Abstract
Setting the adequate dose for voriconazole is challenging due to its variable pharmacokinetics. We investigated the impact of hypoalbuminemia (<35 g/liter) on voriconazole pharmacokinetics in adult intensive care unit (ICU) patients treated with voriconazole (20 samples in 13 patients) as well as in plasma samples from ICU patients that had been spiked with voriconazole at concentrations of 1.5 mg/liter, 2.9 mg/liter, and 9.0 mg/liter (66 samples from 22 patients). Plasma albumin concentrations ranged from 13.8 to 38.7 g/liter. Total voriconazole concentrations in adult ICU patients treated with voriconazole ranged from 0.5 to 8.7 mg/liter. Unbound and bound voriconazole concentrations were separated using high-throughput equilibrium dialysis followed by liquid chromatography-tandem mass spectrometry (LC-MSMS). Multivariate analysis revealed a positive relationship between voriconazole plasma protein binding and plasma albumin concentrations (P < 0.001), indicating higher unbound voriconazole concentrations with decreasing albumin concentrations. The correlation is more pronounced in the presence of elevated bilirubin concentrations (P = 0.05). We therefore propose to adjust the measured total voriconazole concentrations in patients with abnormal plasma albumin and total serum bilirubin plasma concentrations who show adverse events potentially related to voriconazole via a formula that we developed. Assuming 50% protein binding on average and an upper limit of 5.5 mg/liter for total voriconazole concentrations, the upper limit for unbound voriconazole concentrations is 2.75 mg/liter. Alterations in voriconazole unbound concentrations caused by hypoalbuminemia and/or elevated bilirubin plasma concentrations cannot be countered immediately, due to the adult saturated hepatic metabolism. Consequently, increased unbound voriconazole concentrations can possibly cause adverse events, even when total voriconazole concentrations are within the reference range.
INTRODUCTION
Setting an adequate dosing regimen for voriconazole remains challenging given the nonlinear pharmacokinetic profile in adults (1). The extreme intra- and interpatient variability in plasma concentrations in the context of established exposure-response/toxicity relationships has triggered the need for therapeutic drug monitoring (TDM) in daily practice (1–4). Extensive pharmacokinetic research has revealed several altering covariates, including CYP450-mediated drug-drug interactions (5), genetic polymorphism associated with the CYP2C19 enzyme (6), age (7–9), liver disease (10), coadministration of drug with food (11, 12) or enteral feeding (13), and switching from intravenous to oral administration (1, 14). As a result, inadequate responses or severe toxic events have been reported.
Recently, plasma protein binding (PPB) has been investigated as an additional factor influencing the pharmacokinetics (PK) of antimicrobial agents (15–18). Since hypoalbuminemia occurs in approximately 40% of critically ill patients (18), the potentially negative effects of altered protein binding of antimicrobials may be common (19, 20). Unbound drug concentrations can differ among patients and underlying disorders, resulting in different responses to therapy or toxicity, as only the unbound drug concentration exhibits pharmacological activity (21). Hypoalbuminemia usually results in higher unbound drug concentrations in plasma (16). Because the temporary increase in the unbound plasma concentration is reversed by the rapid distribution and elimination of the drug via the liver or the kidneys, this phenomenon is expected to be clinically relevant only for highly protein-bound drugs (PPB above 70%) (16). However, for drugs such as voriconazole, with nonlinear pharmacokinetics (6–8), the elevated unbound drug concentration in plasma caused by decreased plasma albumin concentrations cannot be instantly metabolized and eliminated. This can be explained by its saturated metabolism and the fact that only 2% of voriconazole is excreted unchanged in urine (22). Although voriconazole PPB is only ±50% (23), this saturated metabolism is hypothesized to cause clinically relevant variations in unbound fractions in cases of hypoalbuminemia, potentially resulting in an increased risk for toxic adverse events, even with a total voriconazole trough concentration (VTC) within the reference range of 1 or 2 up to 5.5 mg/liter (24–26).
In the past, equations were developed to correct measured total concentrations of antiepileptics in the function of the higher free fraction in cases of hypoalbuminemia. These formulae are applied in daily clinical practice, the formula for valproic acid being the Hermida Tutor formula (27) and that for phenytoin being the Sheiner Tozer or Winter-Tozer formula (28, 29), as both drugs are characterized by high PPB and saturated metabolism. This renders patients with hypoalbuminemia prone to toxicity even with total measured concentrations within the reference interval (30).
In this study, the potential influence of hypoalbuminemia on voriconazole pharmacokinetics, which has thus far not been addressed in detail, was investigated.
MATERIALS AND METHODS
Patient inclusion.
A prospective PK study (study A) was conducted at the University Hospitals Leuven from July 2012 until August 2013 to assess PPB of voriconazole in medical intensive care unit (ICU) patients. Consecutive patients admitted to the 17-bed medical ICU and with at least 4 consecutive days of voriconazole treatment (Vfend; Pfizer Belgium) for invasive aspergillosis (IA) were eligible (31). Only patients with a documented total VTC of at least 0.4 mg/liter were eligible, as the lower limit of quantification (LLOQ) of our in-house validated liquid-chromatography–tandem mass spectrometry (LC-MSMS) method (32) used to quantify voriconazole concentrations is 0.2 mg/liter (32) and PPB is about 50% in patients without hypoalbuminemia, as previously reported by our group (23).
At the same time, we conducted an in vitro study (study B) in which plasma samples from ICU patients without IA but with plasma albumin concentrations ranging from 10 to 35 g/liter (reference range, 35 to 52 g/liter) were spiked with voriconazole, resulting in 3 final concentrations (5 μM, 10 μM, and 30 μM, corresponding to 1.5 mg/liter, 2.9 mg/liter, and 9.0 mg/liter). Plasma albumin concentrations were determined with colorimetry using bromocresol green (33). Both studies were conducted in accordance with the Declaration of Helsinki. Approval from the local Ethical Committee and written informed consent from each subject were obtained.
High-throughput equilibrium dialysis followed by LC-MSMS.
All heparinized blood samples from study A and study B patients were centrifuged (10 min, 1,500 × g, 20°C) immediately after sampling through an arterial catheter, and plasma was stored at −20°C until analysis. In study B, spiking with voriconazole was carried out in batch just before equilibrium dialysis. Unbound and bound fractions of voriconazole were separated using high-throughput equilibrium dialysis (HT-ED), as described elsewhere (23). HT-ED is a robust method to separate unbound and bound voriconazole concentrations without an impact of environmental temperature, frozen storage, freeze-thaw cycles, and total voriconazole concentrations. Mass balance was checked, and no volume shift was observed. Voriconazole PPB was determined in 235 samples of blank plasma spiked with voriconazole, resulting in a median PPB of 47.6% (interquartile range [IQR], 45.3% to 50%). More importantly, these results were compared with the PPB of voriconazole measured in plasma from critically ill patients (20 samples from 13 patients) treated with voriconazole, resulting in a median PPB of 49.6% (IQR, 42.5% to 52.5%) (P = 0.35).
In brief, plasma was separated from phosphate-buffered saline (PBS) in a 96-well plate with a semipermeable membrane (molecular weight cutoff [WMCO], 12 to 14) through which unbound voriconazole can permeate. Equilibrium was reached after 4 h at 37°C. After reaching equilibrium, voriconazole concentrations in both the plasma and the buffer compartment were determined using a validated LC-MSMS method (32), with a LLOQ of 0.2 mg/liter, between-run precision, expressed as coefficient of variation, below 12%, and accuracy, expressed as a percentage of the theoretically added concentration, of between 89% and 109%. Linearity from 0.06 to 20.0 mg/liter was demonstrated.
The percentage of unbound voriconazole was calculated as follows (34):
| (1) |
where UF% stands for the percentage of unbound voriconazole and [voriconazole]PBS and [voriconazole]plasma represent the concentrations of voriconazole at equilibrium in the buffer compartment and in the plasma compartment, respectively.
Data collection.
Baseline data in the following categories were collected from the patient's electronic medical records and from the laboratory records: age, gender, weight, acute physiology and chronic health evaluation II (APACHE II) score at admission, underlying condition, diagnosis upon admission, in-hospital mortality, and 28-day mortality (defined as mortality within 28 days after discharge from the ICU). Treatment details consisted of the voriconazole daily dose and route of administration and coadministered drugs at the day of sampling, with special focus on those with high (above 70%) PPB, such as vitamin K antagonists, aspirin, nonsteroid anti-inflammatory drugs (NSAIDs), phenytoin, and valproic acid (35). Biochemical findings on the day of sampling included C-reactive protein (CRP) concentrations, albumin and α-1-acid glycoprotein (AAG) plasma concentrations, total plasma protein concentrations, blood urea nitrogen, creatinine plasma concentrations, creatinine clearance (Chronic Kidney Disease-Epidemiology Collaboration [CKD-EPI]), total and direct bilirubin plasma concentrations, and total VTCs. Also, liver function tests (plasma concentrations of alanine transaminase [ALT], aspartate transaminase [AST], bilirubin, gamma-glutamyltransferase [γGT], and alkaline phosphatase) were performed to detect abnormalities possibly caused by voriconazole. Any type of neurological adverse events was also monitored.
Statistical analysis.
Statistical analysis was performed using SPSS 20.0 for Windows (SPSS Inc. 2011, Chicago, IL). P < 0.05 was considered significant. Data were described with means ± standard deviations (SD) or medians and IQR according to the data distribution. Univariate correlations were investigated using scatterplots combined with Spearman's rank correlation coefficients. Multivariate analysis was conducted using linear mixed models with random intercept. Spearman's rank correlation coefficients were used instead of Pearson correlation coefficients because no assumptions of linearity were made in advance. However, because linear mixed models were used to create the prediction formula, Pearson correlation coefficients could also be used in this analysis.
RESULTS
Table 1 displays the baseline demographic and clinical and biochemical data for all patients included in both studies. In study A, 20 plasma samples were obtained from 13 patients. All patients received voriconazole for the treatment of IA (11 cases of probable and 2 cases of possible IA according to the revised criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group [EORTC/MSG]) (31). Risk factors for IA included the following: high-dose corticosteroids for chronic obstructive lung disorders (n = 5), lung transplantation (n = 1), radiotherapy for bronchus carcinoma (n = 1), kidney transplantation (n = 2), acute myeloid leukemia (n = 2), multiple organ failure following pancreatitis (n = 1), and vasculitis treated with immunosuppressive therapy (n = 1). In the in vitro study (study B), 66 plasma samples from 22 patients without aspergillosis were used. Plasma albumin levels ranged from 10 to 14.9 g/liter (2 patients), 15 to 19.9 g/liter (5 patients), 20 to 24.9 g/liter (5 patients), 25 to 29.9 g/liter (5 patients), and 30 to 35 g/liter (5 patients).
TABLE 1.
Demographics and clinical and biochemical characteristics at day of sampling of patients treated with voriconazole and patients with hypoalbuminemia not treated with voriconazole whose plasma samples were spiked with voriconazole
| Demographic or characteristic | Value(s) or resulta |
||
|---|---|---|---|
| Study A | Study B | Total | |
| Demographics | |||
| No. of patients | 13 | 22 | 35 |
| Median [IQR] age (yrs) | 62 [57.5–71.5] | 66 [60–74] | 65 [58–73] |
| Median [IQR] wt (kg) | 68 [61–82.5] | 71 [60.3–80] | 68 [62–80] |
| No. (%) of males | 7 (53.8) | 14 (63.6) | 21 (60) |
| Mean APACHE II score ± SD | 26.8 ± 6.9 | 23.5 ± 10.0 | 25.3 ± 8.3 |
| No. (%) of cases of in-hospital mortality | 6 (46.2) | 9 (40.9) | 15 (42.9) |
| No. (%) of cases of 28-day mortality | 6 (46.2) | 8 (36.4) | 15 (42.9) |
| Clinical and biochemical characteristics | |||
| Total no. of samples | 20 | 66 | 86 |
| No. (%) of samples from patients with i.v. VRC administrationb | 20 (100) | Not applicable | Not applicable |
| Median [range] albumin plasma concn (g/liter) (35–52)b,e | 31.1 [13.8–38.7] | 23.7 [14.4–33.7] | 26.7 [13.8–38.7] |
| Median [range] AAG plasma concn (g/liter) (0.51–1.17)b,e | 1.8 [1.1–3.0] | 1.4 [0–2.4] | 1.47 [0–3.0] |
| Mean total plasma protein concn ± SD (g/liter) (66–87)b,e | 56.6 ± 7.3 (n = 18) | 53.4 ± 12.4 (n = 63) | 54.2 ± 11.5 |
| Median [IQR] total bilirubin plasma concn (mg/liter) (<1)b,e | 0.41 [0.28–1.69] (n = 12) | 0.75 [0.42–1.93] | 0.67 [0.42–1.80] (n = 85) |
| Median [IQR] blood urea nitrogen (mg/dl) (<50)b,e | 70 [44.8–112.5] | 54.5[37–146] | 57.5 [37–132.5] |
| Median [IQR] creatinine plasma concn (mg/dl) (F, 0.51–0.95; M, 0.67–1.17)b,e | 0.95 [0.52–1.57] | 1.14 [0.67–1.99] (n = 57) | 1.13 [0.67–1.81] (n = 77) |
| CKD-EPI eGFR [ml/(min × 1.73m2)]b | 84 [37.5–108.3] | 66 [40–93] | 74 [40–100] |
| Median [IQR] CRP plasma concn (mg/liter) (<5)b,e | 47.2 [21.6–116.9] | 72.1 [48.2–89.6] | 71.5 [30.8–92.5] |
| Median [IQR] VRC daily dose (mg/day)b | 580 [465–800] | Not applicable | Not applicable |
| Median [IQR] VRC daily dose (mg/kg/day)b | 8 [7.5–8.3] | Not applicable | Not applicable |
| Median [IQR] no. of days of VRC treatment at day of sampling | 6 [4–13] | Not applicable | Not applicable |
| Median [IQR] total VRC plasma concn (mg/liter)b | 2.4 [1.5–4.5] | 1.5; 2.9; 9.0 (spiking expt) | 2.9 [1.5–9.0] |
| Median [IQR] unbound VRC plasma concn (mg/liter) | 0.71 [0.51–1.46] | 0.86 [0.45–2.66] | 0.85 [0.46–2.55] |
| Median [IQR] bound VRC plasma concn (mg/liter) | 1.52 [1.07–2.57] | 1.63 [0.85–5.35] | 1.62 [0.87–5.07] |
| Median [IQR] % protein binding | 49.6 [42.5–52.5] | 49.1 [42.6–52.3] | 49.1 [42.6–52.4] |
| Median [IQR] no. of coadministered drugs with PPB > 70%b | 2.5 [2–3]c | 4.5 [1–6]d | 3 [2–5] |
Study A, patients admitted to the ICU and treated with voriconazole; study B, patients admitted to the ICU and not treated with voriconazole (plasma spiked with 1.5, 2.9, and 9 mg/liter). i.v., intravenous; eGFR, estimated glomerular filtration rate; VRC, voriconazole; CRP, C-reactive protein; AAG, α-1-acid glycoprotein; n, number of samples.
Day of sampling.
In study A, no coadministration of NSAIDs, phenytoin, valproic acid, coumarins, or aspirin was documented.
In study B, administration of aspirin was documented in 7 patients and of acenocoumarol and phenytoin in 1 patient each. No coadministration of NSAIDs or valproic acid was documented.
The values in parentheses represent reference values according to the University Hospitals Leuven.
Voriconazole PPB correlated strongly with the plasma albumin concentration in the univariate analysis (for study A, R = 0.61, P < 0.01; for study B, R = 0.71, P < 0.01; for the combined data set, R = 0.67, P < 0.01) as shown in Fig. 1. No correlations and weak or moderate correlations with total bilirubin plasma concentration were seen in the univariate analysis (for study A, R = −0.17, P = 0.49; for study B, R = −0.42, P < 0.01; for the combined data set, R = −039, P < 0.01) or for age (for study A, R = −0.01, P = 0.966; for study B, R = −0.09, P < 0.49; for the combined data set, R = −0.10, P = 0.36), AAG plasma concentration (for study A, R = 0.049, P = 0.838; for study B, R = 0.27, P = 0.03; for the combined data set, R = 0.23, P = 0.03), CRP (for study A, R = −0.27, P = 0.251; for study B, R = −0.09, P < 0.45; for the combined data set, R = −0.15, P = 0.17), and total VTC (for study A, R = −0.40, P = 0.09; for study B, R = −0.04, P = 0.77; for the combined data set, R = −0.12, P = 0.26). Pearson correlation coefficients were very similar to the Spearman's rank correlation coefficients (data not shown).
FIG 1.
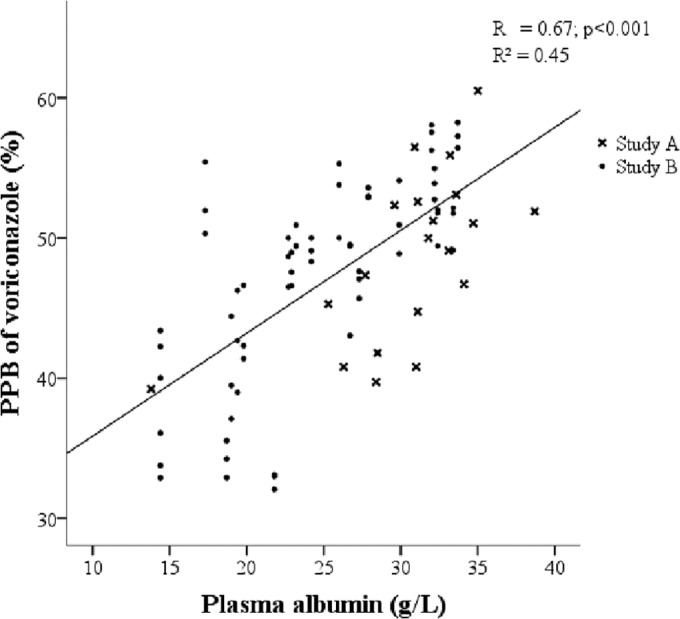
Positive correlation between the percentages of voriconazole plasma protein binding (PPB) and plasma albumin concentrations (in grams/liter) according to the univariate analysis of the combined set of data from study A (patients admitted to the ICU and treated with voriconazole) and study B (patients admitted to the ICU and not treated with voriconazole; plasma spiked with 1.5, 2.9, and 9 mg/liter voriconazole).
A multiple linear mixed model using the combined data set from study A and study B provided the following results: voriconazole PPB was influenced by plasma albumin concentrations (P < 0.001) and total bilirubin concentrations (P = 0.04). The number of drugs with a PPB higher than 70% did not significantly influence the final PPB of voriconazole (P = 0.36). According to our model, voriconazole PPB decreases by 0.67% if the plasma albumin concentration decreases by 1 g/liter (P < 0.001) and total bilirubin concentrations remain stable. An increase of 1 mg/dl in the total bilirubin concentration with stable plasma albumin concentrations results in a decrease in PPB of 0.19% (P < 0.001).
Based on these results, the bound voriconazole fraction can be predicted with the following equations:
| (2) |
| (3) |
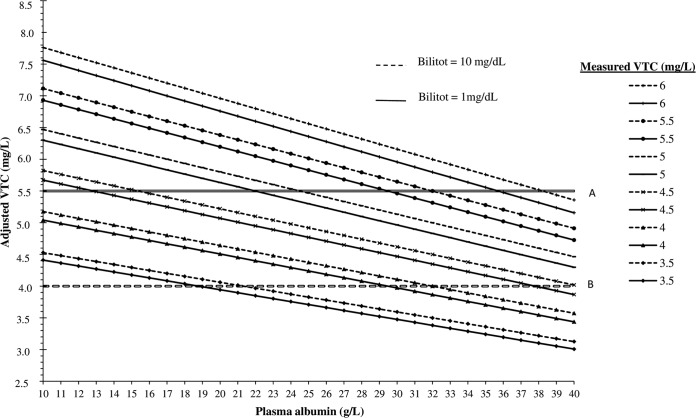
where VRC PPB is the percentage of voriconazole protein binding, total VTC represents the total voriconazole trough level expressed in mg/liter, HPA represents the human plasma albumin concentration expressed in g/liter, and Bilitot represents the total bilirubin plasma concentration expressed in mg/dl. Assuming that the level of voriconazole PPB is 50% (23) on average and assuming an upper limit of 5.5 mg/liter (26) for total VTCs associated with a relatively low probability of toxicity, the upper limit for nontoxic unbound voriconazole concentrations is 2.75 mg/liter. These assumptions, together with the above-mentioned formula, resulted in the data presented in Fig. 2, in which the clinical applicability of our proposed formula is demonstrated for two values of total bilirubin plasma concentrations and a broad range of measured VTCs.
FIG 2.
Adjusted total voriconazole trough concentrations (VTCs) based on plasma albumin concentrations (HPA), total bilirubin plasma concentrations (Bilitot), and measured VTCs. VTC = total voriconazole trough concentration (mg/liter). Adjusted VTCs were calculated with the following formula: adjusted VTC = [(100 − VRC PPB)/100] × measured VTC × 2 (where VRC PPB = 30.5015 + 0.668 × HPA − 0.1867 × Bilitot, based on the multivariate analysis of the combined data set of plasma from ICU patients treated with VRC and plasma from ICU patients not treated with VRC, spiked with 1.5, 2.9, and 9 mg/liter of VRC. Solid diagonal lines represent adjusted VTCs for measured VTCs ranging from 3.5 to 6 mg/liter, when Bilitot equals 1 mg/dl. Dotted diagonal lines represent adjusted VTCs for measured VTCs ranging from 3.5 to 6 mg/liter, when Bilitot equals 10 mg/dl. Solid horizontal line A represents an upper limit of 5.5 mg/liter for total VTCs associated with a relatively low probability of toxicity. Dashed horizontal line B represents an upper limit of 4 mg/liter for total VTCs associated with a relatively low probability of toxicity. VRC, voriconazole.
DISCUSSION
To the best of our knowledge, this is the first report to show a positive correlation between voriconazole PPB and plasma albumin concentration (P < 0.001).
Apart from the plasma albumin concentration, the total bilirubin plasma concentration also influences voriconazole PPB significantly (P = 0.04), probably because it is largely (up to 90%) protein bound, causing displacement of drugs from albumin (36).
We present a formula to adjust measured VTCs in individual cases, based on plasma albumin and total bilirubin concentrations. Similar formulas were developed previously to estimate unbound concentrations of phenytoin and valproic acid, two antiepileptic drugs with high PPB (above 70%) based on plasma albumin concentrations (27, 28), as total measured plasma concentrations could not always explain adverse events seen in patients (37–41).
Although the total bilirubin plasma concentration is included in the formula, Fig. 2 shows that it mainly influences voriconazole PPB in the case of total bilirubin plasma concentrations above 5 mg/dl. A statistically significant correlation between voriconazole PPB and total bilirubin concentrations was found in the multivariate analysis of the combined data set, but the correlation disappeared with total bilirubin concentrations above 5 mg/dl (P = 0.32; data not shown). Despite the warning not to use voriconazole in cases of Child-Pugh C liver cirrhosis with bilirubin plasma concentrations above 3 mg/liter (Vfend labeling [http://labeling.pfizer.com/ShowLabeling.aspx?id=618]), higher bilirubin concentrations are often seen in ICU patients in situations of acute disease, including patients with severe sepsis, ischemic hepatitis, or bile duct obstruction (42). The exact impact of bilirubin on voriconazole PPB should be further confirmed, e.g., by in vitro spiking experiments using plasma from patients with different plasma albumin and total bilirubin plasma concentrations.
We believe that our hypothesis and formula are clinically relevant. First, measured total VTCs should be interpreted with caution with regard to the reference range, especially in patients with profound hypoalbuminemia and total VTCs close to the upper limit of the therapeutic reference range. Dose adjustments should be considered and our proposed formula should be applied for patients with adverse events potentially related to an increased unbound voriconazole plasma concentration. In our center, we selected 2 to 5.5 mg/liter as the reference range for total VTCs (24–26, 43, 44). However, as there is still no clear consensus about the reference range of VTCs (24–26, 43, 45–49), the same conclusions can be drawn when simulating the worst-case scenario with an upper limit of 4 mg/liter (45–48), as shown in Fig. 2. Second, one could question whether free drug monitoring for voriconazole should be implemented in daily practice in a manner comparable to what has recently been discussed for vancomycin (50). However, the most important issue for voriconazole administration is to avoid subtherapeutic VTCs and therapeutic failure, especially in a Caucasian population most often presenting with wild-type CYP2C9/CYP2C19 metabolism, rather than to avoid toxicity associated with supratherapeutic VTCs (51). Therefore, this process would be too time-consuming, due to an additional step of, e.g., equilibrium dialysis or other separating techniques which should be built in the analytical process (23), making our proposed formula more practical to use. Still, our findings are definitely relevant, because even if few patients would suffer from adverse events due to increased elevated unbound voriconazole concentrations, it would be beneficial to prevent therapy cessation or to indicate a need to switch to another antifungal agent. Moreover, in other populations in which toxicity is the main driver of therapeutic drug monitoring, as described for a Korean randomized controlled trial (52), adjusting measured total VTCs based on plasma albumin concentrations might avoid hepato- and neurotoxicity, being the most common side effects requiring a switch to an alternative antifungal therapy. It is possible that neurotoxicity can occur in patients with hypoalbuminemia and total VTCs within the reference range because of the increased unbound voriconazole concentration which can penetrate the blood-brain barrier. Otherwise, higher penetration of increased unbound voriconazole concentrations through the blood-brain barrier could also improve the therapeutic effect in cases of an invasive fungal infection in the central nervous system. Third, given the extremely variable pharmacokinetic profile of voriconazole, the discovery of additional PK-altering factors may be of great value in unraveling the rather unpredictable behavior of voriconazole in the human body. Finally, conflicting information has been published in the past about the clinical relevance of increased unbound drug concentrations in cases of hypoalbuminemia (30, 53). However, in these studies, the drugs that were the subject of debate were mostly drugs with linear pharmacokinetics. The influence of hypoalbuminemia on protein binding characteristics of antimicrobial agents was demonstrated in critically ill patients (15, 16) for highly protein-bound drugs (PPB above 70%) (16) such as ceftriaxone (PPB, ±95%) (19), flucloxacilline (PPB, above 90%) (15), carbamazepine (PPB, 70% to 80%) (54), phenytoin (PPB, ±90%) (28, 55, 56), and valproic acid (PPB, 80% to 90%) (27, 57). For drugs with low to moderate PPB (30 to 70%) and linear pharmacokinetics, changes in PPB have little consequence in clinical practice, as small increases in unbound drug concentrations are immediately metabolized and eliminated (16). To the best of our knowledge, we are the first to show the influence of hypoalbuminemia on the PPB of voriconazole, a drug with a nonlinear PK profile due to a saturated metabolism combined with a narrow therapeutic range and moderate PPB. Recently, unbound voriconazole concentrations were also investigated with ultrafiltration (UF) by Florent et al. A correlation was seen between the unbound voriconazole fraction and albumin plasma concentrations below 25 g/liter. Overall, no clear correlation with plasma albumin concentrations was measurable (58). Remarkably, the voriconazole PPB values were highly deviant from our previously published in vitro data, from the data published by Roffey et al. (59), and from the results in this study, which were all generated by ED, in our opinion still the gold standard in determining protein binding of drugs (34, 60–62). Moreover, the most important factors possibly influencing the performance of the ED procedure, such as environmental temperature, pH, mass balance, and volume shift, were ruled out (23), resulting in a robust and reliable method for determining voriconazole PPB. We believe that the differences in PPB might potentially be explained by the use of different methods for the separation of unbound and bound voriconazole concentrations. It has previously been shown that UF may underestimate unbound drug concentrations compared to ED (63).
Although highly protein-bound drugs are able to expel other drugs from albumin, thereby inducing elevated unbound plasma concentrations (64), no significant correlation between voriconazole PPB and the amount of coadministered drugs with a PPB of more than 70% could be found in our study population. Apart from a high PPB, drugs should have affinity for the same albumin binding places to expel voriconazole from the plasma protein (65). In study A, none of the included patients received nonsteroidal anti-inflammatory drugs (NSAIDs) (PPB above 90%), aspirin (PPB above 99%), phenytoin (PPB of ±90%) (66), valproic acid (PPB of 80% to 90%) (67), or vitamin K antagonists (PPB above 90%), drugs especially known for their ability to expel drugs from albumin (35). In study B, 7 patients received aspirin, and only 1 patient received the vitamin K antagonist acenocoumarol. The role of coadministration of these specific highly protein-bound drugs in voriconazole PPB should be elucidated in the future both in a real-life setting and in in vitro spiking experiments with voriconazole and albumin and known concentrations of the displacing agents.
The most important limitation of this experiment was the relatively small data set. However, to our knowledge, this is the first report identifying the role of protein binding and hypoalbuminemia in the variable PK profile of voriconazole, and as the correlation was already clearly significant in our rather limited data set, we believe that larger data sets would only confirm this. A prospective experiment in which plasma albumin concentrations and unbound voriconazole plasma concentrations are closely monitored in all patients treated with voriconazole, suffering from adverse events due to voriconazole, irrespective of the total VTCs, would be interesting to document the clinical relevance.
The fact that we combined real-life samples with spiked plasma is not really a limitation. No significant differences between the PPB values of the two groups were found, a result which had already been shown in our previous work (23). Moreover, all samples were taken in a medical ICU population and were subject to identical sample handling procedures. Premarketing studies determining the PPB characteristics of drugs are often similarly performed on blank plasma spiked with the investigational drug (59).
Only adult ICU patients were included in this study. Most likely, this phenomenon will also be seen in adult non-critically ill patients presenting with severe hypoalbuminemia treated with voriconazole. Apart from plasma albumin and total bilirubin plasma concentrations, no additional factors influencing voriconazole PPB, specifically associated with or encountered in ICU patients, were identified in the multivariate analysis. It would be interesting to confirm our results in, e.g., a cohort of non-critically ill patients with hematological disease. In the pediatric population, we do not expect a clinically relevant influence of hypoalbuminemia on voriconazole PPB. Similarly to drugs with linear pharmacokinetics and moderate to low protein binding, elevated unbound concentrations due to hypoalbuminemia would probably be quickly metabolized and cleared due to the additional activity of the flavin-containing mono-oxygenase 3 (FMO3) enzyme system, responsible for the linear voriconazole pharmacokinetics in children, within the reference range (7, 8).
Finally, no data are available for plasma albumin concentrations above 40 g/liter. However, because hyperalbuminemia with values above 50 g/liter is not often seen in clinical practice, we do not expect large differences from the adjusted total voriconazole concentrations in the case of albumin concentrations just below 40 g/liter.
Conclusion.
Although voriconazole PPB is documented to be moderate (±50%) in patients with normal plasma albumin levels, hypoalbuminemia can alter voriconazole PPB, probably due to the saturated hepatic metabolism. Increased unbound voriconazole plasma concentrations in patients with profound hypoalbuminemia can possibly cause adverse events, even when total voriconazole plasma concentrations are within the reference range. Likewise, measured total voriconazole concentrations should be adjusted via the proposed formula, in patients suffering from hypoalbuminemia and showing adverse events potentially related to voriconazole, especially in those with severe hypoalbuminemia.
ACKNOWLEDGMENTS
K. Vanstraelen has received travel support from Gilead, MSD, and Pfizer and has received lecture honoraria from Pfizer. J. Wauters has received research grants from MSD and Pfizer and has received travel support from MSD and Pfizer. J. Maertens has received research grants from Gilead, MSD, Pfizer, and Astellas, has received travel support from Gilead, MSD, Pfizer, and Astellas, and has received lecture honoraria from Gilead, MSD, Pfizer, and Astellas. K. Lagrou has received research grants from Gilead, MSD, and Pfizer, has received travel support from Gilead, MSD, and Pfizer, and has received lecture honoraria from Gilead, MSD, and Pfizer. I. Spriet has received research grants from MSD and Pfizer, has received travel support from Gilead, MSD, and Pfizer, and has received lecture honoraria from Gilead, MSD, and Pfizer. We have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article apart from those disclosed.
Footnotes
Published ahead of print 2 September 2014
REFERENCES
- 1.Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546–2553. 10.1128/AAC.46.8.2546-2553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trifilio S, Ortiz R, Pennick G, Verma A, Pi J, Stosor V, Zembower T, Mehta J. 2005. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 35:509–513. 10.1038/sj.bmt.1704828. [DOI] [PubMed] [Google Scholar]
- 3.Purkins L, Wood N, Greenhalgh K, Eve MD, Oliver SD, Nichols D. 2003. The pharmacokinetics and safety of intravenous voriconazole - a novel wide-spectrum antifungal agent. Br. J. Clin. Pharmacol. 56(Suppl 1):2–9. 10.1046/j.1365-2125.2003.01992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh TJ, Karlsson MO, Driscoll T, Arguedas AG, Adamson P, Saez-Llorens X, Vora AJ, Arrieta AC, Blumer J, Lutsar I, Milligan P, Wood N. 2004. Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration. Antimicrob. Agents Chemother. 48:2166–2172. 10.1128/AAC.48.6.2166-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasqualotto AC, Shah M, Wynn R, Denning DW. 2008. Voriconazole plasma monitoring. Arch. Dis. Child. 93:578–581. 10.1136/adc.2007.118844. [DOI] [PubMed] [Google Scholar]
- 6.Hyland R, Jones BC, Smith DA. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos 31:540–547. 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 7.Yanni SB, Annaert PP, Augustijns P, Bridges A, Gao Y, Benjamin DK, Jr, Thakker DR. 2008. Role of flavin-containing monooxygenase in oxidative metabolism of voriconazole by human liver microsomes. Drug Metab. Dispos 36:1119–1125. 10.1124/dmd.107.019646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanni SB, Annaert PP, Augustijns P, Ibrahim JG, Benjamin DK, Jr, Thakker DR. 2010. In vitro hepatic metabolism explains higher clearance of voriconazole in children versus adults: role of CYP2C19 and flavin-containing monooxygenase 3. Drug Metab. Dispos 38:25–31. 10.1124/dmd.109.029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolton MJ, Ray JE, Chen SC, Ng K, Pont LG, McLachlan AJ. 2012. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob. Agents Chemother. 56:4793–4799. 10.1128/AAC.00626-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiler S, Zoller H, Graziadei I, Vogel W, Bellmann-Weiler R, Joannidis M, Bellmann R. 2007. Altered pharmacokinetics of voriconazole in a patient with liver cirrhosis. Antimicrob. Agents Chemother. 51:3459–3460. 10.1128/AAC.00791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purkins L, Wood N, Kleinermans D, Greenhalgh K, Nichols D. 2003. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br. J. Clin. Pharmacol. 56(Suppl 1):17–23. 10.1046/j.1365-2125.2003.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams D. 2012. The effect of enteral nutrition supplements on serum voriconazole levels. J. Oncol. Pharm. Pract. 18:128–131. 10.1177/1078155210396576. [DOI] [PubMed] [Google Scholar]
- 13.Mohammedi I, Piens MA, Padoin C, Robert D. 2005. Plasma levels of voriconazole administered via a nasogastric tube to critically ill patients. Eur. J. Clin. Microbiol. Infect. Dis. 24:358-360. 10.1007/s10096-005-1325-7. [DOI] [PubMed] [Google Scholar]
- 14.Driscoll TA, Yu LC, Frangoul H, Krance RA, Nemecek E, Blumer J, Arrieta A, Graham ML, Bradfield SM, Baruch A, Liu P. 2011. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised children and healthy adults. Antimicrob. Agents Chemother. 55:5770–5779. 10.1128/AAC.00531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulldemolins M, Roberts JA, Wallis SC, Rello J, Lipman J. 2010. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. J. Antimicrob. Chemother. 65:1771–1778. 10.1093/jac/dkq184. [DOI] [PubMed] [Google Scholar]
- 16.Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. 2011. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 50:99–110. 10.2165/11539220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki K, Chuang VT, Maruyama T, Otagiri M. 2013. Albumin-drug interaction and its clinical implication. Biochim. Biophys. Acta 1830:5435–5443. 10.1016/j.bbagen.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, Roberts JA. 2013. Protein binding of beta-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob. Agents Chemother. 57:6165–6170. 10.1128/AAC.00951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mimoz O, Soreda S, Padoin C, Tod M, Petitjean O, Benhamou D. 2000. Ceftriaxone pharmacokinetics during iatrogenic hydroxyethyl starch-induced hypoalbuminemia: a model to explore the effects of decreased protein binding capacity on highly bound drugs. Anesthesiology 93:735-743. 10.1097/00000542-200009000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. 2001. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 47:421–429. 10.1093/jac/47.4.421. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, Hope WW, Farkas A, Neely MN, Schentag JJ, Drusano G, Frey OR, Theuretzbacher U, Kuti JL. 2014. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect. Dis. 14:498–509. 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnelly JP, De Pauw BE. 2004. Voriconazole-a new therapeutic agent with an extended spectrum of antifungal activity. Clin. Microbiol. Infect. 10(Suppl 1):107–117. 10.1111/j.1470-9465.2004.00838.x. [DOI] [PubMed] [Google Scholar]
- 23.Vanstraelen K, Wauters J, deLoor H, Vercammen I, Annaert P, Lagrou K, Spriet I. 2014. Protein-binding characteristics of voriconazole determined by high-throughput equilibrium dialysis. J. Pharm. Sci. 103:2565–2570. 10.1002/jps.24064. [DOI] [PubMed] [Google Scholar]
- 24.Troke PF, Hockey HP, Hope WW. 2011. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob. Agents Chemother. 55:4782–4788. 10.1128/AAC.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolton MJ, Mikus G, Weiss J, Ray JE, McLachlan AJ. 18 February 2014. Understanding variability with voriconazole using a population pharmacokinetic approach: implications for optimal dosing. J. Antimicrob. Chemother. 10.1093/jac/dku031. [DOI] [PubMed] [Google Scholar]
- 26.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211. 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 27.Hermida J, Tutor JC. 2005. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J. Pharmacol. Sci. 97:489–493. 10.1254/jphs.FPE04007X. [DOI] [PubMed] [Google Scholar]
- 28.Dager WE, Inciardi JF, Howe TL. 1995. Estimating phenytoin concentrations by the Sheiner-Tozer method in adults with pronounced hypoalbuminemia. Ann. Pharmacother. 29:667–670. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GD, Pak C, Doane KW, Griffy KG, Temkin NR, Wilensky AJ, Winn HR. 1997. Revised Winter-Tozer equation for normalized phenytoin concentrations in trauma and elderly patients with hypoalbuminemia. Ann. Pharmacother. 31:279–284. [DOI] [PubMed] [Google Scholar]
- 30.Musteata FM. 2012. Calculation of normalized drug concentrations in the presence of altered plasma protein binding. Clin. Pharmacokinet. 51:55–68. 10.2165/11595650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821. 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauwels S, Vermeersch P, Van Eldere J, Desmet K. 2012. Fast and simple LC-MS/MS method for quantifying plasma voriconazole. Clin. Chim. Acta 413:740–743. 10.1016/j.cca.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 33.McGinlay JM, Payne RB. 1988. Serum albumin by dye-binding: bromocresol green or bromocresol purple? The case for conservatism. Ann. Clin. Biochem. 25(Pt 4):417–421. [DOI] [PubMed] [Google Scholar]
- 34.Wan H, Rehngren M. 2006. High-throughput screening of protein binding by equilibrium dialysis combined with liquid chromatography and mass spectrometry. J. Chromatogr. A 1102:125–134. 10.1016/j.chroma.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Hardman JG, Limbird LE, Goodman Gilman A. (ed). 2001. Goodman & Gilman's the pharmacological basis of therapeutics, 10th ed., p 1924–2023 R.R. Donnelley and Sons, Chicago, IL. [Google Scholar]
- 36.Weiss JS, Gautam A, Lauff JJ, Sundberg MW, Jatlow P, Boyer JL, Seligson D. 1983. The clinical importance of a protein-bound fraction of serum bilirubin in patients with hyperbilirubinemia. N. Engl. J. Med. 309:147–150. 10.1056/NEJM198307213090305. [DOI] [PubMed] [Google Scholar]
- 37.De Schoenmakere G, De Waele J, Terryn W, Deweweire M, Verstraete A, Hoste E, Rottey S, Lameire N, Colardyn F. 2005. Phenytoin intoxication in critically ill patients. Am. J. Kidney Dis. 45:189–192. 10.1053/j.ajkd.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Lindow J, Wijdicks EF. 1994. Phenytoin toxicity associated with hypoalbuminemia in critically ill patients. Chest 105:602–604. 10.1378/chest.105.2.602. [DOI] [PubMed] [Google Scholar]
- 39.Kemper EM, van Kan HJ, Speelman P, de Gans K, Beijnen JH, Schellens JH. 2007. Severe phenytoin intoxication in patients with hypoalbuminaemia. Ned. Tijdschr. Geneeskd. 151:138–141 (In Dutch.) [PubMed] [Google Scholar]
- 40.Thakral A, Shenoy R, Deleu D. 2003. Acute visual dysfunction following phenytoin-induced toxicity. Acta Neurol. Belg. 103:218–220. [PubMed] [Google Scholar]
- 41.Rambeck B, Schnabel R, May T, Jurgens U, Villagran R. 1990. Postmortem serum protein binding and brain concentrations of antiepileptic drugs in autoptic specimens from 45 epileptic patients. Ther. Drug Monit. 12:533–540. 10.1097/00007691-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Angus DC, van der Poll T. 2013. Severe sepsis and septic shock. N. Engl. J. Med. 369:2063. 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- 43.Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. 2012. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob. Agents Chemother. 56:5503–5510. 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueda K, Nannya Y, Kumano K, Hangaishi A, Takahashi T, Imai Y, Kurokawa M. 2009. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int. J. Hematol. 89:592–599. 10.1007/s12185-009-0296-3. [DOI] [PubMed] [Google Scholar]
- 45.Hagiwara E, Shiihara J, Matsushima A, Enomoto T, Tagawa A, Sekine A, Tsuchiya N, Baba T, Shinohara T, Endo T, Sogo Y, Nishihira R, Komatsu S, Kato T, Ogura T, Takahashi H. 2009. Usefulness of monitoring plasma voriconazole concentration in patients with chronic necrotizing pulmonary aspergillosis. Nihon Kokyuki Gakkai Zasshi 47:93–97 (In Japanese.) [PubMed] [Google Scholar]
- 46.Hamada Y, Seto Y, Yago K, Kuroyama M. 2012. Investigation and threshold of optimum blood concentration of voriconazole: a descriptive statistical meta-analysis. J. Infect. Chemother. 18:501–507. 10.1007/s10156-011-0363-6. [DOI] [PubMed] [Google Scholar]
- 47.Hamada Y, Tokimatsu I, Mikamo H, Kimura M, Seki M, Takakura S, Ohmagari N, Takahashi Y, Kasahara K, Matsumoto K, Okada K, Igarashi M, Kobayashi M, Mochizuki T, Nishi Y, Tanigawara Y, Kimura T, Takesue Y. 2013. Practice guidelines for therapeutic drug monitoring of voriconazole: a consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 19:381–392. 10.1007/s10156-013-0607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki Y, Tokimatsu I, Sato Y, Kawasaki K, Goto T, Hashinaga K, Itoh H, Hiramatsu K, Kadota J. 2013. Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity. Clin. Chim. Acta 424:119–122. 10.1016/j.cca.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 49.Pascual A, Csajka C, Buclin T, Bolay S, Bille J, Calandra T, Marchetti O. 2012. Challenging recommended oral and intravenous voriconazole doses for improved efficacy and safety: population pharmacokinetics-based analysis of adult patients with invasive fungal infections. Clin. Infect. Dis. 55:381–390. 10.1093/cid/cis437. [DOI] [PubMed] [Google Scholar]
- 50.Berthoin K, Ampe E, Tulkens PM, Carryn S. 2009. Correlation between free and total vancomycin serum concentrations in patients treated for Gram-positive infections. Int. J. Antimicrob. Agents 34:555–560. 10.1016/j.ijantimicag.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Bertilsson L. 1995. Geographical/interracial differences in polymorphic drug oxidation. Current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin. Pharmacokinet. 29:192–209. [DOI] [PubMed] [Google Scholar]
- 52.Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, Song KH, Choe PG, Kim NJ, Jang IJ, Oh MD, Yu KS. 2012. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin. Infect. Dis. 55:1080–1087. 10.1093/cid/cis599. [DOI] [PubMed] [Google Scholar]
- 53.D'Arcy PF, McElnay JC. 1982. Drug interactions involving the displacement of drugs from plasma protein and tissue binding sites. Pharmacol. Ther. 17:211–220. 10.1016/0163-7258(82)90012-2. [DOI] [PubMed] [Google Scholar]
- 54.Bertilsson L. 1978. Clinical pharmacokinetics of carbamazepine. Clin. Pharmacokinet. 3:128–143. 10.2165/00003088-197803020-00003. [DOI] [PubMed] [Google Scholar]
- 55.Perucca E. 1980. Plasma protein binding of phenytoin in health and disease: relevance to therapeutic drug monitoring. Ther. Drug Monit. 2:331–344. [PubMed] [Google Scholar]
- 56.von Winckelmann SL, Spriet I, Willems L. 2008. Therapeutic drug monitoring of phenytoin in critically ill patients. Pharmacotherapy 28:1391–1400. 10.1592/phco.28.11.1391. [DOI] [PubMed] [Google Scholar]
- 57.Bowdle AT, Patel IH, Levy RH, Wilensky AJ. 1980. Valproic acid dosage and plasma protein binding and clearance. Clin. Pharmacol. Ther. 28:486–492. 10.1038/clpt.1980.192. [DOI] [PubMed] [Google Scholar]
- 58.Florent A, Gandia P, Seraissol P, Chatelut E, Houin G. 9 May 2014. Determination of plasma unbound fraction of voriconazole in patients treated with a prophylactic or a curative treatment. Ther. Drug Monit. 10.1097/FTD.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 59.Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, Smith DA, Walker DK, Wood N. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731–741. 10.1124/dmd.31.6.731. [DOI] [PubMed] [Google Scholar]
- 60.Howard ML, Hill JJ, Galluppi GR, McLean MA. 2010. Plasma protein binding in drug discovery and development. Comb. Chem. High Throughput Screen. 13:170–187. 10.2174/138620710790596745. [DOI] [PubMed] [Google Scholar]
- 61.Sebille B. 1990. Methods of drug protein binding determinations. Fundam. Clin. Pharmacol. 4(Suppl 2):151s–161s. 10.1111/j.1472-8206.1990.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 62.Sebille B, Zini R, Madjar CV, Thuaud N, Tillement JP. 1990. Separation procedures used to reveal and follow drug-protein binding. J. Chromatogr. 531:51–77. 10.1016/S0378-4347(00)82280-X. [DOI] [PubMed] [Google Scholar]
- 63.Barré J, Chamouard JM, Houin G, Tillement JP. 1985. Equilibrium dialysis, ultrafiltration, and ultracentrifugation compared for determining the plasma-protein-binding characteristics of valproic acid. Clin. Chem. 31:60–64. [PubMed] [Google Scholar]
- 64.Johnson GJ, Kilpatrick CJ, Bury RW, Fullinfaw RO, Moulds RF. 1989. Unbound phenytoin plasma concentrations in patients comedicated with sodium valproate–the predictive value of plasma albumin concentration. Br. J. Clin. Pharmacol. 27:843–849. 10.1111/j.1365-2125.1989.tb03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koch-Weser J, Sellers EM. 1976. Drug therapy. Binding of drugs to serum albumin (second of two parts). N. Engl. J. Med. 294:526–531. [DOI] [PubMed] [Google Scholar]
- 66.Richens A. 1979. Clinical pharmacokinetics of phenytoin. Clin. Pharmacokinet. 4:153–169. 10.2165/00003088-197904030-00001. [DOI] [PubMed] [Google Scholar]
- 67.Gugler R, Mueller G. 1978. Plasma protein binding of valproic acid in healthy subjects and in patients with renal disease. Br. J. Clin. Pharmacol. 5:441–446. 10.1111/j.1365-2125.1978.tb01652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]