Abstract
Antivirals against enterovirus 71 (EV71) are urgently needed. We demonstrate that the novel enteroviral protease inhibitor (PI) SG85 and capsid binder (CB) vapendavir efficiently inhibit the in vitro replication of 21 EV71 strains/isolates that are representative of the different genogroups A, B, and C. The PI rupintrivir, the CB pirodavir, and the host-targeting compound enviroxime, which were included as reference compounds, also inhibited the replication of all isolates. Remarkably, the CB compound pleconaril was devoid of any anti-EV71 activity. An in silico docking study revealed that pleconaril—unlike vapendavir and pirodavir—lacks essential binding interactions with the viral capsid. Vapendavir and SG85 (or analogues) should be further explored for the treatment of EV71 infections. The data presented here may serve as a reference when developing yet-novel inhibitors.
TEXT
Enterovirus 71 (EV71) is a nonenveloped, single-stranded, positive-sense RNA virus that belongs to the family Picornaviridae. The virus is, together with coxsackie A viruses (CVA), the major causative agent of hand, foot, and mouth disease (HFMD), a mild and self-limiting disease that affects mostly children younger than 5 years old. However, in some patients the virus may cause severe, potentially lethal complications such as aseptic meningitis, encephalitis, pulmonary edema, and viral myocarditis (1, 2). In recent years, EV71 has been shown to cause in parts of Asia large outbreaks of HFMD that are associated with severe neurological conditions such as encephalitis and acute flaccid paralysis (3).
Medical care of patients with EV71 infections is symptomatic and depends on the clinical stage of the disease. Patients with uncomplicated HFMD can use paracetamol for pain relief, whereas severe cases of HFMD, i.e., those with central nervous system (CNS) involvement, may be treated by administration of intravenous immunoglobulin (IVIG) (4, 5). When the brainstem is affected, intravenous fluid therapy and the use of inotropes to support cardiac function should be considered. Phase III clinical vaccine trials have recently been completed (6–8). There are, however, no antivirals available for the treatment or prophylaxis of EV71 infections. Such anti-EV71 drugs are urgently needed.
Since EV71 consists of different (sub)genogroups, it will be important to have a representative panel of isolates against which the activity of novel compounds can be assessed. Marked differences in susceptibility of enteroviruses to antiviral drugs have been reported. For example, the capsid binder pleconaril is active against most rhino- and coxsackievirus strains but is, however, completely inactive against other rhino- and enteroviruses (9, 10).
Six enterovirus inhibitors were included in this study: (i) the novel 3C protease inhibitor (PI) SG85 (11) and the PI rupintrivir (12); (ii) the host cell-targeting compound enviroxime (13); and (iii) three capsid binding compounds, i.e., pleconaril, pirodavir, and vapendavir (14–16). Vapendavir is currently in clinical development for the treatment or prophylaxis of rhinovirus infections in patients at risk of rhinovirus-mediated exacerbation of their underlying respiratory disease(s) (NCT01175226). The potential antiviral activity of these compounds against a panel of 21 EV71 strains or isolates was assessed in a cell-based multicycle cytopathic effect (CPE) reduction assay using an [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] (MTS) readout, as described previously (17). The EV71 strains were selected such that all three genogroups, A, B, and C, were represented in the panel as determined on the basis of their VP1 sequence (see Fig. S1 in the supplemental material).
The novel PI SG85 potently inhibited the replication of all 21 EV71 strains, with 50% effective concentrations (EC50s) varying between 0.039 μM and 0.200 μM (Table 1; also see Table S1 in the supplemental material). Rupintrivir did so with EC50s ranging between 0.003 μM and 0.012 μM. All isolates proved markedly sensitive to the antiviral activity of these two PIs, although strains belonging to subgenogroup B5 proved somewhat less sensitive than those belonging to subgenogroups C2 and C4. Enviroxime, which was included as a reference compound (and which inhibits viral replication by targeting cellular phosphoinositol 4-kinase IIIβ [PI4KIIIβ], a kinase essential for picornavirus replication [18]) inhibited the replication of all EV71 strains with EC50s between 0.070 μM and 0.458 μM.
TABLE 1.
Effect of selected enterovirus inhibitors on the replication of EV71a
| Genogroup | EC50 (μM) |
|||||
|---|---|---|---|---|---|---|
| 3C protease inhibitors |
PI4KIIIβ inhibitor enviroxime | Capsid binding compounds |
||||
| SG85 | Rupintrivir | Vapendavir | Pirodavir | Pleconaril | ||
| A | 0.080 ± 0.015 | 0.006 ± 0.003 | 0.098 ± 0.026 | 0.842 ± 0.325 | 0.361 ± 0.209 | >262 |
| B2 | 0.200 ± 0.070 | 0.012 ± 0.004 | 0.148 ± 0.072 | 0.671 ± 0.321 | 0.727 ± 0.223 | >262 |
| B5 | 0.184 ± 0.026 | 0.010 ± 0.001 | 0.198 ± 0.078 | 0.498 ± 0.236 | 0.484 ± 0.170 | >262 |
| C2 | 0.069 ± 0.032 | 0.005 ± 0.002 | 0.248 ± 0.166 | 0.957 ± 0.074 | 0.491 ± 0.102 | >262 |
| C4 | 0.117 ± 0.012 | 0.007 ± 0.001 | 0.196 ± 0.096 | 0.739 ± 0.248 | 0.513 ± 0.090 | >262 |
Data are the means ± standard deviations of all EV71 strains included in the virus panel which group to the designated genogroup (1 isolate for genogroup A, 1 for B2, 6 for B5, 3 for C2, and 10 for C4). Per strain, at least 3 independently obtained EC50s were used.
A remarkable difference in activity was noted for the capsid binding compounds vapendavir, pirodavir, and pleconaril. Whereas vapendavir and the analogue pirodavir inhibited EV71 replication of all isolates (average EC50s of 0.7 μM for vapendavir and 0.5 μM for pirodavir), pleconaril was completely devoid of any antiviral activity. The antiviral activity of the pleconaril batch that was used for this study was confirmed against coxsackievirus A9 (strain Bozek) and poliovirus (type 3 Sabin) (with EC50s of 0.027 μM and 0.341 μM, respectively, which are comparable to published values) (14, 19).
Conflicting data exist regarding the antiviral activity of pleconaril against EV71. In one study, antiviral activity of pleconaril was reported in EV71-infected mice (20). Other studies, however, reported a lack of in vitro anti-EV71 activity of pleconaril (21–23). Moreover, inconsistent data on the potential efficacy of pleconaril in the treatment of enteroviral infections in humans have been reported (24–26). These incompatible data were one of the reasons to perform this study. We now present conclusive evidence that pleconaril is inactive against EV71 strains of all three genogroups. Hence, pleconaril should no longer be considered for the (compassionate) treatment of enteroviral infections, caused by EV71. We recently established a mouse model of EV71-induced encephalitis in adult SCID mice (unpublished results). This model will be ideally suited to assess whether compounds such as vapendavir and SG85 have, in contrast to pleconaril, any protective activity in vivo against EV71 infections.
To explain the marked difference in susceptibility of EV71 to pleconaril (lack of activity) on the one hand and vapendavir and pirodavir (robust activity) on the other hand, a modeling study was performed. The potential interaction of the compounds in the pocket under the floor of the receptor binding canyon was explored (detailed methods are given in the supplemental material). Docking studies revealed that vapendavir and pirodavir have stronger binding interactions with the viral capsid at the opening of the canyon than pleconaril. When the interactions of pirodavir and vapendavir were compared to those of WIN51711 (the capsid binding compound which was cocrystallized with the EV71 capsid), a remarkable similarity was noted. All three molecules extend their binding in the direction of the pore and anchor via a hydrogen bond (either with Asp112 or with Ile113) (Fig. 1). In contrast, pleconaril appears unable to reach that far in the EV71 pocket; hence, anchoring is not possible, which may explain the lack of antiviral activity. Knowledge of the precise interactions between the viral capsid and capsid binding compounds may help to develop novel and yet more potent antivirals. A novel class of EV71 capsid binders was recently reported which were designed based on the crystal structure of the EV71 capsid (27). In that particular study, cocrystals of the EV71 capsid with the compounds revealed an interaction with Asp112 and/or Ile113, underlining the importance of these interactions and corroborating the findings of our modeling study.
FIG 1.
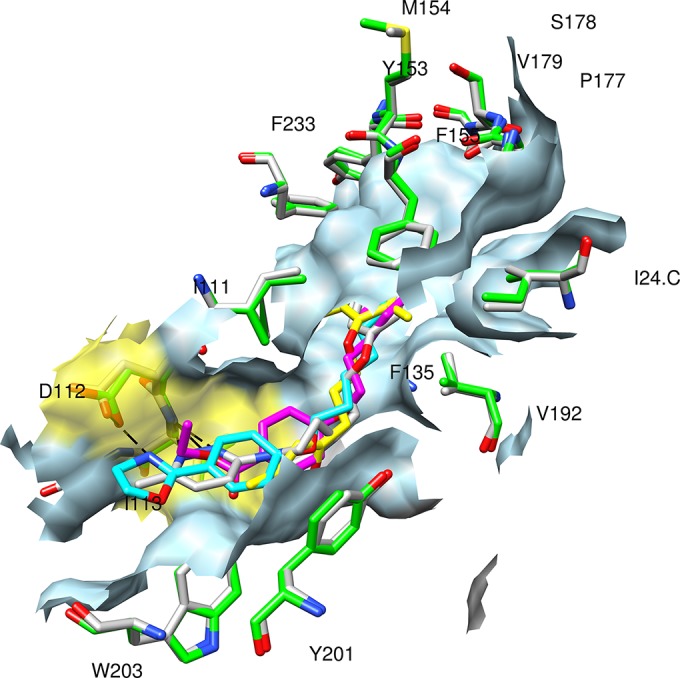
Docking result of pirodavir (magenta carbons), pleconaril (yellow carbons), and vapendavir (gray carbons) in the EV71 homologue model VP1 pocket (created from 3ZFE, green carbons in residues, light blue surface), superimposed onto the canyon of the 3ZFF structure (residues having gray carbons) containing the WIN51711 inhibitor (cyan). The surface of the opening of the canyon is colored yellow; residue labels have been added for residues in hydrophobic contact with the inhibitors.
In conclusion, we established a reference panel of EV71 isolates representative for the different (sub)genogroups. The novel capsid binder vapendavir (currently under clinical study for the treatment of rhinovirus infections in high-risk patients), its analogue pirodavir, and the novel PI SG85 efficiently inhibit EV71 replication. In contrast, pleconaril was completely devoid of activity against EV71, which was explained in a molecular modeling study. This information will be important for the design of novel EV71 capsid binding compounds. Vapendavir and SG85 (or analogues) may be further developed for the treatment of EV71 infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kim Donckers for expert technical assistance and Cathy De Meyer for excellent editorial assistance.
The work presented here was supported by the European Union 7th Framework Program project SILVER (260644), the European Union 7th Framework Program EUVIRNA Marie Curie Initial Training Network (264286), the KU Leuven Geconcentererde Onderzoeksacties (GOA), and IUAP Belvir Belspo.
Footnotes
Published ahead of print 8 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03328-14.
REFERENCES
- 1.Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, Shih SR, Ning HC, Hwang MS, Wang HS, Lee CY. 1999. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 354:1682–1686. 10.1016/S0140-6736(99)04434-7. [DOI] [PubMed] [Google Scholar]
- 2.Wong KT, Munisamy B, Ong KC, Kojima H, Noriyo N, Chua KB, Ong BB, Nagashima K. 2008. The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J. Neuropathol. Exp. Neurol. 67:162–169. 10.1097/nen.0b013e318163a990. [DOI] [PubMed] [Google Scholar]
- 3.Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y, Cowling BJ, Varma JK, Farrar JJ, Leung GM, Yu H. 2014. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect. Dis. 14:308–318. 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao RY, Han JF, Jiang T, Tian X, Yu M, Deng YQ, Qin ED, Qin CF. 2011. In vitro and in vivo characterization of a new enterovirus type 71-specific human intravenous immunoglobulin manufactured from selected plasma donors. J. Clin. Virol. 51:246–249. 10.1016/j.jcv.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Frange P, Michon J, Fromantin I, Franck N, Safar E, Escande MC, Desguerre I, Orbach D. 2007. Enterovirus 71 meningoencephalitis during chemotherapy in a child with metastatic osteosarcoma. J. Pediatr. Hematol. Oncol. 29:566–568. 10.1097/MPH.0b013e3180f61bbc. [DOI] [PubMed] [Google Scholar]
- 6.Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, Hu YM, Wu X, Liu P, Zhu LY, Gao F, Jin H, Chen YJ, Dong YY, Liang YC, Shi NM, Ge HM, Liu L, Chen SG, Ai X, Zhang ZY, Ji YG, Luo FJ, Chen XQ, Zhang Y, Zhu LW, Liang ZL, Shen XL. 2013. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 381:2024–2032. 10.1016/S0140-6736(13)61049-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, Hu Y, Ji T, Song L, Liang Q, Zhang B, Gao Q, Li J, Wang S, Hu Y, Gu S, Zhang J, Yao G, Gu J, Wang X, Zhou Y, Chen C, Zhang M, Cao M, Wang J, Wang H, Wang N. 2014. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N. Engl. J. Med. 370:818–828. 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 8.Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, Jiang L, Dong C, Che Y, Huang T, Jiang Z, Xie Z, Wang L, Liao Y, Liang Y, Nong Y, Liu J, Zhao H, Na R, Guo L, Pu J, Yang E, Sun L, Cui P, Shi H, Wang J, Li Q. 2014. An inactivated enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 370:829–837. 10.1056/NEJMoa1303224. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser L, Crump CE, Hayden FG. 2000. In vitro activity of pleconaril and AG7088 against selected serotypes and clinical isolates of human rhinoviruses. Antiviral Res. 47:215–220. 10.1016/S0166-3542(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 10.Schmidtke M, Hammerschmidt E, Schuler S, Zell R, Birch-Hirschfeld E, Makarov VA, Riabova OB, Wutzler P. 2005. Susceptibility of coxsackievirus B3 laboratory strains and clinical isolates to the capsid function inhibitor pleconaril: antiviral studies with virus chimeras demonstrate the crucial role of amino acid 1092 in treatment. J. Antimicrob. Chemother. 56:648–656. 10.1093/jac/dki263. [DOI] [PubMed] [Google Scholar]
- 11.Tan J, George S, Kusov Y, Perbandt M, Anemuller S, Mesters JR, Norder H, Coutard B, Lacroix C, Leyssen P, Neyts J, Hilgenfeld R. 2013. 3C protease of enterovirus 68: structure-based design of Michael acceptor inhibitors and their broad-spectrum antiviral effects against picornaviruses. J. Virol. 87:4339–4351. 10.1128/JVI.01123-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DA, Dragovich PS, Webber SE, Fuhrman SA, Patick AK, Zalman LS, Hendrickson TF, Love RA, Prins TJ, Marakovits JT, Zhou R, Tikhe J, Ford CE, Meador JW, Ferre RA, Brown EL, Binford SL, Brothers MA, DeLisle DM, Worland ST. 1999. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc. Natl. Acad. Sci. U. S. A. 96:11000–11007. 10.1073/pnas.96.20.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLong DC, Reed SE. 1980. Inhibition of rhinovirus replication in in organ culture by a potential antiviral drug. J. Infect. Dis. 141:87–91. 10.1093/infdis/141.1.87. [DOI] [PubMed] [Google Scholar]
- 14.Pevear DC, Tull TM, Seipel ME, Groarke JM. 1999. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 43:2109–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andries K, Dewindt B, Snoeks J, Willebrords R, van Eemeren K, Stokbroekx R, Janssen PA. 1992. In vitro activity of pirodavir (R 77975), a substituted phenoxy-pyridazinamine with broad-spectrum antipicornaviral activity. Antimicrob. Agents Chemother. 36:100–107. 10.1128/AAC.36.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson KG, Brown RN, Cameron R, Chalmers DK, Hamilton S, Jin B, Krippner GY, Luttick A, McConnell DB, Reece PA, Ryan J, Stanislawski PC, Tucker SP, Wu WY, Barnard DL, Sidwell RW. 2003. An orally bioavailable oxime ether capsid binder with potent activity against human rhinovirus. J. Med. Chem. 46:3181–3184. 10.1021/jm0202876. [DOI] [PubMed] [Google Scholar]
- 17.De Palma AM, Heggermont W, Leyssen P, Purstinger G, Wimmer E, De CE, Rao A, Monforte AM, Chimirri A, Neyts J. 2007. Anti-enterovirus activity and structure-activity relationship of a series of 2,6-dihalophenyl-substituted 1H,3H-thiazolo[3,4-a]benzimidazoles. Biochem. Biophys. Res. Commun. 353:628–632. 10.1016/j.bbrc.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 18.van der Schaar HM, van der Linden L, Lanke KH, Strating JR, Purstinger G, de Vries E, de Haan CA, Neyts J, van Kuppeveld FJ. 2012. Coxsackievirus mutants that can bypass host factor PI4KIIIbeta and the need for high levels of PI4P lipids for replication. Cell Res. 22:1576–1592. 10.1038/cr.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Palma AM, Purstinger G, Wimmer E, Patick AK, Andries K, Rombaut B, De Clercq E, Neyts J. 2008. Potential use of antiviral agents in polio eradication. Emerg. Infect. Dis. 14:545–551. 10.3201/eid1404.070439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, Zhou F, Gu B, Ding C, Feng D, Xie F, Wang J, Zhang C, Cao Q, Deng Y, Hu W, Yao K. 2012. In vitro and in vivo evaluation of ribavirin and pleconaril antiviral activity against enterovirus 71 infection. Arch. Virol. 157:669–679. 10.1007/s00705-011-1222-6. [DOI] [PubMed] [Google Scholar]
- 21.Thibaut HJ, Leyssen P, Puerstinger G, Muigg A, Neyts J, De Palma AM. 2011. Towards the design of combination therapy for the treatment of enterovirus infections. Antiviral Res. 90:213–217. 10.1016/j.antiviral.2011.03.187. [DOI] [PubMed] [Google Scholar]
- 22.Shia KS, Li WT, Chang CM, Hsu MC, Chern JH, Leong MK, Tseng SN, Lee CC, Lee YC, Chen SJ, Peng KC, Tseng HY, Chang YL, Tai CL, Shih SR. 2002. Design, synthesis, and structure-activity relationship of pyridyl imidazolidinones: a novel class of potent and selective human enterovirus 71 inhibitors. J. Med. Chem. 45:1644–1655. 10.1021/jm010536a. [DOI] [PubMed] [Google Scholar]
- 23.Wildenbeest JG, van den Broek PJ, Benschop KS, Koen G, Wierenga PC, Vossen AC, Kuijpers TW, Wolthers KC. 2012. Pleconaril revisited: clinical course of chronic enteroviral meningoencephalitis after treatment correlates with in vitro susceptibility. Antivir. Ther. 17:459–466. 10.3851/IMP1936. [DOI] [PubMed] [Google Scholar]
- 24.Rotbart HA, Webster AD. 2001. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin. Infect. Dis. 32:228–235. 10.1086/318452. [DOI] [PubMed] [Google Scholar]
- 25.Hayden FG, Herrington DT, Coats TL, Kim K, Cooper EC, Villano SA, Liu S, Hudson S, Pevear DC, Collett M, McKinlay M. 2003. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin. Infect. Dis. 36:1523–1532. 10.1086/375069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abzug MJ, Cloud G, Bradley J, Sanchez PJ, Romero J, Powell D, Lepow M, Mani C, Capparelli EV, Blount S, Lakeman F, Whitley RJ, Kimberlin DW. 2003. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr. Infect. Dis. J. 22:335–341. 10.1097/01.inf.0000059765.92623.70. [DOI] [PubMed] [Google Scholar]
- 27.De Colibus L, Wang X, Spyrou JA, Kelly J, Ren J, Grimes J, Puerstinger G, Stonehouse N, Walter TS, Hu Z, Wang J, Li X, Peng W, Rowlands DJ, Fry EE, Rao Z, Stuart DI. 2014. More-powerful virus inhibitors from structure-based analysis of HEV71 capsid-binding molecules. Nat. Struct. Mol. Biol. 21:282–288. 10.1038/nsmb.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


