable on a molar basis (Fig. 1). However, we have noted that developing (rather than preformed) S. aureus biofilms were 10-fold more susceptible to GML (D. J. Hess, M. J. Henry-Stanley, and C. L. Wells, submitted for publication). Others reported that GML had greater bactericidal activity for planktonic cells than lauric acid, that GML was more effective on a molar basis than lauric acid in inhibiting TSST-1 production, and that 500 μg/ml GML eliminated detectable growth in biofilms cultivated on tampon fibers (4). Using planktonic cells, Ruzin and Novick (17) reported similar inhibitory effects of GML and lauric acid on staphylococcal exoproteins, suggesting that lauric acid might be responsible for the inhibitory effects of GML. Thus, the relative activities of GML and lauric acid are likely model dependent.
The present study is the first to investigate possible synergy between GML and antibiotics in a biofilm model. Here, an additive bactericidal effect was noted when either GML or lauric acid was combined with GEN, i.e., 1 μg/ml GEN eliminated all detectable biofilm bacteria when combined with 0.3 to 0.5 mM GML or lauric acid (Fig. 1). This result is noteworthy considering that 0.35 mM GML is equivalent to 100 μg/ml, and in other studies involving planktonic cells, the MIC of GML was reported to be ≥10 μg/ml (3, 4, 25). Synergistic activity of GML with coenzyme Q1 (26) and lauric arginate (18) has been reported. Thus, using GML in combination with other agents may help combat biofilm drug resistance.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Services Health Grant R01 GM095553 from the National Institutes of Health and in part by funds from the Department of Surgery, University of Minnesota, Minneapolis, MN.
Footnotes
Published ahead of print 2 September 2014
REFERENCES
- 1.Code of Federal Regulations. 2010. Title 21. Food and drugs. Chapter I. Food and Drug Administration. Subchapter B. Food for human consumption. Part 184. Direct food substances affirmed as generally recognized as safe. Subpart B. Listing of specific substances affirmed as GRAS. 21 CFR 184.1505 - mono- and diglycerides http://cfr.vlex.com/vid/184-1505-mono-and-diglycerides-19707498.
- 2.Thormar H, Isaacs CE, Brown HR, Barshatzky MR, Pessolano T. 1987. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 31:27–31. 10.1128/AAC.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlievert PM, Deringer JR, Kim MH, Projan SJ, Novick RP. 1992. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob. Agents Chemother. 36:626–631. 10.1128/AAC.36.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlievert PM, Peterson ML. 2012. Glycerol monolaurate antibacterial activity in broth and biofilm cultures. PLoS One 7(7):e40350. 10.1371/journal.pone.0040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fux CA, Costerton JW, Stewart PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34–40. 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Stoodley P. 2009. Evolving concepts in biofilm infections. Cell. Microbiol. 11:1034–1043. 10.1111/j.1462-5822.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 7.Bjarnsholt T. 2013. The role of bacterial biofilms in chronic infections. APMIS Suppl. 1–51. 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 8.Flemming H-C, Neu TR, Wozniak DJ. 2007. The EPS matrix: the “house of biofilm cells.” J. Bacteriol. 189:7945–7947. 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flemming H-C, Wingender J. 2010. The biofilm matrix. Nat. Rev. Microbiol. 8:623–633. 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 10.Cos P, Tote K, Horemans T, Maes L. 2010. Biofilms: an extra hurdle for effective antimicrobial therapy. Curr. Pharm. Des. 16:2279–2295. 10.2174/138161210791792868. [DOI] [PubMed] [Google Scholar]
- 11.Fux CA, Wilson S, Stoodley P. 2004. Detachment characteristics and oxacillin resistance of Staphylococcus aureus biofilm emboli in an in vitro catheter infection model. J. Bacteriol. 186:4486–4491. 10.1128/JB.186.14.4486-4491.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanks RMQ, Donegan NP, Graber ML, Buckingham SE, Zegans ME, Cheung AL, O'Toole GA. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 73:4596–4606. 10.1128/IAI.73.8.4596-4606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells CL, Henry-Stanley MJ, Barnes AMT, Dunny GM, Hess DJ. 2011. Relationship between antibiotic susceptibility and ultrastructure of Staphylococcus aureus biofilms on surgical suture. Surg. Infect. 12:297–305. 10.1089/sur.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; approved standard M100-S16, 16th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Erlandsen SL, Kristich CJ, Dunny GM, Wells CL. 2004. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: dependence on cationic dyes. J. Histochem. Cytochem. 52:1427–1435. 10.1369/jhc.4A6428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess DJ, Henry-Stanley MJ, Wells CL. 2011. Gentamicin promotes Staphylococcus aureus biofilms on silk suture. J. Surg. Res. 170:302–308. 10.1016/j.jss.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruzin A, Novick RP. 2000. Equivalence of lauric acid and glycerol monolaurate as inhibitors of signal transduction in Staphylococcus aureus. J. Bacteriol. 182:2668–2671. 10.1128/JB.182.9.2668-2671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noll KS, Prichard MN, Khaykin A, Sinko PJ, Chikindas ML. 2012. The natural antimicrobial peptide subtilosin acts synergistically with glycerol monolaurate, lauric arginate, and ε-poly-l-lysine against bacterial vaginosis-associated pathogens but not human lactobacilli. Antimicrob. Agents Chemother. 56:1756–1761. 10.1128/AAC.05861-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pechous R, Ledala N, Wilkinson BJ, Jayaswal RK. 2004. Regulation of the expression of cell wall stress stimulon member gene msrA1 in methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 48:3057–3063. 10.1128/AAC.48.8.3057-3063.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess DJ, Henry-Stanley MJ, Barnes MT, Dunny GM, Wells CL. 2012. Ultrastructure of a novel bacterial form located in Staphylococcus aureus in vitro and in vivo catheter-associated biofilms. J. Histochem. Cytochem. 60:770–776. 10.1369/0022155412457573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn GA, Maloy AP, Banat MM, Banat IM. 2013. A comparison of effects of broad-spectrum antibiotics and biosurfactants on established bacterial biofilms. Curr. Microbiol. 67:614–623. 10.1007/s00284-013-0412-8. [DOI] [PubMed] [Google Scholar]
- 22.Daddi Oubekka S, Briandet R, Fontaine-Aupart MP, Steenkeste K. 2012. Correlative time-resolved fluorescence microscopy to assess antibiotic diffusion-reaction in biofilms. Antimicrob. Agents Chemother. 56:3349–3358. 10.1128/AAC.00216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rani SA, Pitts B, Stewart PS. 2005. Rapid diffusion of fluorescent tracers into Staphylococcus epidermidis biofilms visualized by time lapse microscopy. Antimicrob. Agents Chemother. 49:728–732. 10.1128/AAC.49.2.728-732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderl JN, Zahller J, Roe F, Stewart PS. 2003. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 47:1251–1256. 10.1128/AAC.47.4.1251-1256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland KT, Taylor D, Farrell AM. 1994. The effect of glycerol monolaurate on growth of, and production of toxic shock syndrome toxin-1 and lipase by, Staphylococcus aureus. J. Antimicrob. Chemother. 33:41–55. 10.1093/jac/33.1.41. [DOI] [PubMed] [Google Scholar]
- 26.Schlievert PM, Merriman JA, Salgado-Pabon W, Mueller EA, Spaulding AR, Vu BG, Chang-Smith O, Kohler PL, Kirby JR. 2013. Menaquinone analogs inhibit growth of bacterial pathogens. Antimicrob. Agents Chemother. 57:5432–5437. 10.1128/AAC.01279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]