Abstract
Ciprofloxacin is used in neonates with suspected or documented Gram-negative serious infections. Currently, its use is off-label partly because of lack of pharmacokinetic studies. Within the FP7 EU project TINN (Treat Infection in NeoNates), our aim was to evaluate the population pharmacokinetics of ciprofloxacin in neonates and young infants <3 months of age and define the appropriate dose in order to optimize ciprofloxacin treatment in this vulnerable population. Blood samples were collected from neonates treated with ciprofloxacin and concentrations were quantified by high-pressure liquid chromatography–mass spectrometry. Population pharmacokinetic analysis was performed using NONMEM software. The data from 60 newborn infants (postmenstrual age [PMA] range, 24.9 to 47.9 weeks) were available for population pharmacokinetic analysis. A two-compartment model with first-order elimination showed the best fit with the data. A covariate analysis identified that gestational age, postnatal age, current weight, serum creatinine concentration, and use of inotropes had a significant impact on ciprofloxacin pharmacokinetics. Monte Carlo simulation demonstrated that 90% of hypothetical newborns with a PMA of <34 weeks treated with 7.5 mg/kg twice daily and 84% of newborns with a PMA ≥34 weeks and young infants receiving 12.5 mg/kg twice daily would reach the AUC/MIC target of 125, using the standard EUCAST MIC susceptibility breakpoint of 0.5 mg/liter. The associated risks of overdose for the proposed dosing regimen were <8%. The population pharmacokinetics of ciprofloxacin was evaluated in neonates and young infants <3 months old, and a dosing regimen was established based on simulation.
INTRODUCTION
Ciprofloxacin, a synthetic fluoroquinolone, can be used to treat sepsis caused by multiple resistant organisms (1). It is not considered a first-line treatment in current guidelines for neonatal sepsis but is used in severe infections caused by Enterobacter spp. resistant to standard treatment and when there is a major risk of meningitis and secondary cerebral abscess (2, 3). In a recent European survey, ciprofloxacin was used “off-label” in 25% of neonatal intensive care units, mainly in cases of culture-proven bacterial sepsis due to multidrug-resistant organisms that are sensitive to ciprofloxacin (4).
After intravenous administration, ciprofloxacin is widely distributed in most bodily fluids and tissues, with a high penetration in the cerebrospinal fluid (CSF) and central nervous system. Glomerular filtration and tubular secretion are the main mechanisms of renal excretion, and >65% of ciprofloxacin is excreted unchanged by the kidney (5). In adults, the area under the concentration-time curve from 0 to 24 h (AUC0−24)/MIC ratio appears to be the best predictor of microbiological and clinical outcome. A target AUC0−24/MIC value of 125 was required for treating Gram-negative infections (6, 7).
Since data in neonates are limited, the present study was conducted to assess the population pharmacokinetics of ciprofloxacin in neonates and young infants <3 months of age and to use these data to calculate an optimal dosing regimen of ciprofloxacin for use in these patients.
MATERIALS AND METHODS
Study design.
The trial was a prospective, open label pharmacokinetic study of ciprofloxacin, conducted in the neonatal intensive care unit of the Liverpool women's hospital and in the pediatric intensive care unit of the Alder Hey Children's Hospital, Liverpool, United Kingdom. Inclusion and exclusion criteria are presented in Fig. 1. The study was approved by the institutional ethics board and independent ethics board of the TINN project (EudraCT 2010-019955-23). It was also monitored by an independent safety monitoring board (DSMB).
FIG 1.
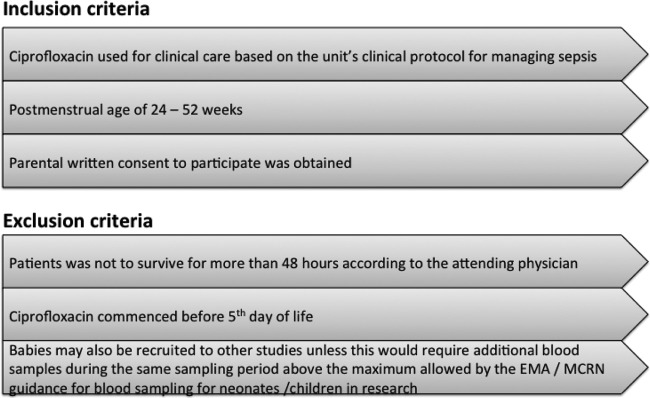
Inclusion and exclusion criteria.
Dosing regimen and pharmacokinetic sampling.
Ciprofloxacin (generic; Peckforton Pharmaceuticals, Crewe, United Kingdom) was administered as an intravenous infusion either over either 30 or 60 min by using a syringe pump connected to microbore tubing at a dose of 10 mg/kg/dose twice daily (BID) for neonates with a postmenstrual age (PMA) of <40 weeks and three times daily (TID) or BID for young infants with a PMA of ≥40 weeks.
Ciprofloxacin pharmacokinetics was assessed on day 1 (D1) or D2 of treatment and again between D5 and D7. The total number of study-specific blood samples was restricted to six per participant with a maximum of three on each sampling day. Patients were randomly assigned to one of the two predefined three-time point schedules (Table 1). Precise infusion and sample times were recorded. The blood volume of samples obtained for pharmacokinetic analyses was 0.2 ml per sample. Scavenged samples were also obtained from blood remaining after routine biochemical tests. Only samples with validated sampling information were included. Blood samples were refrigerated and centrifuged (2,500 × g at 4C for 10 min), and serum or plasma was stored at −70°C. Samples were shipped on dry ice to the Department of Pediatric Pharmacology at Robert Debré Hospital, where they were stored at −70°C prior to analysis.
TABLE 1.
Pharmacokinetic sampling schedule
| Group | Sampling timea |
|||||
|---|---|---|---|---|---|---|
| Ciprofloxacin administered twice daily | ||||||
| A | End of infusion* | T3 | T8 | |||
| B | T2 | T6 | T12 | |||
| Ciprofloxacin administered three times daily | ||||||
| C | End of infusion* | T3 | T8 | |||
| D | T2 | T4 | T8 | |||
The sampling times are indicated with reference to the start of ciprofloxacin infusion. *, For infants weighing <1,000 g, samples were taken randomly on two specific times to minimize blood loss and to ensure representation of each period.
Analytical method of ciprofloxacin and creatinine.
The analytical method of ciprofloxacin has been reported previously (8). Briefly, ciprofloxacin concentrations were determined using high-performance liquid chromatography with mass spectrometry with ciprofloxacin-d8 as an internal standard. The calibration curve ranged from 25 to 3,000 ng/ml. The inter- and intraday coefficients of variation of controls were 4.1 and 2.4%, respectively. The lower limit of quantification was 25 ng/ml. Serum creatinine concentrations were measured by an adapted Jaffé method using the Architect C system (Abbott Diagnostics, Abbott Park, IL).
Population pharmacokinetic modeling of ciprofloxacin.
Pharmacokinetic analysis was carried out using the nonlinear mixed effects modeling program NONMEM v7.2 (Icon Development Solutions, San Antonio, TX). First-order conditional estimation method with interaction was used to estimate pharmacokinetic parameters and their variability.
The interindividual variability of the pharmacokinetic parameters was estimated by using an exponential model and was expressed as follows: θi = θmean*eηi, where θi represents the parameter value of the ith subject, θmean is the typical value of the parameter in the population, and ηi is the variability between subjects, which is assumed to follow a normal distribution with a mean of zero and variance ω2.
Covariate analysis followed a forward and backward selection process. The likelihood ratio test was used to test the effect of each variable on model parameters. The effects of current weight, birth weight, gestational age, postnatal age, postmenstrual age, serum creatinine concentration (collected within ≤48 h of pharmacokinetic sampling), and comedication were investigated as potential variables affecting pharmacokinetic parameters. During the first step of covariate model building, a covariate was included if a significant (P < 0.05, χ2 distribution with one degree of freedom) decrease (reduction > 3.84) in the objective function value (OFV) from the basic model and a reduction in the variability of the pharmacokinetic parameter were obtained. All of the significant covariates were then added simultaneously into a “full” model. Subsequently, each covariate was independently removed from the full model. If the increase in the OFV was higher than 6.635 (P < 0.01, χ2 distribution), the covariate was considered significantly correlated with the pharmacokinetic parameter and was therefore retained in the final model.
Model validation was based on graphical and statistical criteria. Goodness-of-fit plots, including observed (DV) versus population prediction (PRED), DV versus individual prediction (IPRED), conditional weighted residuals (CWRES) versus time, and CWRES versus PRED, were initially used for diagnostic purposes (9). The stability and performance of the final model was also assessed by means of a nonparametric bootstrap with resampling and replacement. Resampling was repeated 500 times, and the values of estimated parameters from the bootstrap procedure were compared to those estimated from the original data set. The entire procedure was performed in an automated fashion, using PsN (v2.30) (10). The final model was also evaluated graphically and statistically by normalized prediction distribution errors (NPDE) and prediction-corrected visual predictive check (pcVPC) (11, 12). One-thousand data sets were simulated using the final population model parameters. The NPDE results were summarized graphically by default as provided by the NPDE R package (v1.2) (13): (i) QQ-plot of the NPDE and (ii) histogram of the NPDE. The NPDE is expected to follow an “N(0,1)” distribution. For pcVPC, observed and simulated dependent variables were normalized based on the typical population prediction for the median independent variable in the bin. The 95% confidence intervals for the median and the 5th and 95th percentiles of the prediction-corrected simulated concentrations were calculated, plotted against the time, and compared to the prediction-corrected observed concentrations.
Ciprofloxacin penetration into the CSF.
Assessment of the ciprofloxacin penetration into the CSF was evaluated by the CSF/serum ciprofloxacin concentration ratio. Because serum ciprofloxacin concentrations were not obtained concurrently with CSF sample collection, serum concentrations at the time of CSF sample collection were calculated via Bayesian estimation using the final model.
Dosing regimen optimization.
Monte Carlo simulations were performed using the parameter estimates obtained from the final model in order to define optimal dosing regimen able to attain the target AUC/MIC value of 125 h in ca. 80% of patients. To ensure comparable safety profiles, the percentage of patients below the reported maximum AUC was also considered. The pediatric dose of ciprofloxacin was simulated on a mg/kg basis according to different age groups. Thus, various mg/kg dosing regimens (5, 7.5, 10, 12.5, and 15 mg/kg/dose BID) were simulated in each neonatal group. One-thousand simulations were performed using the original data set, and AUC0−24 at steady state was calculated for each simulated patient. The target attainment rate was then calculated for each dosing regimen to define the optimal dose regimen in each neonatal group.
RESULTS
Study population.
Sixty-four patients were initially included from February 2011 to June 2012. All of the patients fulfilled the inclusion and exclusion criteria, and informed consent was obtained from all patients. Four patients were excluded from the pharmacokinetic analysis for the following reasons: two patients were withdrawn from the study, one patient was treated with dialysis, and one patient received ciprofloxacin within 36 h of inclusion. Finally, 60 newborns were included for the population pharmacokinetic analysis. Of these, 7 received ciprofloxacin at 5 mg/kg/dose BID, 6 were administered ciprofloxacin at 10 mg/kg/dose TID, and 47 were given ciprofloxacin at 10 mg/kg/dose BID. No patients discontinued the ciprofloxacin treatment due to adverse events, and no drug-related adverse events were shown to have a causal association with ciprofloxacin therapy. The trial flow is presented in Fig. 2.
FIG 2.
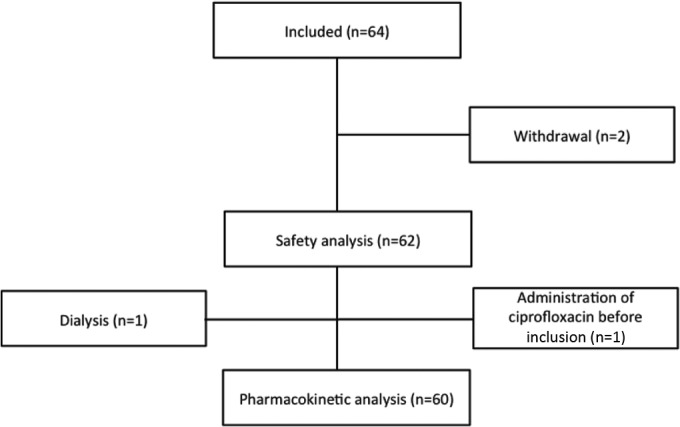
Trial flow chart.
The mean ± the standard deviation postmenstrual age (PMA) and weight of the 60 patients at the time of study were 35.7 ± 6.5 weeks (range, 24.9 to 47.9) and 2,060 ± 1,020 g (range, 700 to 4,200 g), respectively. The PMA and current weight were all normally distributed (P = 0.4 and P = 0.2, respectively [Kolmogorov-Smirnov test]). A summary of patient characteristics is presented in Table 2.
TABLE 2.
Baseline characteristics in 60 neonates and infants
| Characteristicsa | No. of patients | Mean (SD) | Median (range) |
|---|---|---|---|
| Total patients | 60 | ||
| Gender (male/female) | 39/21 | ||
| Race (Caucasian/Asian/unknown) | 53/5/2 | ||
| IUGR | 3 | ||
| GA (wks) | 30.4 (5.8) | 27.9 (23.3–42.0) | |
| PMA (wks) | 35.7 (6.5) | 36.5 (24.9–47.9) | |
| PNA (days) | 38 (30) | 27 (5–121) | |
| Birth wt (g) | 1,518 (884) | 1,115 (540–3850) | |
| Current wt (g) | 2,060 (1020) | 1,955 (700–4200) | |
| Serum creatinine concn (μmol/liter) | 52 (32) | 41 (22–164) | |
| Ciprofloxacin treatment | |||
| Duration (days) | 5 (4) | 5 (1–17) | |
| Dose (mg/dose) | 18.9 (10.1) | 18.7 (4.5–40.0) | |
| Dose (mg/kg/dose) | 9.1 (1.6) | 9.7 (4.4–11.0) | |
| Comedication | |||
| Inotropic agents | 22 | ||
| Teicoplanin | 41 | ||
| Diuretics | 30 | ||
| Caffeine | 15 | ||
| Amoxicillin-clavulanic acid | 12 | ||
| Nystatin | 12 | ||
| Colistin-tobramycin-amphotericin B | 10 |
IUGR, intrauterine growth restriction; GA, gestational age at birth; PMA, postmenstrual age; PNA, postnatal age.
Model building.
For population modeling, 430 ciprofloxacin concentrations (265 pharmacokinetic and 165 scavenged samples) were available. The ciprofloxacin concentrations of pharmacokinetic and scavenged samples ranged from 450 to 15,976 and from 52 to 10,961 ng/ml, respectively. The concentration versus time profile is shown in Fig. 3.
FIG 3.
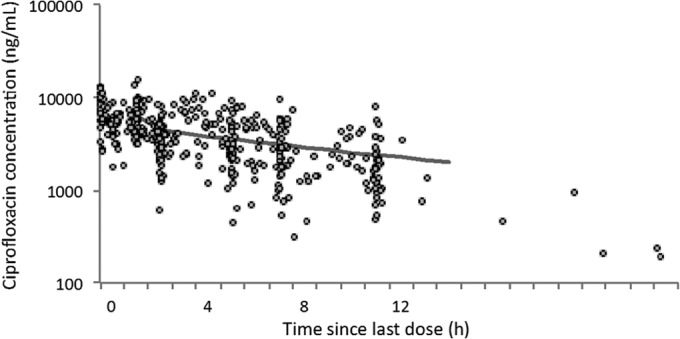
Ciprofloxacin concentrations versus time. The solid line represents the population prediction of a typical patient.
A two-compartment model with first-order elimination fitted the data. The OFV value and residual variability of the two-compartment model were lower than those of the one-compartment model. The model was parameterized in terms of the central volume of distribution (V1), the peripheral volume of distribution (V2), the intercompartment clearance (Q), and the clearance (CL) of ciprofloxacin. Interindividual variability was best described by an exponential model and was then estimated for V1, V2, and CL. Interoccasion variability on CL was coupled to interindividual variability by an additive model, respectively. A proportional model best described residual variability.
Covariate analysis.
The allometric size approach was used by incorporating a priori the current weight into the basic model (allometric coefficients of 0.75 for CL and Q and of 1 for V1 and V2), which caused a significant drop in the OFV of 113.9 points. Postmenstrual age was identified as the most important covariate on CL, associated with a drop in the OFV of 55.6 U. However, gestational age and postnatal age together proved to be superior (ΔOFV 66.7 U) to postmenstrual age alone. A further decrease in the OFV of 17.1 U was achieved by implementing the serum creatinine concentration on clearance. The model was further improved by introducing the coadministration of inotropic agents (ΔOFV 7.5 U) as a third covariate on clearance. For V1, only the coadministration of inotropic agents caused a significant drop in the OFV of 3.9 points in the forward selection process. However, it was not retained in the model after the backward selection process. A detailed presentation of the covariate analysis results is presented in Table 3. Size explained 31.2%, renal maturation explained 25.6%, renal function explained 5.7%, and coadministration of inotropic agents explained 2.4% of the ciprofloxacin CL variability. The η shrinkages were 5.4% for CL, 25.7% for V1, and 25.0% for V2, respectively. The ε shrinkage was 13.6%.
TABLE 3.
Covariate analysis
| Characteristica | Pharmacokinetic parameter(s) | Objective function value | IIVb CL (%) |
|---|---|---|---|
| Structural model | 6,569.0c | 98.3 | |
| Allometric model | CL, V1, V2, Q | ||
| Current body wt | 6,455.1 | 67.6 | |
| Impact of age | V1 | ||
| GA | 6,454.9 | ||
| PNA | 6,451.9 | ||
| PMA | 6,453.2 | ||
| Impact of age | V2 | ||
| GA | 6,455.1 | ||
| PNA | 6,452.3 | ||
| PMA | 6,453.7 | ||
| Impact of renal maturation | CL | ||
| Birth wt | 6,442.0 | ||
| GA | 6,438.7 | ||
| PNA | 6,425.2 | ||
| PMA | 6,399.5 | 43.6 | |
| Birth wt and PNA | 6,396.2 | ||
| GA and PNA | 6,388.4 | 42.5 | |
| Impact of renal function | CL | ||
| Serum creatinine | 6,429.3 | ||
| Impacts of renal maturation and renal function | CL | ||
| GA, PNA, and serum creatinine | 6,371.3 | 36.9 | |
| Impacts of renal maturation, renal function, and comedication | CL | ||
| GA, PNA, serum creatinine, and diuretics | 6,371.4 | ||
| GA, PNA, serum creatinine, and caffeine | 6,369.2 | ||
| GA, PNA, serum creatinine, and teicoplanin | 6,371.0 | ||
| GA, PNA, serum creatinine, and amoxicillin-clavulanic acid | 6,366.8 | ||
| GA, PNA, serum creatinine, and nystatin | 6,370.9 | ||
| GA, PNA, serum creatinine, and inotropic agents | 6,363.8 | 34.5 |
GA, gestational age; PNA, postnatal age; PMA, postmenstrual age.
IIV, interindividual variability.
OFV values of model with significant improvement are indicated in boldface.
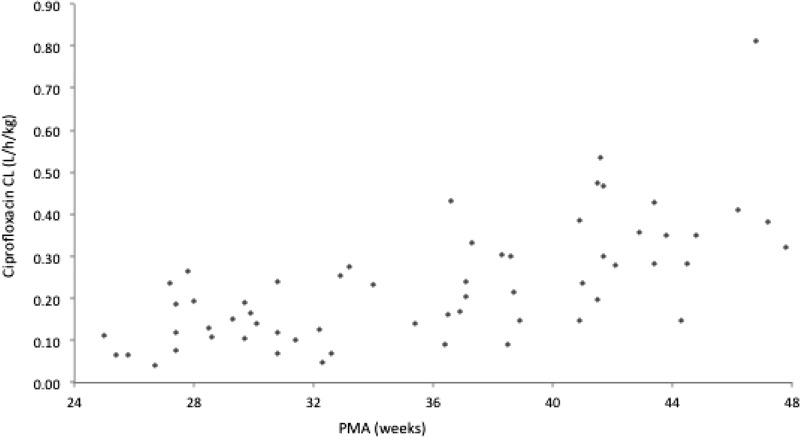
Table 4 summarizes parameter estimates of the final pharmacokinetic model. The median (range) of estimated weight-normalized CL and volume distribution at steady state (sum of V1 and V2) were 0.20 (0.04 to 0.81) liters/h/kg and 2.02 (0.40 to 3.55) liters/kg, respectively. The AUC0−24 at steady state for the evaluated dose regimen ranged from 35 to 291 mg·h/liter. Ciprofloxacin CL increased allometrically with current weight in neonates and young infants, decreased with increasing creatinine concentration, and showed a 29% decrease with the coadministration of inotropic agents. The relationship between ciprofloxacin weight-normalized CL (liters/kg) versus postmenstrual age is shown in Fig. 4.
TABLE 4.
Population pharmacokinetic parameters of ciprofloxacin and bootstrap results
| Parametera | Full data set |
Bootstrap |
||
|---|---|---|---|---|
| Final estimate | RSE (%) | Median | 5th–95th percentile | |
| V1 (liters) | ||||
| V1 = θ1 × (CW/1955) | ||||
| θ1 | 1.97 | 17.7 | 1.82 | 0.78–2.59 |
| V2 (liters) | ||||
| V2 = θ2 × (CW/1955) | ||||
| θ2 | 1.93 | 21.9 | 1.97 | 1.38–3.02 |
| Q (liters/h) | ||||
| Q = θ3 × (CW/1955)0.75 | ||||
| θ3 | 2.5 | 32.6 | 2.62 | 1.02–5.41 |
| CL (liters/h) | ||||
| CL = θ4 × (CW/1955)0.75 × Fage × RF × Finotrope | ||||
| θ4 | 0.366 | 6.0 | 0.365 | 0.323–0.407 |
| Fage = (GA/27.9)θ5 × (PNA/27)θ6 | ||||
| θ5 | 2.11 | 11.9 | 2.09 | 1.60–2.57 |
| θ6 | 0.494 | 10.8 | 0.492 | 0.386–0.606 |
| RF = EXP[(CREA-42) × θ7] | ||||
| θ7 | −0.00335 | 46.0 | −0.00331 | −0.00753 to −0.00063 |
| Finotrope | ||||
| θ8 | 0.708 | 10.9 | 0.719 | 0.572–0.869 |
| Interindividual variability (%) | ||||
| V1 | 48.1 | 63.6 | 49.6 | 26.2–77.2 |
| V2 | 49.3 | 68.3 | 51.2 | 15.8–76.9 |
| CL | 33.2 | 19.9 | 31.3 | 25.3–37.4 |
| Interoccasion variability (%) | ||||
| CL | 16.4 | 55.6 | 16.6 | 9.2–26.2 |
| Residual variability (%) | 19.3 | 28.2 | 18.7 | 14.8–23.1 |
V1, central volume of distribution; V2, peripheral volume of distribution; Q, intercompartment clearance; CL, clearance; RF, renal function; CW, current weight in grams; Finotrope, scaling factor applied for patients coadministered with inotropic agents; CREA, serum creatinine concentration in μmol/liter; GA, gestational age in weeks; PNA, postnatal age in days. In our population, 1,955 g, 27.9 weeks, 27 days, and 42 μmol/liter were the median current weight (at the day of the study), gestational age, postnatal age, and serum creatinine concentration values, respectively.
FIG 4.
Ciprofloxacin CL versus PMA.
Model evaluation.
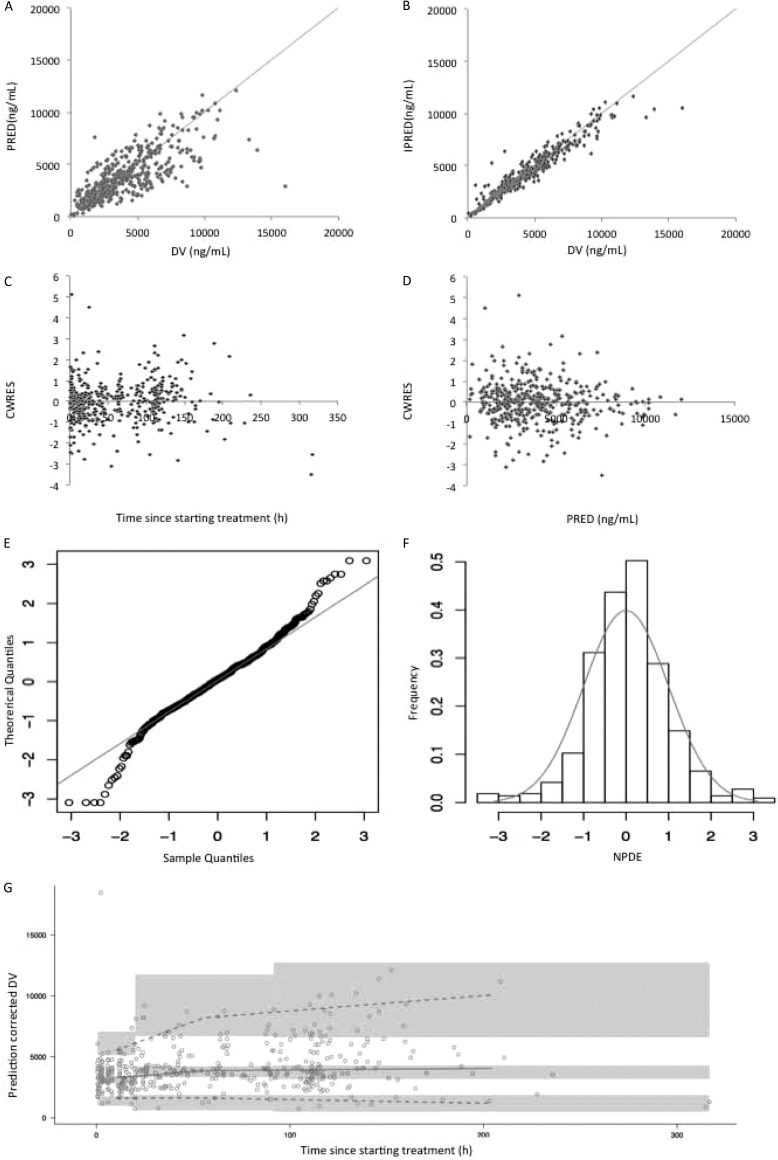
Model diagnostics showed acceptable goodness-of-fit for the final model of ciprofloxacin. As shown in Fig. 5A and B, the predictions are unbiased. In the diagnostic plots of CWRES versus time and PRED, no trends were observed (Fig. 5C and D). In addition, the median parameter estimates resulting from the bootstrap procedure closely agreed with the respective values from the final population model, indicating that the final model is stable and can redetermine the estimates of population pharmacokinetic parameters (Table 4). The NPDEs are presented in Fig. 5E and F. NPDE distribution and histogram met well the theoretical N(0,1) distribution and density, indicating a good fit of the model to the individual data. The mean and variance of the NPDE were 0.05 (Wilcoxon signed-rank test, P = 0.19) and 0.91 (Fisher variance test, 0.17), respectively. The pcVPC is shown in Fig. 5G. The prediction-corrected observed concentrations fit well the simulated concentrations, confirming the predictive performance of the developed model.
FIG 5.
Model evaluation for ciprofloxacin. (A) Population predicted (PRED) versus observed concentrations (DV); (B) individual predicted (IPRED) versus DV; (C) conditional weighted residuals (CWRES) versus time; (D) CWRES) versus PRED; (E) QQ-plot of the distribution of the normalized prediction distribution errors (NPDE) versus the theoretical N(0,1) distribution; (F) histogram of the distribution of the NPDE, with the density of the standard Gaussian distribution overlaid; (G) prediction-corrected visual predictive check. The circles represent the prediction-corrected observed concentrations. The solid line represent the median prediction-corrected observed concentrations and semitransparent gray field represents simulation-based 95% confidence intervals for the median. The observed 5th and 95th percentiles are indicated by dashed lines, and the 95% intervals for the model-predicted percentiles are indicated as corresponding semitransparent gray fields.
Ciprofloxacin penetration into the CSF.
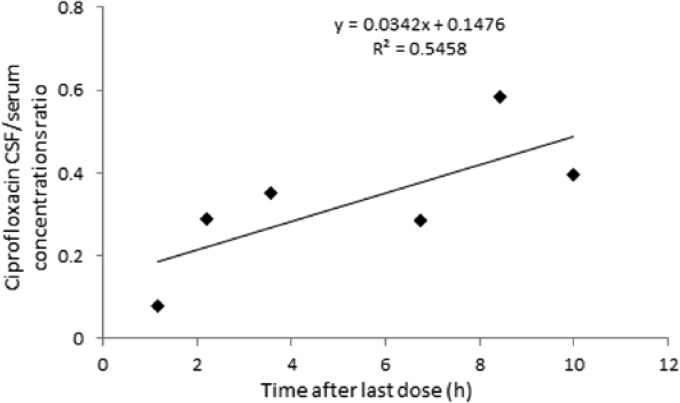
The concentrations of ciprofloxacin in six CSF samples ranged from 187 to 1,650 ng/ml, respectively. The median value of CSF/serum concentration ratio was 0.32 (range, 0.08 to 0.58). A trend for correlation between the CSF collection time and the CSF/serum concentration ratios was demonstrated, suggesting less elimination from (or diffusion in) the CSF than the systemic circulation (Fig. 6).
FIG 6.
Ciprofloxacin CSF/serum concentration ratio versus time.
Dosing regimen optimization.
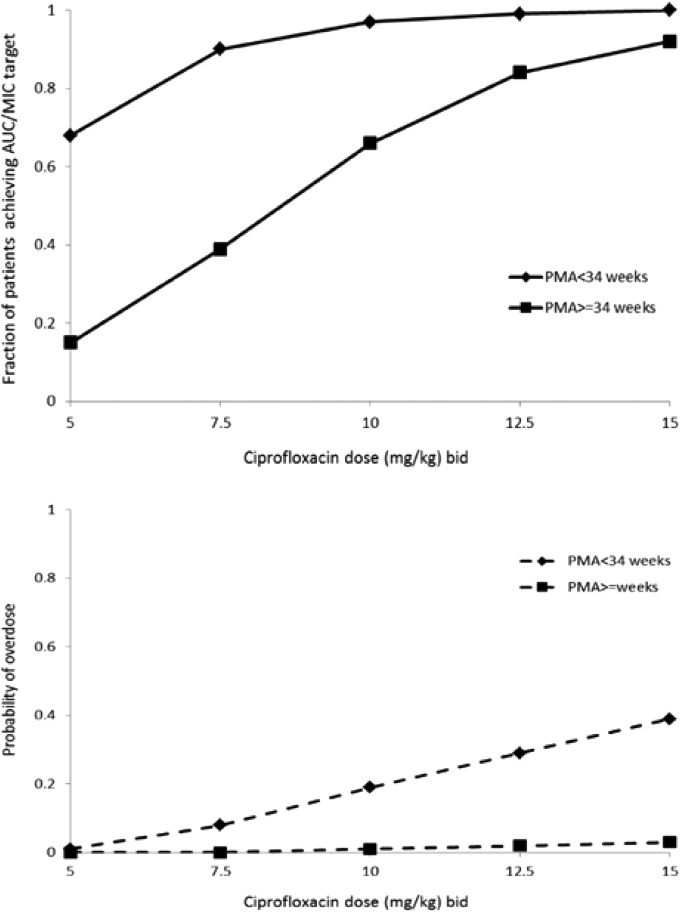
The target attainment rates as a function of dose and age groups for a standard MIC susceptibility breakpoint of 0.5 mg/liter are shown in Fig. 7. A cutoff point of a PMA of 34 weeks was selected to separate age groups based on visual inspection of the plot showing CL versus PMA (Fig. 4).
FIG 7.
Target attainment rates and probability of overdose. The target attainment rates for the 100 simulated trials for MIC value of 0.5 mg/liter is presented as a function of the dose and age group. The AUC/MIC target is 125. Overdose is defined as an AUC over the maximum reported value of 291 mg·h/liter.
A total of 90% of simulated newborns with a PMA of <34 weeks and 84% of newborns with a PMA of ≥34 weeks achieved the target AUC/MIC values of 125 h when treated at doses of 7.5 and 12.5 mg/kg BID, respectively. The associated risks of overdose (AUC > 291 mg·h/liter, the maximal value reported in the present study, did not show short-term adverse events) for the proposed dosing regimen were <8%. Higher doses will be required for patients with more resistant bacterial strains.
DISCUSSION
This is the first population pharmacokinetic study of ciprofloxacin conducted in a cohort of neonates and young infants. It was undertaken to estimate ciprofloxacin pharmacokinetic parameters and to evaluate the impact of demographic, clinical, and biological factors on ciprofloxacin disposition. Our results show that a two-compartment model with first-order elimination with gestational age, postnatal age, current weight, serum creatinine concentration, and coadministration of inotropic agents was optimal for data modeling.
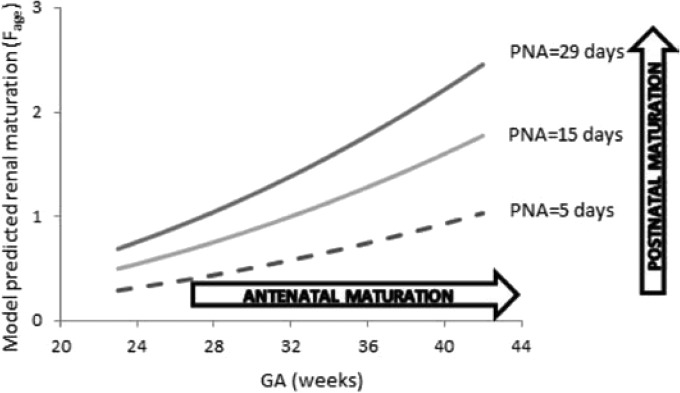
Ciprofloxacin is a fluoroquinolone targeting bacterial DNA gyrase enzyme, a member of the class of type II topoisomerases that displays in vitro activity against most Gram-negative and many Gram-positive pathogenic bacteria, many of which are resistant to a wide range of antibiotics. Ciprofloxacin has a complex safety profile. A range of adverse drug reactions, including arthropathies, without identified long-term effects, need to be balanced with its efficacy (14–16). Potential neuroprotective effects were demonstrated in a juvenile animal model subjected to Escherichia coli sepsis (17). Increase in multidrug-resistant sepsis forces the use of ciprofloxacin (18, 19). However, dosages vary widely from one country to another and from one center to another within the same country (4), since only a few pharmacokinetic studies have been conducted in preterm and term neonates (20–22). Since ciprofloxacin is mainly eliminated by the renal route, renal anatomical and functional maturation is expected to have a major influence on ciprofloxacin clearance and dosing in neonates and young infants. When the current weight is taken into account using an allometric scaling approach, the independent impact of gestational and postnatal ages on clearance illustrates the influence of both antenatal and postnatal renal maturation on ciprofloxacin clearance (Fig. 8); this includes the expected consequences of the normal pattern of renal function ontogeny (23).
FIG 8.
Model-based predicted impact of renal maturation on the clearance of ciprofloxacin in neonates. The influence of both gestational age (GA; representing antenatal maturation) and postnatal age (PNA; representing postnatal maturation) on renal maturation is depicted graphically.
Renal function, as reflected by serum creatinine concentrations, was significantly and independently correlated with ciprofloxacin clearance in the present study. The relation of serum creatinine to the glomerular filtration rate (GFR) at birth is complicated. There was no unique equation to describe the relationship between renal function and drug clearance in neonates. We have demonstrated that the variation in the serum creatinine measurement had a considerable impact on the transferability of a published creatinine-based dosing regimen of renal excreted drug to different clinical settings (24). The influence of residual maternally derived creatinine seems to be limited in our study, since neonates at ≥5 days of age were included. Ciprofloxacin clearance decreased with the coadministration of inotropic drugs. A possible explanation is that inotropic agents are often given when there is decreased blood pressure, which will result in decreased GFR. The same effect was observed by Seay et al. (25), who reported that the coadministration of dopamine induced a 28% decrease in vancomycin clearance in neonates. The use of inotropic agents may be also a surrogate for underlying hemodynamic instability and altered renal hemodynamics, resulting in decreased drug elimination, as suggested by previous investigators (25). We were unable to demonstrate any significant effect of ventilation or intrauterine growth retardation on ciprofloxacin disposition. Clearly, the impact of these variables on ciprofloxacin neonatal pharmacokinetics is complex, probably even more in critically ill neonates. In addition, our study is probably not able to demonstrate their full impact.
In adults, the best surrogate for the ciprofloxacin pharmacokinetic-pharmacodynamic relationship is the AUC0−24/MIC ratio. A target AUC0−24/MIC value of 125 h was required against Gram-negative infections (6, 7). According to regulatory guidelines (26–28), the pharmacokinetic-pharmacodynamic relationship for most anti-infective drugs can be assumed to be similar across all age groups, including neonates, making ciprofloxacin a good example for demonstrating that modeling and simulation approaches can be used to establish optimal dosage recommendations in neonates. Dosage administration in neonates is commonly based on an mg/kg basis according to the different age groups (preterm or term neonates), and a standard mg/kg dose is calculated accordingly in each group. In the present study and in order to reach “optimal but practical dosage recommendations” for clinicians, two groups of patients were defined, newborns with a PMA of <34 weeks or a PMA of ≥34 weeks, and the dose required to achieve the predefined AUC/MIC target was adapted to body weight (i.e., in mg/kg) in the two age groups. The simulation approach demonstrates that doses of 7.5 mg/kg for newborns with a PMA of <34 weeks and 12.5 mg/kg for newborns with a PMA of ≥34 weeks given twice daily allowed 90 and 84% of the patients, respectively, to achieve the AUC/MIC target, with a standard EUCAST MIC susceptibility breakpoint of 0.5 mg/liter (29). For this optimal dosing regimen, the associated risks of overdose, which was defined as the simulated AUC over the maximum reported value of 291 mg·h/liter, were low (<8%), which supports a balanced efficacy and safety profile associated with the recommended dosing regimens. Clearly, organisms with a higher MIC would require a higher AUC to achieve the same outcome (6, 30). In the United States, the Clinical Laboratory Standards Institute's breakpoint is even higher with a, MIC of >1 mg/liter (31) and, as a consequence, a double dose (with assumption of linear pharmacokinetics) would be required to achieve the same AUC/MIC target.
The pharmacokinetic model of ciprofloxacin was developed and internally validated. External validation was not performed because of the limited number of patients currently exposed to this drug. Ultimately, a patient-tailored dose based on modeling and simulation has to be evaluated in clinical practice to confirm its clinical benefits.
Conclusion.
A population pharmacokinetic model of ciprofloxacin was developed in neonates and young infants, showing a low clearance of the drug, compared to older children and adults. Gestational age at birth, postnatal age, current weight, serum creatinine concentration, and the coadministration of inotropic agents had significant impact on ciprofloxacin pharmacokinetics. The ciprofloxacin dosing regimen in neonates and young infants <3 months old was established based on population pharmacokinetics analysis.
ACKNOWLEDGMENTS
We thank all the children and their families for participating in this study. This work is part of the TINN network (Collaborative Project) supported by the European Commission under the Health Cooperation Work Program of the 7th Framework Program (grant agreement 223614). We acknowledge the UK National Institute for Health Research for supporting the delivery of the trial and the clinical teams at the Liverpool Women's NHS FT and Alder Hey Children's NHS FT Liverpool UK.
E.J.A., C.L.G., B.K., J.N.V.D.A., and M.A.T. designed the study; all authors contributed to the final version of the protocol; H.H., W.Z., C.L.G., and E.J.-A. developed the eCRF, H.H. designed S.P., H.H., T.N., S.M., S.P., and M.A.T. organized the trial and recruited patients; W.Z. and E.J.-A. set up the analytical method; W.Z., C.L.G., and E.J.-A. performed the population pharmacokinetic analysis; W.Z. and E.J.-A. drafted the first version of the manuscript; significant contributions to the final version were provided by C.L.G., J.N.V.D.A., and G.L.K.
The TINN consortium includes the following individuals: Wei Zhao, Boris Matrot, Jorge Gallego, and Evelyne Jacqz-Aigrain, French National Institute of Health and Medical Research; Helen Hill, Tim Neal, Tony Nunn, and Mark Turner, University of Liverpool; Helen Sammons and Imti Choonara, University of Nottingham; Natacha Edme and Jerome Weinbach, INSERM-Transfert; Charlotte Castellan, Kim-An Nguyen, and Behrouz Kassai, Claude Bernard University Lyon 1; Paolo Manzoni, Sant'Anna Hospital; John Van Den Anker and Rene Kornelisse, ERASMUS-Medical Center; Julia Stingl, University of Ulm; Jean-Paul Langhendries, Centre Hospitalier Chrétien; Bart Van Overmeire, University of Antwerp; Chantal Le Guellec, François Rabelais University; Valérie Biran, May Fakhoury, Florentia Kaguelidou, Pierre-Henri Jarreau, and Corinne Alberti, Assistance Publique-Hôpitaux de Paris; Patrick Chevarier, Clininfo SA; Chiara Pandolfini and Maurizio Bonati, Istituto di Ricerche Farmacologiche Mario Negri; and Anders Rane, Karolinska Institute.
Footnotes
Published ahead of print 25 August 2014
REFERENCES
- 1.Gendrel D, Chalumeau M, Moulin F, Raymond J. 2003. Fluoroquinolones in paediatrics: a risk for the patient or for the community? Lancet Infect. Dis. 3:537–546. 10.1016/S1473-3099(03)00736-9. [DOI] [PubMed] [Google Scholar]
- 2.Gray JW, Patel M. 2011. Management of antibiotic-resistant infection in the newborn. Arch. Dis. Child. Educ. Pract. Ed. 96:122–127. 10.1136/adc.2010.199653. [DOI] [PubMed] [Google Scholar]
- 3.Kaguelidou F, Turner MA, Choonara I, Jacqz-Aigrain E. 2011. Ciprofloxacin use in neonates: a systematic review of the literature. Pediatr. Infect. Dis. J. 30:e29–e37. 10.1097/INF.0b013e3181fe353d. [DOI] [PubMed] [Google Scholar]
- 4.Pandolfini C, Kaguelidou F, Sequi M, Jacqz-Aigrain E, Choonara I, Turner MA, Manzoni P, Bonati M. 2013. Wide intra- and inter-country variability in drug use and dosage in very-low-birth-weight newborns with severe infections. Eur. J. Clin. Pharmacol. 69:1031–1036. 10.1007/s00228-012-1415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vance-Bryan K, Guay DR, Rotschafer JC. 1990. Clinical pharmacokinetics of ciprofloxacin. Clin. Pharmacokinet. 19:434–461. 10.2165/00003088-199019060-00003. [DOI] [PubMed] [Google Scholar]
- 6.Forrest A, Nix DE, Ballow CH, Goss TF, Birmingham MC, Schentag JJ. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073–1081. 10.1128/AAC.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zanten AR, Polderman KH, van Geijlswijk IM, van der Meer GY, Schouten MA, Girbes AR. 2008. Ciprofloxacin pharmacokinetics in critically ill patients: a prospective cohort study. J. Crit. Care 23:422–430. 10.1016/j.jcrc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Grondin C, Zhao W, Fakhoury M, Jacqz-Aigrain E. 2011. Determination of ciprofloxacin in plasma by micro-liquid chromatography-mass spectrometry: an adapted method for neonates. Biomed. Chromatogr. 25:827–832. 10.1002/bmc.1523. [DOI] [PubMed] [Google Scholar]
- 9.Hooker AC, Staatz CE, Karlsson MO. 2007. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm. Res. 24:2187–2197. 10.1007/s11095-007-9361-x. [DOI] [PubMed] [Google Scholar]
- 10.Lindbom L, Ribbing J, Jonsson EN. 2004. Perl-speaks-NONMEM (PsN): a Perl module for NONMEM related programming. Comput. Methods Programs. Biomed. 75:85–94. 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Brendel K, Comets E, Laffont C, Laveille C, Mentré F. 2006. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 23:2036–2049. 10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects modes. AAPS J. 13:143–151. 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comets E, Brendel K, Mentré F. 2008. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput. Methods Programs Biomed. 90:154–166. 10.1016/j.cmpb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Adefurin A, Sammons H, Jacqz-Aigrain E, Choonara I. 2011. Ciprofloxacin safety in paediatrics: a systematic review. Arch. Dis. Child. 96:874–880. 10.1136/adc.2010.208843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sendzik J, Lode H, Stahlmann R. 2009. Quinolone-induced arthropathy: an update focusing on new mechanistic and clinical data. Int. J. Antimicrob. Agents 33:194–200. 10.1016/j.ijantimicag.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Drossou-Agakidou V, Roilides E, Papakyriakidou-Koliouska P, Agakidis C, Nikolaides N, Sarafidis K, Kremenopoulos G. 2004. Use of ciprofloxacin in neonatal sepsis: lack of adverse effects up to one year. Pediatr. Infect. Dis. J. 23:346–349. 10.1097/00006454-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Loron G, Olivier P, See H, Le Saché N, Angulo L, Biran V, Brunelle N, Besson-Lescure B, Kitzis MD, Pansiot J, Bingen E, Gressens P, Bonacorsi S, Baud O. 2011. Ciprofloxacin prevents myelination delay in neonatal rats subjected to Escherichia coli sepsis. Ann. Neurol. 69:341–351. 10.1002/ana.22190. [DOI] [PubMed] [Google Scholar]
- 18.Shane AL, Stoll BJ. 2013. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am. J. Perinatol. 30:131–142. 10.1055/s-0032-1333413. [DOI] [PubMed] [Google Scholar]
- 19.Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, Jr, Smith PB, Manzoni P, Jacqz-Aigrain E, Kaguelidou F, Cohen-Wolkowiez M. 2012. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early. Hum. Dev. 88:S69–S74. 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal P, Dutta S, Garg SK, Narang A. 2004. Multiple dose pharmacokinetics of ciprofloxacin in preterm babies. Indian Pediatr. 41:1001–1007. [PubMed] [Google Scholar]
- 21.Peltola H, Väärälä M, Renkonen OV, Neuvonen PJ. 1992. Pharmacokinetics of single-dose oral ciprofloxacin in infants and small children. Antimicrob. Agents Chemother. 36:1086–1090. 10.1128/AAC.36.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Oever HL, Versteegh FG, Thewessen EA, van den Anker JN, Mouton JW, Neijens HJ. 1998. Ciprofloxacin in preterm neonates: case report and review of the literature. Eur. J. Pediatr. 157:843–845. 10.1007/s004310050949. [DOI] [PubMed] [Google Scholar]
- 23.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. 2003. Developmental pharmacology: drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 349:1157–1167. 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W, Kaguelidou F, Biran V, Zhang D, Allegaert K, Capparelli EV, Holford N, Kimura T, Lo YL, Peris JE, Thomson A, van den Anker JN, Fakhoury M, Jacqz-Aigrain E. 2013. External evaluation of population pharmacokinetic models of vancomycin in neonates: the transferability of published models to different clinical settings. Br. J. Clin. Pharmacol. 75:1068–1080. 10.1111/j.1365-2125.2012.04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seay RE, Brundage RC, Jensen PD, Schilling CG, Edgren BE. 1994. Population pharmacokinetics of vancomycin in neonates. Clin. Pharmacol. Ther. 56:169–175. 10.1038/clpt.1994.120. [DOI] [PubMed] [Google Scholar]
- 26.Food and Drug Administration. 2014. Guidance for industry—exposure-response relationships: study design, data analysis, and regulatory applications. U.S. Food and Drug Administration, Washington, DC: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072109.pdf. [Google Scholar]
- 27.European Medicines Agency. 2014. Guideline on evaluation of medicinal products indicated for treatment of bacterial infections (CPMP/EWP/558/95 rev2). European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003417.pdf. [Google Scholar]
- 28.European Medicines Agency. 2014. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. Document CPMP/EWP/2655/99 European Medicines Agency, London, United Kingdom. [Google Scholar]
- 29.EUCAST. 2014. Breakpoint tables for interpretation of MICs and zone diameters, version 4.0 EUCAST, Växjö, Sweden. [Google Scholar]
- 30.Lipman J, Gous AG, Mathivha LR, Tshukutsoane S, Scribante J, Hon H, Pinder M, Riera-Fanego JF, Verhoef L, Stass H. 2002. Ciprofloxacin pharmacokinetic profiles in paediatric sepsis: how much ciprofloxacin is enough? Intensive Care Med. 28:493–500. 10.1007/s00134-002-1212-y. [DOI] [PubMed] [Google Scholar]
- 31.CLSI. 2011. Standards for antimicrobial susceptibility testing. CLSI, Wayne, PA. [Google Scholar]