Abstract
Twenty-three 3-nitrotriazole-based and 2-nitroimidazole-based amides and sulfonamides were screened for antitubercular (anti-TB) activity in aerobic Mycobacterium tuberculosis H37Rv by using the BacTiter-Glo (BTG) microbial cell viability assay. In general, 3-nitrotriazole-based sulfonamides demonstrated anti-TB activity, whereas 3-nitrotriazole-based amides and 2-nitroimidazole-based amides and sulfonamides were inactive. Three 3-nitrotriazole-based sulfonamides (compounds 4, 2, and 7) demonstrated 50% inhibitory concentration (IC50), IC90, and MIC values of 0.38, 0.43, and 1.56 μM (compound 4), 0.57, 0.98, and 3.13 μM (compound 2), and 0.79, 0.87, and 3.13 μM (compound 7), respectively. For 3-nitrotriazole-based sulfonamides, anti-TB activity increased with lipophilicity, whereas the one-electron reduction potential (E1/2) did not play a role. 2-Nitroimidazole-based analogs, which were inactive in the BTG assay, were significantly more active in the low-oxygen assay and more active than the 3-nitrotriazoles. All active nitrotriazoles in the BTG assay were similarly active or more potent (lower MIC values) against resistant strains, with the exception of compounds 2, 3, 4, and 8, which demonstrated greater MIC values against isoniazid-resistant strains. Five 3-nitrotriazole-based sulfonamides demonstrated activity in infected murine J774 macrophages, causing log reductions similar to those seen with rifampin. However, some compounds caused toxicity in uninfected macrophages. In conclusion, the classes of 3-nitrotriazole-based amides and sulfonamides merit further investigation as potential antitubercular agents.
INTRODUCTION
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, is the second leading cause of death worldwide. According to the World Health Organization (WHO), 8.6 million people developed TB and 1.3 million died from the disease in 2012 (1). Infection by M. tuberculosis represents an escalating threat for global health due to potential coinfection with HIV and the increasing prevalence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB. In addition, one-third of the world's population is infected with latent, nonproliferating persistent (NRP) M. tuberculosis and has a 10% lifetime risk of developing active TB (2). NRP TB fosters tolerance to many drugs and may explain the long treatment time required to eliminate infection (3).
The WHO recently called for the implementation of directly observed treatment short courses (DOTS), in which treatment compliance is monitored by health care workers. However, this approach risks failure because it requires at least 6 months of treatment. Thus, the development of novel drugs, especially drugs that target NRP M. tuberculosis, as well as the development of improved combination regimens could result in shortening the length and cost of treatment, increased patient compliance, and prevention or slowed emergence of drug resistance.
Nitroimidazoles are one of the most promising classes of new antitubercular drugs for clinical development against NRP M. tuberculosis, with at least two candidates in clinical trials: OPC-67683 (Otsuka Pharmaceutical Co. Ltd.), a 5-nitroimidazooxazole, and PA-824 (PathoGenesis; now Novartis), a 5-nitroimidazooxazine (4, 5). Both compounds exhibit early bactericidal activity in patients (6, 7). Metronidazole, a 5-nitroimidazole antibiotic used to treat anaerobic bacterial infections, was one of the first compounds to show selective bactericidal activity against NRP M. tuberculosis, whereas no activity was seen in aerobic, actively proliferating bacilli (8, 9). Latent bacilli subsist in a state of low metabolic activity within granulomatous lesions formed in the infected lungs; these lesions are highly hypoxic (10). Thus, metronidazole, albeit a drug with moderate activity, kills anaerobic bacilli due to a hypoxia-activated mechanism (11). This is the rationale for using nitroheterocyclic compounds (12) as well as other bioreductive prodrugs (13, 14) known to be activated under hypoxia as drugs against latent TB.
We previously showed that the 2-nitroimidazole-linked chloroquinoline NLCQ-1 (15) and its analog, NLCQ-2, have significant and selective in vitro bactericidal activities against dormant M. tuberculosis (16). The activities were comparable to that of the nitroimidazopyran PA-824. Both NLCQ-1 and NLCQ-2 were originally developed as weak DNA-intercalating bioreductive prodrugs to target solid tumors upon hypoxic activation by reductive enzymes (15, 17).
In the present study, we investigated 3-nitrotriazole-based amides and sulfonamides as potential antitubercular agents, in the hope that such compounds could potentially target both replicating and NRP M. tuberculosis; 2-nitroimidazole analogs were tested in parallel for comparison purposes. 3-Nitrotriazole-based amides, sulfonamides, amines, and piperazines have demonstrated excellent antitrypanosomal activities in vitro and in vivo (18–21). We have shown that the activities of these compounds against Trypasoma cruzi (the causative parasite of Chagas disease) and Trypanasoma brucei brucei (one of the causative parasites of human African trypanosomiasis) are mediated via 2-electron reduction of the compounds by oxygen-insensitive type I nitroreductase (NTR), which is specific to the trypanosomatids (18, 19, 21). Since trypanosomatids are not hypoxic and 2-electron-mediated reductions are associated with aerobic conditions, the activation of nitrotriazoles, at least in these parasites, occurs under aerobic conditions. Therefore, it is plausible that a similar activation mechanism of 3-nitrotriazole-based compounds occurs in replicating M. tuberculosis.
MATERIALS AND METHODS
Tested compounds.
The compounds were synthesized in our laboratory as has been described elsewhere (18). Stock solutions of each compound in dimethyl sulfoxide (DMSO; 10 mM) were prepared and checked by liquid chromatography-mass spectrometry/UV analysis (LC-MS/UV) for purity, at 254 nm. Compound solubility in assay media was determined by using a Nephelostar Galaxy microtiter plate reader. A reading of >29,000 was selected to identify compounds that precipitated in media at the highest tested concentration of 100 μM.
Amikacin sulfate (Sigma) at 13.1 mM in sterile water was used as the positive control. Six known inhibitors of M. tuberculosis (isoniazid [INH], rifampin [RMP], pyrimethamine [PMA], ethambutol [ETB], and cycloserine [CSR]) were tested in parallel as additional references.
Bacterial strains, growth conditions, and media.
M. tuberculosis H37Rv (ATCC 27294; ATCC, Manassas, VA) was used for the initial screening. Strains used in secondary screenings included M. tuberculosis H37Rv (SRI 1345), INH-resistant M. tuberculosis (SRI 1369), RMP-resistant M. tuberculosis (SRI 1367), and ofloxacin (OFX)-resistant M. tuberculosis (SRI 4000). Minimal bactericidal concentration (MBC) determinations, low-oxygen recovery assays (LORAs), and intracellular drug screening assays were conducted using only M. tuberculosis H37Rv (SRI 1345).
Permanent frozen (−80°C) stocks of H37Rv were prepared in Middlebrook 7H9 broth (Becton Dickinson) supplemented with 0.2% glycerol (Becton Dickinson), 0.05% Tween 80 (Becton Dickinson), and 10% ADC enrichment (albumin, dextrose, catalase; Becton Dickinson). A contamination check on the thawed cultures was performed with Trypticase soy agar plates and incubation for 3 to 4 days.
All manipulations of M. tuberculosis bacteria were conducted in accordance with the Biosafety in Microbiological and Biomedical Laboratories 5th edition, in biosafety level 3 (BSL3) containment laboratories.
Mycobacterium tuberculosis assay.
For the M. tuberculosis high-throughput screening (HTS) assay, black, clear-bottom, 384-well microtiter plates and 7H12 broth were used. Compounds stocks in DMSO were diluted in assay medium (1% final DMSO concentration) to create 10 serial dilutions to a 2× final test compound concentration, and 25-μl aliquots of these diluted compounds were transferred to 384-well plates. Amikacin was included at 0.17 μM (its approximate MIC) as an indicator of proper assay performance and at 3.20 μM, a concentration which completely inhibits growth and was used as background, to calculate percent inhibition by the test compounds for each plate. Plates containing test compounds, controls, and references were transferred into the BSL3 facility for bacteria addition and incubation. The bacterial stock was diluted to 1 × 105 to 2 × 105 CFU/ml in Middlebrook 7H12 broth supplemented with 0.1% Casitone, 5.6 μg/ml palmitate, 0.5% bovine serum albumin, and 4 μg/ml catalase, and 25 μl was plated over the compounds by using a Thermo Scientific Matrix WellMate. Historical 50% inhibitory concentration (IC50) and IC90 values for the amikacin control were 0.102 ± 0.07 μM and 0.141 ± 0.08 μM (means ± standard deviations; n = 366), respectively. Plates were incubated for 7 days at 37°C with approximately 95% humidity. Autofluorescence of test compounds was determined by prereading the high-dose plate for fluorescence using a PerkinElmer Envision plate reader at 535-nm excitation and 590-nm emission.
After incubation, Promega BacTiter-Glo (BTG) microbial cell viability reagent (25 μl) was added, the plates were incubated for an additional 20 min at room temperature and sealed with PerkinElmer clear TopSeal A, and luminescence was read from the top using a PerkinElmer Envision plate reader. The BTG reagent lyses the bacteria for ATP quantification based on luciferase production as the endpoint (22).
Data were analyzed using the IDBS Activity Base software. Results for the individual wells were expressed as the percent inhibition, which was calculated from the median cell counts in the wells, as follows: {[median cells in control wells − median cells in high-dose amikacin wells) − (median cells in test wells − median cells in high-dose amikacin wells)]/(median cells in control wells − median cells in high-dose amikacin wells)} × 100.
Dose-response curves were generated by using a four-parameter logistic fit (Excel Fit, equation 205) with the maximum and minimum locked at 100 and 0. From these curves, MIC, IC90, and IC50 values were calculated. Compounds with activities outside the tested ranges were reported as either less than the minimum concentration or greater than the maximum concentration, as appropriate.
Secondary assays.
Subsequent to initial dose-response testing, active compounds were subjected to secondary assays for evaluation of antimycobacterial activity in a low-throughput format. These assays included determinations of MICs in sensitive and resistant strains (INH-resistant, RMP-resistant, and OFX-resistant M. tuberculosis), MBC determinations, LORA, intracellular (macrophage) drug screening, and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) cell proliferation tests. Listed below are abbreviated summaries of each assay.
MIC determinations.
The broth microdilution assay format, following the guidelines established by the Clinical and Laboratory Standards Institute, was routinely utilized for MIC testing. Briefly, testing was conducted using 96-well, U-bottom microplates with an assay volume of 0.2 ml/well. First, the test medium, Middlebrook 7H9 broth supplemented with oleic acid-albumin-dextrose-catalase enrichment (BD BioSciences, Sparks, MD), was added (0.1 ml/well) to each well. The test compounds were added (0.1 ml/well) to appropriate wells at twice the intended starting concentration and serially diluted 2-fold across the plate. The plates were then inoculated with a targeted concentration of 1.0 × 106 CFU/ml M. tuberculosis and incubated at 37°C for 7 days in approximately 90% humidity. Following incubation, the plates were read visually, and individual wells scored for turbidity, partial clearing, or complete clearing. Testing was conducted in duplicate, and the following controls were included in each test plate: (i) medium only (sterility control); (ii) organism in medium (negative control); (iii) rifampin or isoniazid (positive control). The reported MICs are the lowest concentrations of drug that visually inhibited growth of the organism.
MBC.
The MBC was determined subsequent to MIC testing by subculturing diluted aliquots from wells that failed to exhibit macroscopic growth. The sample aliquots were inoculated onto Middlebrook 7H10 agar plates and subsequently incubated for 16 to 21 days at 37°C. Once growth was readily apparent, the bacterial colonies were enumerated. The MBC was defined as the lowest concentration of compound that exhibited 99.9% killing. MBC values greater than 16× the MIC typically indicate antimicrobial tolerance.
LORA.
For NRP M. tuberculosis, the LORA was used (23). For measurements carried out at the UIC, M. tuberculosis H37Rv containing a plasmid with an acetamidase promoter driving a bacterial luciferase gene was adapted to low-oxygen conditions. Briefly, microplates were prepared in the same manner as for the MIC testing format. The microplate cultures were placed under anaerobic conditions (oxygen concentration less than 0.16%), incubated at 37°C for 10 days, and then transferred to an ambient gaseous condition (5% CO2-enriched air) incubator for a 28-h “recovery.” The numbers of CFU (determined by subculture onto Middlebrook 7H11 agar) during the 10-day incubation did not increase and remained essentially unchanged. After the 28-h aerobic recovery, 100 μl of culture was transferred to white 96-well microtiter plates for determination of luminescence. For measurements not conducted at the UIC, the plates were placed under anaerobic conditions in a MACS MIC automated jar gassing system and incubated for 7 days at 37°C. The plates were subsequently transferred to an ambient gaseous condition (5% CO2) for 7 days, after which an absorbance reading was taken via an Envision plate reader. Testing was conducted in duplicate in all cases, and average values were recorded.
Intracellular drug activity.
Briefly, the murine J774 cell line was propagated in RPMI 1640 supplemented with l-glutamine and fetal bovine serum (FBS) at 37°C in the presence of 5% CO2. For infection studies, J774 cells were transferred to 12-well tissue culture plates in 1-ml volumes at a density of 2.0 × 105 in the presence of 10% FBS. After overnight incubation, the medium was replaced with fresh medium containing 1% FBS to stop macrophage division while maintaining cell viability. Twenty-four hours later, the macrophage monolayer was enumerated for the total number of cells per well to determine the infection ratio. The medium was replaced with 1 ml of fresh medium with 1% FBS containing M. tuberculosis at a multiplicity of infection (MOI) of 5 mycobacteria/macrophage. The cells were infected for 4 h, after which time nonphagocytosed mycobacteria were washed from the monolayers and fresh medium was added. Drugs were then added, using 3 concentrations of each, and infection was allowed to proceed for 7 days. At 0 and 7 days, the macrophages were lysed with sodium dodecyl sulfate, treated with DNase, diluted, and plated onto 7H10 agar to determine the cell numbers or CFU. Each drug concentration was tested in duplicate, and rifampin was used as the positive control. A drug cytotoxicity control plate assay (MTT proliferation) was also conducted in parallel with uninfected macrophages.
One-electron reduction E1/2 determination.
Half-wave potential values (E1/2) for 3-nitrotriazoles were determined by using cyclic voltammetry in acetonitrile. The E1/2 value provides information about the ease of the one-electron reversible reduction of these compounds according to the reaction shown in Fig. 1.
FIG 1.
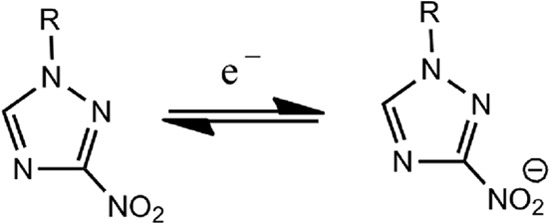
Chemical reaction.
All measurements were performed in a jacketed, one-compartment cell, using an Au working electrode, Pt counter electrode, and Ag pseudoreference electrode with 25°C water running through the cell's outer jacket via a circulating water bath. The electrolyte solution was 0.10 M TBAPF6 in acetonitrile. After a blank cyclic voltammogram (CV) was taken, ferrocene (Fc) was added to act as an internal potential reference. In most measurements, 5 μl of a 100 mM stock solution of the triazole compound in acetonitrile was added to the electrolyte solution. All CVs were recorded at 0.1 V/s. The E1/2 values were determined in V and relative to Fc.
RESULTS
Antitubercular activity data from the initial screening.
The chemical structures and the results from the initial screening of all tested compounds against H37Rv M. tuberculosis, by using the BTG assay, are shown in Table 1. From 10 3-nitrotriazole-based sulfonamides (compounds 1 to 10), seven (compounds 1 to 5, 7, and 8) were active against rapidly proliferating (RP) M. tuberculosis, with IC50s of 0.38 to 11.84 μM and IC90 values of 0.43 to 13.59 μM (0.17 to 4.94 μg/ml). MIC values were approximated, since they were determined as the first actual point in the plateau phase of the dose-response growth curves and were not calculated from the fitted curves (Fig. 2). Therefore, in most cases, the reported MIC values are greater than the actual ones and the IC90 values more accurately reflect the antitubercular potencies of the compounds. Among the 3-nitrotriazole-based sulfonamides, chlorothiophenesulfonamide compounds 2 and 4 and the trifluoromethoxy-phenylsulfonamide compound 7 demonstrated the greatest potency as antitubercular agents, with IC50 and IC90 values at nM concentrations and MIC values at low μM (<4 μM) concentrations (Table 1). It was noticeable that all of them had a 4-methylene linker between the 3-nitrotriazole ring and the sulfonamide group. The 3-nitrotriazole-based sulfonamides, compounds 6, 9, and 10, that did not show activity against RP M. tuberculosis had lower clogP values (low lipophilicity; clogP is the log of the ratio of the concentrations of an unionized solute in octanol versus water, i.e., the log of the partition coefficient, and is a measure of lipophilicity) than the active ones. Therefore, lipophilicity seems to play an important role in the antitubercular activity, and this was more apparent when we compared pairs of similar compounds, such as 1 and 2, 3 and 4, or 3 and 5. Thus, in the first two cases, the more lipophilic compound demonstrated a lower IC90 value, whereas in the last case compounds 3 and 5, with the same lipohilicity, demonstrated similar anti-TB activity. No correlation existed between activity and redox potential (E1/2) in this subgroup of compounds (e.g., compound 4 versus compound 10). When we compared the active 3-nitotriazole-based sulfonamides to reference compounds tested in parallel, we observed that compounds 2, 4, and 7 were more potent antitubercular agents than CSR, ETB, and PMA but less potent than amikacin, INH, and RMP (Table 1).
TABLE 1.
Antitubercular activity of tested compounds against H37Rv M. tuberculosis (BTG assay) and their physical propertiesa
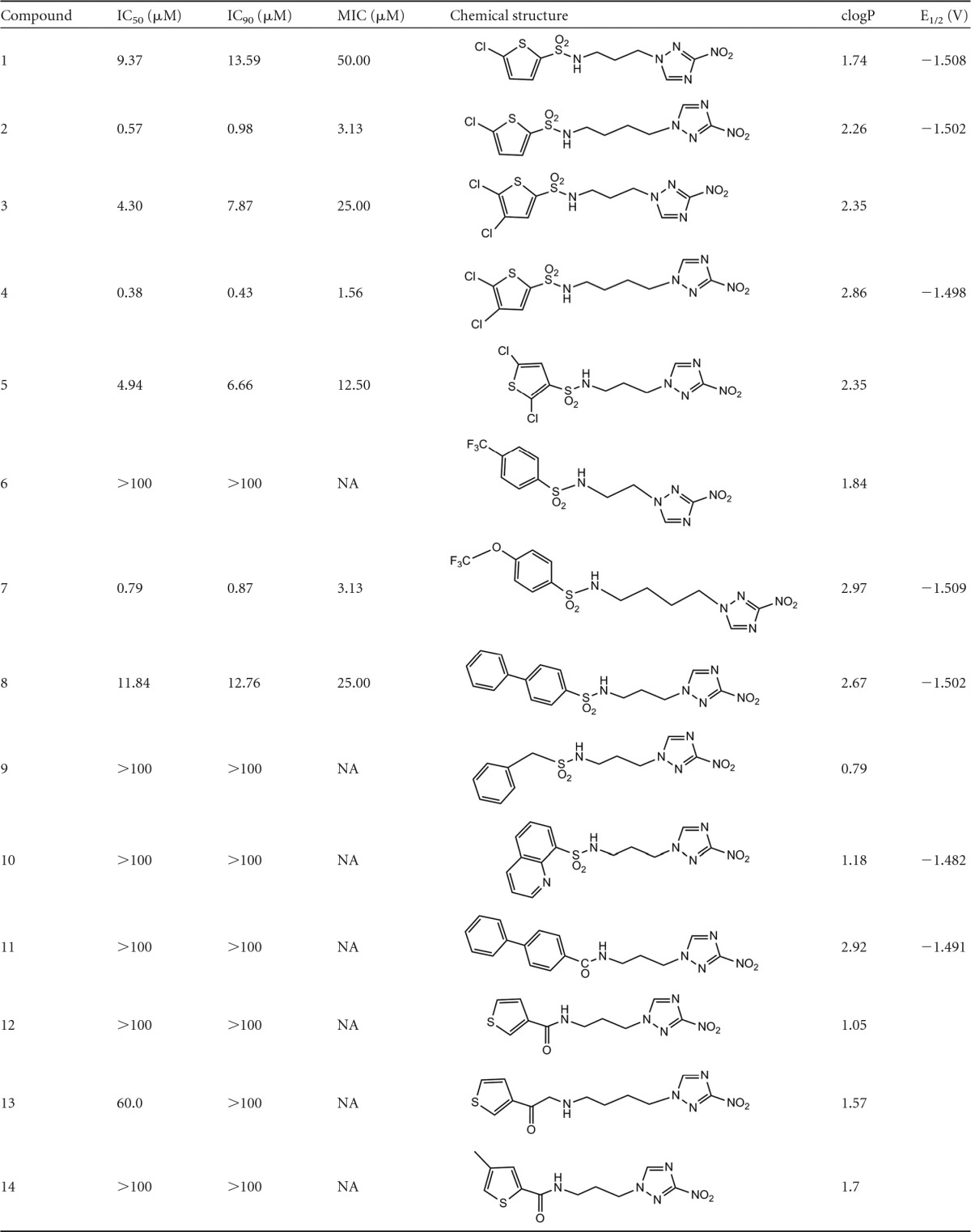
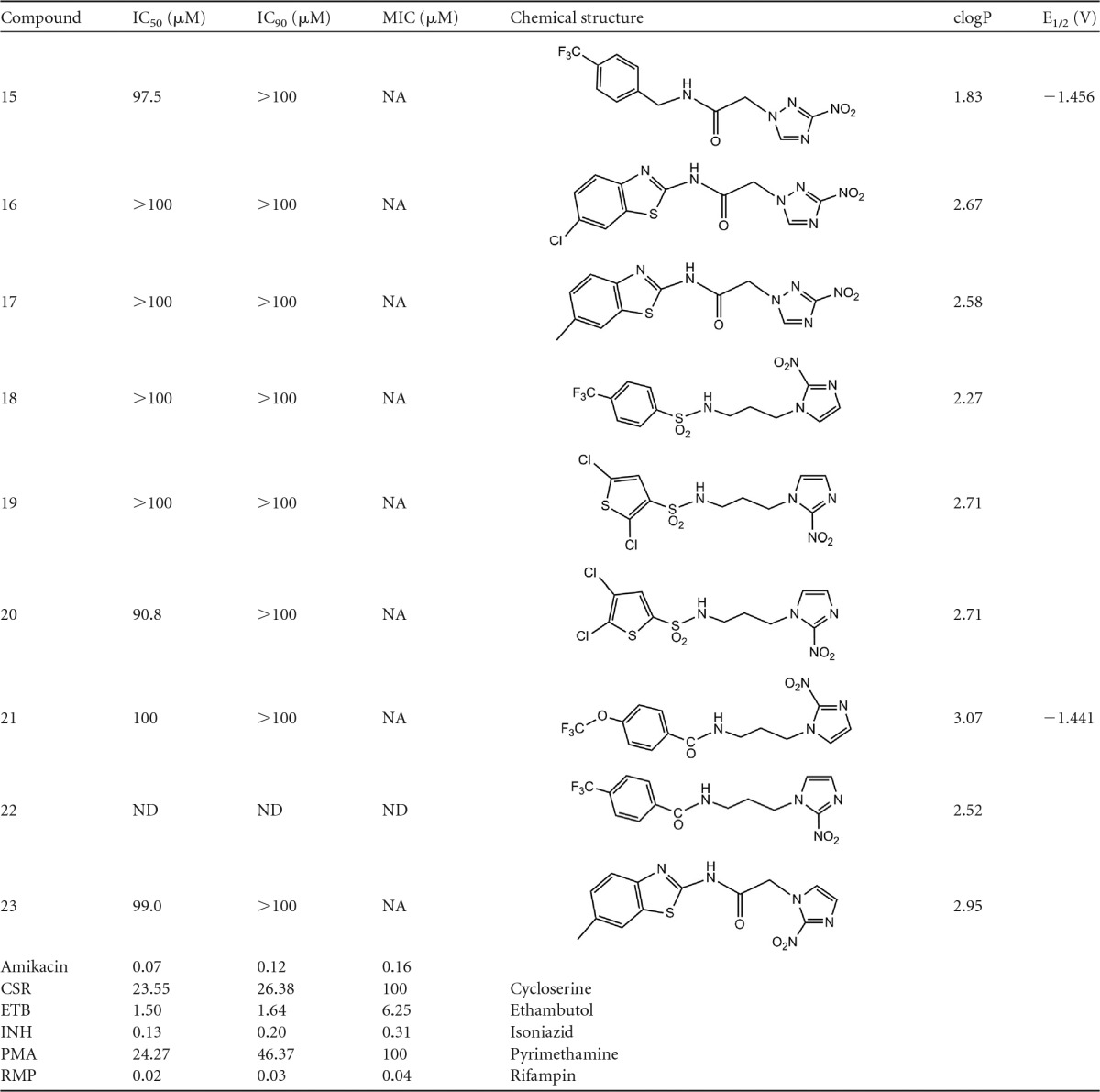
NA, not applicable; ND, not determined. clogP values were predicted by using the Marvin calculator (Chemaxon).
FIG 2.
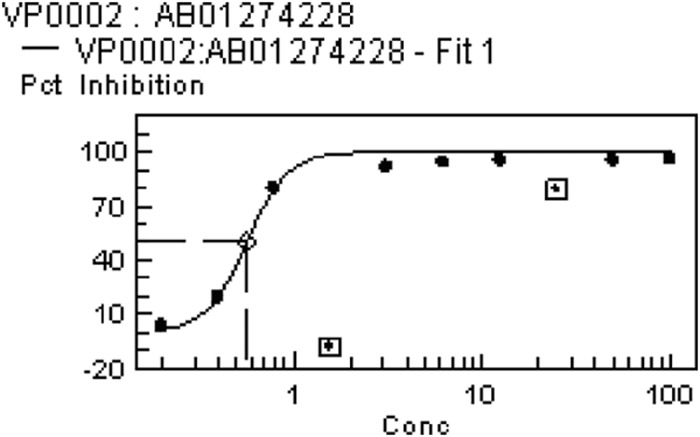
Representative plot of data obtained in the BTG assay for compound 2. MIC values from the BTG assay were not calculated from the fitted curve but represent the first point in the plateau phase.
3-Nitrotriazole-based amides 11 to 17, as well as 2-nitroimidazole-based sulfonamides and amides 18 to 23, did not demonstrate activity against RP M. tuberculosis, since their IC90 values were >100 μM.
Antitubercular activity against NRP M. tuberculosis (LORA).
Active compounds and several inactive compounds in the initial screening against RP M. tuberculosis (Table 1) were then tested against NRP M. tuberculosis by using the low-oxygen assay (23). The data of this secondary screening are shown in Table 2. Data from Table 1 are included for comparison purposes. Several compounds, indicated with an asterisk, were screened in the Institute for TB Research at the UIC.
TABLE 2.
Comparison of antitubercular activities of selected compounds against M. tuberculosis via aerobic (BTG) or anaerobic (LORA) testa
| Compound ID | IC90 (μM) BTG | MIC (μM) based on: |
|
|---|---|---|---|
| BTG | LORA | ||
| 2 | 0.98 | 3.13 | 68.40 |
| 3 | 7.87 | 25.00 | 64.76 |
| 4 | 0.43 | 1.56 | 82.25* |
| 7 | 0.87 | 3.13 | 140.83* |
| 8 | 12.76 | 25.00 | 64.60 |
| 19 | >100 | NA | 41.82* |
| 20 | >100 | NA | 27.14* |
| 21 | >100 | NA | 30.17* |
| 22 | ND | ND | 18.13* |
| 23 | >100 | NA | 25.24* |
| RMP | 0.03 | 0.04 | 1.90 |
| INH | 0.2 | 0.31 | >934* |
| PA-824 | 1.34* | ||
Values are averages of duplicate measurements. NA, not applicable; ND, not determined. *, the compound was tested at the Institute of TB Research at the UIC.
Active compounds in the BTG assay were considerably less active in the LORA. Thus, the most active compounds, 2, 4, and 7, had LORA MIC values ca. 70-, 191-, and 162-fold greater, respectively, than their IC90 values in the BTG assay (Table 2). In general, all 3-nitrotriazole-based compounds tested in the LORA had LORA MIC values 2.6- to 53-fold greater than their BTG MIC values (Table 2). To the contrary, all 2-nitroimidazole-based compounds in Table 2 (compounds 19 to 23) demonstrated lower LORA MIC values (<50 μM; 6.2 to 16.1 μg/ml) than their IC90 values in the BTG assay. Therefore, 2-nitroimidazole-based compounds seem to be more active against NRP than RP M. tuberculosis. However, none of these compounds (19 to 23) was more potent than RMP or PA-824, but they were significantly more efficacious (>22- to 51-fold) than INH (Table 2).
Antitubercular activity against drug-resistant M. tuberculosis strains.
Five 3-nitrotriazole-based compounds with activity against RP M. tuberculosis (compounds 2, 3, 4, 7, and 8) and one 2-nitroimidazole-based amide with activity in the LORA (compound 21) were subjected to secondary testing against sensitive and drug-resistant M. tuberculosis strains, as shown in Table 3. There are some variations in the IC50 and MIC values in Tables 1 and 3 due to the different methods applied (see Materials and Methods) and variations of H37Rv strains among labs (24). The MIC values in Table 3 do not always represent the concentration required for 100% growth inhibition, since the plateau in the growth curves did not always reach this value. Therefore, the greatest growth inhibition obtained in each case is provided in Table 3.
TABLE 3.
Antitubercular activity of selected compounds against sensitive and resistant M. tuberculosis strains
| Compound | Inhibitory concn (μM) of compound in M. tuberculosis straina |
|||||||
|---|---|---|---|---|---|---|---|---|
| H37RV |
INH resistant |
RPM resistant |
OFX resistant |
|||||
| IC50 | MIC (% inhibition) | IC50 | MIC (% inhibition) | IC50 | MIC (% inhibition) | IC50 | MIC (% inhibition) | |
| 2 | 1.59 | 8.44 (85) | 1.53 | 17.1 (85) | 0.85 | 8.28 (97) | ≪0.85 | 4.57 (92) |
| 3 | 3.68 | 16.19 (97) | 20.44 | 32.4 (62) | 3.73 | 16.11 (94) | 3.24 | 7.98 (82) |
| 4 | 0.34 | 1.95 (102) | 1.30 | 3.9 (72) | 0.325 | 1.95 (100) | 0.275 | 1.95 (98) |
| 7 | 1.64 | 7.53 (83) | 1.22 | 3.67 (81) | 1.69 | 7.41 (93) | 0.95 | 8.14 (86) |
| 8 | 3.15 | 8.32 (100) | 26.69 | 64.6 (77) | 3.31 | 8.01 (97) | 4.32 | 8.01 (90.4) |
| 21 | 71.66 | 558.7 (84) | 31.67 | 558.7 (80) | 31.25 | 279.3 (85) | 45.0 | 279.3 (77) |
| RMP | 0.06 (81) | 0.06 (67) | NA | 0.237 (75) | ||||
| INH | NA | NA | 0.146 (86) | NA | ||||
All values are averages of duplicate measurements. The INH resistance level relative to that of the wild-type strains was 100%, 83%, and 17% at 1.46 μM, 7.30 μM, and 36.5 μM, respectively. NA, not applicable.
RMP- and OFX-resistant strains did not develop cross-resistance to the 3-nitrotriazole-based chlorothiophene-2-sulfonamides compounds 2, 3, and 4. In fact, RMP-resistant M. tuberculosis was ca. 2-fold more sensitive to compound 2 than H37Rv bacilli (IC50, 0.85 versus 1.59 μM), and the MIC value represents 97% inhibition of growth compared to 85% for H37Rv. Similarly, the OFX-resistant strain was >2-fold more sensitive to compound 2 than the H37Rv bacilli, based on both IC50 and MIC values (Table 3).
RMP- and OFX-resistant strains did not develop cross-resistance to the 3-nitrotriazole-based arylsulfonamides 7 and 8, based on similar IC50 and MIC values and the percent growth inhibition observed (Table 3). Interestingly, RMP- and OFX-resistant strains were ca. 2-fold more sensitive to the 2-nitroimidazole-based arylamide 21 (compare IC50 and MIC values in Table 3). So, in general, RMP- and OFX-resistant strains were similarly sensitive or more sensitive than H37Rv strains for all compounds tested. The OFX-resistant strain was about 4-fold less sensitive to RMP than the H37Rv strain, implying that the tested compounds have a different mechanism of action than that of RMP (Table 3).
With regard to the INH-resistant strain, however, we saw some differences among the compounds tested. Based on the MIC values and percent inhibition at the MIC, this strain was 2-fold less sensitive than H37Rv for compounds 2, 3, and 4, about 2-fold more sensitive for compound 7, about 8-fold less sensitive for compound 8, and similarly sensitive for compound 21, although for the latter the strain appeared to be 2-fold more sensitive than H37Rv based on the IC50s (Table 3).
Activity and toxicity in infected macrophages.
The same compounds tested for activity against resistant strains (Table 3) were also tested for intracellular activity in infected J774 macrophages at three 10-fold-different concentrations based on their MIC values. A log reduction in the burden of the intracellular bacilli was observed for all tested compounds (Table 4). Interestingly, all 3-nitrotriazoles except for compound 7 produced a >1-log reduction (>90% inhibition) at the lowest concentration, which was lower than the MIC value in Table 3. However, no dose-response log reduction was observed for any of the tested compounds, except for the reference compound RMP (Table 4). The best log reduction achieved (similar to that of RMP at the highest concentration tested, 12.2 μM) was for the 3-nitrotriazole-based chlorothiophene-2-sulfonamide, compound 2. Thus, a reduction of 1.72 logs was observed (>97% inhibition) at 2.74 μM of compound 2, a concentration close to its MIC value, as reported in Table 1.
TABLE 4.
Antitubercular activity of selected compounds in infected macrophages and toxicity of compounds for J774 macrophagesa
| Compound | Log reduction (concn of compound [μM])b | % viability (concn of compound [μM]) | SIc (IC50/IC90) | ||
|---|---|---|---|---|---|
| 2 | 1.72 (2.74) | 1.78 (27.4) | 1.75 (274) | 32 (274) | 180 |
| 3 | 1.30 (1.94) | 1.31 (19.4) | 0.94 (194) | 24 (194) | 13 |
| 4 | 1.18 (1.88) | 1.16 (18.8) | 1.45 (188) | 91 (188) | 795 |
| 7 | 0.69 (2.44) | 1.03 (24.4) | 1.00 (244) | 19 (244) | 129 |
| 8 | 1.36 (1.94) | 1.55 (19.4) | 1.56 (194) | 70 (194) | 21 |
| 21 | 0.36 (2.79) | 0.31 (27.9) | 0.24 (279) | 38 (279) | 9 |
| RMP | 0.76 (0.122) | 1.11 (1.22) | 2.0 (12.2) | 86 (12.2) | 694 |
The viability of J774 cells was <50% for compounds 2, 3, 7, and 21 at their highest tested concentrations (Table 4), and this might explain why we did not see a dose-response log reduction in bacilli. Compounds 4 and 8 were not toxic in J774 cells at the highest concentration tested, providing 91 and 70% viability at 188 and 194 μM, respectively. When we estimated the IC50s of the compounds in macrophages and calculated a selectivity index (SI) by using the ratio of the estimated IC50 in J774 cells and the corresponding IC90 value in Table 1, we observed that compounds 2, 4, and 7 demonstrated good selectivity (Table 4). In the case of compound 21, which was moderately active against NRP M. tuberculosis, we obtained an SI of 9 by using the LORA MIC value of 30.17 μM (Table 2). It is worth mentioning that the dichlorothiophene-2-sulfonamide, comound 4, besides not being toxic to the macrophages (SI, 795), demonstrated the lowest IC50, IC90, and MIC values against RP bacilli in both primary and secondary assays (Tables 1 and 3). An SI of 694 was calculated for RMP.
Bactericidal activity.
A compound is considered to be bactericidal if the MBC/MIC ratio is ≤4 (25). None of the compounds in Table 3 satisfied this criterion (data not shown), and therefore the compounds were considered bacteriostatic.
DISCUSSION
We have shown that a new class of nitrocompounds, 3-nitro-1,2,4-triazole-based sulfonamides, forms a potential scaffold with aerobic antitubercular activity. Further improvements might provide activity against NRP bacilli as well. Since the number of active 3-nitrotriazole-based sulfonamides was limited, adequate structure-activity relationships (SARs) cannot be discussed. However, it is clear that antitubercular activity under aerobic conditions in this subgroup decreases with decreasing length of the linkage between the nitrotriazole ring and the sulfamido group. Thus, a 10- and 100-fold reduction occurred in the IC90 value, respectively, when we moved from a 2-methylene to a 3-methylene and 4-methylene linker, respectively (compare compound 6 to compounds 1, 3, 5, and 8 and to compounds 2, 4, and 7, respectively). A second chlorine in the thiophene ring slightly improved aerobic antitubercular activity (compare compounds 3 and 5 to 1 and compare compound 4 to 2). As mentioned earlier, this improvement in activity could be related to increased lipophilicity (Table 1). The position of the sulfamido group in the thiophene ring does not seem to play a significant role in the antitubercular activity (compare compounds 5 and 3); however, this is not conclusive, since both of these compounds share the same clogP value (Table 1).
Several 2-nitroimidazole-based amides and sulfonamides demonstrated moderate but selective activity against NRP M. tuberculosis, and their anaerobic MIC values were better than that of metronidazole (MIC = 62.5 μM) (26). The LORA MIC values of all active 2-nitroimidazole-based amides or sulfonamides were in the same concentration range; therefore, no extensive SARs can be discussed (Table 2). It is noticeable that compound 20, with the sulfonamide group in the 2-position of the thiophene ring, was slightly more active than its analog, compound 19 with the sulfonamide group in the 3-position, and this was not due to lipophilicity, since both analogs had the same clogP value (Tables 1 and 2). The p-trifluoromethoxy group in the amide of compound 21 resulted in almost a 2-fold decrease in its anaerobic activity compared to analog 22, which has a p-trifluoromethyl group instead (Tables 1 and 2).
In general, “single”-drug-resistant M. tuberculosis strains were not cross-resistant to 3-nitrotriazole-based sulfonamides, which demonstrated intracellular anti-TB activity as well.
3-Nitrotriazole-based compounds are excellent substrates of the oxygen-insensitive type I NTR, which contains flavin mononucleotide (FMN) as a coenzyme and activates nitrocompounds via a series of 2-electron reductions (18, 19, 21). In M. tuberculosis bacilli, an F420-dependent nitroreductase (in Rv3547), Ddn, is responsible for the activation of PA-824 and similar nitroimidazoles (27). The F420 deazaflavin coenzyme is an obligate two-electron donor (28). Moreover, the aerobic, but not the hypoxic, anti-TB activities of PA-824 analogs were proportional to their affinities to Ddn (26). Therefore, we hypothesized that this enzymatic system is involved in the activation of 3-nitrotriazoles and their aerobic antitubercular activity.
With regard to the anaerobic activity of PA-824, it was shown that Ddn converts PA-824 into the corresponding des-nitroimidazole and reactive nitrogen species, including nitric oxide (NO), which are the major effectors of PA-824 anaerobic activity (29). Thus, PA-824 analogs may act as intracellular NO donors and could augment a killing mechanism intrinsic to the innate immune system (26).
NLCQ-1, which is activated under hypoxia by cytochrome P450 reductase (17), is also activated by inducible nitric oxide synthase (iNOS) (R. Cowen, unpublished data), an enzyme with structural similarities to cytochrome P450 reductase. iNOS is induced in infected macrophages and, in the presence of oxygen, converts l-arginine to l-citrulline, with the concomitant release of NO. However, this release is limited under hypoxia. Therefore, it is possible that 2-nitroimidazoles, like the ones presented, could be activated in macrophages by iNOS to toxic metabolites that kill M. tuberculosis, especially in hypoxic granulomas (10). In the present study, no intracellular activity was demonstrated by compound 21, the only 2-nitroimidazole studied in macrophages; however, the in vitro studies do not resemble the hypoxic environment of the in vivo situation.
Ddn does not activate all nitroimidazoles. Thus, the 5-nitroimidazole metronidazole, which demonstrates moderate activity against NRP M. tuberculosis, is not a substrate for Ddn (26). In addition, a series of antitubercular and structurally similar nitrofurans appear to be reduced via a nitroreductase different from Ddn (30). Therefore, another oxygen-sensitive nitroreductase might be responsible for the reduction and activity against NRP bacilli, as observed with the 2-nitroimidazoles studied. Recently, it was found that Rv2032 (a member of the Acg family) is one of the most induced genes in the hypoxic model of tuberculosis dormancy (31). The product of Rv2032 is a nitroreductase and could be responsible for the activation of nitrocompounds under hypoxic conditions.
In conclusion, two 3-nitro-1,2,4-triazole-based chlorothiophenesulfonamides, compounds 2 and 4, have been identified to have significant aerobic anti-M. tuberculosis activity, including activity in infected macrophages, and minimal toxicity. Moreover, compound 2 demonstrates good metabolic stability and Caco-2 permeability (unpublished data), similar to compound 1 (21). Recently, we demonstrated that compound 4 completely prevents the formation of biofilm at 10 μM, the lowest tested concentration (unpublished data). Therefore, in vivo evaluations of compounds 2 and 4, as well as investigation of their mechanisms of action, are warranted.
ACKNOWLEDGMENTS
Antimycobacterial data were provided by NIH NIAID through research and development contracts (contract number HHSN272201100012I). The work was also supported by internal funding from the Radiation Medicine Department of NorthShore University HealthSystem.
Footnotes
Published ahead of print 2 September 2014
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis report 2013. WHO, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/gtbr13_executive_summary.pdf?ua=1. [Google Scholar]
- 2.Dye C, Lonnroth K, Jaramillo E, Williams BG, Raviglione M. 2009. Trends in tuberculosis incidence and their determinants in 134 countries. Bull. World Health Organ. 87:683–691. 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boshoff HI, Barry CE., III 2005. Tuberculosis: metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 3:70–80. 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 4.National Library of Medicine. 2012. Safety and pharmacokinetics (PK) in multidrug-resistant (MDR) refractive tuberculosis. National Library of Medicine, National Institutes of Health, Bethesda, MD: http://clinicaltrials.gov/ct2/show/NCT01131351. [Google Scholar]
- 5.National Library of Medicine. 2014. PA-824-CL-007: phase IIa evaluation of early bactericidal activity in pulmonary tuberculosis. National Library of Medicine, National Institutes of Health, Bethesda, MD: http://clinicaltrials.gov/ct2/show/NCT00567840. [Google Scholar]
- 6.Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, Geiter LJ, Wells CD, Paccaly AJ, Donald PR. 2011. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int. J. Tuberc. Lung Dis. 15:949–954. 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 7.Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, Donald PR, van Niekerk C, Whitney K, Rouse DJ, Laurenzi MW, Ginsberg AM, Spigelman MK. 2010. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob. Agents Chemother. 54:3402–3407. 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wayne LG, Sramek HA. 1994. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 38:2054–2058. 10.1128/AAC.38.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., III 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76:2333–2340. 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barry CE, III, Boshoff HI, Dowd CS. 2004. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr. Pharm. Des. 10:3239–3262. 10.2174/1381612043383214. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee T, Boshoff H. 2011. Nitroimidazoles for the treatment of TB: past, present and future. Future Med. Chem. 3:1427–1454. 10.4155/fmc.11.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chopra S, Koolpe GA, Tambo-ong AA, Matsuyama KN, Ryan KJ, Tran TB, Doppalapudi RS, Riccio ES, Iyer LV, Green CE, Wan B, Franzblau SG, Madrid PB. 2012. Discovery and optimization of benzotriazine di-N-oxides targeting replicating and nonreplicating Mycobacterium tuberculosis. J. Med. Chem. 55:6047–6060. 10.1021/jm300123s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vicente E, Villar R, Burguete A, Solano B, Pérez-Silanes S, Aldana I, Maddry JA, Lenaerts AJ, Franzblau SG, Cho S, Monge A, Goldman RC. 2008. Efficacy of quinoxaline-2-carboxylate 1,4-di-N-oxide derivatives in experimental tuberculosis. Antimicrob. Agents Chemother. 52:3321–3326. 10.1128/AAC.00379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadopoulou MV, Bloomer WD. 2003. NLCQ-1 (NSC 709257): exploiting hypoxia with a weak DNA-intercalating bioreductive drug. Clin. Cancer Res. 9:5714–5720. [PubMed] [Google Scholar]
- 16.Papadopoulou MV, Bloomer WD, McNeil MR. 2007. NLCQ-1 and NLCQ-2, two new agents with activity against dormant Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 29:724–727. 10.1016/j.ijantimicag.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulou MV, Ji M, Rao MK, Bloomer WD. 2003. Reductive metabolism of the nitroimidazole-based hypoxia-selective cytotoxin NLCQ-1 (NSC 709257). Oncol. Res. 14:21–29. 10.3727/000000003108748577. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulou MV, Bloomer W, Rosenzweig HS, Chatelain E, Kaiser M, Wilkinson S, McKenzie C, Ioset JR. 2012. Novel 3-nitro-1H-1,2,4-triazole-based amides and sulfonamides as potential anti-trypanosomal agents. J. Med. Chem. 55:5554–5565. 10.1021/jm300508n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadopoulou MV, Bourdin-Trunz B, Bloomer WD, McKenzie C, Wilkinson SR, Prasittichai C, Brun R, Kaiser M, Torreele E. 2011. Novel 3-nitro-1,2,4-triazole-based aliphatic and aromatic amines as anti-Chagasic agents. J. Med. Chem. 54:8214–8223. 10.1021/jm201215n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Kaiser M, Chatelain E, Ioset J-R. 2013. Novel 3-nitro-1H-1,2,4-triazole-bearing piperazines and 2-amino-benzothiazoles as anti-Chagasic agents. Bioorg. Med. Chem. 21:6600–6607. 10.1016/j.bmc.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Ashworth R, Wilkinson SR, Kaiser M, Andriani G, Ana Rodriguez R. 2013. Novel 3-nitro-1H-1,2,4-triazole-based compounds as potential anti-Chagasic drugs: in vivo studies. Future Med. Chem. 5:1763–1776. 10.4155/fmc.13.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter DM, Lim DV. 2010. Rapid detection and identification of bacterial pathogens by using an ATP bioluminescence immunoassay. J. Food Prot. 73:739–746. [DOI] [PubMed] [Google Scholar]
- 23.Cho SH, Warit S, Wan B, Hwang CH, Pauli GF, Franzblau SG. 2007. Low-oxygen-recovery assay for high-throughput screening of compounds against nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 51:1380–1385. 10.1128/AAC.00055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ioerger TR, Feng Y, Ganesula K, Chen X, Dobos KM, Fortune S, Jacobs WR, Jr, Mizrahi V, Parish T, Rubin E, Sassetti C, Sacchettini JC. 2010. Variation among genome sequences of H37Rv strains of Mycobacterium tuberculosis from multiple laboratories. J. Bacteriol. 192:3645–3653. 10.1128/JB.00166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White EL, Suling WJ, Ross LJ, Seitz LE, Reynolds RC. 2002. 2-Alkoxycarbonylaminopyridines: inhibitors of Mycobacterium tuberculosis FtsZ. J. Antimicrob. Chemother. 50:111–114. 10.1093/jac/dkf075. [DOI] [PubMed] [Google Scholar]
- 26.Kim P, Zhang L, Manjunatha UH, Singh R, Patel S, Jiricek J, Keller TH, Boshoff HI, Barry CE, III, Dowd CS. 2009. Structure-activity relationships of antitubercular nitroimidazoles. 1. Structural features associated with aerobic and anaerobic activities of 4- and 5-nitroimidazoles. J. Med. Chem. 52:1317–1328. 10.1021/jm801246z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manjunatha UH, Boshoff H, Dowd CS, Zhang L, Albert TJ, Norton JE, Daniels L, Dick T, Pang SS, Barry CE., III 2006. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103:431–436. 10.1073/pnas.0508392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bair TB, Isabelle DW, Daniels L. 2001. Structures of coenzyme F(420) in Mycobacterium species. Arch. Microbiol. 176:37–43. 10.1007/s002030100290. [DOI] [PubMed] [Google Scholar]
- 29.Singh R, Manjunatha U, Boshoff HIM, Ha YH, Niyomrattanakit P, Ledwidge R, Dowd CS, Lee IY, Kim P, Zhang L, Kang S, Keller TH, Jiricek J, Barry CE., III 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392–1395. 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurdle JG, Lee RB, Budha NR, Carson EI, Qi J, Scherman MS, Cho SH, McNeil MR, Lenaerts AJ, Franzblau SG, Meibohm B, Lee RE. 2008. A microbiological assessment of novel nitrofuranylamides as anti-tuberculosis agents. J. Antimicrob. Chemother. 62:1037–1045. 10.1093/jac/dkn307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chauviac F-X, Bommer M, Yan J, Parkin G, Daviter T, Lowden P, Raven EL, Thalassinos K, Keep NH. 2012. Crystal structure of reduced MsAcg, a putative nitroreductase from Mycobacterium smegmatis and a close homologue of Mycobacterium tuberculosis Acg. J. Biol. Chem. 287:44372–44383. 10.1074/jbc.M112.406264. [DOI] [PMC free article] [PubMed] [Google Scholar]


