Abstract
Tenofovir (TFV) is a reverse transcriptase inhibitor used in microbicide preexposure prophylaxis trials to prevent HIV infection. Recognizing that changes in cytokine/chemokine secretion and nucleotidase biological activity can influence female reproductive tract (FRT) immune protection against HIV infection, we tested the hypothesis that TFV regulates immune protection in the FRT. Epithelial cells, fibroblasts, CD4+ T cells, and CD14+ cells were isolated from the endometrium (Em), endocervix (Cx), and ectocervix (Ecx) following hysterectomy. The levels of proinflammatory cytokines (macrophage inflammatory protein 3α [MIP-3α], interleukin 8 [IL-8], and tumor necrosis factor alpha [TNF-α]), the expression levels of specific nucleotidases, and nucleotidase biological activities were analyzed in the presence or absence of TFV. TFV influenced mRNA and/or protein cytokines and nucleotidases in a cell- and site-specific manner. TFV significantly enhanced IL-8 and TNF-α secretion by epithelial cells from the Em and Ecx but not from the Cx. In contrast, in response to TFV, IL-8 secretion was significantly decreased in Em and Cx fibroblasts but increased with fibroblasts from the Ecx. When incubated with CD4+ T cells from the FRT, TFV increased IL-8 (Em and Ecx) and TNF-α (Cx and Ecx) secretion levels. Moreover, when incubated with Em CD14+ cells, TFV significantly increased MIP-3α, IL-8, and TNF-α secretion levels relative to those of the controls. In contrast, nucleotidase biological activities were significantly decreased by TFV in epithelial (Cx) and CD4+ T cells (Em) but increased in fibroblasts (Em). Our findings indicate that TFV modulates proinflammatory cytokines, nucleotidase gene expression, and nucleotidase biological activity in epithelial cells, fibroblasts, CD4+ T cells, and CD14+ cells at distinct sites within the FRT.
INTRODUCTION
With almost 2.5 million new infections in 2011, HIV remains a pandemic and continues to spread. Sexual contact remains the main mechanism of transmission worldwide, with infection rates in young women 2-fold higher than those in young men (1). Despite ongoing efforts, questions remain as to the site of HIV entry in the female reproductive tract (FRT). The FRT is divided into the upper (endocervix, endometrium, and fallopian tubes) and lower (vagina and ectocervix) tracts. It is a unique mucosal surface that combines reproductive function with a host defense against incoming pathogens (2, 3). The epithelial cells of the FRT form an active barrier between the external environment and the underlying tissue that constitutes the first line of defense. In response to invading pathogens, the epithelial cells secrete cytokines, chemokines, and antimicrobials, which play a vital role in initiating the innate immune response (4). The predominant cell types in the subepithelial tissue or stroma are the stromal fibroblasts. They are essential structural components of the reproductive tissue that regulate tissue morphology, epithelial growth and development, and epithelial barrier permeability (5).
We previously demonstrated that 10% to 20% of all cells in the FRT are immune cells, distributed throughout the lower and upper tracts (6). These immune cells include the HIV target cells that will be infected first after sexual contact and will be protected by microbicides. However, FRT epithelial cells and fibroblasts also interact and respond to microbicide treatment, which may enhance or inhibit HIV infection through secreted immune factors that alter the mucosal tissue environment.
To prevent sexual transmission of HIV and other sexually transmitted infections (STIs), recent trials have tested microbicides that can be applied topically or taken orally (7, 8). As a nucleotide analogue, tenofovir (TFV) is an effective reverse transcriptase inhibitor (9, 10). The active form, which has anti-HIV activity, is TFV diphosphate (TFV-DP) (11). After entering the cell, TFV is converted into the active form following two steps of phosphorylation, which leads to inhibition of viral replication (12). TFV, which can be taken orally or administered topically in the vagina in a gel form, has been tested in clinical trials as a microbicide for preexposure prophylaxis (PrEP) (13). While the CAPRISA (Centre for the AIDS Program of Research in South Africa) 004 trial resulted in a 39% reduction in HIV infection (7), the VOICE (Vaginal and Oral Interventions to Control the Epidemic) trial was discontinued due to a lack of efficacy (14). While a lack of adherence was highlighted as the main contributing factor for the lack of protection in the VOICE trial, a better understanding of the effects of TFV on the HIV target cells in the FRT and the mucosal environment that supports them will help improve and optimize future clinical trials.
5′-nucleotidases (NT5), which catalyze the dephosphorylation of nucleotide phosphates, control the intracellular levels of nucleotides and nucleosides (15). Multiple 5′-nucleotidases have been isolated and characterized, and five of them (NT5C1A, NT5C1B, NT5C2, NT5C3, and NT5C3L) are present in the cytoplasm. NT5E is bound to the extracellular part of the plasma membrane, and NT5M is present in the mitochondrial matrix (16). Results from clinical and in vitro studies suggest that the activation of nucleoside analogs, such as TFV or emtricitabine, can be regulated by 5′-nucleotidase activity (16). We recently demonstrated that CD4+ T cells and macrophages isolated from blood upregulate nucleotidase activity in response to TFV (17). Additionally, we demonstrated the expression of nucleotidase genes in epithelial cells and fibroblasts from all FRT tissues (18). However, the role of nucleotide analogs in the regulation of 5′-nucleotidase expression and activity in HIV target cells from the FRT mucosal tissue environment has not been explored.
Additionally, TFV and other reverse transcriptase inhibitors (RTI) have been shown to modulate serum and cervical vaginal lavage cytokines and chemokines, as well as the response of immune cells from peripheral blood in humans and mice (19–22). Overall, these results show that RTI differ in their effects on immune responses. Our previous work demonstrated that TFV stimulates the production of interleukin 8 [IL-8] from CD4+ T cells and of macrophage inflammatory protein 3α (MIP-3α) and IL-8 from macrophages derived from blood (17). However, the effect of TFV on cytokine production by FRT cells has not been explored. Since there is an increased risk of HIV-1 transmission with proinflammatory conditions in the FRT (23–25), it is important to evaluate the effects of TFV on cytokine and chemokine production in FRT cells.
In this study, we focus on key topical microbicides by evaluating the immunomodulatory effects of TFV on HIV target cells (CD4+ T cells and macrophages) and non-target cells (epithelial cells and fibroblasts) from different sites in the human FRT. We found that TFV upregulated cytokines and nucleotidase activity in a cell- and site-specific manner. Our results should help aid the design of future clinical trials with microbicides.
MATERIALS AND METHODS
Study subjects.
All investigations involving human subjects were carried out according to the principles expressed in the Declaration of Helsinki and with the approval from the Institutional Review Board Committee for the Protection of Human Subjects (CPHS), Dartmouth-Hitchcock Medical Center. Written informed consent was obtained from each patient before surgery.
Source of tissue.
Human reproductive tissues were obtained from women (35 to 75 years of age) undergoing hysterectomy at Dartmouth-Hitchcock Medical Center (Lebanon, NH). Tissues from endometrium (Em), endocervix (Cx), and ectocervix (Ecx) were collected from patients with benign conditions such as fibroids and prolapse. As determined by a pathologist, all tissue samples were distal from the site of pathology and were without pathological lesions.
Isolation and culture of FRT epithelial cells and fibroblasts.
Epithelial cells and fibroblasts were isolated as described previously (18). Briefly, tissues were minced into 1- to 2-mm fragments under sterile conditions and subjected to enzymatic digestion for 2 h at 37°C. After digestion, the cells were dispersed through a 250-mm mesh screen (Small Parts, Miami Lakes, FL, USA), and epithelial sheets were separated from stromal cells by filtration through a 20-mm nylon mesh filter (Small Parts). Epithelial cell sheets were retained on the filter while the fibroblasts passed through. Fibroblasts were collected, analyzed for cell number and viability, and plated in 75-mm flasks containing complete medium. The medium was changed every 2 days, and when the fibroblasts were confluent, they were trypsinized with 0.05% trypsin-EDTA (Cellgro, Manassas, VA) and plated onto a 24-well plate at a density 2 × 105 cells/well. Epithelial cells were recovered by rinsing and backwashing the filter and then were analyzed for cell number and viability. Epithelial cells were cultured in human extracellular matrix (Becton Dickinson, Franklin Lakes, NJ)-coated Falcon cell culture inserts on 24-well plates (Fisher Scientific, Pittsburgh, PA) as previously described (18). The medium was changed every 2 days; 300 μl of complete medium was added in the apical compartment, and 500 μl of complete medium was added in the basolateral chamber. The tight junction formation of cultured epithelial cell monolayers was assessed by periodically measuring transepithelial resistance (TER) using an EVOM electrode and Voltohmmeter (World Precision Instruments, Sarasota, FL, USA), as described previously (18).
Isolation of CD4+ T cells and CD14+ cells from FRT.
Following tissue digestion and filtration, a dead-cell removal kit (Miltenyi Biotec, Auburn, CA) was used according to the manufacturer's instructions to remove dead cells from the stromal cell suspension. CD4+ T cells were then isolated using the CD4+ T cell isolation kit (Miltenyi Biotec) following instructions, with minor modifications as previously described (26). This negative bead selection depletes CD8, CD14, CD16, CD19, CD36, CD56, CD123, T cell receptor gamma/delta (TCR-γ/δ), and CD235a. In addition, antifibroblast microbeads (Miltenyi Biotec) were added to deplete stromal fibroblasts in the cell suspension. Two rounds of negative selection were performed to obtain >90% pure CD4+ T cells. CD14+ cells were isolated by positive selection with magnetic beads (Miltenyi Biotec) following the manufacturer's instructions. Two rounds of selection were performed to obtain >90% purity (26).
TFV treatment.
TFV in a powder form was obtained from the AIDS Research and Reference Reagent Program (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH; catalog number 10199). A stock concentration of TFV (10 mg/ml) was prepared by adding 1 ml of phosphate-buffered saline (PBS) to 10 mg of TFV powder. A final concentration of 1 mg/ml (3.5 mM) of TFV was used in all experiments, based on our previous dose-response studies (17).
Cell viability assay.
The viability of epithelial cells, fibroblasts, CD4+ T cells, and CD14+ cells upon treatment with TFV was quantified using the CellTiter 96 AQueous One Solution cell proliferation assay (Promega, Madison, WI, USA) according to the manufacturer's instructions, as mentioned previously (17). Cell viability was also measured using the Trypan blue (HyClone Laboratories, Inc., Logan, UT) method. We found no changes in viability with TFV treatment on all cells measured, covering times up to 24 h with 1 mg/ml of TFV.
RNA isolation and quantitative RT-PCR analysis.
Real-time (RT) PCR was done with a two-step protocol as described previously (18). The 5′-nucleotidases measured were ecto-5′-nucleotidase (NT5E), cytosolic 5′-nucleotidase 1A (NT5C1A), cytosolic 5′-nucleotidase 1B (NT5C1B), cytosolic 5′-nucleotidase II (NT5C2), cytosolic 5′-nucleotidase III (NT5C3L), cytosolic 5′(3′)-deoxyribonucleotidase (NT5C), and mitochondrial 5′(3′)-deoxyribonucleotidase (NT5M) and proinflammatory cytokines measured were MIP-3α, IL-8, and tumor necrosis factor alpha (TNF-α). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene for macrophages, β-actin was the housekeeping gene for epithelial cells and fibroblasts, and RPL13A was used for CD4+ T cells (27). PCR was done as described previously (18). The averages of cycle threshold (CT) values for each set of duplicate reactions were calculated, and a difference in CT values (ΔCT) was determined for each gene by taking the mean CT of each gene of interest and subtracting the mean CT for the housekeeping gene β-actin/GAPDH/RPL13A for each cDNA sample. The relative expression level of each gene was calculated using the formula 2−ΔCT.
Measurement of 5′-nucleotidase biological activity assay.
5′-nucleotidase biological activity was measured using a 5′-nucleotidase kit (Diazyme Laboratories, Poway, CA, USA), modified to adapt it from a serum to a cellular base assay as previously described (18). Briefly, 0.2 × 105 to 0.4 × 105 epithelial cells, 1 × 105 fibroblasts, 0.3 × 105 to 0.5 × 105 CD4+ T cells, and 0.3 × 105 to 0.5 × 105 CD14+ cells were used per well. After treatment with TFV (1 mg/ml) or PBS (control) for 24 h, cells were washed, permeabilized, and added to 96-well plates along with appropriate kit reagents as described by Shen et al. (18). Data were expressed as activity per million cells.
ELISAs.
TFV (1 mg/ml) or PBS as a control was added in 0.2 × 105 to 0.4 × 105 epithelial cells for 24 h, 1 × 105 fibroblasts for 24 h and 48 h, and 0.3 × 105 to 0.5 × 105 CD4+ T and CD14+ cells for 24 h. Supernatants were recovered and centrifuged (10,000 × g) for 10 min to remove cellular debris. MIP-3α, IL-8, and TNF-α secretion levels were measured by enzyme-linked immunosorbent assays (ELISAs) (all from R&D Systems Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis.
GraphPad Prism 5.0 software was used to analyze the data. The nonparametric test, Mann-Whitney U test, or Wilcoxon paired test was performed to compare the two groups. A two-sided P value of <0.05 was considered statistically significant in all cases. The Wilcoxon signed-rank test was performed to compare the column medians to a hypothetical value of 1 (the controls were, by definition, all equal to 1).
RESULTS
TFV upregulates cytokine gene expression in epithelial cells and fibroblasts from the FRT.
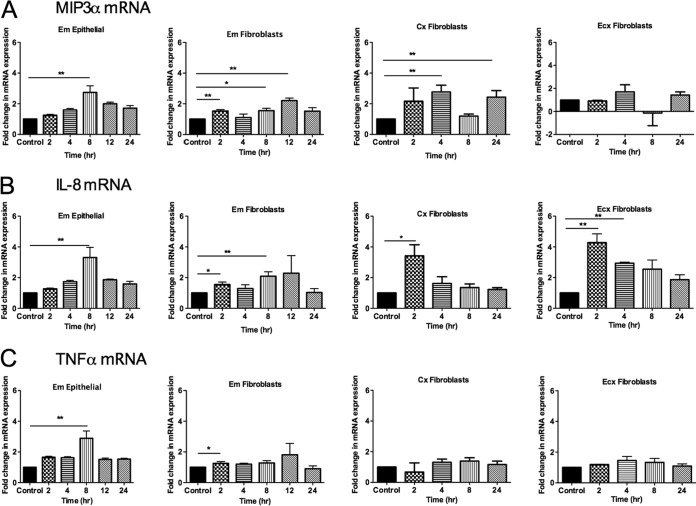
To evaluate the effect of TFV on epithelial cells and fibroblasts from different sites in the FRT, cells were treated with 1 mg/ml TFV from 2 to 24 h, and the gene expression levels of MIP-3α, IL-8, and TNF-α were analyzed. For epithelial cells, TFV was added to the apical chamber of cell inserts. A dose of 1 mg/ml of TFV was chosen based in our previous dose-response studies (17). This dose was below the clinical dose of 80 mg for vaginal deposition in a 24-h period used in the CAPRISA 004 trial (7). The genes analyzed were determined based on our previous studies (17). Due to low epithelial cell recovery in the Cx and Ecx, only epithelial cells from the Em were examined for gene expression in the presence of TFV. As seen in Fig. 1, the responses to TFV varied with the FRT site analyzed. Epithelial cells and fibroblasts from Em significantly increased MIP-3α mRNA message levels (Fig. 1A). The maximum expression occurred at 8 h (2.7-fold) in Em epithelial cells and at 12 h (2.2-fold) in Em fibroblasts. Fibroblasts from the Cx also significantly upregulated MIP-3α (a 2.8-fold increase at 4 h and a 2.5-fold increase after 24 h of treatment). In contrast, TFV had no significant effect on MIP-3α expression in fibroblasts from Ecx.
FIG 1.
Effects of TFV on cytokine gene expression in epithelial cells and fibroblasts from the FRT. Epithelial cells were isolated from endometrium, and fibroblasts were isolated from endometrium, endocervix, and ectocervix. Cells were treated with 1 mg/ml of TFV. RNA was isolated, and RT-PCR was performed to compare the levels of mRNA of MIP-3α, IL-8, and TNF-α at different time points. Each TFV-treated sample was compared with its own control at the same time point, and fold changes in mRNA expression were calculated by converting each control to 1. Bars represent means ± SEM from 6 to 8 independent experiments with different patients. *, P < 0.05; **, P < 0.01. Shown are expression levels of the MIP3α gene (A), IL-8 gene (B), or TNF-α gene (C) from Em epithelial cells and Em, Cx, and Ecx fibroblasts.
As seen in Fig. 1B, TFV treatment increased IL-8 expression in epithelial cells and fibroblasts from Em and in fibroblasts from the Cx and Ecx. In Em epithelial cells, IL-8 message levels were significantly elevated at 8 h (3-fold) and returned toward baseline at 12 h, while fibroblasts initially upregulated IL-8 mRNA within 2 h (1.5-fold). Maximum expression occurred at 8 h (2-fold) in Em fibroblasts. A significant increase in the expression of IL-8 was also observed at 2 h in Cx (2.8-fold) and Ecx (4.2-fold) fibroblasts in the presence of TFV.
Figure 1C shows that TNF-α was upregulated by TFV in Em epithelial cells and fibroblasts. Similar to that seen with MIP-3α and IL-8, TFV significantly enhanced the expression of TNF-α at 8 h in Em epithelial cells (2.9-fold) and at 2 h in Em fibroblasts (1.3-fold). In contrast to Em fibroblasts, no significant changes were observed in TNF-α gene expression in fibroblasts from the Cx and Ecx. These results indicate that TFV selectively modulates the expression of proinflammatory cytokine genes and that responses vary with cell type, site in the FRT, and duration of TFV treatment.
Differential effect of TFV on MIP-3α, IL-8, and TNF-α secretion levels by FRT epithelial cells and fibroblasts.
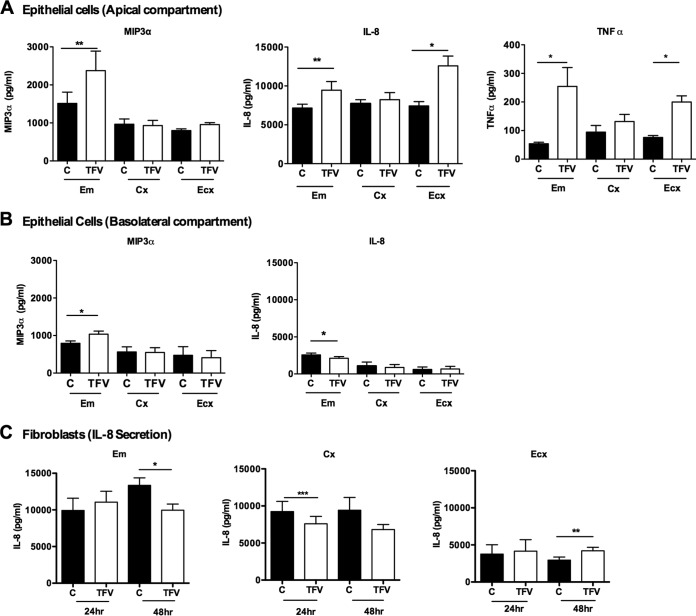
To determine if the effects of TFV on cytokine gene expression translate into protein production, we measured the secretion levels of MIP-3α, IL-8, and TNF-α by epithelial cells and fibroblasts from Em, Cx, and Ecx. As seen in Fig. 2, incubation of Em epithelial cells with TFV (1 mg/ml) for 24 h significantly increased the apical secretion levels of MIP-3α (P = 0.0078) (Fig. 2A, left), IL-8 (P = 0.0039) (Fig. 2A, middle), and TNF-α (P = 0.048) (Fig. 2A, right) relative to control cells. Similarly, there was a significant (P = 0.03) increase in the basolateral secretion level of MIP-3α (Fig. 2B, left), but a significant (P = 0.043) decrease was observed in the secretion level of IL-8 (Fig. 2B, right) by Em epithelial cells. Incubation of Ecx epithelial cells with TFV (1 mg/ml) for 24 h also significantly increased the apical secretion level of IL-8 (P = 0.003) (Fig. 2A, middle) and TNF-α (P = 0.0035) (Fig. 2A, right) but had no effect on the Cx epithelial cell secretion level of either cytokine. The basolateral secretion levels of TNF-α were below the detection limit (15.6 pg/ml) for all samples.
FIG 2.
Effect of TFV on MIP-3α, IL-8, and TNF-α secretion from FRT epithelial cells and fibroblasts. Epithelial cells and fibroblasts were isolated from endometrium, endocervix, and ectocervix. Cells were treated with 1 mg/ml of TFV for 24 h. Secretions of MIP-3α, IL-8, and TNF-α were analyzed from the apical and basolateral surfaces of Em, Cx, and Ecx epithelial cells and from culture supernatants of Em, Cx, and Ecx fibroblasts by ELISA. Bars represent means ± SEM from 6 to 8 independent experiments with different patients. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (A) Secretion of MIP3α, IL-8, and TNF-α by apical surface of FRT Em, Cx and Ecx; (B) secretion of MIP3α, IL-8, and TNF-α by basolateral surface of FRT Em, Cx and Ecx; (C) secretion of IL-8 by Em, Cx and Ecx fibroblasts. C, control.
We also evaluated the effects of TFV on proinflammatory cytokine protein secretion by Em, Cx, and Ecx fibroblasts. In contrast to epithelial cells, no differences were observed in the levels of MIP-3α secretion by Em, Cx, or Ecx fibroblasts when cells were incubated with TFV (data not shown). Interestingly, in response to TFV, IL-8 secretion was significantly decreased at 48 h in Em fibroblasts (P = 0.0078) and at 24 h in Cx fibroblasts (P = 0.0001) (Fig. 2C, left and middle). In contrast, a significant increase in the secretion of IL-8 was observed in Ecx fibroblasts at 48 h (P = 0.01) (Fig. 2C, right). The TNF-α secretion level did not change in fibroblasts from Cx and Ecx and was below the detection limit in Em (data not shown).
In Fig. 2, the protein secretion levels are expressed in pg/ml. The insert system utilizes two different volumes of media for the culture of epithelial cells (500 μl for the basolateral compartment and 300 μl for the apical compartment). When we corrected for volume differences, we found that the secretion level of IL-8 in the apical compartment of Em, Cx, and Ecx epithelial cells is higher than in the basolateral compartment in both control and TFV-treated cells (Fig. 2A, middle, and Fig. 2B, right). In all cases, irrespective of whether results are expressed per ml or per compartment, the TFV effects were significantly different. These calculations indicate that IL-8 is preferentially secreted into the apical compartment by Em, Cx, and Ecx epithelial cells.
Effect of TFV on the expression and secretion of MIP-3α, IL-8, and TNF-α by CD4+ T cells and CD14+ cells from the upper and lower FRT.
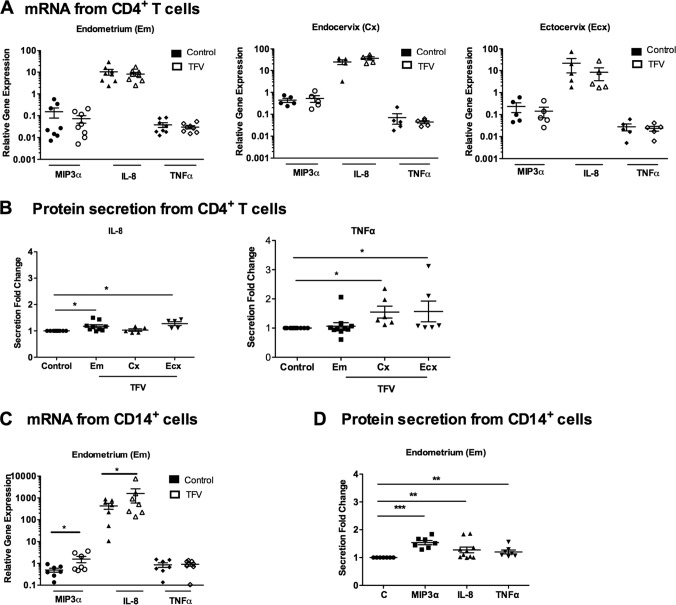
In the current study, we examined the effect of TFV on CD4+ T cells from the Em, Cx, and Ecx. CD4+ T cells were isolated by magnetic bead separation and treated with 1 mg/ml TFV for 24 h. RNA was isolated, and real-time PCR was performed to analyze the effect of TFV on the gene expression of MIP-3α, IL-8, and TNF-α. As seen in Fig. 3A, at 24 h, there were no significant changes in the expression levels of MIP-3α, IL-8, and TNF-α mRNA in CD4+ T cells from Em, Cx, and Ecx compared to the controls.
FIG 3.
Effect of TFV on MIP-3α, IL-8, and TNF-α in CD4+ T cells and CD14+ cells. CD4+ T cells were isolated from Em, Cx, and Ecx, and CD14+ cells were isolated from Em. Cells were treated with 1 mg/ml of TFV for 24 h. RNA was isolated, and RT-PCR was performed to compare the levels of mRNA of MIP-3α, IL-8, and TNF-α. mRNA was expressed as a relative expression compared to untreated samples. Secretions of MIP-3α and IL-8 were analyzed from culture supernatants by ELISA. Each TFV-treated sample was compared with its own control, and fold changes in protein secretion were calculated by converting each control to 1. Bars represent means ± SEM from 6 to 8 independent experiments with different patients. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (A) Expression of MIP3α, IL-8, and TNF-α from Em, Cx, and Ecx CD4+ T cells in the presence of TFV; (B) secretion of IL-8 and TNF-α from Em, Cx, and Ecx CD4+ T cells; (C) expression of MIP3α, IL-8, and TNF-α from CD14+ cells from Em in the presence of TFV; (D) secretion of MIP3α, IL-8, and TNF-α from CD14+ cells from Em in the presence of TFV.
Since changes in gene expression do not necessarily correlate with changes in protein secretion, we then determined the effects of TFV on cytokine/chemokine protein secretion. As seen in Fig. 3B, incubation with TFV (1 mg/ml) significantly increased the secretion level of IL-8 (Fig. 3B, left) by CD4+ T cells from the Em (P = 0.0196) and Ecx (P = 0.0194); no significant changes were observed in CD4+ T cells from the Cx. Incubation with TFV significantly increased the TNF-α secretion level by CD4+ T cells from the Cx (P = 0.03) and the Ecx (P = 0.03) but not from the Em (Fig. 3B, right). In all cases, MIP-3α secretion levels were below the detection limit (15.6 pg/ml) in secretions of CD4+ T cells from all three tissues. These results indicate that TFV selectively regulates the secretion of proinflammatory cytokines by CD4+ T cells from the Em, Cx, and Ecx in the FRT.
In other studies, we found that TFV (1 mg/ml) upregulated the gene expression of MIP-3α and IL-8 in macrophages derived from blood monocytes (17). As seen in Fig. 3C, to determine whether TFV had comparable effects on FRT cells, we examined the effect of TFV on CD14+ cells isolated from the FRT. Due to low cell numbers in the Cx and Ecx, we were only able to analyze CD14+ cells from the Em. CD14+ cells were isolated from Em and treated with TFV (1 mg/ml) for 24 h, and real-time PCR was performed to analyze the effect of TFV on the mRNA expression of MIP-3α, IL-8, and TNF-α. As seen in Fig. 3C, CD14+ cells from the Em significantly increased MIP-3α (P = 0.0156) and IL-8 (P = 0.0154) mRNA message levels at 24 h in response to TFV compared to those of the control; no significant changes were observed for TNF-α.
To determine the effects of TFV on cytokine/chemokine protein secretion, we measured MIP-3α, IL-8, and TNF-α in the cell culture. As seen in Fig. 3D, incubation of CD14+ cells with TFV significantly increased MIP-3α (P = 0.0156), IL-8 (P = 0.0039), and TNF-α (P = 0.0265) secretion after 24 h. These results suggest that TFV modulates the expression and secretion of proinflammatory cytokine genes in CD14+ cells from Em.
Site-dependent effect of TFV on 5′-nucleotidase gene expression in FRT CD4+ T cells.
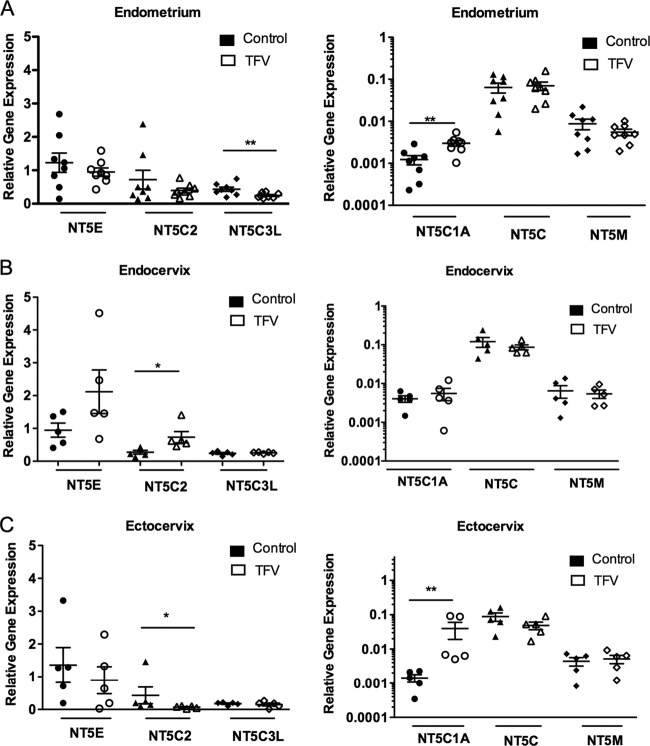
We next examined the effect of TFV on the regulation of nucleotidase gene expression in CD4+ T cells from the FRT. The cells were treated with 1 mg/ml of TFV for 24 h. Differential responses to TFV were observed in CD4+ T cells from the different FRT sites. As seen in Fig. 4, analysis of constitutive expression of nucleotidase genes in CD4+ T cells from the Em, Cx, and Ecx indicated that the expression levels of NT5E, NT5C2, and NT5C3L are higher than those of NT5C1A, NT5C, and NT5M. Therefore, the results are presented in two different graphs with different scales to show the expression levels of the genes.
FIG 4.
Site-dependent effect of TFV on 5′-nucleotidase gene expression in FRT CD4+ T cells. CD4+ T cells were isolated from Em, Cx, and Ecx, and cells were treated with 1 mg/ml of TFV for 24 h. RNA was isolated, and RT-PCR was performed to compare the levels of mRNA of the nucleotidase genes NT5E, NT5C2, NT5C3L, NT5C1A, NT5C, and NT5M in the presence of TFV from CD4+ T cells in Em (A), Cx (B), and Ecx (C). mRNA was expressed as a relative expression compared to untreated samples. Five to 9 independent experiments with different patients were done. *, P < 0.05; **, P < 0.01.
As shown in Fig. 4A (left), TFV incubation with CD4+ T cells from the Em significantly (P = 0.0078) decreased the expression level of NT5C3L and significantly (P = 0.0078) increased NT5C1A gene expression (Fig. 4A, right). In CD4+ T cells from Cx, TFV increased the expression level of NT5C2 (P = 0.049) (Fig. 4B, left) but had no effect on the 5 other genes examined. In CD4+ T cells from the Ecx, NT5C1A expression was increased (P = 0.0049) (Fig. 4C, right), while NT5C2 was decreased (P = 0.049) (Fig. 4C, left). These results suggest that TFV can specifically modulate the expression of nucleotidase genes in CD4+ T cells from the different FRT sites.
The effect of TFV on nucleotidase gene expression was also analyzed in epithelial cells and fibroblasts from Em, Cx, and Ecx. When epithelial cells and fibroblasts from 5 to 7 tissues were treated with TFV, no significant differences in the expression of nucleotidase genes were observed relative to controls (data not shown).
Effect of TFV on 5′-nucleotidase gene expression in CD14+ cells.
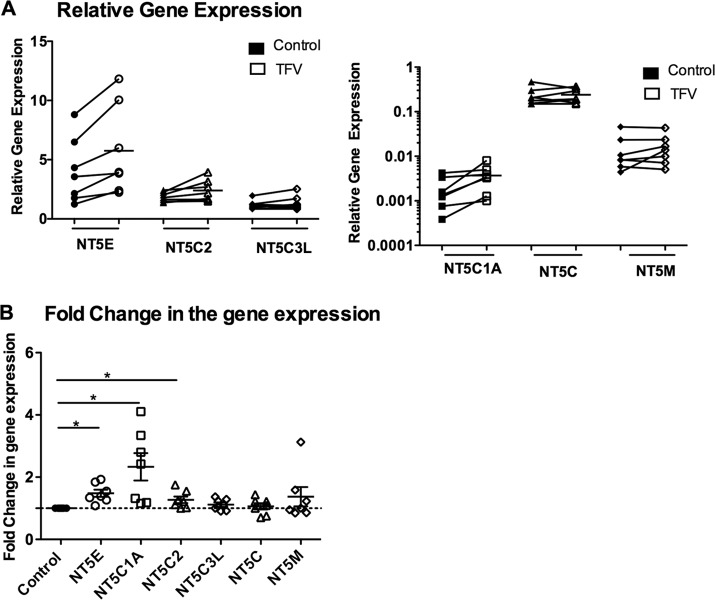
Previously, we observed that TFV increases the expression of NT5E nucleotidase genes in monocyte-derived macrophages (17). Here, we explored whether TFV has any effect on the modulation of nucleotidase genes in CD14+ cells from Em. As seen in Fig. 5A, gene expression in the presence of TFV showed no significant changes relative to controls. To control for patient-to-patient variability, samples were normalized, and TFV-treated cells were compared to untreated controls. As seen in Fig. 5B, a significant increase was found for NT5E (P = 0.01), NT5C1A (P = 0.01), and NT5C2 (P = 0.03).
FIG 5.
Effect of TFV on 5′-nucleotidase gene expression in CD14+ cells. CD14+ cells were isolated from Em and treated with 1 mg/ml of TFV for 24 h. RNA was isolated, and RT- PCR was performed to compare the levels of mRNA of the nucleotidase genes NT5E, NT5C2, NT5C3L, NT5C1A, NT5C, and NT5M. Six to 9 independent experiments with different patients were done. *, P < 0.05. Relative mRNA expression (A) and fold change in the mRNA expression (B) of NT5E, NT5C2, NT5C3L, NT5C1A, NT5C, and NT5M gene expression in the presence of TFV from CD14+ cells in Em. Each TFV-treated sample was compared with its own control, and fold changes in mRNA expression were calculated by converting each control to 1.
Effect of TFV on 5′-nucleotidase biological activity in epithelial cells, fibroblasts, CD4+ T cells, and CD14+ cells from the FRT.
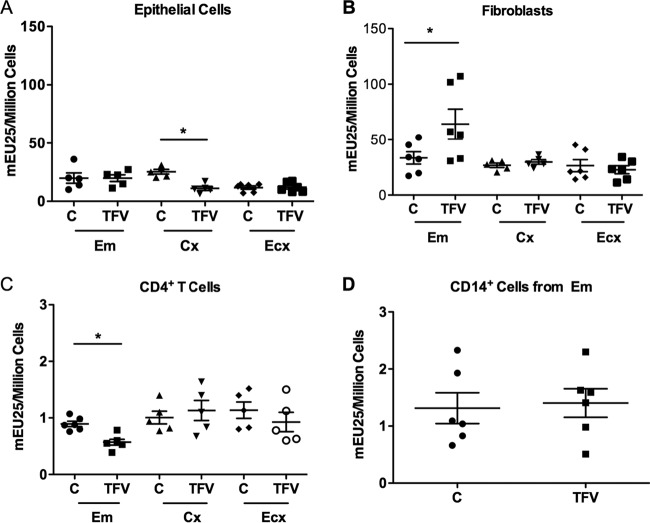
We previously reported that nucleotidase biological activity can be measured in cells from the FRT (18). To determine whether TFV modulates 5′-nucleotidase biological activity, we used a modified Diazyme 5′-nucleotidase assay (18). Cells were isolated from the FRT and treated with TFV (1 mg/ml) prior to measuring 5′-nucleotidase activity after 24 h in culture. Nucleotidase activity remained unchanged in epithelial cells from Em and Ecx in the presence of TFV, while a significant (P = 0.048) decrease was observed in the Cx (Fig. 6A). In fibroblasts, a significant increase in the biological activity was observed in the Em in the presence of TFV (P = 0.0312) (Fig. 6B); no changes were observed in fibroblasts from the Cx or Ecx (Fig. 6B).
FIG 6.
Effect of TFV on 5′-nucleotidase activity in epithelial cells, fibroblasts, CD4+ T cells, and CD14+ cells. 5′-nucleotidase biological activity was measured (see Materials and Methods) in epithelial cells, fibroblasts, and CD4+ T cells from Em, Cx, and Ecx and from CD14+ cells from Em after 24 h with 1 mg/ml of TFV treatment. Values are expressed as milli-enzyme units (mEU) per 1 million cells. Bars represent means ± SEM from 5 or 6 separate experiments with different donors. *, P < 0.05. Epithelial cells (A) fibroblasts (B), CD4+ T cells (C), and CD14+ cells (D) from Em, Cx, and Ecx after 24 h with 1 mg/ml of TFV treatment.
When CD4+ T cells from the FRT were examined, we found a significant decrease (P = 0.031) in the activity of 5′-nucleotidase in the presence of TFV in cells from the Em (Fig. 6C). In contrast, no changes were observed in the 5′-nucleotidase activities in CD4+ T cells from the Cx or Ecx (Fig. 6C) or in CD14+ cells from Em (Fig. 6D).
DISCUSSION
In the present study, we investigated the effects of TFV on cytokine/chemokine production and 5′-nucleotidase gene expression and biological activity on cells from different sites in the FRT. To our knowledge, this is the first study to analyze the effects of TFV on primary HIV target cells, epithelial cells, and fibroblasts isolated from the endometrium, endocervix, and ectocervix of the FRT. This study extends our previous work with cells derived from blood and establishes a suitable system for topical microbicide evaluation. Our findings suggest that the proinflammatory effects of TFV on HIV target cells, epithelial cells, and fibroblasts in the FRT may compromise microbicide effectiveness against HIV.
Our studies indicated that the net effects of TFV in the FRT most likely involve epithelial cells, fibroblasts, and HIV target cells. We found significant increases in the secretion of MIP-3α, IL-8, and TNF-α from the apical compartment of Em and Ecx epithelial cells. In contrast, TFV had no effect on epithelial cells from the Cx, suggesting differential responsiveness to TFV at different sites in the FRT. Interestingly, the apical response to TFV in epithelial cells, which is directed toward the lumen, was different from the response found in the basolateral compartment, which affects the underlying tissue and HIV-1 target cells. TFV increased MIP-3α secretion into the basolateral compartment of Em epithelial cells but decreased IL-8 secretion, suggesting a more fine-tuned response toward the tissue.
FRT site-specific responses to TFV treatment were also found in fibroblasts; IL-8 secretion was decreased in the Em and Cx but increased in the Ecx. Additionally, while TFV increased IL-8 secretion toward the lumen, the levels of IL-8 in the tissue are decreased due to interaction with epithelial cells and fibroblasts.
Although epithelial cells and fibroblasts are not productively infected by HIV, they most likely play an important role in the infection process through the secretion of proinflammatory cytokines, including MIP-3α and IL-8, that have complex implications for HIV acquisition. While Ghosh et al. (28) previously demonstrated the anti-HIV activity of MIP-3α in vitro, MIP-3α is also a chemoattractant for HIV target cells, including CD4+ T cells and dendritic cells (29, 30). The blockade of MIP-3α in nonhuman primates was shown to prevent systemic dissemination of simian immunodeficiency virus (SIV) infection following vaginal challenge (31). Therefore, the findings in our study suggest that the TFV upregulation of MIP-3α apical and basolateral secretions by epithelial cells may result in increased chemotaxis of target cells and increased HIV susceptibility. Furthermore, IL-8 is also a chemoattractant molecule for CD4+ T cells and neutrophils (32). Previous studies demonstrated that IL-8 increased HIV replication in macrophages and CD4+ T cells and increased viral replication in peripheral blood lymphocytes and ectocervical explants (33, 34).
In addition to effects on non-target cells, we found that TFV treatment induced a proinflammatory response in HIV target cells from the FRT. IL-8 was upregulated in CD4+ T cells from Em and Ecx, while TNF-α secretion was increased in Cx and Ecx. In CD14+ cells from the Em, TFV increased secretion of MIP-3α, IL-8, and TNF-α. An analysis of TFV effects on cells isolated from blood in our previous study also showed increased IL-8 secretion by CD4+ T cells and increased IL-8 and MIP-3α secretion by monocyte-derived macrophages (17); no changes in TNF-α secretion were found in HIV target cells from blood in response to TFV.
In the present study, we found that TFV has a cell-specific and FRT site-specific effect on the gene expression and biological activity of nucleotidases. 5′-nucleotidases are a group of cytosolic enzymes that catalyze the dephosphorylation of nucleotide phosphates to regulate cellular nucleotide and nucleoside levels (15) and therefore are implicated in the regulation of nucleotide and drug metabolism (16, 35). We found that TFV increased NT5C1A mRNA in CD4+ T cells in Em and Ecx and NT5C2 gene in Cx but decreased NT5C3L in Em and NT5C2 in Ecx. These changes in nucleotidase gene expression are different from our observations in a previous study with blood CD4+ T cells (17), in which no changes were detected. In CD14+ cells from the Em, we observed an increase in NT5E, NT5C1A, and NT5C2, different from the monocyte-derived macrophages in which we detected an increase in NT5E and a decrease in NT5M (17). Overall, our findings demonstrate that cells from blood and the FRT behave differently in response to TFV exposure.
Consistent with previous findings, nucleotidase gene expression did not correlate with the observed changes in nucleotidase activity (17, 18). 5′-nucleotidase biological activity in the presence of TFV significantly decreased in epithelial cells from the Cx, increased in fibroblasts from the Em, decreased in CD4+ T cells from the Em, and remained unchanged in CD14+ cells. The nucleotidase biological activity assay detects the total activity of all the nucleotidases, not all of which contribute equally to biological activity. This assay preferentially detects the activity of nucleotidases that prefer purine-based substrates, such as NT5E, NT5C1A, and NT5C2, which are the 5′-nucleotidases expected to interact with TFV as a purine analog. Nucleotidases with a preference for pyrimidine bases, such as NT5C, NT5C3L, and NT5M, contribute only marginally to the total activity detected. Clinical and in vitro studies suggest that nucleotide analog activation, such as TFV or emtricitabine, can be inhibited by an increase in nucleotidase activity (16). Therefore, the decrease in biological activity might be expected to maintain or increase intracellular TFV-DP concentrations, the active form of TFV. A decrease in nucleotidase activity in epithelial cells lowers TFV availability for HIV target cells (less TFV is inactivated, and it is retained in active form inside the cells). In contrast, increased nucleotidase activity in fibroblasts results in increased availability for target cells. Therefore, the alterations in nucleotidase activity found in our study may be relevant to drug inactivation, resulting in a decrease in the effectiveness of TFV in specific cell types and FRT sites.
To date, clinical trials for HIV prevention using TFV in gel form directly applied into the FRT have shown mixed results. In the CAPRISA 004 study, using 1% TFV gel before and after sex, partial success was achieved, with 39% protection against HIV-1 acquisition (7). In the VOICE trial, however, daily application of vaginal TFV gel was ineffective, and the study was interrupted (14). Although the lack of adherence was a major contributing factor for the lack of effects, other biological factors may also contribute, and the direct effects of TFV on HIV target cells and the tissue mucosal environment in the FRT are largely unknown. Our studies indicate that TFV has profound effects on HIV target cells and non-target cells in the FRT at multiple levels that can affect TFV effectiveness and the consequent protection against HIV infection. While TFV has had a favorable treatment and prevention history and is the licensed microbicide for preexposure prophylaxis, the induction of proinflammatory factors observed in our study is worrisome. Further studies are needed to optimize the dosage and timing of TFV application in relation to sexual intercourse and for the exploration of alternative formulations that preferentially reach HIV target cells, to prevent or minimize the generation of a proinflammatory environment in the FRT that would increase the risk for HIV acquisition. Recognizing that new trials (e.g., FACTS 001) are under way to test the efficacy of TFV in preventing HIV infection, and that future studies are considering TFV application in vaginal rings, etc., to minimize the lack of compliance (36), the results presented in our study are relevant and should help optimize the design of future studies.
In conclusion, our study demonstrates that TFV modulates cytokine production, as well as the gene expression and biological activity, of 5′-nucleotidases in HIV target cells and the mucosal tissue environment in the FRT. Knowledge on how TFV affects these cells at different sites of the FRT will improve the design of new preventive trials that optimize the amount and timing of TFV in a way that will protect the immune cells without compromising immune protection in the FRT.
ACKNOWLEDGMENTS
This work was supported by NIH contract HHSN272201000001C, which was indirectly funded by Advanced BioScience Laboratories, Inc., and by NIH grants AI102838 and AI071761 (to C.R.W.).
We thank Richard Rossoll and Mickey Patel for technical assistance.
We declare that we have no competing interests.
N.B. designed and performed the majority of the experiments, analyzed the data, and wrote the paper. M.R.G. helped in data analysis and manuscript writing, Z.S. and S.G.C. performed some experiments, J.E.B. developed the nucleotidase biological activity assay, and J.V.F. contributed to the writing of this manuscript. C.R.W. helped in designing experiments, data analysis, and manuscript writing. We all read and approved the manuscript.
Footnotes
Published ahead of print 18 August 2014
REFERENCES
- 1.UNAIDS. 2012. UNAIDS report on the global AIDS epidemic: 2012. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 2.Barnhart KT, Izquierdo A, Pretorius ES, Shera DM, Shabbout M, Shaunik A. 2006. Baseline dimensions of the human vagina. Hum. Reprod. 21:1618–1622. 10.1093/humrep/del022. [DOI] [PubMed] [Google Scholar]
- 3.Wira CR, Fahey JV. 2008. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS 22:1909–1917. 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, Hill ET, Fahey JV. 2011. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 4:335–342. 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuno A, Nasu K, Yuge A, Matsumoto H, Nishida M, Narahara H. 2009. Decidualization attenuates the contractility of eutopic and ectopic endometrial stromal cells: implications for hormone therapy of endometriosis. J. Clin. Endocrinol. Metab. 94:2516–2523. 10.1210/jc.2009-0207. [DOI] [PubMed] [Google Scholar]
- 6.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. 1997. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am. J. Reprod. Immunol. 38:350–359. 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174. 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, Mujugira A, Mugo N, Ndase P, Hendrix C, Celum C. 2014. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J. Acquir. Immune Defic. Syndr. 66:340–348. 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balzarini J, Hao Z, Herdewijn P, Johns DG, De Clercq E. 1991. Intracellular metabolism and mechanism of anti-retrovirus action of 9-(2-phosphonylmethoxyethyl)adenine, a potent anti-human immunodeficiency virus compound. Proc. Natl. Acad. Sci. U. S. A. 88:1499–1503. 10.1073/pnas.88.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Clercq E. 2007. Acyclic nucleoside phosphonates: past, present and future: bridging chemistry to HIV, HBV, HCV, HPV, adeno-, herpes-, and poxvirus infections: the phosphonate bridge. Biochem. Pharmacol. 73:911–922. 10.1016/j.bcp.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK. 2004. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292:191–201. 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 12.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. 2011. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J. Antimicrob. Chemother. 66:240–250. 10.1093/jac/dkq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. 2012. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS 26:F13–F19. 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 14.Microbicide Trials Network. 25 November 2011. MTN statement on decision to discontinue use of tenofovir gel in VOICE, a major HIV prevention study in women. Microbicide Trials Network, Pittsburgh, PA. [Google Scholar]
- 15.Bianchi V, Pontis E, Reichard P. 1986. Interrelations between substrate cycles and de novo synthesis of pyrimidine deoxyribonucleoside triphosphates in 3T6 cells. Proc. Natl. Acad. Sci. U. S. A. 83:986–990. 10.1073/pnas.83.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunsucker SA, Mitchell BS, Spychala J. 2005. The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol. Ther. 107:1–30. 10.1016/j.pharmthera.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Biswas N, Rodriguez-Garcia M, Crist SG, Shen Z, Bodwell JE, Fahey JV, Wira CR. 2013. Effect of tenofovir on nucleotidases and cytokines in HIV-1 target cells. PLoS One 8:e78814. 10.1371/journal.pone.0078814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Z, Fahey JV, Bodwell JE, Rodriguez-Garcia M, Rossoll RM, Crist SG, Patel MV, Wira CR. 2013. Estradiol regulation of nucleotidases in female reproductive tract epithelial cells and fibroblasts. PLoS One 8:e69854. 10.1371/journal.pone.0069854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enomoto L, Anderson PL, Li S, Edelstein CL, Weinberg A. 2011. Effect of nucleoside and nucleotide analog reverse transcriptase inhibitors on cell-mediated immune functions. AIDS Res. Hum. Retroviruses 27:47–55. 10.1089/aid.2010.0067. [DOI] [PubMed] [Google Scholar]
- 20.Melchjorsen J, Risor MW, Sogaard OS, O'Loughlin KL, Chow S, Paludan SR, Ellermann-Eriksen S, Hedley DW, Minderman H, Ostergaard L, Tolstrup M. 2011. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J. Acquir. Immune Defic. Syndr. 57:265–275. 10.1097/QAI.0b013e3182185276. [DOI] [PubMed] [Google Scholar]
- 21.Sachdeva RK, Wanchu A, Bagga R, Malla N, Sharma M. 2010. Effect of non-nucleoside reverse transcriptase inhibitors on cytokine, chemokine, and immunoglobulin profiles in serum and genital secretions of HIV-infected women. J. Interferon Cytokine Res. 30:299–310. 10.1089/jir.2009.0056. [DOI] [PubMed] [Google Scholar]
- 22.Zidek Z, Kmonickova E, Holy A. 2007. Secretion of antiretroviral chemokines by human cells cultured with acyclic nucleoside phosphonates. Eur. J. Pharmacol. 574:77–84. 10.1016/j.ejphar.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Carreno MP, Krieff C, Irinopoulou T, Kazatchkine MD, Belec L. 2002. Enhanced transcytosis of R5-tropic human immunodeficiency virus across tight monolayer of polarized human endometrial cells under pro-inflammatory conditions. Cytokine 20:289–294. 10.1006/cyto.2002.2009. [DOI] [PubMed] [Google Scholar]
- 24.Levinson P, Kaul R, Kimani J, Ngugi E, Moses S, MacDonald KS, Broliden K, Hirbod T. 2009. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 23:309–317. 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 25.Naranbhai V, Abdool Karim SS, Altfeld M, Samsunder N, Durgiah R, Sibeko S, Abdool Karim Q, Carr WH. 2012. Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J. Infect. Dis. 206:993–1001. 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Garcia M, Barr FD, Crist SG, Fahey JV, Wira CR. 23 April 2014. Phenotype and susceptibility to HIV infection of CD4 Th17 cells in the human female reproductive tract. Mucosal Immunol. 10.1038/mi.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mane VP, Heuer MA, Hillyer P, Navarro MB, Rabin RL. 2008. Systematic method for determining an ideal housekeeping gene for real-time PCR analysis. J. Biomol. Tech. 19:342–347. [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. 2009. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am. J. Reprod. Immunol. 62:60–71. 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. 2002. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut 51:818–826. 10.1136/gut.51.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schutyser E, Struyf S, Van Damme J. 2003. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 14:409–426. 10.1016/S1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 31.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038. 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. 1994. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 56:559–564. [PubMed] [Google Scholar]
- 33.Lane BR, Lore K, Bock PJ, Andersson J, Coffey MJ, Strieter RM, Markovitz DM. 2001. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J. Virol. 75:8195–8202. 10.1128/JVI.75.17.8195-8202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narimatsu R, Wolday D, Patterson BK. 2005. IL-8 increases transmission of HIV type 1 in cervical explant tissue. AIDS Res. Hum. Retroviruses 21:228–233. 10.1089/aid.2005.21.228. [DOI] [PubMed] [Google Scholar]
- 35.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. 2006. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 112:358–404. 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Garcia M, Patel MV, Wira CR. 2013. Innate and adaptive anti-HIV immune responses in the female reproductive tract. J. Reprod. Immunol. 97:74–84. 10.1016/j.jri.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]