Abstract
Teicoplanin is frequently administered to treat Gram-positive infections in pediatric patients. However, not enough is known about the pharmacokinetics (PK) of teicoplanin in children to justify the optimal dosing regimen. The aim of this study was to determine the population PK of teicoplanin in children and evaluate the current dosage regimens. A PK hospital-based study was conducted. Current dosage recommendations were used for children up to 16 years of age. Thirty-nine children were recruited. Serum samples were collected at the first dose interval (1, 3, 6, and 24 h) and at steady state. A standard 2-compartment PK model was developed, followed by structural models that incorporated weight. Weight was allowed to affect clearance (CL) using linear and allometric scaling terms. The linear model best accounted for the observed data and was subsequently chosen for Monte Carlo simulations. The PK parameter medians/means (standard deviation [SD]) were as follows: CL, [0.019/0.023 (0.01)] × weight liters/h/kg of body weight; volume, 2.282/4.138 liters (4.14 liters); first-order rate constant from the central to peripheral compartment (Kcp), 0.474/3.876 h−1 (8.16 h−1); and first-order rate constant from peripheral to central compartment (Kpc), 0.292/3.994 h−1 (8.93 h−1). The percentage of patients with a minimum concentration of drug in serum (Cmin) of <10 mg/liter was 53.85%. The median/mean (SD) total population area under the concentration-time curve (AUC) was 619/527.05 mg · h/liter (166.03 mg · h/liter). Based on Monte Carlo simulations, only 30.04% (median AUC, 507.04 mg · h/liter), 44.88% (494.1 mg · h/liter), and 60.54% (452.03 mg · h/liter) of patients weighing 50, 25, and 10 kg, respectively, attained trough concentrations of >10 mg/liter by day 4 of treatment. The teicoplanin population PK is highly variable in children, with a wider AUC distribution spread than for adults. Therapeutic drug monitoring should be a routine requirement to minimize suboptimal concentrations.
(This trial has been registered in the European Clinical Trials Database Registry [EudraCT] under registration number 2012-005738-12.)
INTRODUCTION
Gram-positive infections are an important cause of morbidity and mortality in neonatal and pediatric intensive care units (1, 2). A significant rise in infections caused by methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative staphylococci (CoNS) has led to increased use of glycopeptides in the last decade (3, 4). Both vancomycin and teicoplanin are used for treatment of invasive infections caused by Gram-positive organisms, especially those that are resistant to β-lactam antibiotics (5, 6). The currently recommended regimen for teicoplanin in adults is 3 loading doses of 400 mg every 12 hours followed by a maintenance dose of 400 mg/day. In contrast, children receive 3 loading doses of 10 mg/kg of body weight every 12 hours followed by a maintenance dose of 10 mg/kg daily (7). However, there is a relative paucity of information to justify these regimens in children and even less information to identify optimal dosing strategies.
Regulatory authorities such as the European Medicines Agency (EMA) have developed strategies to facilitate the safe and effective use of medicines in neonates and children (8). EMA supports the extrapolation of information from adults to children provided there are adequate safety data in the latter and the pharmacodynamics can reasonably assumed to be the same in both populations. This approach requires the development of robust population pharmacokinetic (PK) models in both adults and children, which facilitates the design of regimens that enable drug exposures in both populations to be matched (8).
Teicoplanin is largely used without routinely measuring concentrations in the majority of pediatric patients. In our pediatric hospital setting, we have observed anecdotal cases of clinical failures with teicoplanin therapy and have observed “MIC creep” for CoNS with MICs at the breakpoint (4 mg/liter) (unpublished data). To further investigate the clinical pharmacology of teicoplanin and to provide an insight into effective regimens for children, we performed a population PK study. The specific objectives of this study were to (i) describe the population PK of teicoplanin in children in a hospital setting, (ii) explore the percentage of patients attaining a predose minimum concentration (Cmin) of >10 mg/liter, (iii) define the area under the concentration-time curve (AUC) distributions following the administration of currently recommended pediatric regimens, and (iv) compare the extent of variability in drug exposure to that observed in adults.
MATERIALS AND METHODS
Study design, pediatric patient population, and sample collection.
An open-label, hospital-based PK study using a sparse blood sampling strategy was conducted. Patients 0 to 16 years of age were recruited from Alder Hey Children's Hospital, which has a catchment area of approximately 7.6 million people. The study was approved by the Medicines and Healthcare Products Regulatory Agency (clinical trial authorization number 21362/00003/001-0001) and the National Research Ethics Service and Regional Committee. The trial was registered with the European Clinical Trials Database Registry (EudraCT; 2012-005738-12).
Participants were screened and recruited according to three age categories to ensure a representative sample was obtained. Such an approach is consistent with the European Medicines Agency E11 Guidance for Clinical Trials of Investigational Products (CTIMP) in pediatrics (9). The following age categories were used: 1 to 23 months, 2 to 11 years, and 11 to 16 years. A 4-month prospective feasibility assessment conducted before the trial indicated that the pediatric intensive-care unit, the oncology unit, and the intermediate-care unit had the higher prescription rates of teicoplanin, so these units were chosen for targeted screening and recruitment of participants. All patients who received teicoplanin and were likely to survive >72 h were eligible for the study. Written informed consent was obtained from parents and/or legal guardians.
Teicoplanin was used at the discretion of the treating physician. The dosage regimen for children >1 month of age was 10 mg/kg every 12 h for 3 loading doses followed by 10 mg/kg once daily. Teicoplanin was infused over 5 min in children. The duration of treatment was also at the discretion of the treating physician.
Blood samples (0.2 ml) were obtained throughout the first and last dose interval (1, 3, 6, and 24 h postdose). The sampling period was up to a maximum of 264 h for some patients and up to 144 to 168 h for the majority of patients (Fig. 1). If the first dose administration occurred before informed consent was obtained, a predose sample was obtained. When possible, a washout sample was collected 24 h after the last dose was administered. Samples were centrifuged at 1,500 × g for 10 min, and serum was stored at −80°C prior to analysis. Demographic and other variables (height, weight, and serum creatinine level) with a potential impact on the PK of teicoplanin and/or influence on the determination of teicoplanin (e.g., concomitant medications) were also collected for each patient.
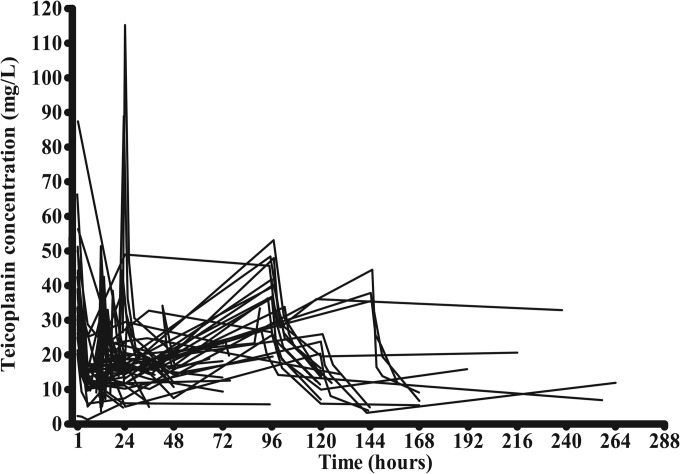
FIG 1.
Teicoplanin serum concentrations in 39 patients. Patients were dosed according to recommended regimens. Children more than 1 month old: 3 loading doses every 12 h, then once daily.
Adult patient population.
Adult patients with normal renal function who were previously treated with chemotherapy because of acute lymphocytic or acute nonlymphocytic leukemia and subsequently were developing febrile neutropenia were recruited to a prospective observational PK study, as previously reported by Pea et al. (10). All patients received teicoplanin for the first 72 h. Subsequently, if a Gram-positive organism susceptible to teicoplanin was isolated and/or resolution of fever was documented within 72 h, teicoplanin therapy was continued for at least 8 days. There were two dosing groups. The standard-dosage group received standard loading and maintenance dosages of teicoplanin (400 mg every 12 h for 3 doses followed by 400 mg once daily), and the high-dosage group received a higher loading regimen (800 mg followed by 400 mg every 12 h on day 1; 600 mg plus 400 mg every 12 h on day 2) followed by a high maintenance regimen (400 mg every 12 h on day 3 and thereafter). Teicoplanin was infused over 15 min. Blood samples were collected 1 h after the first dose to assess the peak level and at 12, 24, 48, 72, 96, 120, and 144 h to estimate terminal elimination.
Demographic data were analyzed with SPSS Statistics, version 21 (IBM Corporation, Armonk, NY [http://www-01.ibm.com/software/analytics/]).
Measurement of teicoplanin concentrations.
A fluorescence polarization immunoassay (FPIA) (Thermo Fisher Scientific, Germany) was used to quantify teicoplanin concentrations in serum. This is a homogeneous particle-enhanced turbidimetric immunoassay that utilizes quantitative microsphere system (QMS) technology and was implemented on an automated analyzer (Abbott Architect ci4100). The assay is based on competition between the drug in the sample and the drug coated onto a microparticle for antibody binding sites of the teicoplanin antibody reagent. A concentration-dependent agglutination inhibition curve was obtained with the minimum and maximum rates of agglutination at the highest and lowest teicoplanin concentrations, respectively. The limit of quantification (LOQ) was <3.0 mg/liter. The dynamic range was 3 to 100 mg/liter, and overall precision was <6%.
Population pharmacokinetic models.
All data were analyzed using a nonparametric population modeling methodology (a nonparametric adaptive grid [NPAG]) with the population pharmacokinetic software program Pmetrics (version 1.2.6; University of Southern California, Los Angeles, CA [http://www.lapk.org/pmetrics.php]) (11) for R (version 3.1.0, Institute for Statistics and Mathematics, Vienna, Austria [http://www.r-project.org/]) (12). The inverse of the estimated assay variance was used as the weighting function for all models.
Three structural models were explored and used in this study. The first represented a standard two-compartment PK model with time-delimited zero-order intravenous infusion and first-order elimination from central compartment. The model is described by the differential equations 1 and 2 below:
| (1) |
| (2) |
where X (1) and X (2) represent the amount of teicoplanin in milligrams (mg) in the central (c) and peripheral (p) compartments, respectively. R (1) is the rate of infusion of drug into the central compartment in mg per hour. The central compartment has volume (Vc) in liters, from which there is clearance (SCL) in liters per hour. The central and peripheral compartments are connected by the first-order rate constant from the central to peripheral compartment (Kcp) and the first-order rate constant from peripheral to central compartment (Kpc) in h−1.
The effects of weight and serum creatinine on the population PK of teicoplanin were explored. The Bayesian estimates for clearance and volume of distribution from each patient were obtained from the standard model (above) and plotted against weight and serum creatinine, using both linear and logarithmic scales. Since both linear and logarithmic relationships between clearance and weight appeared tenable, linear and allometric models that incorporated weight as a covariate were developed. The linear model took the following form:
| (3) |
| (4) |
where SCLslope represents the slope of the linear relationship between clearance and weight. The other terms and relationships are the same as described for the standard model. The intercept of the linear relationship between clearance and weight was initially included in the structural model, but estimates from preliminary runs were approximately zero. Consequently, in the linear model, SCL = SCLslope × weight.
Since a relationship between the log10-transformed estimates for weight and clearance from the standard model was also apparent, the performance of an allometric power model was investigated. Such models have been widely used to determine the effect of size on the pharmacokinetics of various compounds in children and neonates (13, 14). The allometric scaling exponent in equation 5 was fixed at 0.75. In addition, only clearance and not volume appeared to have a relationship with weight, so clearance was normalized to a 70-kg adult, as described elsewhere (14). The differential equations describing the allometric model are as follows:
| (5) |
| (6) |
where SCLstd represents the normalized estimate for clearance in a 70-kg individual, and the other parameters are as described above.
For the adult data, a standard 2-compartment structural model was used.
Model evaluation, comparison, and performance.
For each model, scatter plots of the observed-predicted relationships for each patient and the population as a whole were examined. Goodness of fit was evaluated on the basis of a visual inspection of the data, coefficient of determination of a linear regression of the observed versus predicted values in the scatter plot after the Bayesian step, and the slopes and intercepts of the regression. The log-likelihood values of each model were used to compare models. Statistical comparisons were made using the likelihood ratio test, where twice the likelihood difference was evaluated against a χ2 distribution with the appropriate number of degrees of freedom. Predictive performance was based upon the weighted-mean error and the bias-adjusted weighted-mean-squared error.
Monte Carlo simulation.
Monte Carlo simulations were performed using the linear model for children and the standard model for adults. The structural model was implemented within the simulation module of the pharmacokinetic program ADAPT 5 (15). The covariance matrix was inserted into subroutine PRIOR. Another subroutine within ADAPT 5 (courtesy of David D'Argenio, University of Southern California) enabled teicoplanin to be administered to each of 5,000 simulated patients on a weight basis (in mg/kg body weight). For each simulated patient, the weight-based dose of teicoplanin was converted internally to an absolute dose of teicoplanin (in mg) by multiplying by the simulated weight. Thus, the simulation process mimicked drug administration as it occurred at the bedside in which the dose of teicoplanin was planned on an mg/kg basis, but the absolute amount of drug administered to each patient was determined with reference to weight (14). To ensure consistency with the clinical trial, teicoplanin was infused over 5 min to simulated children >1 month of age with fixed weights of 10, 25, and 50 kg. For adults, the same methodology was used and applied to their dosage regimen and infusion time.
Both normal and log-normal parameter distributions were explored and discriminated on the basis of their ability to recapitulate the original parameter means and their dispersions. All calculations were performed at a steady state between day 4 and day 5 after initiation of treatment. The area under the concentration-time curve from 96 to 120 h (AUC96–120) was determined by integration and the proportion of patients achieving the desired exposure to the drug. A Cmin of >10 mg/liter was used as the PK target throughout the study, as this was suggested to be the current surrogate for efficacy for both adults and children for most indications (16).
RESULTS
Demographics.
A description of demographic data by the age categories is presented in Table 1. A total of 39 patients recruited over 8 months (between April and December 2013) contributed 49 treatment episodes. Eight patients contributed >1 episode (6 patients contributed 2 episodes and 2 patients contributed 3 episodes). The mean age (standard deviation [SD]) was 4 years (4.3 years). The mean weight at inclusion to the study (SD) was 17.27 kg (13.3 kg), and 53.8% (n = 21) of the patients were male. The mean height (SD) was 97.68 cm (34.76 cm) but was only recorded in 30 patients. Patients had a mean serum creatinine at the start of treatment (SD) of 54.5 μmol/liter (50.08 μmol/liter), which was recorded in only 32 patients.
TABLE 1.
Demographics of patients according to age categoriesa
| Age category | n (% total) | Age (yr) | Weight (kg) | Height (cm) | Creatinine (μmol/liter) |
|---|---|---|---|---|---|
| 1–23 mo | 16 (41.02) | 0.25 (0.45) | 6.26 (2.04) | 65.84b (11.93) | 71.54 (75.68) |
| 2–11 yr | 20 (51.3) | 5.4 (2.23) | 21.18 (7.14) | 112.41c (14.36) | 43.3e(13.9) |
| 11–16 yr | 3 (7.7) | 14.7 (0.58) | 49.97 (10.6) | 166.9 (3.59) | 55 (8.48) |
| Total | 39 (100) | 4 (4.3) | 17.27 (13.3) | 97.7d (34.8) | 54.5f (50.1) |
Data are expressed as means (SD).
n = 11.
n = 16.
n = 30.
n = 13.
n = 32.
The majority of patients were being nursed in the oncology unit (43.6%). The next largest population group included patients who had undergone cardiac surgery and were being treated in the intensive care unit (33.3%). The remaining participants were being treated for general medical (20.5%) and cardiac medical (2.6%) conditions, in which teicoplanin was often used to treat catheter-associated bloodstream infections.
Thirty-three adult patients, 11 in the standard-dosage group and 22 in the high-dosage group, were analyzed, and 54.5% of them were male. The patients' mean age (SD) was 47.2 years (13.9 years). The mean weight (SD) was 67.7 kg (13.6 kg), and the mean creatinine level at the start of treatment (SD) was 69.41 μmol/liter (23.78 μmol/liter). All the patients had been previously treated with chemotherapy because of acute lymphocytic or acute nonlymphocytic leukemia and subsequent development of febrile neutropenia.
Pharmacokinetic data.
A total of 306 pharmacokinetic (PK) serum samples were collected, and 298 PK samples (mean, 7.6 samples per patient) were included in the analysis for the children. Eight observations were excluded from the analysis for several reasons. In one patient, a single concentration obtained immediately at the end of 1 h infusion was substantially higher than the usual peak observed with other patients and was inconsistent with other observations from the same patient. On further investigation, this patient was found to have a single lumen line that was used for drug administration and sampling. The remaining seven observations were excluded because of incorrect or absent time records.
As seen in Fig. 1, the teicoplanin concentration-time profile for the 39 pediatric patients was highly variable. Overall, 21 patients (53.85%) had serum concentrations of <10 mg/liter, the serum concentration frequently used to guide dosage adjustment.
Population pharmacokinetic models.
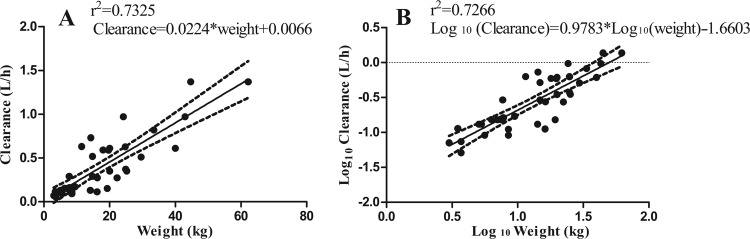
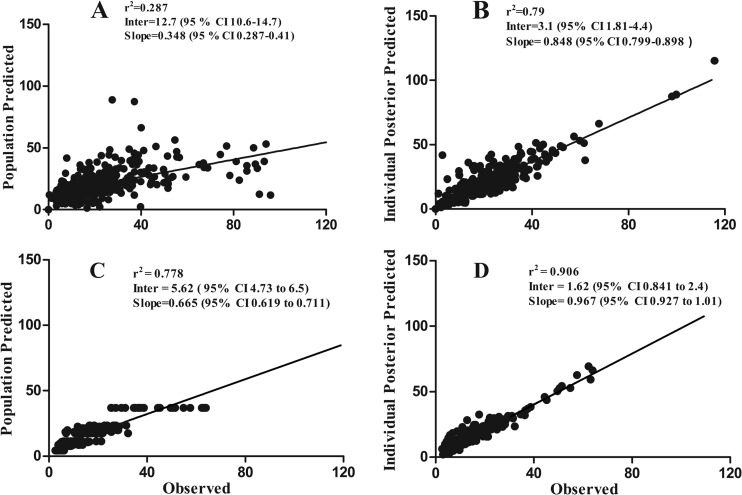
The population parameter estimates from each of the models developed are summarized in Table 2. The relationship between the Bayesian estimates of clearance, obtained using the median population parameter values from the standard model, and weight is shown in Fig. 2. For the three models, the fit of the data was acceptable (r2 = 0.742 to 0.814), with comparable measures of bias and precision. However, the better (more positive) log-likelihood value for both the linear and allometric models suggested that the inclusion of weight as a covariate explained a significant portion of the observed variance. The linear model was finally chosen to account for the observed data (r2 = 0.79), as in Fig. 3A and B. For adults, the data from both dosing groups (n = 33) were examined using a standard 2-compartment model, as shown in Fig. 3B and C. The model diagnostics are shown in Table 3.
TABLE 2.
Estimated population parameters
| Parameter and modela | Mean | Median | SD |
|---|---|---|---|
| Standard model | |||
| SCL (liters/h) | 0.396 | 0.279 | 0.347 |
| Vc (liters) | 4.259 | 2.592 | 3.597 |
| Kcp (h−1) | 3.344 | 0.434 | 7.742 |
| Kpc (h−1) | 4.424 | 0.252 | 9.742 |
| Linear model | |||
| SCLslope (liters/h/kg) | 0.023 | 0.019 | 0.010 |
| Vc (liters) | 4.138 | 2.282 | 4.143 |
| Kcp (h−1) | 3.876 | 0.474 | 8.156 |
| Kpc (h−1) | 3.994 | 0.292 | 8.930 |
| Allometric model | |||
| SCLstd (liters/h/70 kg) | 0.045 | 0.040 | 0.020 |
| Vc (liters) | 3.447 | 1.975 | 3.579 |
| Kcp (h−1) | 4.897 | 0.564 | 9.054 |
| Kpc (h−1) | 4.227 | 0.228 | 9.638 |
| Standard for adults | |||
| SCL (liters/h) | 1.166 | 1.097 | 0.376 |
| Vc (liters) | 7.925 | 7.669 | 1.849 |
| Kcp (h−1) | 1.179 | 1.158 | 0.449 |
| Kpc (h−1) | 0.154 | 0.155 | 0.087 |
SCL (linear model) = SCLslope × weight; SCL (allometric model) = SCLstd × ([weight/70]0.75); Vc, volume of distribution in central compartment; Kcp, first-order rate constant from the central to peripheral compartment; Kpc, first-order rate constant from peripheral to central compartment.
FIG 2.
Evaluation of relationships between the Bayesian PK parameter estimates from the standard model for clearance and weight of patients. (A) Linear relationship between Bayesian clearance estimates and weight. (B) Linear relationship of the log-transformed values of Bayesian clearance estimates versus weight. Dotted lines represent the 95% confidence interval (CI) of the regression line.
FIG 3.
Observed versus predicted plots for the population and Bayesian posterior values in the linear model for children (n = 39) (A and B, respectively) and for the population and Bayesian posterior values in the standard model for adults (n = 33) (C and D).
TABLE 3.
Model diagnostics
| Model | Log likelihood | r2a | Slope (95% CI)a | Intercept (95% CI)a |
|---|---|---|---|---|
| Standard | −987.60 | 0.814 | 0.839 (0.793–0.884) | 3.67 (2.49–4.85) |
| Linear | −979.212 | 0.79 | 0.848 (0.799–0.898) | 3.1 (1.81–4.4) |
| Allometric | −983.428 | 0.742 | 0.917 (0.857–0.978) | 3.15 (1.68–4.61) |
| Standard, adults | −501.381 | 0.906 | 0.967 (0.927–1.01) | 1.62 (0.841–2.4) |
Relative to the regression line fitted for the observed versus predicted values after the Bayesian step. CI, confidence interval.
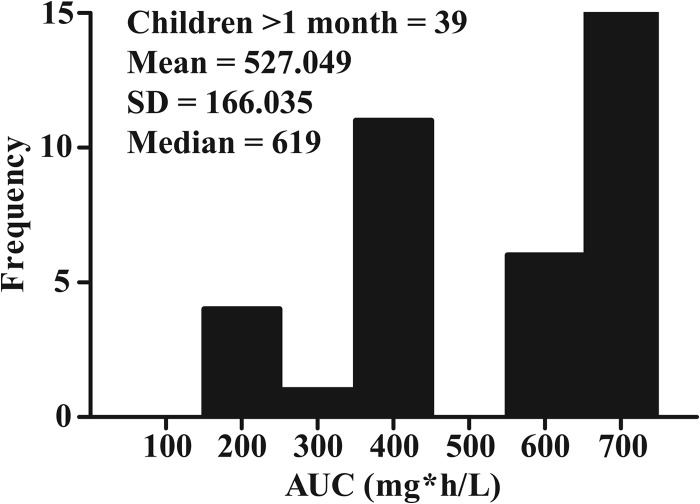
The Bayesian estimates for the AUCs from each of the 39 pediatric patients are shown in Fig. 4. The mean (SD) total population (n = 39) AUC was 527.049 mg · h/liter (166.035 mg · h/liter).
FIG 4.
AUC distributions from the Bayesian posterior estimates from the linear model in children.
Monte Carlo simulations.
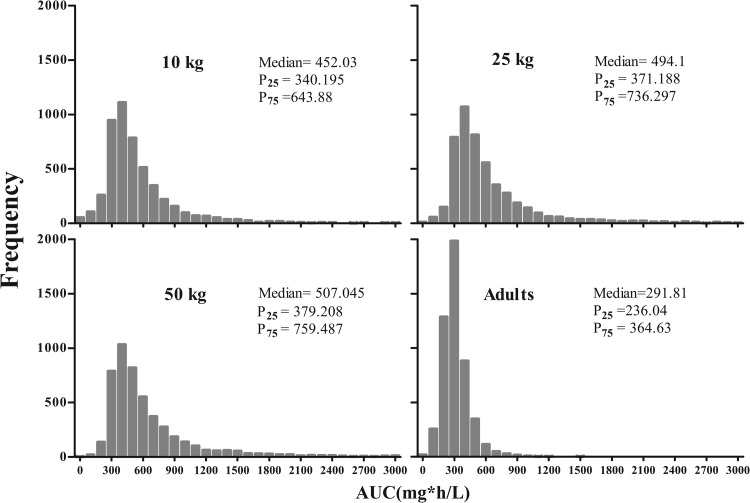
Monte Carlo simulations were performed with the linear model in children (n = 5,000). The extent of the predicted variability in serum teicoplanin concentrations and the resultant AUCs within a simulated human population receiving the current dosage regimen for children >1 month of age, at steady state (between days 4 and 5 of treatment) and for the fixed weights 10 kg (1 to 23 months), 25 kg (2 to 11 years), and 50 kg (11 to 16 years), were obtained. Based on these simulations, only 30.04% (median AUC96–120, 507.04 mg · h/liter), 44.88% (median AUC96–120, 494.1 mg · h/liter), and 60.54% (median AUC96–120, 452.03 mg · h/liter) of patients weighing 50, 25, and 10 kg, respectively, attained trough concentrations of >10 mg/liter by day 4 of treatment. For adults, simulations (n = 5,000) were performed with the standard model and current recommended regimen of three loading doses of 400 mg every 12 h and 400 mg once daily thereafter. The median AUC96–120 was 291.81 mg · h/liter, with 25th and 75th percentiles of 236.04 and 364.63 mg · h/liter, respectively. The simulated AUC distributions for children and adults are shown in Fig. 5. A total of 24.8% of simulated adult patients attained trough concentrations >10 mg/liter by 96 h of treatment.
FIG 5.
AUC distributions based on Monte Carlo simulations for children at fixed weights of 10, 25, and 50 kg and for adults (not fixed weight) with measures of medians, 25th and 75th percentiles (P25 and P75).
DISCUSSION
Teicoplanin is a glycopeptide antibiotic agent that has bactericidal activity against Gram-positive aerobic and anaerobic bacteria (17). It is widely used for the treatment of invasive infections such as septicemia, intravascular device-associated infections, endocarditis, and septic arthritis caused by methicillin-resistant Gram-positive pathogens. Despite its extensive use, there are comparatively few pharmacokinetic-pharmacodynamic (PK-PD) data for teicoplanin compared with vancomycin. There is even less information on the PK of teicoplanin in neonates and children, and evidence for currently recommended regimens is scant.
We conducted a PK study in children and developed a population PK model to quantify interpatient variability. We encountered a series of findings that can be used to improve the use of teicoplanin in children. Of greatest concern was the low proportion of patients attaining minimum serum concentrations (Cmin >10 mg/liter) at steady state with the current recommended dosages. This is consistent with previous PK studies of teicoplanin in children (18–20). For example, Dufort et al. (18) reported that 55.6% of febrile neutropenic patients did not achieve a Cmin of >10 mg/liter. Similarly, Sanchez et al. (19) revealed that 89% of patients had low concentrations (Cmin <10 mg/liter) in a study of critically ill children and infants. The mean estimate of clearance in our study (0.023 liters/h/kg) was similar to that published by Dufort et al. (0.029 liters/h/kg) (18) and slightly lower than that reported by Sanchez et al. (0.045 liters/kg/h) (19). Our findings are also in agreement with those of a retrospective study showing that among 340 treatments with teicoplanin in pediatric patients of different ages (92 neonate/infant episodes, 69 toddler episodes, 62 school-age-children episodes, and 117 adolescent episodes), the initial Cmin values at day 2 to 4 after 10 to 15 mg/kg every 12 h for 3 loading doses and every 24 h thereafter were <10 mg/liter in 14.1% of cases (20).
Previous studies investigated the impact of age on the pharmacokinetics of teicoplanin in children. Reed et al. (21) reported, for example, a trend of clearance decreasing with increasing age in a population of 12 children (2.4 to 11 years old), but there were no statistically significant associations between PK parameters and age. Similarly, in a population of 13 children (2 to 12 years old), Terragna et al. (22) found no significant linear correlation between elimination half-life and age. In contrast, Tarral and colleagues (23) separately investigated two populations of children (6 children with a mean age of 7 years and 4 neonates with a mean age of 8.5 days). They showed that volume of distribution was higher in neonates than in children (0.6 versus 0.54 liter/kg body weight), and clearance was higher in older children than in neonates (0.028 versus 0.016 liter/h/kg). Finally, Lukas et al. (24) conducted a population PK analysis in 20 infants and children (4 months to 10 years of age) in the intensive-care unit, showing differences among two identified subpopulations separated around 12 months of age. In younger infants (<12 months), 92% of concentrations were at target minimum levels (10 mg/liter) versus 65% of concentrations in older children (≥12 months). Interestingly, in this study, linear and nonlinear models of CL and V with weight failed, despite weight showing a linear relationship with CL and V but only in children <12 months old. Instead, weight scaling of CL and V was successful. The population split in the two age categories gave a successful model in this study. Notably, only 4 patients <12 months old contributed to the model. We did not conduct a split analysis by age to avoid losing robustness of the predicted pharmacokinetic variability in the Monte Carlo simulations by decreasing sample sizes (25). In our study, by contrast, only the influence of weight related to clearance in a linear model improved the description of our data across childhood. Volume of distribution, however, did not show a tenable relationship with weight or age.
In the present study, children had more variability in drug exposures (quantified as AUC distributions at steady state) than the adults. Adults had a mean AUC of approximately 300 mg · h/liter, although a previous study reported a higher mean AUC of 550 mg · h/liter (26). In our study, children across different weights achieved a median AUC of 619 mg · h/liter, which compares with the drug exposures reported in adults. The relatively higher pediatric PK variability supports the use of routine therapeutic drug monitoring (TDM) to enable active adjustment of teicoplanin dosages. Collectively, our results suggest that the current dosage regimen in children is probably adequate in terms of the median drug exposures attained (AUC at steady state), but TDM should be considered to minimize the number of patients with suboptimal drug exposure. Such an approach may improve safety and efficacy and prevent the emergence of antimicrobial resistance, although further studies are required to examine this issue.
A number of issues and challenges have prevented the more extensive use of teicoplanin. The first relates to the composition and synthesis of the compound, which is a mixture of six related subcomponents (A2-1, A2-2, A2-3, A2-4, A2-5, and A3-1). The A3-1 component is the core glycopeptide that is common to all teicoplanin-like compounds (27). Concern has been expressed related to the composition of generic teicoplanin products and its potential impact on pharmacodynamics. A second issue relates to problems measuring concentrations in clinical samples. A number of analytical methods have been used (e.g., Bacillus subtilis bioassay, solid-phase enzyme receptor assay [SPERA], fluorescence polarization immunoassay [FPIA], and high-performance liquid chromatography [HPLC]). Importantly, the readouts from these different modalities differ. Thus, the conclusions of PK-PD studies are dependent on the analytical method that was used (28). A third issue is a relative lack of knowledge of the pharmacodynamics of teicoplanin. A Cmin of >10 mg/liter has been associated with higher clinical cure rates compared with values of 5 mg/liter (16). Higher trough serum concentrations (e.g., 15 to 20 mg/liter) are recommended for the treatment of endocarditis caused by Staphylococcus aureus (29). A preclinical pharmacodynamic study suggests a maximum teicoplanin peak free drug concentration (fCmax)/MIC ratio of at least 2 to 3 is required for efficacy (30). Other small clinical studies have suggested that target AUCs as high as 750 mg · h/liter are required to eradicate or cure MRSA infections with an MIC of 1 mg/liter (31, 32). There is an urgent need to further evaluate the pharmacodynamics of teicoplanin.
This study had limitations. First, the study participants largely did not have renal impairment. Teicoplanin is eliminated via the kidneys with clearance of the unbound drug by glomerular filtration with minimal tubular reabsorption and renal secretion (17). The impact of reduced renal function on clearance and therefore dosing could not be assessed. Finally, we could not correlate drug exposure with clinical outcomes in this study because there were too few documented infections.
In conclusion, the population PK of teicoplanin is highly variable in children. TDM should be considered a component of routine care, although further work is required to identify drug exposure targets. The population PK model can now be used to construct algorithms for individualized dosing, as we described previously (33, 34).
ACKNOWLEDGMENTS
We thank the NIHR Alder Hey Clinical Research Facility (CRF) and Alder Hey NHS Foundation Trust Research Business Unit for supporting the study. F. Docobo-Pérez is supported by a Sara Borrell postdoctoral fellowship from the Instituto de Salud Carlos III; T.W. Felton is an MRC Clinical Training Fellow supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which is funded by the Medical Research Council (G1000417/94909), ICON, GlaxoSmithKline, AstraZeneca, and the Medical Evaluation Unit; and W.W. Hope is supported by a National Institutes of Health Research (NIHR) Clinician Scientist Fellowship.
We thank CRF research nurses Timothy Henderson, Lindsay Byrne, Hannah Leyland, and Gail Wallace for contributing to the recruitment of patients and data collection.
Footnotes
Published ahead of print 15 September 2014
REFERENCES
- 1.Verstraete E, Boelens J, De Coen K, Claeys G, Vogelaers D, Vanhaesebrouck P, Blot S. 2014. Healthcare-associated bloodstream infections in a neonatal intensive care unit over a 20-year period (1992–2011): trends in incidence, pathogens, and mortality. Infect. Control Hosp. Epidemiol. 35:511–518. 10.1086/675836. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesh MP, Placencia F, Weisman LE. 2006. Coagulase-negative staphylococcal infections in the neonate and child: an update. Semin. Pediatr. Infect. Dis. 17:120–127. 10.1053/j.spid.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Tiemersma EW, Bronzwaer SL, Lyytikainen O, Degener JE, Schrijnemakers P, Bruinsma N, Monen J, Witte W, Grundman H. 2004. Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg. Infect. Dis. 10:1627–1634. 10.3201/eid1009.040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Hoogen A, Gerards LJ, Verboon-Maciolek MA, Fleer A, Krediet TG. 2010. Long-term trends in the epidemiology of neonatal sepsis and antibiotic susceptibility of causative agents. Neonatology 97:22–28. 10.1159/000226604. [DOI] [PubMed] [Google Scholar]
- 5.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45. 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould FK, Denning DW, Elliott TS, Foweraker J, Perry JD, Prendergast BD, Sandoe JA, Spry MJ, Watkin RW, Working Party of the British Society for Antimicrobial Chemotherapy 2012. Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 67:269–289. 10.1093/jac/dkr450. [DOI] [PubMed] [Google Scholar]
- 7.UK EMCe. 20 May 2014. Teicoplanin (Targocid): summary of product characteristics. http://www.medicines.org.uk/emc/medicine/27319/SPC.
- 8.European Medicines Agency. 2006. Regulation (EC) no. 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use. European Medicines Agency, London, United Kingdom. [Google Scholar]
- 9.European Medicines Agency. 2000. ICH topic E 11 clinical investigation of medicinal products in the paediatric population. European Medicines Agency, London, United Kingdom. [Google Scholar]
- 10.Pea F, Viale P, Candoni A, Pavan F, Pagani L, Damiani D, Casini M, Furlanut M. 2004. Teicoplanin in patients with acute leukaemia and febrile neutropenia: a special population benefiting from higher dosages. Clin. Pharmacokinet. 43:405–415. 10.2165/00003088-200443060-00004. [DOI] [PubMed] [Google Scholar]
- 11.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 34:467–476. 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The R Core Team. 2013. R: a language and environment for statistical computing, R project, 3rd ed. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 13.Wurthwein G, Groll AH, Hempel G, Adler-Shohet FC, Lieberman JM, Walsh TJ. 2005. Population pharmacokinetics of amphotericin B lipid complex in neonates. Antimicrob. Agents Chemother. 49:5092–5098. 10.1128/AAC.49.12.5092-5098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hope WW, Seibel NL, Schwartz CL, Arrieta A, Flynn P, Shad A, Albano E, Keirns JJ, Buell DN, Gumbo T, Drusano GL, Walsh TJ. 2007. Population pharmacokinetics of micafungin in pediatric patients and implications for antifungal dosing. Antimicrob. Agents Chemother. 51:3714–3719. 10.1128/AAC.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA. [Google Scholar]
- 16.Harding I, MacGowan AP, White LO, Darley ES, Reed V. 2000. Teicoplanin therapy for Staphylococcus aureus septicaemia: relationship between pre-dose serum concentrations and outcome. J. Antimicrob. Chemother. 45:835–841. 10.1093/jac/45.6.835. [DOI] [PubMed] [Google Scholar]
- 17.Wilson AP. 2000. Clinical pharmacokinetics of teicoplanin. Clin. Pharmacokinet. 39:167–183. 10.2165/00003088-200039030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Dufort G, Ventura C, Olive T, Ortega JJ. 1996. Teicoplanin pharmacokinetics in pediatric patients. Pediatr. Infect. Dis. J. 15:494–498. 10.1097/00006454-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez A, Lopez-Herce J, Cueto E, Carrillo A, Moral R. 1999. Teicoplanin pharmacokinetics in critically ill paediatric patients. J. Antimicrob. Chemother. 44:407–409. 10.1093/jac/44.3.407. [DOI] [PubMed] [Google Scholar]
- 20.Strenger V, Hofer N, Rodl S, Honigl M, Raggam R, Seidel MG, Dornbusch HJ, Sperl D, Lackner H, Schwinger W, Sovinz P, Benesch M, Urlesberger B, Urban C. 2013. Age- and gender-related differences in teicoplanin levels in paediatric patients. J. Antimicrob. Chemother. 68:2318–2323. 10.1093/jac/dkt176. [DOI] [PubMed] [Google Scholar]
- 21.Reed MD, Yamashita TS, Myers CM, Blumer JL. 1997. The pharmacokinetics of teicoplanin in infants and children. J. Antimicrob. Chemother. 39:789–796. 10.1093/jac/39.6.789. [DOI] [PubMed] [Google Scholar]
- 22.Terragna A, Ferrea G, Loy A, Danese A, Bernareggi A, Cavenaghi L, Rosina R. 1988. Pharmacokinetics of teicoplanin in pediatric patients. Antimicrob. Agents Chemother. 32:1223–1226. 10.1128/AAC.32.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarral E, Jehl F, Tarral A, Simeoni U, Monteil H, Willard D, Geisert J. 1988. Pharmacokinetics of teicoplanin in children. J. Antimicrob. Chemother. 21(Suppl A):47–51. [DOI] [PubMed] [Google Scholar]
- 24.Lukas JC, Karikas G, Gazouli M, Kalabalikis P, Hatzis T, Macheras P. 2004. Pharmacokinetics of teicoplanin in an ICU population of children and infants. Pharm. Res. 21:2064–2071. 10.1023/B:PHAM.0000048198.56873.d8. [DOI] [PubMed] [Google Scholar]
- 25.Tam VH, Kabbara S, Yeh RF, Leary RH. 2006. Impact of sample size on the performance of multiple-model pharmacokinetic simulations. Antimicrob. Agents Chemother. 50:3950–3952. 10.1128/AAC.00337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Bambeke F, Van Laethem Y, Courvalin P, Tulkens PM. 2004. Glycopeptide antibiotics: from conventional molecules to new derivatives. Drugs 64:913–936. 10.2165/00003495-200464090-00001. [DOI] [PubMed] [Google Scholar]
- 27.Bernareggi A, Borghi A, Borgonovi M, Cavenaghi L, Ferrari P, Vekey K, Zanol M, Zerilli LF. 1992. Teicoplanin metabolism in humans. Antimicrob. Agents Chemother. 36:1744–1749. 10.1128/AAC.36.8.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMullin CM, White LO, MacGowan AP, Holt HA, Lovering AM, Reeves DS. 1994. Assay of serum teicoplanin concentrations in clinical specimens: a comparison of isocratic high performance liquid chromatography with polarisation fluoroimmunoassay and bioassay. J. Antimicrob. Chemother. 34:425–429. 10.1093/jac/34.3.425. [DOI] [PubMed] [Google Scholar]
- 29.Wilson AP, Gruneberg RN, Neu H. 1994. A critical review of the dosage of teicoplanin in Europe and the USA. Int. J. Antimicrob. Agents 4(Suppl 1):S1–S30. 10.1016/0924-8579(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 30.Knudsen JD, Fuursted K, Raber S, Espersen F, Frimodt-Moller N. 2000. Pharmacodynamics of glycopeptides in the mouse peritonitis model of Streptococcus pneumoniae or Staphylococcus aureus infection. Antimicrob. Agents Chemother. 44:1247–1254. 10.1128/AAC.44.5.1247-1254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanazawa N, Matsumoto K, Ikawa K, Fukamizu T, Shigemi A, Yaji K, Shimodozono Y, Morikawa N, Takeda Y, Yamada K. 2011. An initial dosing method for teicoplanin based on the area under the serum concentration time curve required for MRSA eradication. J. Infect. Chemother. 17:297–300. 10.1007/s10156-010-0105-1. [DOI] [PubMed] [Google Scholar]
- 32.Hagihara M, Umemura T, Kimura M, Mori T, Hasegawa T, Mikamo H. 2012. Exploration of optimal teicoplanin dosage based on pharmacokinetic parameters for the treatment of intensive care unit patients infected with methicillin-resistant Staphylococcus aureus. J. Infect. Chemother. 18:10–16. 10.1007/s10156-011-0272-8. [DOI] [PubMed] [Google Scholar]
- 33.Hope WW, Vanguilder M, Donnelly JP, Blijlevens NM, Bruggemann RJ, Jelliffe RW, Neely MN. 2013. Software for dosage individualization of voriconazole for immunocompromised patients. Antimicrob. Agents Chemother. 57:1888–1894. 10.1128/AAC.02025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felton TW, Roberts JA, Lodise TP, Van Guilder M, Boselli E, Neely MN, Hope WW. 2014. Individualization of piperacillin dosing for critically ill patients: dosing software to optimize antimicrobial therapy. Antimicrob. Agents Chemother. 58:4094–4102. 10.1128/AAC.02664-14. [DOI] [PMC free article] [PubMed] [Google Scholar]