Abstract
Serum penicillin G falls to low levels 2 weeks after injection as benzathine penicillin G (BPG) in young adults. Using Pmetrics and previously reported penicillin G pharmacokinetic data after 1.2 million units were given as BPG to 329 male military recruits, here we develop the first reported population pharmacokinetic model of penicillin G after BPG injection. We simulated time-concentration profiles over a broad range of pediatric and adult weights after alternative doses and dose frequencies to predict the probability of maintaining serum penicillin G concentrations of >0.02 mg/liter, a proposed protective threshold against group A Streptococcus pyogenes (GAS). The final population model included linear absorption into a central compartment, distribution to and from a peripheral compartment, and linear elimination from the central compartment, with allometrically scaled volumes and rate constants. With 1.2 million units of BPG given intramuscularly every 4 weeks in four total doses, only 23.2% of 5,000 simulated patients maintained serum penicillin G trough concentrations of >0.02 mg/liter 4 weeks after the last dose. When the doses were 1.8 million units and 2.4 million units, the percentages were 30.2% and 40.7%, respectively. With repeated dosing of 1.2 million units every 3 weeks and every 2 weeks for 4 doses, the percentages of simulated patients with a penicillin G trough concentration of >0.02 mg/liter were 37.8% and 65.2%, respectively. Our simulations support recommendations for more frequent rather than higher BPG doses to prevent recurrent rheumatic heart disease in areas of high GAS prevalence or during outbreaks.
INTRODUCTION
Studies performed during the 60 years since the original investigations (1) of the persistence of serum penicillin G after parenteral administration of benzathine penicillin G (BPG) generally found that serum concentrations remain above a putative minimum protective level for the prevention and treatment of group A Streptococcus pyogenes (GAS) infections. This minimum threshold differs among authorities but is usually set as >0.01 to 0.03 μg/ml (2) for between 3 and 4 weeks after a dose.
Recently, we reported that the serum penicillin G levels in 329 military trainees fell more rapidly than expected during the 29 days following a 1.2-million-unit intramuscular injection of the only formulation of BPG currently available in the United States (2). That study was prompted by observations of unexpectedly high GAS treatment failures with BPG, defined as a failure to eradicate GAS from the throat (3–6), and previous reports of undetectable serum penicillin G 3 weeks after the same dose (5, 7).
In this report, we develop a population pharmacokinetic (PK) model of penicillin G concentrations following intramuscular administration of 1.2 million units of BPG in healthy young adult males, using data from our prior study. We set three objectives: (i) to find the optimal body size metric, i.e., weight, body surface area (BSA), or allometry, for scaling PK parameter values; (ii) to use the final model for Monte Carlo simulations to explore alternative BPG dosing regimens and their probabilities of maintaining serum penicillin G concentrations of >0.02 μg/ml (as the middle of the typical target range) at all times during the month following an injection of 1.2 million units; and (iii) to understand the kinetic distribution of penicillin outside the serum (i.e., in tissues) in relation to the serum time-concentration profile. These objectives may be relevant to future dosing for adults and children for therapy of both GAS upper respiratory tract infections and secondary rheumatic fever prophylaxis as well as for treatment of syphilis.
MATERIALS AND METHODS
Subjects.
As described previously (2), we enrolled 329 male military trainees in two cohorts and monitored them for 29 days after their enrollment and injection of 1.2 million units of BPG. None of the subjects were allergic to penicillin.
Procedures.
We enrolled the first cohort in January 2007 (165 subjects) and the second in February 2007 (164 subjects). The Naval Health Research Center institutional review board approved the study (protocol no. NHRC.2007.0022), and all subjects provided written informed consent. On day 0 for each subject, we gave a gluteal intramuscular dose of 900 mg of penicillin G as 2 ml of a solution containing 1.2 million units of BPG (L-A Bicillin, catalog no. 1138883, National Drug Code [NDC] 60793070110; Monarch Pharmaceuticals). This was the only BPG dose that each subject received in the study. On day 1, which was sample 1, we withdrew one blood sample from all subjects by venipuncture. Samples 2 and 3 were taken on different days for cohorts of 12 to 18 subjects, to cover the entire time-concentration profile, as shown in Table 1.
TABLE 1.
Sampling schedule for samples 2 and 3a
| Group (n = 12–18) | Day for sample 2 | Day for sample 3 |
|---|---|---|
| 1 | 3 | 16 |
| 2 | 4 | 17 |
| 3 | 6 | 20 |
| 4 | 7 | 21 |
| 5 | 8 | 22 |
| 6 | 9 | 23 |
| 7 | 10 | 24 |
| 8 | 13 | 27 |
| 9 | 14 | 28 |
| 10 | 15 | 29 |
All subjects were dosed on day 0 and sampled on day 1.
Laboratory analysis.
Samples were stored at −70°C for up to 18 months until analysis. These samples were analyzed in batches of 20 in random order to remove any confounding due to penicillin G degradation. We used our previously described and validated liquid chromatography/tandem mass spectrometry (LC/MS/MS) assay (2).
Population PK model building.
For population PK modeling, we used the Non-Parametric Adaptive Grid algorithm (8) in the Pmetrics package for R (version 1.2.7) (9) to fit candidate PK models to the time-concentration data for penicillin G. For all models, we set the time units to be days rather than the more typically used hours.
We began with the simplest structural model, which was a two-compartment model that had a depot/dosing compartment and a central compartment. We then tested a model with an additional peripheral tissue compartment. Although only three samples were obtained from each subject, the distribution of sampling times over the entire time-concentration profile allowed us to at least test these two models. All models had linear rate constants of absorption (Ka) from dosing to the central compartment with a volume of distribution (V), a linear elimination rate constant from the central compartment (Ke), and, when applicable, linear transfer rate constants to and from the peripheral compartment (KCP and KPC, respectively). We assumed that measured penicillin G concentrations represented the central compartment. Finally, we tested the scaling of PK parameters to three different size models: weight (V = V0 · wt), body surface area (V = V0 · BSA), or allometric size (V = V0 · wt, Ke = Ke0 · wt−0.25) (where wt is weight in kg normalized to 70 kg and BSA is the Mosteller body surface area [10] calculated from the height and weight of each subject, normalized to the population mean of 1.94 m2).
In Pmetrics, a portion of the random error in the model is attributed to the drug assay, with a multiplicative error term, γ, to capture process noise associated with measurement of the serum concentration, such as uncertainty in dose times. Pmetrics weights each observation by the reciprocal of this total assay variance (Fisher information), calculated as (γ · SD)2, where SD = C0 + C1 · [PCN] + C2 · [PCN]2 + C3 · [PCN]3 (where SD is the standard deviation, C0 to C3 are coefficients, and [PCN] is the measured penicillin G concentration). We fixed C0 at 0.00025 as half the assay limit of quantification of 0.005 ng/ml, C1 at 0.10, and C2 and C3 at 0 for an assay with a 10% coefficient of variation.
We selected the final model on the basis of minimizing the Akaike information criterion (AIC) (11), which is a function of the likelihood of the model, penalized by the number of parameters in the model. Additionally, we factored bias (mean weighted predicted-observed error) and imprecision (bias-adjusted, mean weighted squared predicted-observed error) into the selection of the final model.
Visual predictive checks.
To provide some assessment of the ability of the final model to accurately represent the study population, we used the simulation module of Pmetrics to create 5,000 sets of PK parameter values by randomly selecting them from the probability-weighted normalized distribution and covariance of values for each parameter in the model, a technique known as Monte Carlo simulation (12). As in the study, each simulated adult was administered 1.2 million units of BPG once intramuscularly, and penicillin G concentrations were simulated daily thereafter for 1 month. We calculated and plotted the 10th, 50th, and 90th percentiles of the concentrations of penicillin G in all 5,000 simulated patients versus time. Superimposed upon these plots were the actual concentrations of penicillin G measured in the 329 actual subjects. This visual predictive check (13) was considered good if the distribution of concentrations in the simulated population was similar to that in the actual population.
Probability of target attainment.
Using the final model, the simulator, and the probability of target attainment (PTA) functions in Pmetrics, we explored various alternative dosing regimens for BPG. For each dosing regimen, we simulated 5,000 patients. Pmetrics allows simulation with covariates, using the correlation matrix of Bayesian posterior parameter values and covariates in the study population, together with the specified mean and standard deviation of any covariates, all to calculate the correct covariate-parameter covariance matrix for simulations. In this case, the only covariate was weight, which we set for simulations to a mean of 50 kg and a standard deviation of 50 kg, truncating simulated values to be within the range of 25 to 110 kg. This resulted in a uniform distribution of weight between our specified limits, i.e., a homogeneously mixed group of sizes corresponding to children, adolescents, and adults. We used the population distributions for the model pharmacokinetic parameters, limiting simulated values to between 0 and 5 times the maximum in order to simulate a more diverse population than the original study population. For each set of simulated pharmacokinetic parameters and weights, we calculated the proportion of simulated patients who maintained a serum penicillin G concentration of ≥0.02 mg/liter at all times during the subsequent 28 days. For doses of >1.2 million units, we assumed that concentrations would increase proportionally to the dose, since we did not have data for higher doses.
Finally, since the final model included a peripheral tissue compartment, we sought to understand how much penicillin G kinetically distributes from the serum to extravascular tissue and if penicillin G accumulates in bodily fluids outside the serum over time with repeated BPG dosing. To do this, we simulated one additional population of 5,000 adults who received 1.2 million units of BPG monthly for 6 months, to achieve steady state. From this population, we simulated the amount of penicillin G in the peripheral compartment at two times: 1 month after the first dose and again after the final (sixth) dose. For each of the 5,000 simulated adults at each of the two time points, we calculated the relative distribution of penicillin G to the peripheral compartment as the ratio of the model-calculated amount of penicillin G in the peripheral compartment to the amount in the central (serum) compartment. We also assessed accumulation of penicillin G in the peripheral compartment over time as the ratio of the amount of penicillin G 1 month after the final dose to the amount 1 month after the first dose.
RESULTS
Characteristics of the study population are shown in Table 2. The distribution of penicillin G concentrations per day is included in Fig. 1 (circles). There was one outlier subject whose measured penicillin G concentration on day 22 was 0.14 μg/ml, versus a mean of 0.006 μg/ml for the other subjects. However, we retained this outlier measurement in the analysis, as we did not have a defined reason to exclude it.
TABLE 2.
Characteristics of study subjects (n = 329)
| Variable | Mean value (SD) | Range |
|---|---|---|
| Age (yr) | 20 (1.9) | 17–32 |
| ht (cm) | 176.8 (7.8) | 144.8–195.6 |
| wt (kg) | 76.7 (11.6) | 50.0–109.1 |
| BSA (m2) | 1.94 (0.17) | 1.53–2.42 |
FIG 1.

Distribution of measured penicillin G concentrations (circles) in the population. Lines are the indicated percentiles of 5,000 simulated concentration-time profiles. The gray shading around the percentile lines represents the 95% confidence interval around each percentile. The dotted horizontal line at 0.02 mg/liter is the suggested minimum protective concentration of penicillin G against group A streptococcus. Note that the majority of measured concentrations fall below this threshold. The dashed horizontal line is the limit of the assay, which is below all measured concentrations but is above some simulated values at the end of the dosing interval. As a visual predictive check (13) of the model, the distribution of the simulated profiles is similar to that of the observed concentrations, suggesting that the model describes the data well.
Population model.
We compared AIC, bias, and imprecision for five models after allowing the population-fitting algorithm to iterate 1,000 times toward the convergent, maximally likely distribution of parameter values. Details of the models and their AIC values are shown in Table 3. On the basis of the AIC, bias, imprecision, and stronger a priori justification for scaling to children, we chose model 5, which had a peripheral tissue compartment and allometrically scaled central compartment volume and elimination from that compartment. We then allowed model 5 to cycle until convergence, which took 9,429 cycles. The final-cycle-estimated γ from the pooled participant concentration data was 1.08. A value of 1.0 indicates that there is no additional process noise in the study, such as errors in the recorded times of sampling, and that the study procedures were carried out very precisely.
TABLE 3.
Model statistics after 1,000 cyclesa
| Model | Parameters | AIC value | Population prediction |
Individual prediction |
||
|---|---|---|---|---|---|---|
| Bias | Imprecision | Bias | Imprecision | |||
| 1 | Ka, Ke, V | −2,362 | 0.054 | 6.15 | −0.39 | 0.65 |
| 2 | Ka, Ke, V, KCP, KPC | −2,435 | 4.81 | 125.28 | −0.01 | 1.18 |
| 3 | Ka, Ke, V = V0 · wt, KCP, KPC | −2,444 | 4.98 | 139.54 | −0.06 | 0.97 |
| 4 | Ka, Ke, V = V0 · BSA, KCP, KPC | −2,441 | 5.05 | 127.92 | 0.08 | 2.20 |
| 5 | Ka, Ke = Ke0/wt0.25, V = V0 · wt, KCP = KCP/wt0.25, KPC = KPC/wt0.25 | −2,440 | 3.51 | 108.65 | −0.01 | 1.16 |
All size-scaled models (models 3 to 5) are similar, but model 5, with allometric scaling for body size, is preferable based on minimization of AIC and favorably low bias and imprecision. AIC, Akaike information criterion, with the lowest value indicating the most likely model; bias, mean weighted error of predictions minus observations; BSA, body surface area in m2 normalized to a mean population BSA of 1.94 m2; imprecision, bias-adjusted mean weighted squared error of predictions minus observations; Ka, absorption from dosing to the central compartment; KCP, transfer from the central to the peripheral compartment; Ke, elimination from the central compartment; KPC, transfer from the peripheral to the central compartment; V, volume of the central compartment; wt, weight in kg normalized to 70 kg.
The population parameter value distributions for the final model are summarized in Table 4, and the full marginal distributions are shown in Fig. 2. The parameter values appeared to most closely approximate a log-normal distribution. The half-life of penicillin G absorption after dosing as BPG is much longer than the half-life of elimination, as would be expected. Using the parameter-value distributions in Table 4, the visual predictive check of 5,000 concentration-time profiles versus the observed penicillin G concentrations in the study population (329 subjects) is shown in Fig. 1. The simulated distribution of concentrations matches the observed distribution well, suggesting that the model describes the study data adequately and can be used for meaningful PTA analysis.
TABLE 4.
Population parameter values after a single dose of 1.2 million units of benzathine penicillin G in adultsa
| Parameter | Unit of measure | Value |
|
|---|---|---|---|
| Mean (SD) | Median | ||
| Ke0 | 1/(day · 70 kg0.25) | 4.11 (2.22) | 3.96 |
| Ka | 1/day | 0.40 (0.25) | 0.32 |
| KCP0 | 1/(day · 70 kg0.25) | 6.91 (6.30) | 4.71 |
| KPC0 | 1/(day · 70 kg0.25) | 0.27 (0.34) | 0.12 |
| V0 | Liters/70 kg | 260.96 (96.45) | 239.43 |
| Calculated from full-fitted concn profiles | |||
| AUC0–∞ | mg · h/liter | 19.33 (6.09) | 18.68 |
| Clearance | Liters/h/kg | 0.68 (0.24) | 0.65 |
| Half-life of absorption | h | 72.55 (202.21) | 50.79 |
| Half-life of elimination | h | 6.03 (4.29) | 4.19 |
| Cmax | mg/liter | 0.14 (0.09) | 0.13 |
| Tmax | h | 22.90 (32.10) | 9.60 |
AUC0–∞, area under the concentration-time curve from 0 h to infinity; Ka, absorption from dosing to the central compartment; Ke, elimination from the central compartment; KCP, transfer from the central to the peripheral compartment; KPC, transfer from the peripheral to the central compartment; V, volume of the central compartment; Cmax, maximum concentration; Tmax, time to maximum concentration.
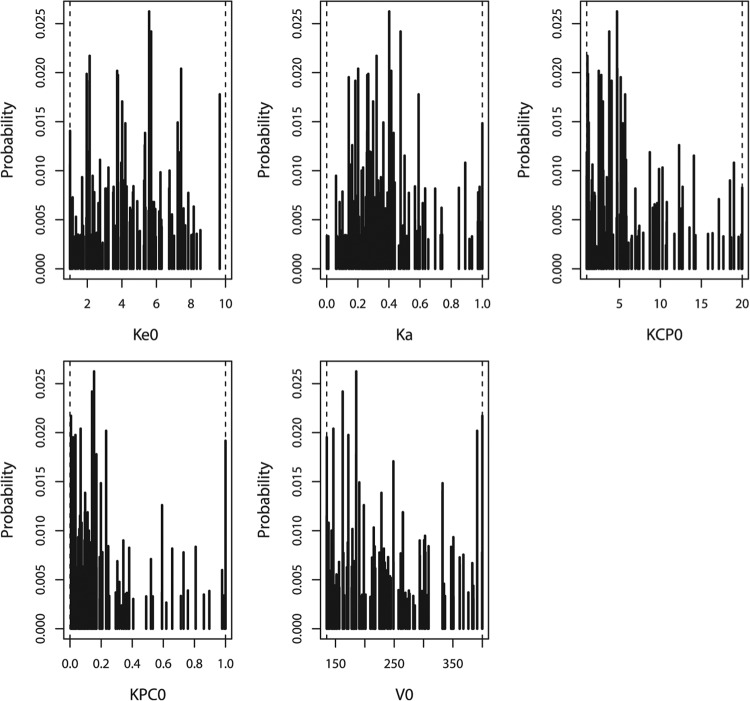
FIG 2.
Marginal distributions of parameter values in the final model. Ke0, elimination of BPG from the central compartment scaled to body weight (h−1 · kg−0.25); Ka, absorption from muscle to the central compartment (h−1); KCP and KPC, distribution of BPG from the central compartment to the peripheral compartment and back (h−1); V0, volume of the central compartment (liters/kg).
Probability of target attainment.
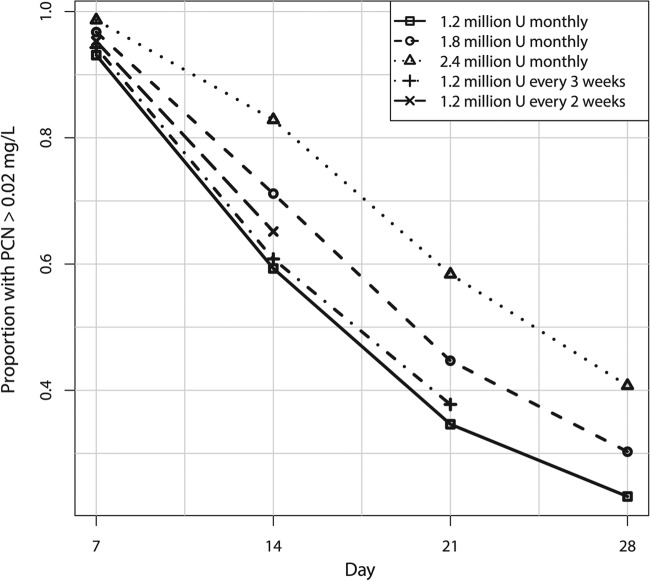
In addition to the standard dose of 1.2 million units of BPG every 4 weeks, we studied simulated doses of 1.8 million or 2.4 million units every 4 weeks as well as 1.2 million units given every 2 or 3 weeks. The results are shown in Table 5 and Fig. 3. All of the regimens resulted in substantial proportions of the study population with concentrations predicted to fall below 0.02 mg/liter of penicillin G in the serum at the end of the dosing interval, with only 23.2% having concentrations above this threshold 4 weeks after the fourth dose of 1.2 million units. The regimen with the highest success rate was 1.2 million units of BPG every 2 weeks, with 65.2% of patients having concentrations of >0.02 mg/liter 2 weeks after the previous dose at steady state.
TABLE 5.
Proportion of 5,000 simulated subjects weighing between 25 and 110 kg with trough serum penicillin G concentrations of >0.02 mg/liter for various dosing regimens
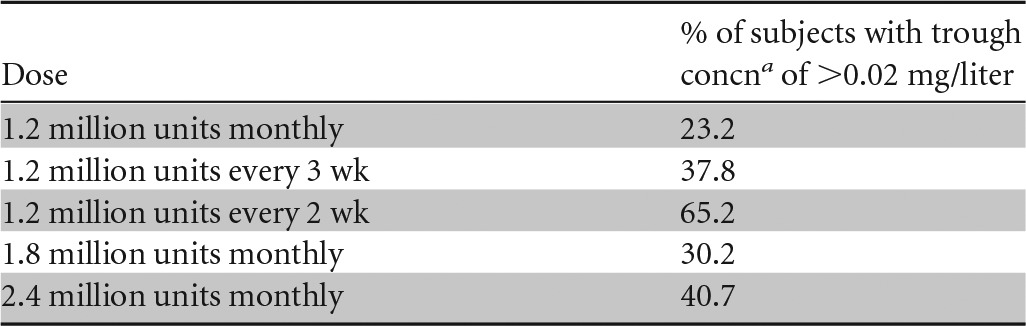
The trough concentration is defined as the serum penicillin G concentration just prior to the next dose.
FIG 3.
Proportions of simulated patients with penicillin G trough concentrations of >0.02 mg/liter for various dosage regimens of BPG on a given day after the fourth dose, i.e., at steady state.
Accumulation and peripheral compartment distribution of penicillin G.
In our model, 4 weeks after the first dose of 1.2 million units of BPG, the serum penicillin G concentration in the simulated adult population (using a median population weight of 74 kg) was a median of 0.005 mg/liter (interquartile range [IQR], 0.001 to 0.01 mg/liter). For comparison as a validation, in the real subjects, this value was very similar: 0.004 mg/liter (IQR, 0.002 to 0.009 mg/liter). In the peripheral compartment of the simulated population, the penicillin G concentration was 0.02 mg/kg of body weight (IQR, 0.005 to 0.04 mg/kg). Note that this does not correspond to an actual concentration in any specific tissue, but it is a weight-normalized amount of drug that has kinetically distributed outside the measurable serum; i.e., this is a mathematical phenomenon used to explain the shape of the observed serum concentrations with respect to time. Nevertheless, we examined the characteristics of the peripheral distribution of penicillin to determine if the drug was likely to be accumulating outside the serum. The median ratio of the peripheral to the serum penicillin G concentration after 4 weeks was 3.8 (IQR, 2.6 to 5.1). The ratio of the peripheral concentration to the serum concentration 4 weeks after the sixth dose was 3.7 (IQR, 2.7 to 5.0), the same as 4 weeks after the first dose, which indicates that the drug amount in peripheral tissues is not larger after six doses than after only one dose. This lack of substantial accumulation over time is further supported by comparing the median ratio of the serum penicillin G concentration 4 weeks after the sixth dose to the serum penicillin G concentration 4 weeks after the first dose, which was 1.23 (IQR, 0.97 to 1.73). In the peripheral compartment, this ratio was 1.08 (IQR, 1.01 to 1.40). In other words, in both serum and peripheral tissues, the penicillin G concentration 4 weeks after the sixth dose is similar to the concentration 4 weeks after the first dose.
Together, these data show that there is very little accumulation of penicillin G in the serum or peripheral tissue compartment over time at the dose used in this study. This is because the drug is nearly fully cleared from the body with monthly dosing, and the weight-normalized amount of penicillin G kinetically distributed to the peripheral compartment is stable with respect to time at ≈2.5 to 5 times the serum concentration.
DISCUSSION
We present the first reported population model of penicillin G administered as BPG. This model was developed with >300 young, active, and healthy adults who each contributed three serum samples covering the period from the first day after administration and weekly thereafter until 1 month after dosing. To explore the dose-exposure relationships with the currently available preparation of BPG, we scaled the penicillin G PK parameters to weight, BSA, and allometric size and found them to be roughly equivalent. In our model, however, allometric scaling was slightly better, and it allowed for better extrapolation from adult to pediatric populations (14–16).
It is clear from our data that with the exception of biweekly BPG dosing, the majority of patients will not sustain serum penicillin G concentrations above the MIC of ≥0.02 mg/liter for GAS during the entire dosing interval. Here we have demonstrated this in young healthy adults, and by modeling and simulation, we extrapolated our conclusion to patients with weights ranging between 25 and 110 kg. This extrapolation appears valid, since our predictions of the proportions of patients with concentrations falling below 0.02 mg/liter 3 to 4 weeks after doses of 1.2 million to 2.4 million units of BPG (Table 4) are in close agreement with observations made by others (3, 17).
Penicillin G is a “time-dependent” beta-lactam antibiotic, which is a class of antibiotics whose antibacterial kill rates are maximized when the serum concentration is above the MIC of the organism for 30 to 70% of the dosing interval (18), depending on the drug and the organism. However, this PK/pharmacodynamic (PD) linkage is really relevant only to drugs that are administered daily or multiple times a day. There is no such time-dependent model of the effects of repository penicillin G administered as BPG on GAS. Prolonged concentrations below the MIC of the organism for 1 to 2 weeks are likely not optimal, as suggested by a study over a 32-week period from November 1956 to June 1957, where the breakthrough rate for GAS infections in military recruits within the month after administration of 1.2 million units of BPG averaged 3.7 per 100 patient-years (19), and almost all GAS infections occurred >2 weeks after injection, when concentrations were lower. In 1996, Lue et al. reported 7.5 and 12.7 breakthroughs per 100 patient-years among children and adults receiving 1.2 million units of BPG every 3 weeks and every 4 weeks, respectively (20); i.e., those with longer interdose intervals had more breakthroughs (P < 0.01). In a comparison of twice-monthly versus once-monthly BPG, Kassem et al. reported 3.7 breakthroughs per 100 patient-years, and 50% of the infections occurred during the third or fourth week after injection, versus only 21% in the first 2 weeks (21). Despite the use of BPG formulations from different manufacturers, which can affect serum penicillin G concentrations (22), these studies clearly suggest that protection against GAS wanes after the first 2 weeks following a dose, which is consistent with our own observations.
Waning serum penicillin G concentrations may also be reflected in a relatively high rate of failure to eradicate GAS from the pharynx. One study found a 37% rate of failure to eradicate pharyngeal GAS 10 to 14 days or 29 to 31 days after a single injection of 600,000 units (if patients weighed <60 lb) or 1.2 million units (if patients weighed >60 lb) of BPG in 271 children who presented with acute pharyngitis and who were culture positive for GAS (6).
Despite this evidence that waning penicillin G concentrations are linked with increased numbers of breakthrough infections and failure to eradicate GAS in those patients with established pharyngeal/throat infections, the overall failure rates in the setting of BPG injections monthly or every 3 weeks are still relatively low compared with the proportion of the population that has concentrations below the MIC threshold of 0.02 mg/liter by 2 weeks after an injection. We have several hypotheses regarding why the ability of BPG to protect against GAS infections appears to be better than one would expect from the associated serum penicillin G concentrations. First, the MIC for any particular GAS isolate may be <0.02 mg/liter, since the MIC for 90% of >4,000 strains was reported to be <0.06 mg/liter in 2004 (23). Only one strain out of 282 had an MIC of >0.012 mg/liter (0.024 mg/liter) in 1992 (24). No isolates had an MIC of >0.01 mg/liter in 1965 (25). More than twice as many patients are expected to maintain serum penicillin G concentrations of >0.01 mg/liter, compared to 0.02 mg/liter for 1 month (data not shown).
The second hypothesis is that the young healthy military males in this study may have cleared penicillin G faster than other populations after injection as BPG. Other patients may retain higher concentrations for longer periods. In one of the only reported clinical studies of the same doses that we used for our simulations (1.2 million, 1.8 million, and 2.4 million units), higher proportions (10 to 20%) of adolescents and young adults maintained therapeutic serum penicillin G concentrations 2, 3, and 4 weeks after injection than the subjects in our study, although that study used a bioassay to measure penicillin G concentrations (26).
The third hypothesis is that tissue penicillin G concentrations are likely to be different in blood (17, 27). Since most GAS infections, such as tonsillitis, occur in tissues other than blood, we attempted to relate the serum kinetics of penicillin G to the kinetics outside the measurable serum compartment over time. Specifically, we tried to address the question of whether there is kinetic accumulation of penicillin G with repeated dosing such that tissue concentrations might persist after the clearance of drug from the serum, extending the functional efficacy of the drug to prevent GAS infection and/or disease. However, our data suggest that there is little residual drug in the serum or peripheral compartments by the end of a 28-day dosing interval, even with monthly dosing; that is, the drug is nearly fully cleared from the body within 1 month. In our model, we did find that the average concentration of penicillin G in the peripheral compartment 1 month after a dose of 1.2 million units of BPG, when normalized to body weight, is nearly 4-fold higher than that in the serum; again, however, this is a kinetic observation, and one cannot draw specific conclusions about the concentrations of penicillin G in any specific tissue. For example, Peloso et al. reported that the average measured concentration ratio of tonsillar to serum penicillin G in children was only about 33% for the first 2 weeks, and by 21 days, 70% of children did not have detectable tonsillar penicillin G despite having measurable serum penicillin G concentrations (17).
The fourth hypothesis is that GAS disease rates depend upon not only the concentration of penicillin G in the serum but also a combination of location- and season-dependent GAS prevalence, individual infection rates, and risk of progression from infection to disease (28–31). Even for a patient with subtherapeutic serum penicillin G concentrations, it is not 100% certain that GAS infection and disease will follow. However, in areas with higher GAS prevalence, more frequent BPG injections are recommended, consistent with our observations of the waning of serum penicillin G levels at the end of the monthly dose interval (4, 20, 32). Future work to combine our PK model with GAS PK/PD modeling (33, 34) and epidemiology may be able to more quantitatively predict optimal BPG dosing frequency based on local GAS prevalence.
In summary, we have developed a population PK model to calculate serum penicillin G exposure after intramuscular BPG dosing in healthy young adults. Although this model also appears to be relevant to children, there is a paucity of actual supportive pediatric PK data (17). With the current dose of 1.2 million units, serum concentrations of penicillin G will fall below a threshold of 0.02 mg/liter in the majority of adults and likely in children after 2 weeks. Higher monthly doses seem unlikely to substantially increase the proportion of patients whose concentrations remain above 0.02 mg/liter for the entire dosing interval, but dosing every 3 weeks or even every 2 weeks will be more successful. However, it may well be that a minimum level of 0.02 mg/liter is unnecessarily high; answering this question will require further mechanistic and epidemiological PK/PD modeling of penicillin G and GAS disease.
ACKNOWLEDGMENTS
This work was supported by NIH grants GM068968 and HD070886 (M.N.) and U10-HD-031323-15 (J.L.B.) as well as Department of Defense grant W911QY-08-P0281 (M.P.B. and D.J.F.). This work was supported by a grant from the Military Vaccine Agency to the Naval Health Research Center (work unit 60501).
The data used in the manuscript are available to collaborators upon request.
The views expressed in this work are those of the authors and do not reflect the official policy of the Department of the Navy, Department of Defense, or the U.S. Government.
We declare that we have no conflicts of interest.
M.N. performed modeling, simulation, and statistical analyses and wrote the manuscript. D.J.F. designed the clinical study and contributed to manuscript preparation. J.L.B. performed the laboratory analysis. M.P.B. performed the initial data analysis and contributed to manuscript preparation. E.L.K. contributed to the study design and manuscript preparation.
Footnotes
Published ahead of print 2 September 2014
REFERENCES
- 1.Stollerman GH, Rusoff JH. 1952. Prophylaxis against group A streptococcal infections in rheumatic fever patients; use of new repository penicillin preparation. JAMA 150:1571–1575. 10.1001/jama.1952.03680160021005. [DOI] [PubMed] [Google Scholar]
- 2.Broderick MP, Hansen CJ, Russell KL, Kaplan EL, Blumer JL, Faix DJ. 2011. Serum penicillin G levels are lower than expected in adults within two weeks of administration of 1.2 million units. PLoS One 6:e25308. 10.1371/journal.pone.0025308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie BJ. 1996. Are the currently recommended doses of benzathine penicillin G adequate for secondary prophylaxis of rheumatic fever? Pediatrics 97:989–991. [PubMed] [Google Scholar]
- 4.Kaplan EL, Berrios X, Speth J, Siefferman T, Guzman B, Quesny F. 1989. Pharmacokinetics of benzathine penicillin G: serum levels during the 28 days after intramuscular injection of 1,200,000 units. J. Pediatr. 115:146–150. 10.1016/S0022-3476(89)80352-X. [DOI] [PubMed] [Google Scholar]
- 5.Bass JW, Longfield JN, Jones RG, Hartmann RM. 1996. Serum levels of penicillin in basic trainees in the U.S. Army who received intramuscular penicillin G benzathine. Clin. Infect. Dis. 22:727–728. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan EL, Johnson DR. 2001. Unexplained reduced microbiological efficacy of intramuscular benzathine penicillin G and of oral penicillin V in eradication of group A streptococci from children with acute pharyngitis. Pediatrics 108:1180–1186. 10.1542/peds.108.5.1180. [DOI] [PubMed] [Google Scholar]
- 7.Broderick MP, Hansen CJ, Faix DJ. 2012. Factors associated with loss of penicillin G concentrations in serum after intramuscular benzathine penicillin G injection: a meta-analysis. Pediatr. Infect. Dis. J. 31:722–725. 10.1097/INF.0b013e31825051d4. [DOI] [PubMed] [Google Scholar]
- 8.Yamada WM, Bartroff J, Bayard DS, Burke J, Van Guilder M, Jelliffe R, Leary R, Neely MN, Kryshchenko A, Schumitzky A. Accessed 1 May 2014 The nonparametric adaptive grid algorithm for population pharmacokinetic modeling. Technical report TR-2014-1. University of Southern California Laboratory of Applied Pharmacokinetics, Los Angeles, CA: http://www.lapk.org/techReports.php. [Google Scholar]
- 9.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 34:467–476. 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosteller RD. 1987. Simplified calculation of body-surface area. N. Engl. J. Med. 317:1098. 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 11.Burnham KP, Anderson DR. 2010. Model selection and multi-model inference. Springer, New York, NY. [Google Scholar]
- 12.Metropolis N, Ulam S. 1949. The Monte Carlo method. J. Am. Stat. Assoc. 44:335–341. 10.1080/01621459.1949.10483310. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson MO, Savic RM. 2007. Diagnosing model diagnostics. Clin. Pharmacol. Ther. 82:17–20. 10.1038/sj.clpt.6100241. [DOI] [PubMed] [Google Scholar]
- 14.Anderson BJ, Holford NHG. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 24:25–36. 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 15.Anderson BJ, Meakin GH. 2002. Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr. Anaesth. 12:205–219. 10.1046/j.1460-9592.2002.00616.x. [DOI] [PubMed] [Google Scholar]
- 16.Anderson BJ, McKee AD, Holford NH. 1997. Size, myths and the clinical pharmacokinetics of analgesia in paediatric patients. Clin. Pharmacokinet. 33:313–327. 10.2165/00003088-199733050-00001. [DOI] [PubMed] [Google Scholar]
- 17.Peloso UC, De Souza, JCR. Botino MA, Miniti A. 2003. Penicillin concentrations in sera and tonsils after intramuscular administration of benzathine penicillin G to children. Pediatr. Infect. Dis. J. 22:1075–1078. 10.1097/01.inf.0000101476.65430.f8. [DOI] [PubMed] [Google Scholar]
- 18.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10. 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 19.McFarland RB, Colvin VG, Seal JR. 1958. Mass prophylaxis of epidemic streptococcal infections with benzathine penicillin G. N. Engl. J. Med. 258:1277–1284. 10.1056/NEJM195806262582601. [DOI] [PubMed] [Google Scholar]
- 20.Lue HC, Wu MH, Wang JK, Wu FF, Wu YN. 1996. Three- versus four-week administration of benzathine penicillin G: effects on incidence of streptococcal infections and recurrences of rheumatic fever. Pediatrics 97:984–988. [PubMed] [Google Scholar]
- 21.Kassem AS, Zaher SR, Abou-Shleib H, El-Kholy AG, Madkour AA, Kaplan EL. 1996. Rheumatic fever prophylaxis using benzathine penicillin G (BPG): two-week versus four-week regimens. Comparison of two brands of BPG. Pediatrics 97:992–995. [PubMed] [Google Scholar]
- 22.Zaher SR, Kassem AS, Abou-Shleib H, El-Kholy AG, Madkour AA, Kaplan EL. 1992. Differences in serum penicillin concentrations following intramuscular injection of benzathine penicillin G (BPG) from different manufacturers. J. Pharm. Med. 2:17–23. [Google Scholar]
- 23.Brown SD, Rybak MJ. 2004. Antimicrobial susceptibility of Streptococcus pneumoniae, Streptococcus pyogenes and Haemophilus influenzae collected from patients across the USA, in 2001-2002, as part of the PROTEKT US study. J. Antimicrob. Chemother. 54(Suppl 1):i7–i15. 10.1093/jac/dkh313. [DOI] [PubMed] [Google Scholar]
- 24.Coonan KM, Kaplan EL. 1994. In vitro susceptibility of recent North American group A streptococcal isolates to eleven oral antibiotics. Pediatr. Infect. Dis. J. 13:630–635. 10.1097/00006454-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Eickhoff TC, Finland M, Wilcox C. 1965. In vitro susceptibility of group A beta hemolytic streptococci to 18 antibiotics. Am. J. Med. Sci. 249:261–268. 10.1097/00000441-196503000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Currie BJ, Burt T, Kaplan EL. 1994. Penicillin concentrations after increased doses of benzathine penicillin G for prevention of secondary rheumatic fever. Antimicrob. Agents Chemother. 38:1203–1204. 10.1128/AAC.38.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan EL, Chhatwal GS, Rohde M. 2006. Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: clinical and pathogenetic implications. Clin. Infect. Dis. 43:1398–1406. 10.1086/508773. [DOI] [PubMed] [Google Scholar]
- 28.Danchin MH, Rogers S, Kelpie L, Selvaraj G, Curtis N, Carlin JB, Nolan TM, Carapetis JR. 2007. Burden of acute sore throat and group A streptococcal pharyngitis in school-aged children and their families in Australia. Pediatrics 120:950–957. 10.1542/peds.2006-3368. [DOI] [PubMed] [Google Scholar]
- 29.Danchin MH, Rogers S, Selvaraj G, Kelpie L, Rankin P, Vorich R, Howson M, Carlin JB, Curtis N, Nolan TM, Carapetis JR. 2004. The burden of group A streptococcal pharyngitis in Melbourne families. Indian J. Med. Res. 119(Suppl):144–147. [PubMed] [Google Scholar]
- 30.Brundage JF, Gunzenhauser JD, Longfield JN, Rubertone MV, Ludwig SL, Rubin FA, Kaplan EL. 1996. Epidemiology and control of acute respiratory diseases with emphasis on group A beta-hemolytic streptococcus: a decade of U.S. Army experience. Pediatrics 97:964–970. [PubMed] [Google Scholar]
- 31.Shaikh N, Leonard E, Martin JM. 2010. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 126:e557–e564. 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 32.Committee on Infectious Diseases. Accessed 1 May 2014 Red book online. American Academy of Pediatrics, Elk Grove, IL: http://aapredbook.aappublications.org/. [Google Scholar]
- 33.Nielsen EI, Cars O, Friberg LE. 2011. Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization. Antimicrob. Agents Chemother. 55:4619–4630. 10.1128/AAC.00182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen EI, Viberg A, Löwdin E, Cars O, Karlsson MO, Sandstrom M. 2007. Semimechanistic pharmacokinetic/pharmacodynamic model for assessment of activity of antibacterial agents from time-kill curve experiments. Antimicrob. Agents Chemother. 51:128–136. 10.1128/AAC.00604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]