Abstract
In light of the in vivo/in vitro discordance among beta-lactams against Gram-negative pathogens, we compared the in vivo pharmacodynamics of humanized ceftaroline against 9 Staphylococcus aureus strains (MICs of 0.13 to 1 mg/liter) from published in vitro studies using standard and high inocula in the murine thigh infection model. Consistent with the in vitro findings, mean reductions of ≥1 log10 CFU were observed for ceftaroline against all strains using both standard and high inocula. These results suggest in vivo/in vitro concordance with no observed inoculum effect.
TEXT
Discordant in vivo and in vitro pharmacodynamics (PD) have been reported among beta-lactams against Gram-negative pathogens (1–4). The discrepancy has been speculated to be due to the unnatural accumulation of enzymes within in vitro pharmacodynamic models and the resultant, rapid hydrolysis of antimicrobials against Enterobacteriaceae with enzyme-mediated resistance (4, 5). However, the in vivo/in vitro paradox has also been observed in other Gram-negative pathogens, such as Pseudomonas aeruginosa, whose mechanisms of resistance are mainly non-enzyme mediated (e.g., efflux pump or outer membrane proteins) (4, 6).
As for Gram-positive pathogens, such as Staphylococcus aureus, while many have been explored in in vivo and in vitro pharmacodynamic models, usage of varied isolates, initial inoculum concentrations, and antimicrobial regimens makes comparisons between in vivo and in vitro models difficult (7–10). Ceftaroline, a cephalosporin with anti-methicillin-resistant S. aureus (MRSA) activity via its high affinity to penicillin-binding protein 2a, is considered a viable option for resistant S. aureus infections. In lieu of the knowledge that increased ceftaroline MICs against S. aureus are mostly associated with mutations in penicillin-binding protein 2a (11, 12), this study aimed to compare the in vivo and in vitro pharmacodynamics of ceftaroline against Gram-positive pathogens with non-enzyme-mediated resistance. To mimic the published in vitro pharmacodynamic studies that tested ceftaroline (active drug) against S. aureus, we evaluated the in vivo pharmacodynamics of a previously determined humanized regimen (simulating ceftaroline pharmacokinetics in humans versus mice) of 600 mg ceftaroline fosamil (a ceftaroline prodrug) every 12 h against nine phenotypically diverse S. aureus isolates in the setting of two different initial inocula in the neutropenic murine thigh infection model.
Commercially available ceftaroline fosamil (lot 0008D36; Forest Pharmaceuticals, Inc., St. Louis, MO) was used for the in vivo studies. Vials were reconstituted and diluted to the desired concentrations according to the manufacturer's specifications.
Nine S. aureus isolates (ceftaroline MICs of 0.13 to 1 mg/liter) that were previously studied in published in vitro pharmacodynamic models (13, 14) were used for the standard-inoculum in vivo studies. Among these, three MRSA isolates were tested for the high-inoculum in vivo studies (Table 1) (14).
TABLE 1.
Phenotypic profiles and corresponding fT>MIC of tested S. aureus isolates in standard- and high-inoculum studiesa
| S. aureus isolate | Isolate no. from referenced in vitro data (reference) | Phenotypeb | Ceftaroline MIC (mg/liter) | fT>MIC (%) |
|---|---|---|---|---|
| 477 | 89608 (13) | MSSA | 0.13 | 100 |
| 478 | 89637 (13) | MSSA | 0.13 | 100 |
| 479 | 83771 (13) | CA-MRSA | 0.5 | 81.7 |
| 480 | 84495 (13) | HA-MRSA | 0.5 | 81.7 |
| 456 | VRS3a (13) | VRSA | 0.5 | 81.7 |
| 435 | NRS4 (13) | VISA | 1 | 60.8 |
| 412c | 412 (14) | MRSA | 1 | 60.8 |
| 449c | 449 (14) | hVISA | 1 | 60.8 |
| 454c | 454 (14) | VISA | 1 | 60.8 |
fT>MIC data from reference 10.
CA, community acquired; HA, health care acquired; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; VISA, vancomycin-intermediate Staphylococcus aureus; VRSA, vancomycin-resistant Staphylococcus aureus; hVISA, heteroresistant vancomycin-intermediate Staphylococcus aureus.
Isolates that were used in both standard- and high-inoculum studies.
The study protocol was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. Animals were maintained and used in accordance with the National Research Council recommendations. The previously established neutropenic murine thigh infection model was employed to evaluate efficacy (10). Briefly, pathogen-free, female ICR mice weighing approximately 25 g were acquired from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Mice were rendered neutropenic with intraperitoneal injections of cyclophosphamide (100 and 150 mg/kg of body weight; Baxter Healthcare Corporation, Deerfield, IL) given 1 and 4 days prior to inoculation, respectively. Three days prior to inoculation, mice were also given a single 5 mg/kg intraperitoneal injection of uranyl nitrate to produce a predictable degree of renal impairment to aid in humanizing the drug regimens (10). Two hours prior to the initiation of the antimicrobial therapy, each thigh was inoculated intramuscularly with 0.1 ml solution containing approximately 1 × 107 CFU/ml of the test isolate for the standard-inoculum and 1 × 109 CFU/ml for the high-inoculum studies.
Two hours postinoculation, subcutaneous doses of ceftaroline fosamil were administered as 0.2-ml subcutaneous injections to groups of three mice as two 12-h regimens over a 24-h period as follows: 20 mg/kg at 0 h, 2 mg/kg at 2.5 h and 4 h, and 1 mg/kg at 6 h and 9 h. This regimen was previously described to simulate the human concentration-time profile of 600 mg intravenous ceftaroline fosamil every 12 h, which achieved a percentage of the dosing interval where a free-drug concentration exceeds the MIC (fT>MIC) of ≥60.8% at an MIC of ≤1 mg/liter (Table 1) (10). The 24-h control groups received normal saline with the same volume, route, and frequency as the treatment regimen. Groups of three untreated mice were assigned to 0-h control groups; they were euthanized by CO2 exposure, followed by cervical dislocation, and harvested at the initiation of therapy. All other animals were harvested 24 h after the initiation of therapy. Mice that failed to survive to 24 h were harvested at the time of expiration. Following sacrifice, each thigh was harvested and homogenized in 5 ml of normal saline. Serially diluted thigh homogenates were plated onto Trypticase soy agar with 5% sheep blood to determine the bacterial density. The efficacy was calculated as the change in bacterial density in log10 CFU at 24 h compared with that at 0 h.
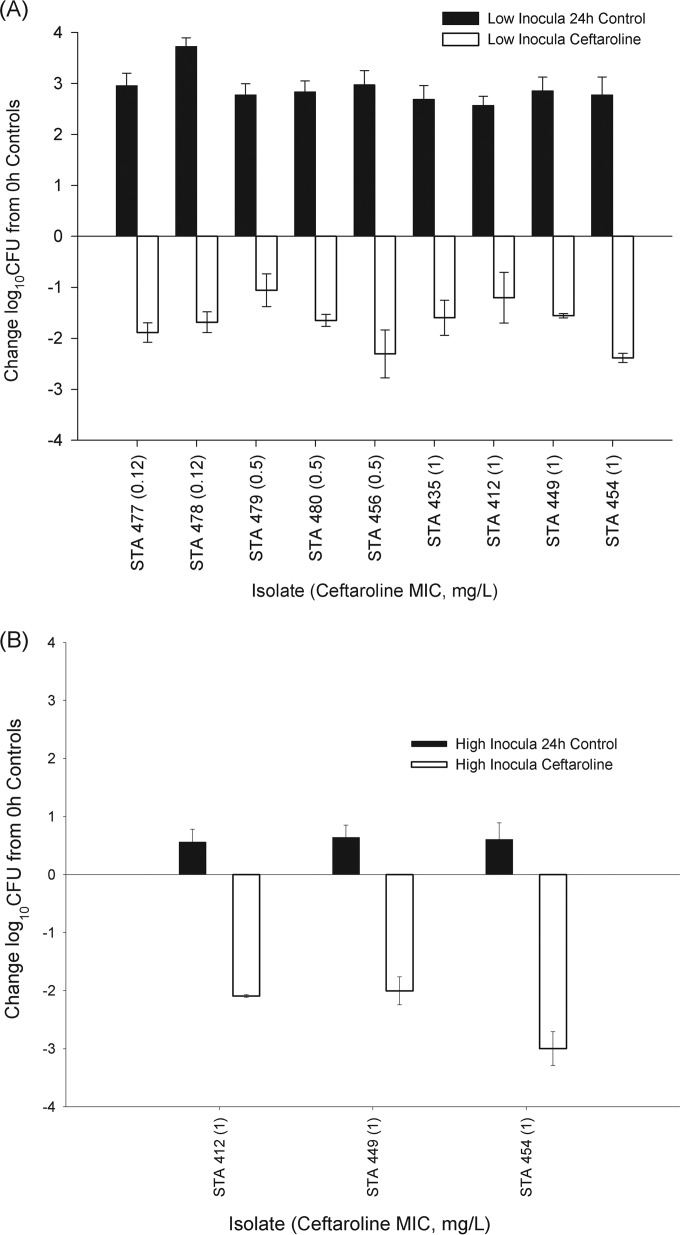
The efficacy of ceftaroline against the nine isolates in the standard-inoculum study is displayed in Fig. 1A. The respective log10 CFU values at 0 h and in the 24-h controls were 5.5 to 5.8 and 8.3 to 9.6. The ceftaroline treatment resulted in mean reductions of 1.1 to 2.4 log10 CFU, with no relationship to the ceftaroline MIC. These observations were consistent with the findings of Zhanel et al., who evaluated the in vitro pharmacodynamics of the humanized regimen of ceftaroline against isolates 477, 478, 479, 480, 456, and 435 at initial inocula of 1 × 106 CFU/ml (Table 1) (13). In their study, human-simulated ceftaroline treatment against all six strains achieved >3 log10 CFU decreases over 24 h.
FIG 1.
Changes in log10 CFU values for a humanized regimen of ceftaroline at 24 h compared with the 0-h controls for a collection of S. aureus isolates tested against standard inocula (A) and high inocula (B). Bars represent means ± standard deviations. STA, Staphylococcus aureus.
For the high-inoculum in vivo study, the corresponding bacterial densities for controls at 0 h and 24 h were 7.4 to 7.6 and 8.0 to 8.1, respectively. The use of high inocula did not affect the ceftaroline efficacy against the three strains tested (Fig. 1B), with mean decreases of 2.0 to 3.0 log10 CFU. These findings recapitulated the results from an in vitro pharmacodynamic study by Bhalodi et al., who tested the same three S. aureus strains (i.e., 412, 449, and 454 [MICs of 1 mg/liter]) at high inocula (14) and showed decreases of >5 log10 CFU after 24 h. While the study of Bhalodi et al. did not evaluate the efficacy using a standard inoculum against these three strains, our data suggest that ceftaroline does not exhibit an inoculum effect. Although previous in vivo and in vitro studies of penicillins and cephalosporins noted an inoculum effect secondary to a reduction in penicillin-binding proteins during the stationary growth phase (15), the absence of an inoculum effect is consistent with the findings of Lee et al. (16), where another anti-MRSA cephalosporin, ceftobiprole, exhibited the smallest inoculum effect against S. aureus compared with those of daptomycin, linezolid, and vancomycin. Of note, although the 24-h treatment groups in the high-inoculum studies showed greater reductions (2.0 to 3.0) in log10 CFU values than those in standard-inoculum studies (1.1 to 2.4), this was an artifact of the elevated initial bacterial load (i.e., 0 h) against which the efficacy was compared.
While previous data have suggested discordance between in vivo/in vitro efficacy for beta-lactams and Gram-negative pathogens, the findings herein suggest in vivo/in vitro concordance for ceftaroline against S. aureus. This concordance extends to both standard and high inocula, in which ceftaroline did not show an inoculum effect.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius, Henry Christensen, Jennifer Hull, Lucinda Lamb, Debora Santini, Sara Robinson, Shawn MacVane, Christina Sutherland, and Pamela Tessier for their assistance with the animal experimentation.
This study was internally funded by the Center for Anti-Infective Research and Development, Hartford Hospital (Hartford, CT).
D.P.N. has received grants and honoraria and participates in the advisory board for Forest Pharmaceuticals, Inc. G.G.Z. has received research grants from Forest. J.L.C. has received an honorarium from Forest. W.S. declares no conflicts of interest.
Footnotes
Published ahead of print 18 August 2014
REFERENCES
- 1.Bulik CC, Christensen H, Li P, Sutherland CA, Nicolau DP, Kuti JL. 2010. Comparison of the activity of a human simulated, high-dose, prolonged infusion of meropenem against Klebsiella pneumoniae producing the KPC carbapenemase versus that against Pseudomonas aeruginosa in an in vitro pharmacodynamics model. Antimicrob. Agents Chemother. 54:804–810. 10.1128/AAC.01190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulik CC, Nicolau DP. 2010. In vivo efficacy of simulated human dosing regimens of prolonged-infusion doripenem against carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4112–4115. 10.1128/AAC.00026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulik CC, Nicolau DP. 2011. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55:3002–3004. 10.1128/AAC.01420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:6137–6146. 10.1128/AAC.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman K, Levasseur P, Girard AM, Borgonovi M, Miossec C, Merdjan H, Drusano G, Shlaes D, Nichols WW. 2014. Activities of ceftazidime and avibactam against β-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob. Agents Chemother. 58:3366–3372. 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fusté E, Lopez-Jimenez L, Segura C, Gainza E, Vinuesa T, Vinas M. 2013. Carbapenem-resistance mechanisms of multidrug-resistant Pseudomonas aeruginosa. J. Med. Microbiol. 62(Part 9):1317–1325. 10.1099/jmm.0.058354-0. [DOI] [PubMed] [Google Scholar]
- 7.LaPlante KL, Leonard SN, Andes DR, Craig WA, Rybak MJ. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamics models. Antimicrob. Agents Chemother. 52:2156–2162. 10.1128/AAC.01046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steed M, Vidaillac C, Rybak MJ. 2011. Evaluation of ceftaroline activity versus daptomycin (DAP) against DAP-nonsusceptible methicillin-resistant Staphylococcus aureus strains in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 55:3522–3526. 10.1128/AAC.00347-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacGowan AP, Noel AR, Tomaselli S, Bowker KE. 2013. Pharmacodynamics of ceftaroline against Staphylococcus aureus studied in an in vitro pharmacokinetic model of infection. Antimicrob. Agents Chemother. 57:2451–2456. 10.1128/AAC.01386-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keel RA, Crandon JL, Nicolau DP. 2011. Efficacy of human simulated exposures of ceftaroline administered at 600 milligrams every 12 hours against phenotypically diverse Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 55:4028–4032. 10.1128/AAC.00372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendes RE, Tsakris A, Sader HS, Jones RN, Biek D, McGhee P, Appelbaum PC, Kosowska-Shick K. 2012. Characterization of methicillin-resistant Staphylococcus aureus displaying increased MICs of ceftaroline. J. Antimicrob. Chemother. 67:1321–1324. 10.1093/jac/dks069. [DOI] [PubMed] [Google Scholar]
- 12.Alm RA, McLaughlin RE, Kos VN, Sader H, Iaconis JP, Lahiri SD. 2014. Analysis of Staphylococcus aureus clinical isolates with reduced susceptibility to ceftaroline: an epidemiological and structural perspective. J. Antimicrob. Chemother. 69:2065–2075. 10.1093/jac/dku114. [DOI] [PubMed] [Google Scholar]
- 13.Zhanel GG, Rossnagel E, Nichol K, Cox L, Karlowsky JA, Zelenitsky S, Noreddin AM, Hoban DJ. 2011. Ceftaroline pharmacodynamics activity versus community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus, heteroresistant vancomycin-intermediate S. aureus, vancomycin-intermediate S. aureus and vancomycin-resistant S. aureus using an in vitro model. J. Antimicrob. Chemother. 66:1301–1305. 10.1093/jac/dkr110. [DOI] [PubMed] [Google Scholar]
- 14.Bhalodi AA, Hagihara M, Nicolau DP, Kuti JL. 2014. In vitro pharmacodynamics of human simulated exposures of ceftaroline and daptomycin against MRSA, hVISA, and VISA with and without prior vancomycin exposure. Antimicrob. Agents Chemother. 58:672–677. 10.1128/AAC.01516-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens DL, Yan S, Bryant AE. 1993. Penicillin-binding protein expression at different growth stages determines penicillin efficacy in vitro and in vivo: an explanation for the inoculum effect. J. Infect. Dis. 167:1401–1405. 10.1093/infdis/167.6.1401. [DOI] [PubMed] [Google Scholar]
- 16.Lee DG, Murakami Y, Nades DR, Craig WA. 2013. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 105 and 107 CFU injected into opposite thighs of neutropenic mice. Antimicrob. Agents Chemother. 57:1434–1441. 10.1128/AAC.00362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]