Abstract
Ceftazidime-avibactam is a β-lactam β-lactamase inhibitor combination under investigation for the treatment of serious Gram-negative infections. When combined with avibactam, a novel non-β-lactam β-lactamase inhibitor, ceftazidime has activity against isolates that produce Ambler class A, class C, and some class D β-lactamases. However, little is known of the in vivo efficacy of the combination against these targeted ceftazidime- and carbapenem-resistant Enterobacteriaceae. Using humanized exposures in the murine thigh model, we evaluated the efficacy of ceftazidime-avibactam against Enterobacteriaceae exhibiting MICs of ≥8 μg/ml to aid in the assignment of interpretive susceptibility criteria. Eighteen clinical Enterobacteriaceae isolates, including nine carbapenem-resistant strains, were evaluated against ceftazidime-avibactam (2,000 mg/500 mg) as a 2-h infusion every 8 h. To highlight the impact of avibactam, 13 select isolates were tested in the neutropenic model against a humanized regimen of 2,000 mg ceftazidime every 8 h (2-h infusion). Additionally, nine isolates were evaluated in immunocompetent animals. The efficacy was evaluated as the change in log10 CFU compared with that of 0-h controls after 24 h. The vast majority (17/18, 94%) of the isolates were resistant to ceftazidime alone. The ceftazidime monotherapy failed to have activity against 10 of 13 isolates, while ceftazidime-avibactam produced reductions in bacterial density against 16 of 18 isolates. Ceftazidime-avibactam (2,000 mg/500 mg) every 8 h (2-h infusion) displayed dependable activity against the Enterobacteriaceae isolates, exhibiting MICs of ≤16 μg/ml (free drug concentration above the MIC [fT>MIC] of ≥62%) and variable activity was noted at an MIC of 32 μg/ml (fT>MIC of 34%). The presence of a functioning immune system enhanced the efficacy for both regimens against all tested isolates. These data support further examination of the use of ceftazidime-avibactam as an effective therapy against infections due to Gram-negative infections, including carbapenem-resistant Enterobacteriaceae.
INTRODUCTION
Antimicrobial resistance among Gram-negative bacilli is a growing problem around the world, complicating the treatment of many severe hospital infections (1). Infections with organisms possessing resistance mechanisms severely limit the number of viable therapeutic options, resulting in poor patient outcomes and increased health care costs (2). A recent CDC report on antibiotic resistance in the United States classified carbapenem-resistant Enterobacteriaceae (CRE) and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae as “urgent” and “serious” threats to the country, respectively (3).
Ceftazidime (CAZ) is an advanced-generation cephalosporin with reliable activity against susceptible Enterobacteriaceae strains. However, the increasing prevalence of ESBL and carbapenemase production among Enterobacteriaceae, such as Klebsiella pneumoniae carbapenemases (KPCs), diminishes the clinical utility of ceftazidime. In a recent surveillance study of resistance among isolates in the United States, 6% of K. pneumoniae isolates were carbapenem nonsusceptible, of which approximately 90% possessed blaKPC (4). Avibactam (AVI), a non-β-lactam β-lactamase inhibitor with activity against a wide variety of β-lactamase-mediated resistance mechanisms, greatly improves the in vitro activity of ceftazidime against Enterobacteriaceae, including those that produce KPC (5–8). Recent in vivo murine infection studies have suggested that the median patient pharmacokinetics of ceftazidime-avibactam (2 g/0.5 g) every 8 h administered as a 2-h infusion would have activity against Pseudomonas aeruginosa isolates with MICs of ≤16 μg/ml and a dilution above the MIC that inhibited ≥90% of the organisms (MIC90) of 8 μg/ml (9–12). However, in vivo efficacy data of ceftazidime-avibactam against the KPC genotype exist for only two KPC K. pneumoniae isolates, for which the pharmacokinetic exposures were not humanized (8).
Given the rising rates of resistance among Gram-negative pathogens and the increasing occurrence of carbapenem-resistant Enterobacteriaceae in a number of common infections, understanding the pharmacodynamic profiles against a distribution of relevant MICs is essential for determining the susceptibility breakpoints for novel therapies. Unlike the various enzymatic (e.g., AmpC β-lactamases) and mutational (e.g., porin channel and efflux pumps) mechanisms of resistance expressed by P. aeruginosa isolates, Enterobacteriaceae species often possess a variety of different classes of β-lactamase enzymes that warrant in vivo evaluation. To further delineate the impact of avibactam, humanized doses of ceftazidime as monotherapy and in combination with avibactam were tested against a collection of contemporary highly ceftazidime- and carbapenem-resistant clinical Enterobacteriaceae isolates. The aim of this study was to assess the efficacy of ceftazidime-avibactam against Enterobacteriaceae isolates with MICs of ≥8 μg/ml to provide in vivo observations that will aid in the determination of optimal susceptibility test interpretive criteria for ceftazidime-avibactam in humans and determine its potential role in the treatment of serious infections.
MATERIALS AND METHODS
Antimicrobial test agents.
Commercially available ceftazidime (lot 104452C; Sandoz Inc., Princeton, NJ, USA) was obtained for the Hartford Hospital Pharmacy Department and utilized for all in vivo studies, while analytical-grade avibactam was provided by AstraZeneca Pharmaceuticals (Waltham, MA, USA). Commercial vials of ceftazidime were reconstituted according to the manufacturer's specifications and diluted as appropriate to achieve the desired concentrations; analytical avibactam powder was used in a quantity sufficient to achieve the desired concentrations and reconstituted immediately prior to use.
Bacterial isolates.
A total of 18 clinical Enterobacteriaceae isolates (2 Enterobacter aerogenes, 3 Enterobacter cloacae, 1 Klebsiella oxytoca, 8 Klebsiella pneumoniae, 1 Proteus mirabilis, 1 Providencia stuartii, and 2 Serratia marcescens) and 2 P. aeruginosa isolates (ceftazidime-avibactam MICs of 4 and 8 μg/ml) were provided by International Health Management Associates, Inc. (Schaumburg, IL, USA), JMI Laboratories (North Liberty, IA, USA), Michael Huband (AstraZeneca Pharmaceuticals), and the Center for Anti-Infective Research and Development (Hartford, CT, USA).
Susceptibility testing.
The MICs of ceftazidime and ceftazidime-avibactam were determined for each isolate using the broth microdilution methodology as outlined by the Clinical and Laboratory Standards Institute (CLSI) (13). For ceftazidime-avibactam, doubling dilutions of ceftazidime were utilized in combination with a fixed 4 μg/ml concentration of avibactam. Staphylococcus aureus ATCC 29213 and ESBL-producing K. pneumoniae ATCC 700603 were used as quality control strains for ceftazidime-avibactam. The MIC studies were conducted in a minimum of 5 replicates, and the modal MIC was reported.
Neutropenic thigh infection model.
Pathogen-free, female ICR mice weighing approximately 25 g were acquired from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and utilized throughout these experiments. This study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. Animals were maintained and used in accordance with the National Research Council recommendations and provided food and water ad libitum. The mice were rendered neutropenic with 100 and 150 mg/kg of body weight intraperitoneal injections of cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, NJ) given 1 and 4 days prior to inoculation, respectively. Three days prior to inoculation, the mice were also given a single 5-mg/kg intraperitoneal injection of uranyl nitrate, which produces a predictable degree of renal impairment, to aid in humanizing the drug regimens (14). Two hours prior to the initiation of antimicrobial therapy, each thigh was inoculated intramuscularly with 0.1 ml of a solution containing approximately 107 CFU/ml of the test isolate. To confirm active β-lactamase production at the initiation of each study, the zone diameter of inhibition was determined for a ceftazidime (30 μg)-containing disc (Sensi-Disc; BD, Sparks, MD) on a lawn of growth of each test isolate made from the same plate used for the inoculum preparation.
Immunocompetent thigh infection model.
The mice utilized in the immunocompetent studies underwent the same procedures as outlined above, except that cyclophosphamide was not given and an inoculum of 108 CFU/ml was used to produce the thigh infection.
Confirmatory pharmacokinetic studies.
A previously validated humanized regimen in mice that simulated the median free drug concentration above the MIC [fT>MIC] profile observed in humans given 2,000 mg ceftazidime every 8 h as a 2-h infusion as monotherapy or combined with 500 mg avibactam every 8 h as a 2-h infusion as developed by our group was used in the current investigation (Table 1) (9). For the free drug calculations, the protein binding values for ceftazidime were 26% for mice and 15% for humans (15, 16). For avibactam, 10% and 8% were used for mice and humans, respectively (data on file, AstraZeneca). Briefly, as previously reported (9), this regimen was developed first using single-dose studies with ceftazidime-avibactam and ceftazidime alone in thigh infections of neutropenic mice. After pharmacokinetic characterization of the single doses, these data were used to determine the regimens in mice that simulated the free drug exposure profile seen in humans for ceftazidime-avibactam and ceftazidime alone as noted above. These studies were conducted over the first interval (i.e., 8 h) for ceftazidime, while ceftazidime-avibactam studies were conducted for all 3 treatment intervals (i.e., 24 h). Confirmatory studies were also undertaken in the immunocompetent animals to ensure similar exposures. In the current analysis, to confirm that target exposures of ceftazidime were achieved, pharmacokinetic studies of human-simulated regimens of ceftazidime alone and in combination with avibactam were conducted prior to the use of these regimens in the pharmacodynamic analyses. Since the same fixed-dose combination of ceftazidime-avibactam was used as previously developed and published (9), pharmacokinetic exposures were confirmed for ceftazidime only to ensure that similar exposures were provided. Briefly, the infected neutropenic mice were administered the humanized regimens, and a group of six mice were euthanized at multiple time points throughout the dosing interval of each regimen. Blood samples were collected via cardiac puncture, and serum samples were stored at −80°C until analysis. Ceftazidime concentrations were analyzed at the Center for Anti-Infective Research and Development (Hartford, CT) using a high-performance liquid chromatography assay (17). Exposures of ceftazidime and avibactam were also confirmed using an internal microbiological response control (see below).
TABLE 1.
fT>MIC profiles for ceftazidime at 2 g every 8 h (2-h infusion) as monotherapy and in combination with avibactam at 500 mg (2-h infusion) in mice and humansa
| MIC (mg/liter) | Ceftazidime ƒT>MIC (%) inb: |
||
|---|---|---|---|
| Humans (CAZ-AVI) | Mice (CAZ) | Mice (CAZ-AVI) | |
| 4 | 100 | 100 | 99 |
| 8 | 96 | 87 | 88 |
| 16 | 63 | 58 | 62 |
| 32 | 24 | 28 | 34 |
| 64 | 0 | 0 | 0 |
Adapted from reference 9. Ceftazidime ƒT>MIC (%) for CAZ and CAZ-AVI regimens were derived from a previous study in neutropenic mice (9). In that study, target concentrations and exposures were similar between immunocompetent and neutropenic animals (data not shown).
CAZ-AVI, ceftazidime-avibactam; CAZ, ceftazidime.
In vivo efficacy.
A total of 18 Enterobacteriaceae isolates were assessed in the efficacy studies. Additionally, two previously studied P. aeruginosa isolates were evaluated in the neutropenic studies for internal validation and quality control utilizing the humanized ceftazidime-avibactam and ceftazidime regimens to confirm pharmacodynamics similar to those previously reported (9). All 20 isolates were evaluated against ceftazidime-avibactam, while 15 select isolates (13 Enterobacteriaceae and 2 P. aeruginosa) were also tested against ceftazidime alone to highlight the pharmacologic effect of avibactam. Nine of the Enterobacteriaceae isolates were also utilized against both regimens in the immunocompetent studies (denoted in Table 2). Beginning 2 h after inoculation, groups of 3 mice were administered human-simulated regimens of ceftazidime or ceftazidime-avibactam. All doses were administered as 0.2-ml subcutaneous injections and consisted of three 8-h dosing intervals (i.e., 24 h). To serve as control animals, an additional group of 3 mice were administered normal saline at the same volume, route, and frequency as those for the treatment regimen. Thighs from all animals were harvested 24 h after the initiation of therapy; mice that failed to survive for 24 h were harvested at the time of expiration. The harvesting procedure for all study mice began with euthanization by CO2 exposure followed by cervical dislocation. After sacrifice, thighs were removed and individually homogenized in normal saline. Serial dilutions of the thigh homogenate were plated on Trypticase soy agar with 5% sheep blood for CFU determination. In addition to the above-mentioned treatment and control groups, another group of 3 infected, untreated mice were harvested at the initiation of dosing and served as 0-h controls. For neutropenic studies, efficacy, designated as the change in bacterial density, was calculated as the change in log10 bacterial CFU obtained for treated mice after 24 h from that of starting densities observed in 0-h control animals. To control for the impact of the host and resulting variability in 24-h control bacterial densities between isolates, efficacy in the immunocompetent model was calculated as the change in log10 CFU obtained for treated mice after 24 h relative to that for the 24-h immunocompetent control mice. Animals with bacterial densities outside of 1.5 times the interquartile range were identified as outliers and excluded from the group averages (18). For the 18 Enterobacteriaceae isolates (ceftazidime-avibactam MICs of 8 to 32 μg/ml), a phenotypic assessment of the in vivo resistance development was conducted by plating the 24-h thigh homogenates from ceftazidime-avibactam-treated animals on Mueller-Hinton agar plates containing ceftazidime concentrations three doubling dilutions higher than the starting MIC of the isolate and a fixed avibactam concentration of 4 μg/ml and incubating overnight. The genesis for these resistance development studies was due to resistant mutants observed on ceftazidime-avibactam drug-containing plates from an in vitro hollow-fiber efficacy study of ceftazidime-avibactam (2 g/0.5 g) against P. aeruginosa isolates at the same starting inoculum of 106 CFU/ml (9).
TABLE 2.
Phenotypic and genotypic data for the 18 Enterobacteriaceae and 2 P. aeruginosa isolates utilized during the in vivo efficacy studiesa
| External isolate no. | CAIRDa isolate no. | Modal MIC (μg/ml) forb: |
β-Lactamase genotype(s) | Isolate(s) utilized in efficacy studyc |
||||
|---|---|---|---|---|---|---|---|---|
| CAZ-AVI | CAZ | Meropenem | CAZ-AVI I− | CAZ I− | CAZ-AVI, CAZ I+ | |||
| NAd | P. aeruginosa 22 | 4 | 64 | 16 | NDe | X | ||
| 3610 | P. aeruginosa 1388 | 8 | 128 | >8 | AmpCf | X | ||
| 853469 | S. marcescens 80 | 8 | 8 | >8 | AmpC | X | X | |
| 3083 | S. marcescens 82 | 8 | 64 | ≤0.125 | ND | X | X | |
| 845662 | K. pneumoniae 479 | 8 | >128 | >8 | SHV-11, KPC-3, OXA-9g | X | X | X |
| 864091 | K. pneumoniae 486 | 8 | >128 | >8 | SHV-11, KPC-2, OXA-9,g TEM-1 | X | X | |
| 249 | K. pneumoniae 496 | 8 | >128 | >8 | KPC-3, SHV-12, TEM-1, OXA-9g | X | ||
| 2930 | E. cloacae 77 | 8 | >128 | 0.5 | ND | X | X | X |
| 871361 | E. aerogenes 50 | 8 | >128 | 0.5 | ND | X | X | X |
| 845664 | K. pneumoniae 480 | 16 | >128 | >8 | SHV-11, KPC-3, TEM-1 | X | X | X |
| 867822 | K. pneumoniae 487 | 16 | >128 | >8 | SHV-11, KPC-3, OXA-9,g TEM-1 | X | X | X |
| 883540 | K. pneumoniae 493 | 16 | >128 | 0.25 | SHV-11, CTX-M-15, OXA-1, OXA-48, TEM-1 | X | X | X |
| 847537 | K. oxytoca 87 | 16 | >128 | >8 | ND | X | X | X |
| 847750 | E. cloacae 74 | 16 | >128 | 0.25 | AmpC, OXA-1, OXA-9, PER-2, TEM-1 | X | ||
| 43399 | E. cloacae 78 | 16 | >128 | 8 | ND | X | X | X |
| ARC3726 | P. stuartii 58 | 16 | >128 | 0.0625 | AmpC, SCO-1, ACC-4, TEM-1 (partial) | X | ||
| 869917 | K. pneumoniae 489 | 32 | >128 | 0.0625 | SHV-11, TEM-1, OXA-1, CTX-M-15, ACC-4 | X | X | X |
| 43475 | K. pneumoniae 497 | 32 | 64 | 2 | ND | X | X | |
| 875448 | E. aerogenes 51 | 32 | >128 | 0.125 | AmpC, CTX-M-27 | X | X | X |
| 856743 | P. mirabilis 19 | 32 | 64 | 4 | ND | X | ||
CAIRD, Center for Anti-Infective Research and Development.
CAZ-AVI, ceftazidime-avibactam; CAZ, ceftazidime.
I−, neutropenic; I+, immunocompetent.
NA, not applicable.
ND, no data (isolates were characterized phenotypically only).
Derepressed, as judged by a qualitative phenotypic nitrocefin hydrolysis test.
OXA-9 but containing an in-frame termination codon.
RESULTS
Bacterial isolates.
The phenotypic and genotypic profiles of the 18 clinical Enterobacteriaceae and 2 P. aeruginosa isolates included in the efficacy studies are shown in Table 2. There was limited variability among the five individual MIC replicates; thus, the modal MIC is reported. The β-lactamase genotype was known for 12 of the isolates.
Confirmation of pharmacokinetics.
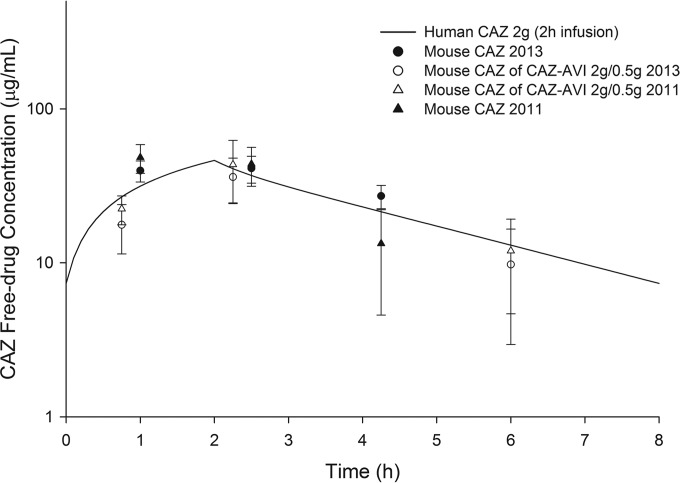
The free drug pharmacokinetic profiles determined in vivo for 2 g ceftazidime when given alone and in combination with 500 mg avibactam are shown in Fig. 1. The ceftazidime concentration-time profile resulted in targets similar to those in previous studies (9). The regimen for ceftazidime-avibactam consisted of 8 doses during the 24-h interval (CAZ-AVI [mg/kg]): 7/0.9, 20/2.5, 7.5/1.25, 1/0.1, 13/1.6, 5/0.8, 13/1.6, and 5/0.8 given at 0 h, 0.75 h, 2.25 h, 4 h, 8.75 h, 10.25 h, 16.75 h, and 18.25 h, respectively, while the ceftazidime regimen consisted of 13 doses during the 24-h interval (CAZ [mg/kg]) 4.5, 16, 13, 4.5, 3, 16, 13, 4.5, 3, 16, 13, 4.5, and 3 given at 0 h, 0.75 h, 2.25 h, 4 h, 6 h, 8.75 h, 8 h, 10.25 h 12 h, 14 h, 16.75 h, 18.25 h, 20 h, and 22 h, respectively (9).
FIG 1.
Free drug concentration-time profile of 2 g ceftazidime (CAZ) in humans and mice alone and in combination with 500 mg avibactam (CAZ-AVI) compared with historical data (9). Symbols and error bars represent means ± standard deviations.
In vivo efficacy.
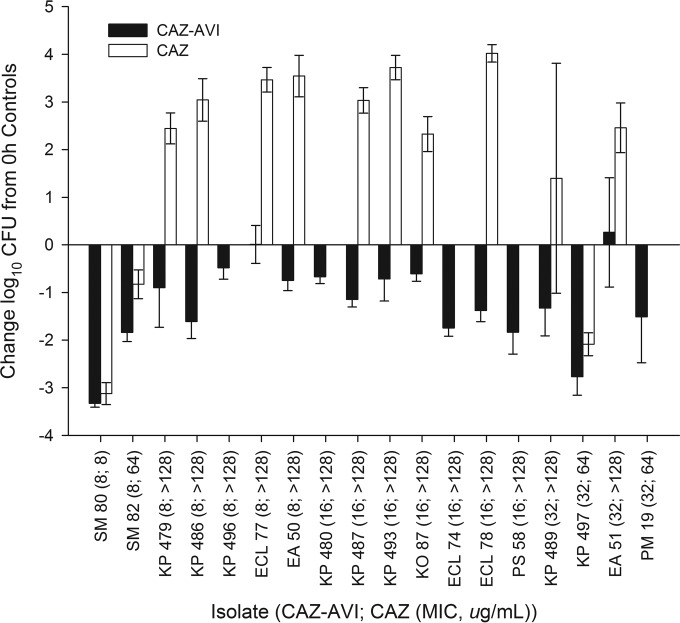
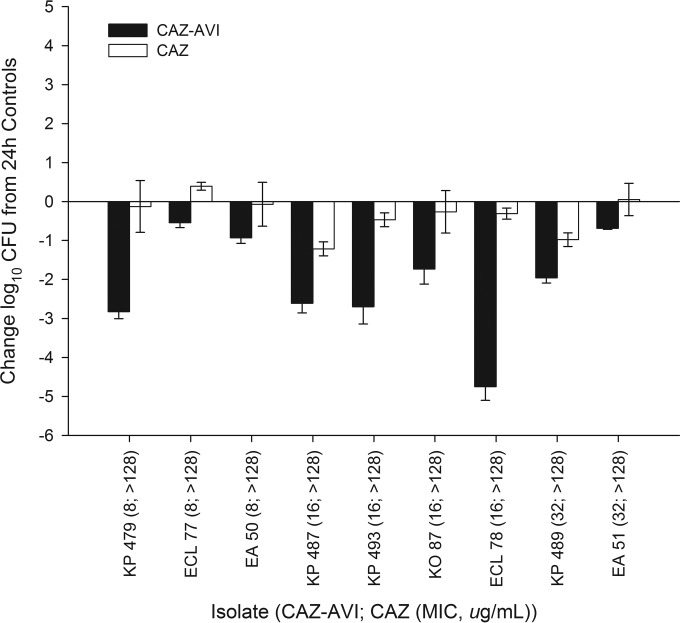
During neutropenic studies, 0-h control mice displayed a mean (± standard deviation [SD]) bacterial density of 5.96 ± 0.30 log10 CFU, which increased to an average of 9.29 ± 0.86 log10 CFU in untreated mice after 24 h. During neutropenic studies, 10 out of 54 (18%) of the 24-h control animals failed to survive to 24 h due to the aggressive virulence of the isolates; thighs of these mice had bacterial densities similar to those of animals that survived and therefore were included in the data analysis. The mean bacterial density in control animals during immunocompetent studies was 7.36 ± 0.16 log10 CFU/ml at 0 h and 7.35 ± 1.22 log10 CFU after 24 h. Three 24-h control and two ceftazidime monotherapy mice failed to survive to 24 h during the immunocompetent studies. No ceftazidime-avibactam-treated animals succumbed to infection. The results of the neutropenic studies are shown in Fig. 2; note that Table 2 shows the isolates against which the ceftazidime monotherapy was not studied. Consistent with the elevated in vitro MICs, the ceftazidime monotherapy produced reductions in bacterial density after 24 h against 3 (23%) of the 13 Enterobacteriaceae isolates tested. The ceftazidime-avibactam therapy resulted in reductions in bacterial density of 0.48 to 3.33 log10 CFU against 13 of 14 isolates with ceftazidime-avibactam MICs of ≤16 μg/ml (fT>MIC of ≥62%), including 6 out of 7 isolates with ceftazidime-avibactam MICs of 8 μg/ml and all 7 isolates with ceftazidime-avibactam MICs of 16 μg/ml. Against isolates with ceftazidime-avibactam MICs of 32 μg/ml (fT>MIC of 34%), the efficacy of ceftazidime-avibactam was shown in 3 of 4 isolates. There was no growth observed after plating of the direct homogenate on ceftazidime-avibactam drug-containing plates, denoting no development of in vivo resistance to ceftazidime-avibactam. Overall, the ceftazidime-avibactam efficacy was enhanced at all MICs in the immunocompetent model, as depicted in Fig. 3, including the two isolates (E. cloacae 77 and E. aerogenes 51) that failed to show bactericidal responses in the neutropenic studies.
FIG 2.
Change in log10 CFU for human-simulated regimens of ceftazidime-avibactam (CAZ-AVI) and ceftazidime (CAZ) alone at 24 h relative to that for 0-h controls for a collection of Enterobacteriaceae isolates tested in neutropenic studies. Error bars represent standard deviations. SM, Serratia marcescens; KP, Klebsiella pneumoniae; ECL, Enterobacter cloacae; EA, Enterobacter aerogenes; KO, Klebsiella oxytoca; PS, Providencia stuartii; PM, Proteus mirabilis.
FIG 3.
Change in log10 CFU for human-simulated regimens of ceftazidime-avibactam (CAZ-AVI) and ceftazidime (CAZ) alone at 24 h relative to that for 24-h controls for Enterobacteriaceae isolates tested in immunocompetent studies. Error bars represent standard deviations.
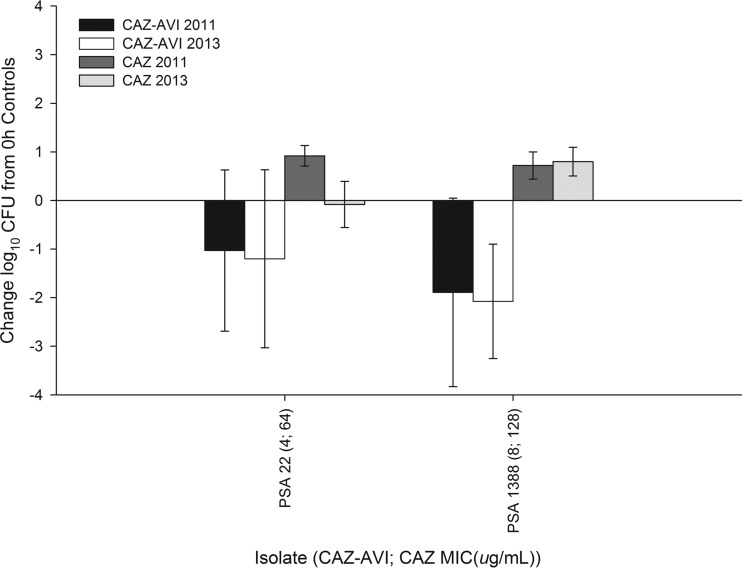
Of note, to ensure internal consistency of the humanized regimens, the in vivo efficacy studies with two P. aeruginosa isolates (22 and 1388) from the previous work by our group were repeated (9). The observed bacterial reductions were comparable to historical data for both ceftazidime-avibactam and ceftazidime alone against these two isolates, as shown in Fig. 4.
FIG 4.
Results of the in vivo efficacy studies utilizing the ceftazidime (CAZ) and ceftazidime-avibactam (CAZ-AVI) regimens at 24 h relative to those for 0-h controls in the neutropenic thigh infection model against two Pseudomonas aeruginosa isolates compared with historical data (9).
DISCUSSION
The utility of ceftazidime, a commonly prescribed third-generation cephalosporin with a broad spectrum of activity, well-characterized pharmacokinetic profile, and proven safety record, has been reduced due to the global spread of Gram-negative bacilli producing β-lactamases such as ESBLs, KPCs, and metallo-β-lactamases (MBLs) (19). However, the addition of the novel non-β-lactam β-lactamase inhibitor avibactam has been shown to effectively improve the activity of the parent cephalosporin against many of these contemporary Gram-negative resistance mechanisms (7, 20, 21). This antibiotic combination is currently in phase III trials to evaluate its efficacy in the treatment of complicated intra-abdominal infections (cIAI) and complicated urinary tract infections, hinging on promising phase II findings (22, 23), as well as hospital-acquired pneumonia. In the current study, a humanized regimen of 2 g ceftazidime combined with 500 mg avibactam was evaluated against a collection of clinical Enterobacteriaceae isolates exhibiting MICs of ≥8 μg/ml to provide support for the susceptibility test interpretive criteria for ceftazidime-avibactam. As predicted based on fT>MIC targets identified for cephalosporins in previous murine infection models (24, 25), ceftazidime-avibactam was active against the majority of these β-lactamase-producing strains, including a large collection of KPC-producing strains that rendered the ceftazidime monotherapy ineffective.
With the use of ceftazidime alone as a comparator, the addition of avibactam dramatically improved the potency of ceftazidime in vitro against the evaluated Enterobacteriaceae isolates by several dilutions (MICs for ceftazidime alone, 8 to >128 μg/ml versus ceftazidime-avibactam MICs of 8 to 32 μg/ml), a finding consistent with the results of other published literature reports. Of 8,640 contemporary Enterobacteriaceae isolates collected from 73 U.S. hospitals, ceftazidime-avibactam inhibited 99.8% at MICs of ≤4 μg/ml (26). Similarly, a recent evaluation of 72 U.S. hospitals from 2012 noted a diverse and increasing prevalence of β-lactamase production among Enterobacteriaceae (27). Of the 118 KPC-producing isolates identified in that study, the ceftazidime MIC50 was >32 μg/ml, while the ceftazidime-avibactam MIC50/MIC90 was 0.5/2 μg/ml. Likewise, against CTX-M-15 producers, the most common ESBL phenotype in the United States, MIC50/MIC90 for ceftazidime and ceftazidime-avibactam were 16/>32 μg/ml and 0.12/0.5 μg/ml, respectively (27). Moreover, of the 136 Enterobacteriaceae pathogens isolated from the cIAI phase II trial with accompanying MIC data, only 3 isolates (2.2%) had ceftazidime-avibactam MICs of >8 μg/ml (22). So while the purpose of the current study was to understand the ceftazidime-avibactam efficacy against organisms that test with MICs of ≥8 μg/ml, as evident by recent surveillance data and the pathogens isolated from patients in phase II trials, the MICs of the isolates used in this study represent the most challenging and are likely to be seen only rarely in current clinical practice.
To highlight the impact of avibactam in vivo, we assessed the activity of ceftazidime alone (2 g every 8 h as a 2-h infusion) against a majority of the tested isolates. As this regimen provided 0% fT>MIC at an MIC of 128 μg/ml, it was not unexpected that ceftazidime alone was unable to achieve activity against any of the 10 isolates with ceftazidime MICs of >128 μg/ml. Against the same isolates, ceftazidime in combination with avibactam showed enhanced activity, consistent with the phenotypic profile. In neutropenic studies, reliable efficacy was observed with ceftazidime-avibactam at MICs of ≤16 μg/ml (fT>MIC of ≥62%), with variable activity against isolates with ceftazidime-avibactam MICs of 32 μg/ml (fT>MIC of 34%). These findings are consistent with the established fT>MIC targets for cephalosporins (50 to 70% fT>MIC) to be predictive of in vivo efficacy (25). The pharmacodynamic target for avibactam is less well defined and may require a critical threshold concentration (28, 29). However, the concentrations of ceftazidime and avibactam in the current study mimicked the human exposures profiles of each compound, suggesting that the 500-mg dose of avibactam is sufficient to restore the efficacy of ceftazidime. To that end, the pharmacodynamic exposures of ceftazidime-avibactam noted to be predictive of efficacy against Enterobacteriaceae isolates in the current study were the same targets that were identified for P. aeruginosa, where reliable activity was observed at MICs of ≤16 μg/ml (fT>MIC of ≥62%) with variable efficacy at an MIC of 32 μg/ml (34% fT>MIC) (9). Of note, with the benefit of functioning granulocytes, ceftazidime-avibactam resulted in a reduction in bacterial density against the lone strain with a ceftazidime-avibactam MIC of 8 μg/ml (E. cloacae 77) that failed to show activity in the neutropenic model.
Using the murine thigh infection model, we found evidence that the addition of avibactam to ceftazidime reestablishes its activity against highly resistant Enterobacteriaceae producing a variety of β-lactamases, including a sizeable collection of KPC-producing strains. With the increasing occurrence of β-lactamase production among clinical infections and few therapeutic options available, our observations support the use of ceftazidime-avibactam (2 g/0.5 g) as a 2-h infusion every 8 h, the median patient pharmacokinetics of which were active in this neutropenic mouse simulation model against Enterobacteriaceae isolates with MICs as high as 16 μg/ml. Future investigations should focus on the clinical applicability of these findings.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius, Henry Christensen, Jennifer Hull, Jami Jain, Lucinda Lamb, Debora Santini, Christina Sutherland, Pamela Tessier, and Wonhee So for their assistance with the animal experimentation and in vitro testing. We also thank Richard Alm for β-lactamase genotype data.
This study was supported by AstraZeneca Pharmaceuticals, Inc. (Waltham, MA, USA) and Forest-Cerexa (a subsidiary of Actavis PLC).
D.P.N. has received research grants and honoraria and participates on the advisory board for AstraZeneca. W.W.N. is an employee of AstraZeneca. S.H.M. and J.L.C. declare no conflicts of interest.
Footnotes
Published ahead of print 15 September 2014
REFERENCES
- 1.Lautenbach E, Polk R. 2007. Resistant gram-negative bacilli: a neglected healthcare crisis? Am. J. Health Syst. Pharm. 64(23 Suppl 14):S3–S21. 10.2146/ajhp070477. [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove SE. 2006. The relationship between antimicrobial resistance and patient outcomes: mortality, length of stay, and health care costs. Clin. Infect. Dis. 42(Suppl 2):S82–S89. 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 4.Kaiser RM, Castanheira M, Jones RN, Tenover F, Lynfield R. 2013. Trends in Klebsiella pneumoniae carbapenemase-positive K. pneumoniae in US hospitals: report from the 2007-2009 SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 76:356–360. 10.1016/j.diagmicrobio.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J. Antimicrob. Chemother. 64:326–329. 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 55:390–394. 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagacé-Wiens PR, Tailor F, Simner P, DeCorby M, Karlowsky JA, Walkty A, Hoban DJ, Zhanel GG. 2011. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob. Agents Chemother. 55:2434–2437. 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endimiani A, Hujer KM, Hujer AM, Pulse ME, Weiss WJ, Bonomo RA. 2011. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55:82–85. 10.1128/AAC.01198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:6137–6146. 10.1128/AAC.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walkty A, DeCorby M, Lagacé-Wiens PR, Karlowsky JA, Hoban DJ, Zhanel GG. 2011. In vitro activity of ceftazidime combined with NXL104 versus Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals (CANWARD 2009 study). Antimicrob. Agents Chemother. 55:2992–2994. 10.1128/AAC.01696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levasseur P, Girard AM, Claudon M, Goossens H, Black MT, Coleman K, Moissec C. 2012. In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 56:1606–1608. 10.1128/AAC.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flamm RK, Stone GG, Sader HS, Jones RN, Nichols WW. Avibactam reverts the ceftazidime MIC90 of European Gram-negative bacterial clinical isolates to the epidemiological cut-off value. J. Chemother., in press. 10.1179/1973947813Y.0000000145. [DOI] [PubMed] [Google Scholar]
- 13.Clinical Laboratory Standards Institute. 2011. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. CLSI publication M07-A8 Clinical Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tawara S, Matsumoto S, Kamimura T, Goto S. 1992. Effect of protein binding in serum on therapeutic efficacy of cephem antibiotics. Antimicrob. Agents Chemother. 36:17–24. 10.1128/AAC.36.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drusano GL, Standiford HC, Fitzpatrick B, Leslie J, Tangtatsawasdi P, Ryan P, Tatem B, Moody MR, Schimpff SC. 1984. Comparison of the pharmacokinetics of ceftazidime and moxalactam and their microbiological correlates in volunteers. Antimicrob. Agents Chemother. 26:388–393. 10.1128/AAC.26.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolau DP, Nightingale CH, Banevicius MA, Fu Q, Quintiliani R. 1996. Serum bactericidal activity of ceftazidime: continuous infusion versus intermittent injections. Antimicrob. Agents Chemother. 40:61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoaglin DC, Iglewicz B, Tukey JW. 1986. Performance of some resistant rules for outlier labeling. J. Am. Stat. Assoc. 81:991–999. 10.1080/01621459.1986.10478363. [DOI] [Google Scholar]
- 19.Bush K. 2010. Alarming beta-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceae. Curr. Opin. Microbiol. 13:558–564. 10.1016/j.mib.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-β-lactamase producers. Antimicrob. Agents Chemother. 57:3299–3306. 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiskirchen DE, Crandon JL, Furtado GH, Williams G, Nicolau DP. 2011. In vivo efficacy of a human-simulated regimen of ceftaroline combined with NXL104 against extended-spectrum-beta-lactamase (ESBL)-producing and non-ESBL-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 55:3220–3225. 10.1128/AAC.00024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. 2013. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J. Antimicrob. Chemother. 68:1183–1192. 10.1093/jac/dks523. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez JA, González Patzán LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, Sable C. 2012. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr. Med. Res. Opin. 28:1921–1931. 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 24.Turnidge JD. 1998. The pharmacodynamics of β-lactams. Clin. Infect. Dis. 27:10–22. 10.1086/514622. [DOI] [PubMed] [Google Scholar]
- 25.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89–96. 10.1016/0732-8893(95)00053-D. [DOI] [PubMed] [Google Scholar]
- 26.Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. 2014. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from U.S. medical centers in 2012. Antimicrob. Agents Chemother. 58:1684–1692. 10.1128/AAC.02429-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. 2014. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine United States census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob. Agents Chemother. 58:833–838. 10.1128/AAC.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borgonovi M, Merdjan H, Girard AM, Levasseur P, Quemin MH, Lowther J, Miossec C, Shlaes D, Drusano GL. 2008. Importance of NXL104 pharmacokinetics (PK) in the pharmacodynamics (PD) of ceftazadime+NXL104 combinations in an in vitro hollow fiber infection model, abstr A-023 Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 29.Nichols P, Levasseur W, Li J, Das S. 2012. A threshold concentration of avibactam (AVI) during the pharmacokinetic decline phase, below which β-lactamase inhibition in Enterobacteriaceae becomes ineffective, abstr A-1760 Abstr. 52nd Intersci Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]