Abstract
Strains of Neisseria gonorrhoeae with mosaic penA genes bearing novel point mutations in penA have been isolated from ceftriaxone treatment failures. Such isolates exhibit significantly higher MIC values to third-generation cephalosporins. Here we report the in vitro isolation of two mutants with elevated MICs to cephalosporins. The first possesses a point mutation in the transpeptidase region of the mosaic penA gene, and the second contains an insertion mutation in pilQ.
TEXT
Increases in MICs and antibiotic resistance have been observed among clinical isolates of Neisseria gonorrhoeae and have resulted in a greater incidence of treatment failure (1–6). β-Lactam resistance results collectively from mutations in four genes—penA (7, 8), ponA (9, 10), mtrR (11–13), and the porBIb allele of the porin gene (14, 15)—and increased cephalosporin resistance requires the presence of a mosaic penA gene (16–18), a variant presumably resulting from gene transfer and recombination (19). Mutations within the mosaic penA gene can result in still greater resistance to cephalosporins (5, 6).
The penA and ponA genes of 11 gonococcal isolates with MICs to penicillin and ceftriaxone ranging from 4.0 to 8.0 μg/ml and 0.008 to 0.060 μg/ml, respectively, were sequenced using GenomeLab DTCS-Quickstart kits (Beckman Coulter, Brea, CA), with product analysis performed on a CEQ3000 instrument, and the data were analyzed using DNAStar software. Isolates with MICs to ceftriaxone ranging from 0.008 to 0.015 μg/ml demonstrated a single additional codon that coded for the insertion of an aspartic acid residue, while all four of the isolates with MICs ranging from 0.03 to 0.06 μg/ml possessed mosaic penA genes. In all of the isolates sequenced, the ponA gene contained a single point mutation (Table 1) as described elsewhere (10). The MtrR phenotype of all of the isolates was established by determining their resistance to 1.2 μg/ml of erythromycin and 600 μg/ml of Triton X-100 (12). Two isolates with nonmosaic penA genes and two with mosaic penA genes (Table 1) were chosen for further study as to their potential to mutate to greater resistance to cephalosporins. Gradient plates of GC base agar with Isovitalex (Becton, Dickinson and Company, Sparks, MD) and either 4.0 or 6.0 μg/ml of cefpodoxime in the agar substratum were seeded with 5 × 1010 CFU of each of the isolates to determine the range of concentrations over which mutants could be selected. Mutants were obtained for both of the mosaic penA strains SPL4 and SPN284 on both concentrations of cefpodoxime. No mutants were obtained for the two nonmosaic penA strains SPN275 and SPN280. Mutants derived from SPL4 and SPN284 were tested for MICs by the agar dilution method (20). The growth patterns observed on the gradient plates and the MICs obtained for the mutants (Table 2) suggested that 3.0 to 3.5 μg/ml of cefpodoxime was the concentration likely to be optimal for the selection of mutants. The actual frequencies of mutation to elevated resistance to cefpodoxime (Cpdr) were determined by seeding GC base agar containing 3.0 μg/ml of cefpodoxime with approximately 2.0 × 109 CFU as determined by viable count on plain GC base agar and were found to be 2.1 × 10−9 Cpdr/CFU for SPL4 and 1.9 × 10−9 Cpdr/CFU for SPN284. By comparison, the frequency of mutation to resistance to rifampin (30 μg/ml) was similarly determined to be 3.0 × 10−9 Rifr/CFU for both SPN284 and SPL4. The MIC values to ceftriaxone for 10 mutants derived from SPL4 were found to be 0.25 to 0.5 μg/ml. Similar results were obtained for most of the mutants derived from SPN284 (Table 2), but a single mutant was found to have an MIC to ceftriaxone of 1.0 μg/ml. Because of the unusually high MIC to ceftriaxone, this mutant, designated SPN284 3-1, and a mutant derived from SPL4 with an MIC to ceftriaxone of 0.5 μg/ml and designated SPL4 3-4 were retained for further characterization. DNA isolated from SPN284 3-1 and from SPL4 3-4 was used to transform the mosaic penA strain 3502 to resistance to 4.5 μg/ml of cefpodoxime as described elsewhere (20, 21). Resistant transformants appeared at a frequency of 2 × 10−3 transformants/donor CFU. Three transformants obtained with SPN284 3-1 DNA and three obtained with SPL4 3-4 DNA exhibited MICs identical to those of the donor strains (Table 1). No detectible level of resistance could be transformed into 28Bl, which lacks the genetic background of 3502 (Table 1). To determine the mutation responsible for increased resistance in SPN284 3-1, DNA from the three transformants was purified and subjected to genomic sequencing at the Broad Institute from modified Illumina (Illumina, Inc., San Diego, CA) libraries (22) using either an Illumina HiSeq 2000 or Gallx instrument. Sequence alignments and analysis were carried out with BWA version 0.5.9-r16 9 (23), SAMtools (24), Galaxy (25–27), and SeaView version 4.2.6 (28, 29). In each transformant, a point mutation that resulted in G→A at position 1444 was found in penA. This mutation caused the substitution G483S. Direct sequencing of the amplified penA gene of SPN284 3-1 confirmed the presence of this mutation (Fig. 1A). To directly demonstrate that this mutation resulted in increased resistance in SPN284 3-1, the penA gene was amplified with specific primers (Table 3) by PCR, and the amplified DNA was used to transform 3502 to resistance to 4.5 μg/ml of cefpodoxime. The transformants, obtained at a frequency of 5 × 10−3 transformants/recipient CFU, were phenotypically indistinguishable from SPN284 3-1 and the initial transformants constructed with native genomic DNA.
TABLE 1.
Properties of the strains used in this study
| Strain (references) | Source | MIC (μg/ml)a |
Genotype |
|||||
|---|---|---|---|---|---|---|---|---|
| Pen | Cro | Cfx | Cpd | Ery/Txb | penA | ponAc | ||
| SPN275 | GISPd isolate | 8.0 | 0.008 | 0.015 | 0.06 | R | Insertion | Leu-Pro |
| SPN280 | GISP isolate | 8.0 | 0.015 | 0.03 | 0.03 | R | Insertion | Leu-Pro |
| SPN284 | GISP isolate | 4.0 | 0.06 | 0.25 | 2.0 | R | Mosaic | Leu-Pro |
| SPL4 | GISP isolate | 8.0 | 0.03 | 0.25 | 2.0 | R | Mosaic | Leu-Pro |
| 3502 | Reference isolate | 4.0 | 0.03 | 0.25 | 1.0 | R | Mosaic | Leu-Pro |
| 28Bl (20, 21) | DGI isolate (CDC, 1974) | 0.015 | 0.008 | 0.015 | 0.06 | S | WTe | WT |
| SPN284 3-1 | Spontaneous mutant of SPN284 | 16.0 | 1.0 | >1.0 | >8.0 | R | Mosaic | Leu-Pro |
| SPL4 3-4 | Spontaneous mutant of SPL4 | 32.0 | 0.5 | 1.0 | >8.0 | R | Mosaic | Leu-Pro |
| 3502T1 | Transformant SPN284 3-1 × 3502 | 32.0 | 1.0 | >1.0 | >8.0 | R | Mosaic | Leu-Pro |
| 3502T2 | Transformant SPN284 3-1 × 3502 | 32.0 | 1.0 | >1.0 | >8.0 | R | Mosaic | Leu-Pro |
| 3502T4 | Transformant SPN284 3-1 × 3502 | 32.0 | 1.0 | >1.0 | >8.0 | R | Mosaic | Leu-Pro |
| 3502L3-1 | Transformant SPL4 3-4 × 3502 | 32.0 | 0.5 | 1.0 | >8.0 | R | Mosaic | Leu-Pro |
| 3502L3-2 | Transformant SPL4 3-4 × 3502 | 32.0 | 0.5 | 1.0 | >8.0 | R | Mosaic | Leu-Pro |
| 3502L3-3 | Transformant SPL4 3-4 × 3502 | 32.0 | 0.5 | 1.0 | >8.0 | R | Mosaic | Leu-Pro |
| 3502L3-4 | Transformant SPL4 3-4 × 3502 | 32.0 | 0.5 | 1.0 | >8.0 | R | Mosaic | Leu-Pro |
Pen, penicillin; Cro, ceftriaxone; Cfx, cefixime; Cpd, cefpodoxime.
Resistance to 600 μg/ml Triton X-100 and 1.2 μg/ml of erythromycin (Ery/Tx) was used to determine the MtrR phenotype (13). R, resistant; S, susceptible.
The genes sequenced show the Leu-Pro substitution at amino acids associated with β-lactam resistance (29).
GISP, Gonococcal Isolate Surveillance Project.
WT, wild type.
TABLE 2.
MICs of mutants selected on cefpodoxime gradient plates
| Cpd concn (μg/ml)a | Strain | No. of isolates tested | MIC (μg/ml)b |
||||
|---|---|---|---|---|---|---|---|
| Pen | Tet | Cro | Cfx | Cpd | |||
| 4.0 | SPL4 | 5 | 32.0–64.0 | 4.0–8.0 | 0.25–0.5 | 0.5 | ≥8.0 |
| SPN284 | 4 | 16.0 | 4.0 | 0.25–0.5 | 0.5 | 8.0 | |
| 6.0 | SPL4 | 8 | 32.0–64.0 | 4.0–8.0 | 0.25–0.5 | 0.5–1.0 | ≥8.0 |
| SPN284 | 3 | 32.0 | 4.0 | 0.25–0.5 | 1.0 | 8.0 | |
Concentration of cefpodoxime (Cpd) in the gradient plate substratum used for in vitro selection of mutants with elevated MICs to cefpodoxime.
Pen, penicillin; Tet, tetracycline; Cro, ceftriaxone; Cfx, cefixime.
FIG 1.
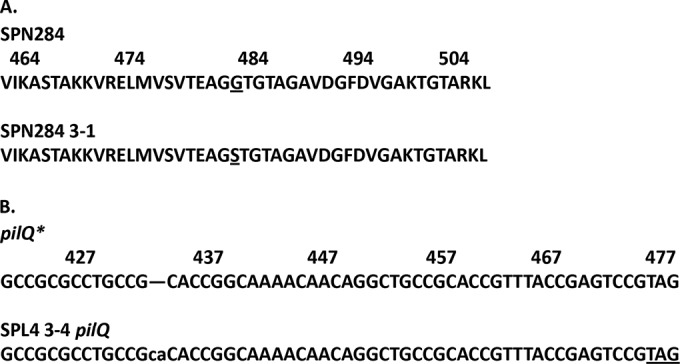
(A) The G→S substitution at position 483 and the flanking amino acid sequence for the protein coded for by the penA gene of SPN284 3-1. The coordinate system used is from reference 20. (B) The 2-bp insertion in the sequence of the pilQ gene of SPL4 3-4 with immediate flanking nucleotide sequence. The alignment was with N. gonorrhoeae pilQ* (accession no. U40596). The highlighted nucleotides denote the termination codon created by the insertion.
TABLE 3.
Primers for amplification of penA and pilQ for transformation and sequencing
| Primer | Sequence |
|---|---|
| ppPNAmut3 | 5′-CGGGCAATACCTTTATGGTGG-3′ |
| ppPNAmut4 | 5′-AGCCAAAGGGCTTAACTTGC-3′ |
| ppPilQ1 | 5′-GGTGTCGGCAACTATTTGGG-3′ |
| ppPilQ4 | 5′-CGGAATGACGGCATTTCGG-3′ |
A similar approach was used to characterize the mutation in SPL4 3-4. Transformants from the cross SPL4 3-4 × 3502 were selected for resistance to 3.4 μg/ml of cefpodoxime. The MIC of four transformants was found to be identical to that of the donor strain. DNA was extracted and subjected to genome sequencing and the data to partial analysis in the Centers for Disease Control and Prevention (CDC) Genomics Sequencing Laboratory. The results showed an identical 2-bp insertion in the pilQ gene of each of the transformants (Fig. 1B). Similar to the previous experiment, the pilQ gene was amplified (Table 3) from the DNAs of both SPL4 3-4 and 3502L3-1. Amplified DNA was used to transform 3502 as described above. The pilQ genes from three transformants from the SPL4 3-4 donor and two transformants from the 3502L3-1 donor, all selected on 3.4 μg/ml of cefpodoxime, were sequenced. In all cases, the same 2-bp insertion was present. All of the 3502 transformants derived from SPL4 3-4 and 3502L3-1 donors and a Pil+ 3502 recipient were phenotypically Pil−, not competent, and did not revert to Pil+ at a detectible frequency regardless of the source of the DNA.
The results show that it was possible to isolate in vitro a mutation in the penA gene of N. gonorrhoeae that caused elevated resistance to at least three different cephalosporins. This previously unrecognized mutation in SPN284 3-1 gave a phenotype similar to those of two strains isolated from clinical sources and was located in the same region as mutations found in H041 and F89 (5, 6, 30). Additionally, mutations in genes other than penA can increase MICs to cephalosporins, as demonstrated by the insertion mutation in pilQ present in SPL4 3-4. Alteration of pilQ was previously shown to increase resistance to penicillin by alteration of the permeability of the outer membrane (31, 32). These results demonstrate that this mutation in pilQ had a similar effect on cephalosporin resistance and increased the MIC to ceftriaxone almost 10-fold. Because mutations in pilQ commonly result in a Pil− phenotype, it is not evident that such mutations will be of consequence, as piliation is required for pathogenesis (33, 34).Since mutations in pilQ can result in complex phenotypes (35), it may be possible to isolate mutations in pilQ that allow for both pathogenesis and for increased cephalosporin resistance.
Finally, it is apparent that caution must be used in selecting alternative cephalosporins and possibly other β-lactam antibiotics for treatment of gonococcal infections. While mutants resistant to slightly elevated concentrations of ceftriaxone or cefixime were difficult to isolate (data not shown), it proved relatively easy to select mutants resistant to moderate concentrations of cefpodoxime that also showed significantly increased resistance to both of the other antibiotics, suggesting that the use of cefpodoxime could adversely affect the value of the other cephalosporins.
Nucleotide sequence accession numbers.
The complete sequences of the penA genes of SPN284 and SPN284 3-1 and the pilQ gene of SPL4 3-4 have been deposited in GenBank under accession no. KM403400, KM403401, and KM452733, respectively.
ACKNOWLEDGMENTS
Y.H.G was supported by grant 1-K08-AL104767 from NIAID, and M.L. was supported by grant U54GM088558 from NIGMS.
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Agency for Toxic Substances and Disease Registry, NIGMS, or NIH.
Footnotes
Published ahead of print 8 September 2014
REFERENCES
- 1.Lo JYC, Ho KM, Leung AOC, Tiu FST, Tsang GKL, Lo ACT, Tapsall JW. 2008. Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection. Antimicrob. Agents Chemother. 52:3564–3567. 10.1128/AAC.00198-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapsall J. 2006. Antibiotic resistance in Neisseria gonorrhoeae is diminishing available treatment options for gonorrhea: some possible remedies. Expert Rev. Anti Infect. Ther. 4:619–628. 10.1586/14787210.4.4.619. [DOI] [PubMed] [Google Scholar]
- 3.Tapsall JW, Ndowa F, Lewis DA, Unemo M. 2009. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev. Anti Infect. Ther. 7:821–834. 10.1586/eri.09.63. [DOI] [PubMed] [Google Scholar]
- 4.Tapsall J, Read P, Carmody C, Bourne C, Ray S, Limnios A, Sloots T, Wiley J. 2009. Two cases of failed ceftriaxone treatment in pharyngeal gonorrhoeae verified by molecular microbiological methods. J. Med. Microbiol. 58:683–687. 10.1099/jmm.0.007641-0. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi M, Goliparian D, Shimuta K, Saiki T, Hoshina S, Iwasaku K, Nakayama S, Kitikawa J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 55:3538–3545. 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unemo M, Goliparian D, Nicholas RA, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antmicrob. Agents Chemother. 56:1273–1280. 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brannigan JA, Tirodimos IA, Zhang Q-Y, Dowson CG, Spratt BG. 1990. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 4:913–919. 10.1111/j.1365-2958.1990.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee S-G, Lee H, Jeong SH, Yong D, Chung GT, Lee YS, Chong Y, Lee K. 2010. Various penA mutations together with mtrR, porB, and ponA mutations in Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone. J. Antimicrob. Chemother. 65:669–675. 10.1093/jac/dkp505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ropp PA, Nicholas RA. 1997. Cloning and characterization of the ponA gene encoding penicillin-binding protein 1 from Neisseria gonorrhoeae and Neisseria meningitides. J. Bacteriol. 179:2783–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ropp PA, Hu M, Olesky M, Nicholas RA. 2002. Mutations in ponA, the gene encoding penicillin-binding protein 1 and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:769–777. 10.1128/AAC.46.3.769-777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folster JP, Dhulipala V, Nicholas RA, Shafer WM. 2007. Differential regulation of ponA and pilMNOPQ expression by the MtrR transcriptional regulatory protein in Neisseria gonorrhoeae. J. Bacteriol. 189:4569–4577. 10.1128/JB.00286-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. 1995. Resistance of Neisseria gonorrhoeae to hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611–622. 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 13.Veal WL, Nicholas RA, Shafer WM. 2002. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J. Bacteriol. 184:5619–5624. 10.1128/JB.184.20.5619-5624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olesky M, Hobbs M, Nicholas RA. 2002. Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:2811–2820. 10.1128/AAC.46.9.2811-2820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill MJ, Simjee S, Al-Hatawi K, Robertson BD, Easmon CSF, Ison CA. 1998. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antmicrob. Agents Chemother. 42:2799–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ameyama S, Onodera S, Takahata M, Minami S, Maki N, Endo K, Goto H, Suzuki H, Oishi Y. 2002. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 46:3744–3749. 10.1128/AAC.46.12.3744-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindberg R, Fredlund H, Nicholas RA, Unemo M. 2007. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porBIb, and ponA. Antimicrob. Agents Chemother. 51:2117–2122. 10.1128/AAC.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito M, Deguchi T, Mizutani KS, Yasuda M, Yokoi S, Ito S-I, Takahashi Y, Ishihara S, Kawamura Y, Ezaki T. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in central Japan. Antimicrob. Agents Chemother. 49:137–143. 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spratt BG, Bowler LD, Zhang QY, Zhou J, Smith JM. 1992. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J. Mol. Evol. 34:115–125. [DOI] [PubMed] [Google Scholar]
- 20.Johnson SR, Sandul AL, Parehk M, Wang SA, Knapp JS, Trees DL. 2003. Mutations causing in vitro resistance to azithromycin in Neisseria gonorrhoeae. Int. J. Antimicrob. Agents 21:414–419. 10.1016/S0924-8579(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 21.Johnson SR, Steiner BM, Perkins GH. 1996. Cloning and characterization of the catalase gene of Neisseria gonorrhoeae: use of the gonococcus as a host organism for recombinant DNA. Infect. Immun. 64:2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher S, Barry A, Abreu J, Minie B, Nolan J, Delory TM, Young G, Fennell TJ, Allen A, Ambroglio L, Berlin AM, Blumenstiel B, Cibulskis K, Friedrich D, Johnson R, Juhn F, Reilly B, Shammas R, Stalker J, Sykes SM, Thompson J, Walsh J, Zimmer A, Zwirko Z, Gabriel S, Nicol R, Nusbaum C. 2011. A scalable, fully automated process for construction of sequence-ready human exome targeted capture libraries. Genome Biol. 12:R1. 10.1186/gb-2011-12-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Durbin R. 2009. Fast and accurate short read alignments with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blankenberg D, von Kuster G, Coraor N, Ananda G, Lazerus R, Mangan M, Nekrutenko A, Taylor J. 2010. Galaxy: a web-based genome analysis tool for experimentalists. Curr. Protoc. Mol. Biol. Chapter 19:Unit 19.10.1-21. 10.1002/0471142727.mb1910s89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giardine B, Riemer C, Hardison RC, Burhans R, Elnitski L, Shah P, Zhang Y, Blankenberg D, Albert I, Taylor J, Miller W, Kent WJ, Nekrutenko A. 2005. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 15:1451–1455. 10.1101/gr.4086505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goecks J, Nekrutenko A, Taylor J. 2010. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 11:R86. 10.1186/gb-2010-11-8-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galtier N, Gouy M, Carter C. 1996. SEAVIEW and PHYLO_Win: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 12:543–548. [DOI] [PubMed] [Google Scholar]
- 29.Gouy M, Guindon S, Gascuel O. 2010. SEAVIEW version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27:221–224. 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 30.Tomberg J, Unemo M, Ohnishi M, Davies C, Nicholas RA. 2013. Identification of amino acids conferring high-level resistance to cephalosporins in the penA gene from Neisseria gonrrhoeae strain H041. Antimicrob. Agents Chemother. 57:3029–3036. 10.1128/AAC.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CJ, Tobiason DM, Thomas CE, Shafer WM, Seifert HS, Sparling PF. 2004. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 186:730–739. 10.1128/JB.186.3.730-739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao S, Tobiason DM, Hu M, Seifert HS, Nicholas RA. 2005. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol. Microbiol. 57:1238–1251. 10.1111/j.1365-2958.2005.04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellogg DS, Jr, Cohen IR, Norins LC, Schroeter AL, Reising G. 1968. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J. Bacteriol. 96:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellogg DS, Peacock WL, Deacon WE, Browh L, Perkle CI. 1963. Neisseria gonorrhoeae. I. Virulence linked to colonial variation. J. Bacteriol. 85:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helm RA, Barnhart MM, Seifert HS. 2007. pilQ missense mutations have diverse effects on PilQ multimer formation, piliation and pilus function in Neisseria gonorrhoeae. J. Bacteriol. 189:3198–3207. 10.1128/JB.01833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


