Abstract
No effective antiviral therapies are currently available to treat disease after infection with yellow fever virus (YFV). A Syrian golden hamster model of yellow fever (YF) was used to characterize the effect of treatment with BCX4430, a novel adenosine nucleoside analog. Significant improvement in survival was observed after treatment with BCX4430 at 4 mg/kg of body weight per day dosed intraperitoneally (i.p.) twice daily (BID). Treatment with BCX4430 at 12.5 mg/kg/day administered i.p. BID for 7 days offered complete protection from mortality and also resulted in significant improvement of other YF disease parameters, including weight loss, serum alanine aminotransferase levels (6 days postinfection [dpi]), and viremia (4 dpi). In uninfected hamsters, BCX4430 at 200 mg/kg/day administered i.p. BID for 7 days was well tolerated and did not result in mortality or weight loss, suggesting a potentially wide therapeutic index. Treatment with BCX4430 at 12 mg/kg/day i.p. remained effective when administered once daily and for only 4 days. Moreover, BCX4430 dosed at 200 mg/kg/day i.p. BID for 7 days effectively treated YF, even when treatment was delayed up to 4 days after virus challenge, corresponding with peak viral titers in the liver and serum. BCX4430 treatment did not preclude a protective antibody response, as higher neutralizing antibody (nAb) concentrations corresponded with increasing delays of treatment initiation, and greater nAb responses resulted in the protection of animals from a secondary challenge with YFV. In summary, BCX4430 is highly active in a hamster model of YF, even when treatment is initiated at the peak of viral replication.
INTRODUCTION
Yellow fever, caused by the enveloped RNA flavivirus yellow fever virus (YFV), causes significant morbidity and mortality in areas of South America and Africa, with case fatality rates of up to 50% for hospitalized patients (1). Although Aedes aegypti, the most common vector of YFV to humans, was largely eradicated from tropical South America during the 1950s and 1960s, the resurgence of this mosquito species has enhanced the transmission of yellow fever (YF) and other viral diseases throughout this region (2). The ecology of YFV includes urban, savannah (intermediate), and jungle (sylvatic) cycles. The jungle cycle involves various mosquito vectors and primate hosts, although the importance of sylvatic maintenance to human transmission is unknown (3). Transmission to humans generally occurs in rural areas, where mosquitoes that have fed on infected nonhuman primates may come into contact with humans (i.e., the intermediate or savannah cycle), which may then be followed by the urban cycle, involving person-to-person transmission, primarily through A. aegypti. Due to its jungle cycle, YF will be maintained as a zoonotic infection, and thus, YFV will continue to pose a threat to human health, despite vaccination efforts and vector control programs.
An effective vaccine for the prevention of YF is available, but its lack of therapeutic utility during outbreaks and the minor risk of vaccine-related adverse events, including acute viral hemorrhagic syndrome (yellow fever vaccine-associated viscerotropic syndrome) (4), further emphasize the need for an effective antiviral therapeutic for YF. Such an effective antiviral would also have utility in treating cases of imported YF and vaccine-associated disease in areas outside the endemic range of YFV.
Unfortunately, there are currently no approved antiviral therapies for YF, and no drugs have demonstrated efficacy in humans with YF (5). Ideally, a compound developed for the treatment of YF would be stable with a long shelf life at ambient temperatures, easy to administer, effective when administered after the onset of clinically evident disease, and safe. From a logistical standpoint, it would also be advantageous if such a drug had broad-spectrum activity against other viral hemorrhagic fever (VHF) pathogens, precluding the need to develop and supply multiple therapeutic agents that treat only a single etiologic agent of VHF. RNA viruses, including other members of the Flaviviridae virus family that are related to YFV (e.g., West Nile virus and dengue virus), may share common replication pathways and similar pathogenic mechanisms. Thus, a drug that targets a common replication pathway might result in an effective treatment for a wide range of viruses.
The novel adenosine analog BCX4430 represents a compound with such broad-spectrum potential (6). This compound targets viral RNA-dependent RNA polymerase, an enzyme critical for the replication of numerous RNA viruses, and causes RNA chain termination after its conversion to the active triphosphate nucleotide form (6). Warren and colleagues (6) demonstrated that BCX4430 is active against several filoviruses in vitro and in animal models and showed unprecedented protection of cynomolgus macaques after lethal challenge with Marburg virus.
Hamsters infected with a hamster-adapted Jimenez strain of YFV have shown utility as a model of YF. The Jimenez strain was isolated in Panama in 1974 from a fatal human case and had undergone one passage in a monkey (Aotus trivirgatus) prior to hamster adaptation (7). The pathology of the Jimenez strain of YF in hamsters replicates many features of human YF, including viscerotropic disease primarily affecting the liver, markedly elevated serum aminotransferase levels, with aspartate aminotransferase (AST) levels higher than those of alanine aminotransferase (ALT), hemorrhagic events, and viremia lasting 2 to 5 days (7–10). Differences include the use of an adapted YFV strain and necrosis that is not restricted to midzonal hepatocytes (1). Mortality is not dependent on virus challenge dose (8), and mortality rates between 50% and 100% have been observed after challenge with the same virus challenge dose. This hamster model has been used to evaluate candidate YF antiviral agents (11–15) and vaccines (16, 17) and was used in the current study.
The primary objective of these studies was to characterize the activity of BCX4430 against YFV. The compound's antiviral activity against this virus was first identified in cell culture. Subsequent in vivo studies confirmed that BCX4430 possesses antiviral activity with acceptable tolerability in a YF hamster model. In summary, BCX4430 represents a promising potential therapeutic for YF and warrants further investigation.
MATERIALS AND METHODS
Animals.
Female Syrian golden hamsters (Charles River Laboratories) with an average weight of 99 g were used after a quarantine period of >48 h. The animals were randomly assigned to cages and individually marked with ear tags.
Facilities.
Experiments were conducted in the AAALAC-accredited biosafety level 3 (BSL3) animal suite at the Utah State University Laboratory Animal Research Center (LARC). All LARC personnel continually receive special training on blood-borne pathogen handling by this university's Environmental Health and Safety Office.
Test article.
BCX4430 is an adenosine analog, developed and supplied by BioCryst Pharmaceuticals, Inc. The compound disrupts viral RNA polymerase activity by causing nonobligate chain termination during viral RNA replication (6). The test article was prepared as a solution in sterile saline that was stable and soluble at a concentration up to 100 mg/ml (296 mM). All dosages were based on an average hamster weight of 100 g. A standard 0.2-ml volume was used for all treatments regardless of dose. Ribavirin was provided by ICN Pharmaceuticals, Inc. (now Valeant Pharmaceuticals) and prepared in sterile saline. Sterile saline was used as a vehicle control. Compounds were prepared just prior to initial administration and were stored at 4°C.
Virus.
Jimenez, a hamster-adapted YFV strain, was obtained as a generous gift from Robert B. Tesh (University of Texas Medical Branch, Galveston, TX). The virus was inoculated into 5 adult female hamsters. The liver of each infected hamster was removed 3 days postinfection (dpi) and homogenized in a 2× volume of sterile phosphate-buffered saline (PBS). This liver homogenate had a titer of 106.0 50% cell culture infectious doses (CCID50)/ml. This virus pool was later titrated for lethality in hamsters and served as the source of virus for these experiments. The 17D vaccine strain of YFV was used for cell culture studies and was prepared by 2 passages in Vero cells (ATCC).
In vitro evaluation of BCX4430.
The antiviral activity of BCX4430 was evaluated in vitro by cytopathic effect (CPE) inhibition assays as previously described (13). Inhibition of virus was determined by visual (microscopic) examination of the cells for cytopathic effect, increase of neutral red (NR) dye uptake (colorimetric determination), and virus yield reduction. Uninfected cells treated with the compound were assayed as described above for toxicity control.
Serum aminotransferase assays.
Sera were collected via ocular sinus bleed 6 dpi. ALT (serum glutamic pyruvic transaminase) reagent (Teco Diagnostics, Anaheim, CA) was used, and the protocol was altered for use in 96-well plates, as described previously (8). Aminotransferase concentrations were determined per the manufacturer's instructions.
Infectious cell culture assay.
Test serum and liver samples were collected for the determination of viral titers. Sera were collected antemortem 4 dpi from anesthetized hamsters via the ocular sinus. Liver samples were collected from a cohort of animals necropsied at 4 dpi, and the tissue was homogenized in 1 ml of cell culture medium. The samples were stored at −80°C until analysis. Thawed samples were serially diluted in 10-fold increments in 96-well microplates of Vero cells (grown to approximately 80% confluence). Four microwells were used per virus dilution of 0.1 ml/well. Ten days later, the presence of a viral cytopathic effect (CPE) was used to calculate the endpoint of infection. Viral titers (CCID50) were calculated by the method of Reed and Muench (18). The weights of the liver samples were included in the calculations for virus titers of these tissues and recorded as CCID50/g tissue, while serum titers were calculated as CCID50/ml.
Fifty percent plaque reduction neutralization titer assay.
Serum samples were heat inactivated at 50°C for 30 min and mixed at various 2-fold dilutions with a known concentration of virus (1:1,600 dilution of stock, approximately 20 PFU/well) and incubated at 4°C overnight. Approximately 18 h later, the virus and serum mixture was added to Vero cells in a single well of a 12-well microplate and incubated with intermittent rocking for 1 h. Then, 1 ml of 0.85% methylcellulose overlay medium with 10% fetal bovine serum (FBS) was added to each well, and the plates were incubated at 37°C for 5 days. After the incubation period, the methylcellulose overlay was removed, and cells were stained with crystal violet containing formaldehyde. Plaques were counted to determine the maximal dilution that reduced the plaque count by 50%.
Experimental design.
For tolerability studies, 3 uninfected hamsters/dose were treated with various doses of BCX4430 for 7 days, and their weight and survival were observed daily for 14 days. For YFV infection and treatment studies, the hamsters were randomly assigned to groups of 10 hamsters each. A 10−4 dilution (102.0 CCID50/ml) of the virus was prepared in minimal essential medium. The hamsters were injected intraperitoneally (i.p.) with 0.2 ml (0.1 ml on each side) of the diluted virus (20 CCID50/animal). BCX4430 was administered i.p. at various doses, and the treatment schedules and treatments were blinded to the technicians. We checked for mortality twice daily for 21 days, and the weight of each hamster was recorded 0, 3, 5, and 6 dpi.
Statistical analysis.
Survival data were analyzed using the Wilcoxon log-rank survival analysis, and all other statistical analyses were performed using one-way analysis of variance (ANOVA) using a Bonferroni group comparison (Prism 5; GraphPad Software, Inc.). Dose response was modeled using dose effect logistic regression in XLSTAT version 2014.1.01.
Ethics statement.
This study, including veterinary care and experimental procedures, was conducted in accordance with the approval of the Institutional Animal Care and Use Committee of Utah State University under approved protocol 1231. The work was done in the AAALAC-accredited and Public Health Service Animal Welfare Assurance-approved Laboratory Animal Research Center of Utah State University.
RESULTS
Effect of BCX4430 against YFV in cell culture.
BCX4430 effectively inhibited the infection of Vero cells with YFV. The 50% effective concentration (EC50) determined by the neutral red uptake assay was 8.3 μg/ml (24.5 μM). In a virus yield assay, the EC90 was 9.33 μg/ml (27.6 μM). The compound concentration that reduces cell viability by 50% (CC50), as determined by visual cytopathic effect and neutral red uptake, was 320 μg/ml (946 μM), yielding in vitro selectivity index values of 38.6 and 34.3 for the EC50 and EC90, respectively. In addition, preliminary in vitro studies have shown that BCX4430 is not incorporated into mammalian DNA or RNA (6), suggesting specificity for viral RNA polymerase. Given the high selectivity index values observed in cell cultures, studies in an in vivo model of YF were warranted.
Maximum tolerated dose of BCX4430.
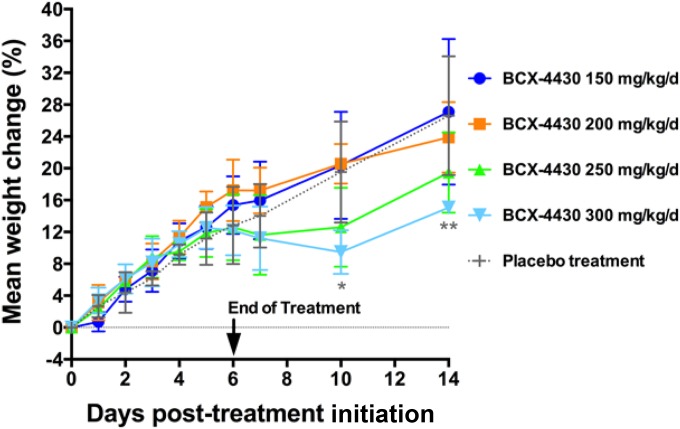
BCX4430 was administered intraperitoneally (i.p.) twice daily (BID) for 7 days at doses of 300, 250, 200, and 150 mg/kg of body weight per day, and the animals were observed for 14 days. Vehicle controls were administered along the same route and on the same treatment schedule. No mortalities were observed in any of the BCX4430 dose groups. Transient weight loss between 5 and 10 days after the initial treatment was observed in animals treated with 300 mg/kg/day, and reduced weight gain was observed in animals treated with 250 mg/kg/day (Fig. 1). The average group weight was significantly reduced at days 10 (P < 0.05) and 14 (P < 0.01) after the initiation of BCX4430 treatment in animals dosed at 300 mg/kg/day compared with those in the vehicle-treated group. Despite the weight effects associated with the two highest doses, all the groups gained weight between 10 and 14 days after treatment initiation (Fig. 1). A steady gain in weight was observed in animals treated with 150 and 200 mg/kg/day, and 200 mg/kg/day was therefore considered the maximum tolerated dose for BCX4430 administered i.p. BID in hamsters.
FIG 1.
BCX4430 is well tolerated in hamsters. Shown is the time course of mean percent weight changes of uninfected hamsters treated with various doses of BCX4430 administered i.p. BID for 7 days.
Efficacy of BCX4430 in the YF hamster model.
To determine the effect of different BCX4430 doses in hamsters infected with YFV, two separate studies were conducted in which the test article or placebo control was administered beginning 4 h prior to virus challenge. In the first study, BCX4430 was administered at doses of 125 and 40 mg/kg/day. In the second study, BCX4430 doses of 125, 40, 12.5, 4, and 1.25 mg/kg/day were administered. In both studies, the hamsters were treated with BCX4430 i.p. BID for 7 days. Ribavirin, administered i.p. BID at a dose of 50 mg/kg/day, was included as a positive control. Disease parameters included survival, weight changes, and serum ALT levels 6 days post-virus infection (dpi). The serum virus titer 4 dpi was also analyzed as a part of the second dose-ranging study.
In the first YFV study, BCX4430 treatment at doses of 125 and 40 mg/kg/day resulted in significant improvements in all YF disease parameters compared to those with vehicle treatment (data not shown). One of 10 animals in the 40-mg/kg/day treatment group and 0/10 animals in the 125-mg/kg/day treatment group succumbed to disease, which was significant (P < 0.001) compared with 10/11 controls. Weight changes and serum levels of ALT were significantly (P < 0.001) decreased in the two BCX4430 groups compared with the vehicle treatment group. Similar results were observed after treatment with the positive-control compound, ribavirin, administered at 50 mg/kg/day using the same dosing regimen as that for BCX4430.
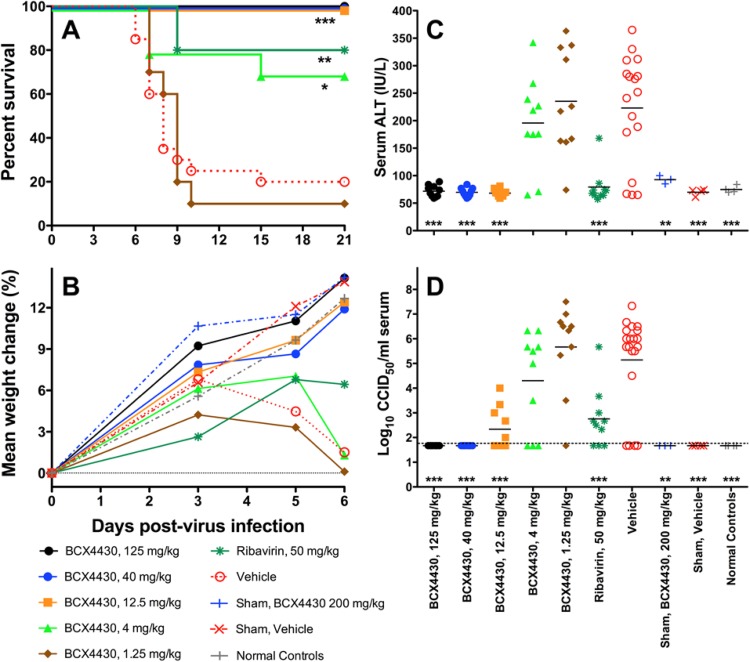
A second treatment study was conducted to explore the minimal effective dose of BCX4430 using doses of 125, 40, 12.5, 4, and 1.25 mg/kg/day. Analogous to the first study, treatment with 125 or 40 mg/kg/day resulted in significant improvements of all the disease parameters compared to those for the vehicle controls (Fig. 2). Compared to the vehicle, BCX4430 doses of ≥4 mg/kg/day significantly improved the survival of hamsters infected with YFV, with complete survival observed in groups treated with doses of ≥12.5 mg/kg/day (Fig. 2A). Logistic regression of the dose responses for survival showed a significant dose effect (−2-log likelihood ln, χ2 = 34.87, P < 0.0001), and the 50% effective dose (ED50) was estimated at 2.8 mg/kg/day (95% confidence interval [CI], 0.7 to 4.6 mg/kg/day). In addition, a significant improvement (P < 0.001) in mean weight change between 3 and 6 dpi was observed in animals treated with doses of ≥12.5 mg/kg/day. The animals treated with lower doses (4 or 1.25 mg/kg/day) had initial weight gains between 0 and 3 dpi but, similar to the vehicle-treated animals, lost weight after 3 or 5 dpi (Fig. 2B). Serum ALT levels 6 dpi in animals treated with BCX4430 at doses of ≥12.5 mg/kg/day were similar to those of uninfected controls, while animals treated with lower BCX4430 doses had elevated ALT concentrations, similar to those of the vehicle-treated animals (Fig. 2C). A dose response was observed in serum virus titers 4 dpi, with significant improvements observed in animals treated with doses of ≥12.5 mg/kg/day (Fig. 2D).
FIG 2.
Dose response of BCX4430 in YFV-infected hamsters. Data represent effect of treatment with different doses of BCX4430 on survival (A), mean percent weight change between 3 and 6 dpi (B), serum ALT level (6 dpi) (C), and serum virus titer (4 dpi) (D) of hamsters infected with YFV. Treatment was administered i.p. BID, beginning 4 h before virus challenge and continuing for 7 days. The dashed line in panel D represents the limit of detection. ***, P < 0.001, **, P < 0.01, and *, P < 0.05, compared with vehicle treatment.
Efficacy of shorter, less frequent dosing regimens.
Once-daily (QD) treatment with BCX4430 was compared with twice-daily (BID) treatment over a 7-day treatment course to determine if less frequent treatment would be as effective at the same overall daily exposure (Table 1). The effect of the shortened treatment regimen (QD or BID) of 4 days' duration, beginning just prior to infection (dosed 4 h before virus challenge to 3 dpi), or 5 days' duration, beginning 2 dpi (dosed 2 to 6 dpi), was also investigated (Table 1). BCX4430 was administered in all the groups at a dose of 12 mg/kg/day, based on the efficacy previously observed in dose-ranging studies.
TABLE 1.
Effect of dosing frequency and treatment duration on the efficacy of BCX4430 at a dose of 12 mg/kg/day in a hamster model of yellow fevera
| Treatment | Freqb | Initiation (h), duration (days) | Toxicity controls |
Hamsters infected and treated with BCX4430 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. alive/total no. | Weight changec (mean ± SDd) (g) | No. alive/total no. | MDDe ± SD | Weight changec (mean ± SD) (g) | Serum ALTf (mean ± SD) (IU/liter) | Virus titerg (mean ± SD) (CCID50/ml) | |||
| BCX4430 | BID | −4, 7 | 3/3 | 13.7 ± 4.0h | 10/10h | >21.0 ± 0.0 | 7.4 ± 1.6h | 76 ± 15h | 3.8 ± 1.1h |
| BCX4430 | QD | −4, 7 | 10/10h | >21.0 ± 0.0 | 5.6 ± 1.9h | 68 ± 10h | 3.1 ± 0.6h | ||
| BCX4430 | BID | −4, 4 | 10/10h | >21.0 ± 0.0 | 4.6 ± 2.3h | 70 ± 7.0h | 3.4 ± 0.8h | ||
| BCX4430 | QD | −4, 4 | 9/10h | 13.0 ± 0.0 | 7.7 ± 3.5h | 67 ± 4.0h | 2.8 ± 0.5h | ||
| BCX4430 | BID | 2, 5 | 10/10h | >21.0 ± 0.0 | 0.5 ± 8.0 | 117 ± 71i | 5.0 ± 1.4 | ||
| BCX4430 | QD | 2, 5 | 8/10i | 11.0 ± 2.8 | − 4.8 ± 7.7 | 151 ± 85 | 5.5 ± 1.4 | ||
| Ribavirin | BID | −4, 7 | 9/10i | 13.0 ± 0.0 | 2.9 ± 3.8i | 79 ± 26h | 3.2 ± 0.9h | ||
| Ribavirin | QD | −4, 7 | 8/9i | 13.0 ± 0.0 | 1.8 ± 4.7j | 103 ± 59h | 3.0 ± 0.7h | ||
| Placebo | BID | −4, 7 | 7/7 | 8.4 ± 2.1h | 7/20 | 7.8 ± 1.5 | − 4.4 ± 7.5 | 189 ± 81 | 5.5 ± 1.8 |
Jimenez strain, 20 CCID50/animal.
Freq, frequency of administration: BID, twice a day; QD, once a day.
Difference in weight in grams between 3 and 6 dpi.
SSD was derived from a single experiment with the number of animals indicated in the “No. alive/total no.” column.
MDD, mean day to death of mice dying prior to 21 dpi.
ALT, alanine aminotransferase.
Serum virus titers on the 4th dpi.
P ≤ 0.001 compared with saline-treated controls.
P ≤ 0.01 compared with saline-treated controls.
P < 0.05 compared with saline-treated controls.
Survival rates between 80% and 100% were observed in all the groups treated with BCX4430 at 12 mg/kg/day, regardless of the dosing frequency, duration of treatment, or time of treatment initiation, and were significantly improved compared with that of the vehicle treatment group (35% survival, P ≤ 0.01; Table 1). No differences in disease parameters were seen between the groups treated QD and BID or between the groups treated for 7 days versus 4 days when treatment was initiated just prior to infection. Weight changes between 3 and 6 dpi, serum ALT levels 6 dpi, and viremia 4 dpi were also significantly improved (P < 0.001) in the groups treated for 7 days or 4 days (Table 1).
A 5-day treatment regimen of BCX4430 administered BID or QD beginning 2 dpi resulted in significantly improved survival compared to the vehicle treatment group, regardless of the dosing frequency (Table 1). A significant reduction in ALT levels was observed in animals treated BID, but not QD, compared with those in the vehicle treatment group (Table 1). Weight changes and virus titers were not significantly improved when treatment was initiated 2 dpi, regardless of the dosing frequency (Table 1).
Ribavirin, administered either BID or QD at a dose of 50 mg/kg/day for 7 days beginning 4 h prior to virus challenge, was included as a positive control for this study. Significant improvements in all the disease parameters were observed with ribavirin, regardless of the dosing frequency (Table 1).
Delayed initiation of BCX4430 treatment.
To investigate the therapeutic efficacy of delayed BCX4430 treatment, initiated after virus infection, hamsters were treated with a dose of 200 mg/kg/day, administered i.p. BID for 7 days beginning at various times after virus challenge. Two separate time course studies were conducted. In the first study, BCX4430 treatment was initiated 4 h prior to or 1, 2, 3, or 4 days after virus challenge, and the second study examined treatment initiation 4, 5, or 6 dpi. The disease parameters, including mortality, weight changes, serum ALT levels, and serum and liver virus titers, were assessed.
Complete protection with a 100% survival rate was observed when animals were treated beginning 4 h before challenge or 1, 2, or 3 dpi (Fig. 3A). Treatment beginning 4 dpi resulted in an 80% survival rate, significantly higher than that observed with vehicle treatment (12.5%, P < 0.001). This result was confirmed in the second study, with an 80% survival rate observed with BCX4430 treatment initiated 4 dpi compared to only a 35% survival rate in the vehicle-treated group (P < 0.01). Treatment initiated ≤3 dpi resulted in significant improvements in the weight changes and serum ALT levels of infected hamsters over those in the vehicle-treated group (P < 0.01) (Fig. 3B and C). Significant improvements in weight changes and ALT levels were observed after a delay of treatment initiation at 4 dpi in the first time course study but not in the second study. These disease parameters, however, were at similar levels for the group treated from 4 through 10 dpi in both studies and were improved over those of the vehicle treatment group, despite the lack of significance in one experiment. The delay of treatment initiation to 5 or 6 dpi did not improve survival or other disease parameters compared with those of the vehicle-treated group (data not shown). In addition, BCX4430 treatment initiated 4 h prior to infection significantly reduced viral titers in the liver and serum to their limits of detection (Fig. 3D and E, respectively) compared to those of the vehicle control group (P < 0.001).
FIG 3.
Efficacy of delayed BCX4430 administration initiated after virus challenge. Data represent the effects of time of BCX4430 treatment initiation on survival (A), mean percent weight change between 3 and 6 dpi (B), serum ALT (6 dpi) (C), liver virus titer (D), and serum virus titer (E) during a YFV infection in a hamster model. Treatments with BCX4430 (200 mg/kg/day) and ribavirin (50 mg/kg/day) were administered i.p. BID for 7 days, starting at the indicated time relative to virus challenge. Liver and serum samples were collected for assay from animals sacrificed at 4 dpi whose treatments began 4 h before virus challenge. The dashed lines in panels D and E represent the limits of detection. ***, P < 0.001, and **, P < 0.01, compared with vehicle treatment.
Ribavirin, which was included as a positive control, protected 90% of infected animals and significantly improved weight changes and serum ALT levels when treatment was initiated 4 h prior to virus challenge (Fig. 3). Compared to vehicle treatment, the delay of ribavirin treatment initiation to 4 dpi was not effective and did not improve any disease parameters (data not shown).
Effect of BCX4430 treatment on rechallenge with YF.
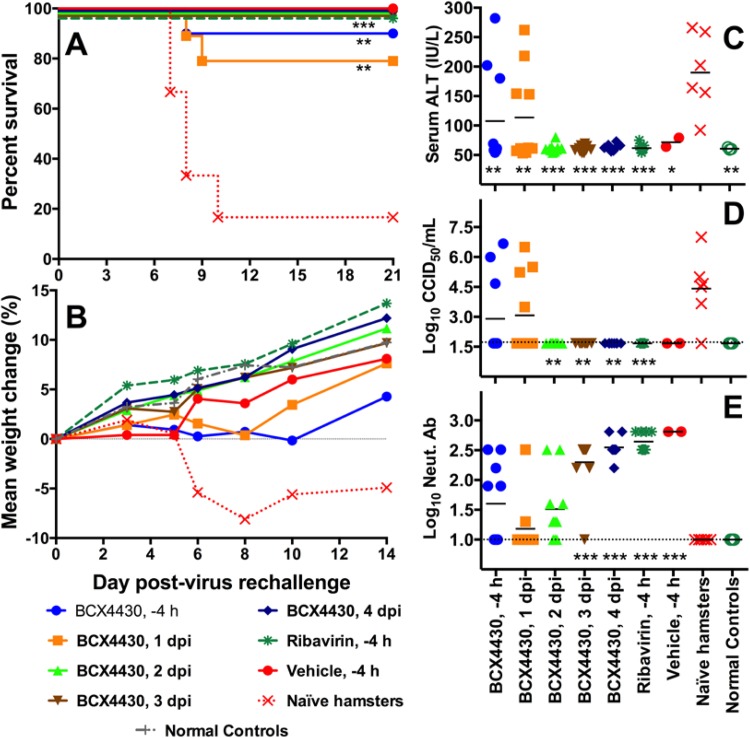
For antiviral therapies, it is important to characterize the effect of highly effective treatment on the development of immunity to the virus in surviving animals. To determine the immune status after treatment, the surviving hamsters previously infected with YFV and treated with 200 mg/kg/day BCX4430, 50 mg/kg/day ribavirin, or vehicle (Fig. 3) were challenged with a secondary YFV infection 28 days after the initial viral challenge (Fig. 4; Table 2).
FIG 4.
Neutralizing antibody development after effective BCX4430 treatment. Data represent the effect of secondary challenge with YFV on survival (A), mean weight change between 3 and 6 dpi (B), serum ALT (6 dpi) (C), serum virus titer (4 dpi) (D), and neutralizing Ab titer (21 dpi) (E) using hamsters that survived primary challenge or naive animals. Surviving animals, previously treated with BCX4430, ribavirin, or vehicle in a therapeutic YF antiviral study (see Fig. 3), were subjected to a secondary virus challenge 28 days after primary challenge (***, P < 0.001, and **, P < 0.01, compared with naive hamsters). The dashed lines in panels D and E represent the limits of detection.
TABLE 2.
Disease parameters of surviving and nonsurviving hamsters treated with BCX4430 4 h prior to or 1 day after secondary virus challenge compared with untreated naive animals after primary virus challenge
| Virus challenge, outcome | No. of animals | Disease parameter (mean ± SD) |
||||
|---|---|---|---|---|---|---|
| Weight change between 3 and 6 dpi (g) | Serum ALT at 6 dpi (IU/liter) | Serum AST at 6 dpi (IU/liter) | Serum virus titer at 4 dpi (CCID50/ml) | Neutralizing Ab titera | ||
| Secondary, survived | 17 | 1.5 ± 6.7 | 90 ± 64 | 100 ± 128 | 2.7 ± 1.8 | 164 ± 137 |
| Secondary, did not survive | 3 | − 16.0 ± 3.1b | 227 ± 31c | 364 ± 48c | 4.9 ± 1.5 | <10 ± 0.0 |
| Primary, survived | 1 | 0.0 ± 0.0 | 63 ± 0.0 | 63 ± 0 | 4.7 ± 0.0 | <10 ± 0.0 |
| Primary, did not survive | 5 | − 12.0 ± 7.5 | 209 ± 52 | 312 ± 95 | 4.4 ± 1.9 | <10 ± 0.0 |
Neutralizing antibody titer present prior to primary or secondary virus challenge.
P < 0.001 compared with values from animals that survived secondary challenge.
P < 0.01 compared with values from animals that survived secondary challenge.
Challenge with YFV resulted in mortality of 5 out of 6 naive control animals (Fig. 4A). Secondary challenge of the two surviving animals from the vehicle group did not result in mortality. Similarly, all surviving animals from the 2-, 3-, and 4-dpi BCX4430 groups survived the YFV rechallenge. The only animals that died as a result of secondary challenge with YFV after surviving the initial infection were those previously treated with BCX4430 initiated early in relation to the initial virus challenge. Specifically, 10% and 20% mortality rates were observed after secondary YFV challenge in animals that had been previously treated with BCX4430 initiated 4 h before challenge and 1 dpi, respectively. Animals that succumbed to secondary challenge exhibited significantly greater weight losses and higher serum ALT and AST levels than the animals from the same BCX4430 treatment groups that survived the secondary infection (Table 2). In animals that did not survive secondary challenge, the average serum virus titers were also higher and the neutralizing antibody (Ab) titers were undetectable, but these differences were not significant compared with levels in the few animals that survived the secondary challenge (Table 2).
Hamsters that survived the secondary virus challenge also experienced an overall weight gain within a 14-day period compared with naive animals that had an average weight loss over the same time, beginning after 3 dpi (Fig. 4B). The extent of weight gain after the secondary challenge was correlated with the time of treatment initiation after the first challenge; similar to the pattern observed in survival, less weight gain was observed with an earlier initiation of BCX4430 treatment. Serum ALT levels showed a similar relationship, with elevated levels in animals that had earlier BCX4430 treatment initiation after the initial virus challenge (Fig. 4C). Viremia on day 4 after the secondary challenge followed a similar pattern to those of weight changes and serum ALT levels (Fig. 4D). The levels of neutralizing antibody present in the serum of hamsters just prior to the secondary virus challenge (28 days after initial YFV challenge) displayed a pattern inverse to that of the disease parameters, with lower average titers in the groups treated with BCX4430 earlier after the initial virus challenge (Fig. 4E).
DISCUSSION
The novel adenosine analog BCX4430 was highly effective in treating hamsters infected with YFV. When considering the activity of the maximum tolerated doses for each compound, the activity of BCX4430 was generally superior to that of the positive-control compound, ribavirin, which has also shown suitable activity in this model (8). Therapy with BCX4430 resulted in significant improvement in survival, weight changes, serum ALT levels, and viremia. The protective effects of BCX4430 were similar or superior to those observed with other compounds previously tested in this model, including T-1106 (12), interferon alfacon-1 (8), 2′-C-methylcytidine (13), and T-705 (14).
The minimal effective dose required to significantly improve survival was 4 mg/kg/day when administered i.p. for 7 days beginning 4 h prior to virus challenge, although a dose of 12 mg/kg/day was required to significantly improve all the disease parameters measured in this experiment. The maximum tolerated dose of BCX4430 administered BID for 7 days was 200 mg/kg/day, as no mortality or adverse changes in weight or behavior were seen at this dose, whereas weight gain was impaired at doses of 250 and 300 mg/kg/day (see Fig. 1). Because Marburg virus was the initial indication planned for BCX4430, long-term tolerability and toxicity studies have not been conducted, and only short-term (∼14-day) treatment has been evaluated. For BCX4430, the ratio of the maximum tolerated dose in uninfected animals to the minimal effective dose for survival in animals infected with YFV is 50 in the hamster. The therapeutic index (ratio of the 50% lethal dose [LD50] to the ED50) is yet to be defined, but given the survival rate of animals treated with 300 mg/kg/day, it is >75-fold. Ribavirin shows a greater degree of toxicity than BCX4430, with an LD50 of about 217 mg/kg in hamsters (19) and a minimum effective dose of approximately 32 mg/kg/day (J. G. Julander, unpublished data). These results give a therapeutic index of only 6.8 for ribavirin administered to hamsters using the same treatment regimen as that for BCX4430.
The i.p. route was utilized in the present study for the administration of BCX4430. Previous studies utilized intramuscular and intravenous routes (6), which were shown to provide exposure levels similar to those of BCX4430. It is anticipated that i.p. administration will provide exposure similar to or lesser than that seen with the other parenteral routes reported by Warren and colleagues (6). Further studies are needed to more fully characterize the pharmacokinetics of BCX4430 administered i.p., but from the present study, it is clear that sufficient exposure is achieved to reduce disease at a daily dose of 12 mg/kg/day.
A decrease in the dosing frequency of BCX4430 to QD with a treatment duration as short as 4 days did not significantly reduce efficacy when drug treatment was initiated just prior to infection. When treatment initiation was delayed to 2 dpi, a 5-day course of BCX4430 administered BID or QD resulted in a significantly reduced mortality rate compared with that of vehicle treatment; however, QD dosing was associated with diminished efficacy, as assessed by other disease parameters, compared with BID dosing. Regardless, the efficacy observed with less frequent QD dosing and shorter 4- or 5-day treatment courses further highlights the clinical potential for BCX4430.
Importantly, BCX4430 was active in the hamster model when administered therapeutically days after YFV challenge. Treatment initiated as late as 4 dpi effectively protected animals from mortality. In the hamster model of YF, virus titers in the sera and livers of infected animals peak 4 dpi, and initial mortality was observed as early as 5 dpi (8). A comparison of this time in hamsters with clinical YF disease in humans roughly corresponds to the time after nonspecific disease signs and just prior to or during the period of intoxication that includes classical YF signs of jaundice, hematemesis, and proteinuria (20). Germane to the timing of pathological events and disease improvement is the finding that treatment with BCX4430 initiated 4 dpi significantly improved serum ALT levels, indicating a reduction of liver damage, and future studies to determine the effect of BCX4430 treatment on liver pathology may be illustrative. Similar protection from mortality was observed after treatment with T-1106, beginning 4 dpi and following a treatment regimen similar to that of BCX4430, but weight changes and serum ALT levels were not improved compared to those with vehicle treatment (12). Weight changes and serum ALT levels were significantly reduced when treatment was initiated 4 dpi in one of two BCX4430 time course studies, although group averages for these parameters were similar between studies. The lack of statistical significance in one experiment may be attributable to the variability inherent in in vivo systems. The therapeutic efficacy of BCX4430 was also superior to that of the positive-control ribavirin treatment. None of the disease parameters were significantly improved by ribavirin using a 7-day treatment regimen with 50 mg/kg/day initiated 4 dpi, although this dose of ribavirin is approximately 3-fold lower than that used for BCX4430. We previously reported the therapeutic efficacy of this dose of ribavirin when treatment was initiated 2 dpi (8), but doses of >50 mg/kg/day are generally less tolerable. Previous studies have reported some protective benefit of ribavirin initiated as late as 5 days after virus challenge (15), but the infection was far less virulent than that in the present study. Further, ribavirin failed to improve viremia in the study by Sbrana et al. (15).
The immune responses of some animals treated early in relation to virus challenge were attenuated, resulting in low neutralizing antibody titers and subsequent morbidity and mortality rates after secondary YFV challenge. We have demonstrated previously that protection from secondary YFV infection is due to the development of neutralizing antibodies (17). The observed immune attenuation is likely the result of a highly effective reduction of viral proliferation associated with early initiation of BCX4430 treatment, so that less antigen is present to stimulate a robust immune response. It is likely that this observation would not be clinically relevant to the treatment of natural infection, as patients would seek medical attention after the onset of disease symptoms and therefore would be exposed to viral antigen several days prior to treatment initiation.
Collectively, these findings suggest that treatment of clinical YF cases with BCX4430 after the onset of symptoms should be associated with the development of protective immunity, as treatment initiated during peak viremia (e.g., 3 or 4 dpi) allowed the development of high neutralizing Ab titers that completely protected against secondary infection challenge.
In summary, we have demonstrated that the novel adenosine analog BCX4430, a direct-acting antiviral drug that disrupts viral RNA-dependent RNA polymerase activity, possesses anti-YFV activity in cell culture and in a hamster model of YF disease using a virulent hamster-adapted strain. Protective immune responses were intact when treatment was begun after virus infection, and the compound was well tolerated with a favorable predicted therapeutic index. Given the activities observed, even when treatment was initiated late in the YFV infection cycle, BCX4430 represents a promising therapeutic candidate for the treatment of clinical YF. The observed preclinical efficacy and tolerability of BCX4430 in the treatment of YF in hamsters strongly support further preclinical and clinical development of this compound.
ACKNOWLEDGMENTS
We thank Isaac Wong for expert technical assistance.
This research was supported in part by grant HHSN272201000039I, Task Order A21, from the Virology Branch, NIAID, NIH.
Footnotes
Published ahead of print 25 August 2014
REFERENCES
- 1.Monath TP. 2008. Treatment of yellow fever. Antiviral Res. 78:116–124. 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Jansen CC, Beebe NW. 2010. The dengue vector Aedes aegypti: what comes next? Microbes Infect. 12:272–279. 10.1016/j.micinf.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Barrett AD, Higgs S. 2007. Yellow fever: a disease that has yet to be conquered. Annu. Rev. Entomol. 52:209–229. 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- 4.Monath TP. 2012. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev. Vaccines 11:427–448. 10.1586/erv.12.6. [DOI] [PubMed] [Google Scholar]
- 5.Julander JG. 2013. Experiment therapies for yellow fever. Antiviral Res. 97:169–179. 10.1016/j.antiviral.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, Honnold S, Bantia S, Kotian P, Chen X, Taubenheim BR, Welch LS, Minning DM, Babu YS, Sheridan WP, Bavari S. 2014. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 508:402–405. 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesh RB, Guzman H, da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. 2001. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus): I. Virologic, biochemical, and immunologic studies. J. Infect. Dis. 183:1431–1436. 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- 8.Julander JG, Morrey JD, Blatt LM, Shafer K, Sidwell RW. 2007. Comparison of the inhibitory effects of interferon alfacon-1 and ribavirin on yellow fever virus infection in a hamster model. Antiviral Res. 73:140–146. 10.1016/j.antiviral.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sbrana E, Xiao SY, Popov VL, Newman PC, Tesh RB. 2006. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus) III. Clinical laboratory values. Am. J. Trop. Med. Hyg. 74:1084–1089. [PubMed] [Google Scholar]
- 10.Xiao SY, Zhang H, Guzman H, Tesh RB. 2001. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus): II. Pathology. J. Infect. Dis. 183:1437–1444. 10.1086/320200. [DOI] [PubMed] [Google Scholar]
- 11.Julander JG, Ennis J, Turner J, Morrey JD. 2011. Treatment of yellow fever virus with an adenovirus-vectored interferon (DEF201) in a hamster model. Antimicrob. Agents Chemother. 55:2067–2073. 10.1128/AAC.01635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julander JG, Furuta Y, Shafer K, Sidwell RW. 2007. Activity of T-1106 in a hamster model of yellow fever virus infection. Antimicrob. Agents Chemother. 51:1962–1966. 10.1128/AAC.01494-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Julander JG, Jha AK, Choi JA, Jung KH, Smee DF, Morrey JD, Chu CK. 2010. Efficacy of 2′-C-methylcytidine against yellow fever virus in cell culture and in a hamster model. Antiviral Res. 86:261–267. 10.1016/j.antiviral.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julander JG, Shafer K, Smee DF, Morrey JD, Furuta Y. 2009. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob. Agents Chemother. 53:202–209. 10.1128/AAC.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sbrana E, Xiao SY, Guzman H, Ye M, Travassos da Rosa AP, Tesh RB. 2004. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am. J. Trop. Med. Hyg. 71:306–312. [PubMed] [Google Scholar]
- 16.Monath TP, Lee CK, Julander JG, Brown A, Beasley DW, Watts DM, Hayman E, Guertin P, Makowiecki J, Crowell J, Levesque P, Bowick GC, Morin M, Fowler E, Trent DW. 2010. Inactivated yellow fever 17D vaccine: development and nonclinical safety, immunogenicity and protective activity. Vaccine 28:3827–3840. 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Julander JG, Trent DW, Monath TP. 2011. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine 29:6008–6016. 10.1016/j.vaccine.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed LJ, Muench CH. 1938. A simple method of estimating fifty percent endpoint. Am. J. Hyg. (Lond.) 27:493–497. [Google Scholar]
- 19.Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW. 2007. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob. Agents Chemother. 51:3168–3176. 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monath TP, Brinker KR, Chandler FW, Kemp GE, Cropp CB. 1981. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am. J. Trop. Med. Hyg. 30:431–443. [DOI] [PubMed] [Google Scholar]