LETTER
Increased acquired resistance to orally administered antibiotics in Escherichia coli has complicated the management of urinary tract infections (UTI) in outpatients (1–3). In this context, nitroxoline (5-nitro-8-hydroxyquinoline) (Fig. 1), an oral antibiotic, has received renewed attention in the management of UTI. Nitroxoline possesses activity in vitro against a variety of microorganisms, including E. coli and other uropathogens (4, 5). The mechanism of action is believed to be chelation of divalent cations required for bacterial RNA polymerase, leading to bacteriostatic activity in most cases (6, 7).
FIG 1.
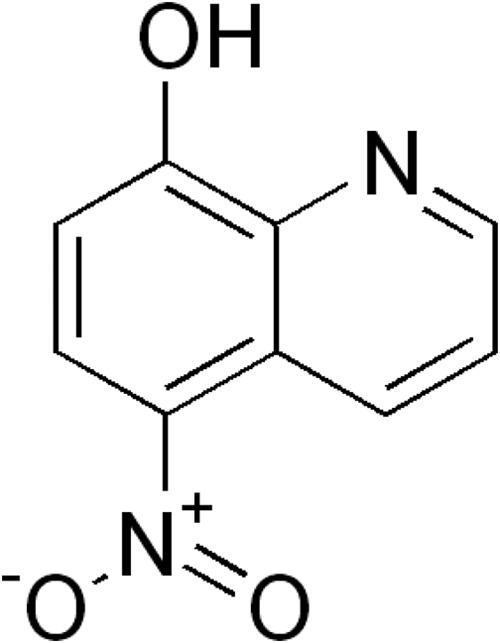
Chemical structure of nitroxoline.
Nitroxoline has received marketing authorization for prophylaxis and treatment of acute and recurrent UTI in various European countries, including Germany. The standard daily dosage of nitroxoline is 250 mg administered every 8 h. The drug is heavily metabolized (>95%) into microbiologically active conjugated and nonconjugated derivatives. Urine recovery is >50% (30% microbiologically active) (7, 8). At subinhibitory concentrations, the drug inhibits the adhesion of uropathogenic E. coli to uroepithelial cells and urinary catheters (9–11). An individual-patient meta-analysis of four randomized controlled clinical trials comparing the safety and efficacy of nitroxoline versus cotrimoxazole or norfloxacin in 466 patients with uncomplicated UTI detected a microbiological eradication rate of >90% for nitroxoline (12).
We determined the in vitro activity of nitroxoline against 499 E. coli isolates recovered from urine samples of outpatients during a surveillance study conducted by the Antimicrobial Resistance Working Party of the Paul Ehrlich Society between October and December of 2010 (13). Twenty-five laboratories were requested each to collect 20 consecutive nonduplicate isolates. Species confirmation and susceptibility testing were performed in a central laboratory (Antiinfectives Intelligence). MICs were determined by the broth microdilution procedure as described in the ISO document ISO 20776-1:2006 (14).
The majority of isolates were obtained from women (85.9%), with approximately 35% of isolates from females aged >65 years. High rates of resistance were observed for amoxicillin (42.9%), cotrimoxazole (30.9%), and ciprofloxacin (19.8%). Two hundred fifty-two (50.5%) isolates were fully susceptible to amoxicillin, amoxicillin-clavulanate, cefuroxime, third-generation cephalosporins (cefixime and cefpodoxime), ciprofloxacin, cotrimoxazole, and fosfomycin, while 143 (28.7%) isolates met the criterion for multidrug resistance (resistance to at least three of the seven antibacterial drugs/drug subclasses). Nineteen (3.8%) isolates were resistant to all drugs/drug subclasses except for fosfomycin, and 1 strain (0.2%) was resistant to all antibiotics. Forty isolates (8%) showed an extended-spectrum β-lactamase phenotype (13).
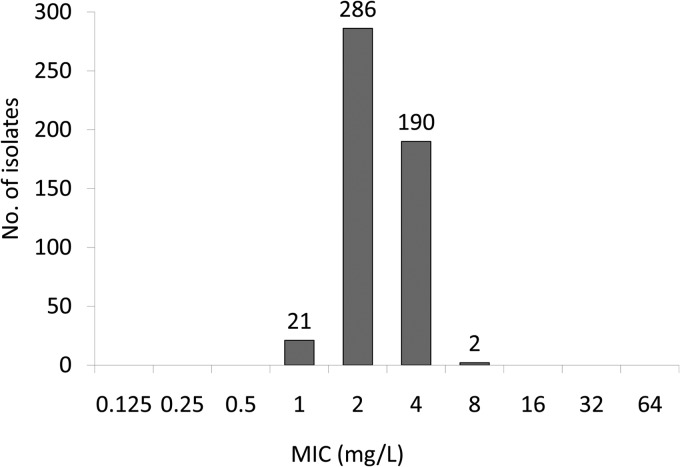
The nitroxoline MICs showed a normal (Gaussian) distribution, with values ranging from 1 to 8 mg/liter (Fig. 2). Based on the MIC50 and MIC90 values, there was no difference in activity between fully susceptible and multidrug resistant isolates (Table 1). The German breakpoint committee (Nationales Antibiotika-Sensitivitätstest-Komitee [NAK]) has recently established a clinical breakpoint (susceptible, ≤16 mg/liter, and resistant, >16 mg/liter) for nitroxoline against E. coli (15). Applying this breakpoint, all 499 isolates were rated susceptible to nitroxoline.
FIG 2.
Susceptibilities of 499 E. coli isolates to nitroxoline.
TABLE 1.
In vitro activity of nitroxoline against subgroups of fully susceptible and multidrug-resistant E. coli isolates
| Group (no. of isolates) | Nitroxoline MIC (mg/liter) |
|
|---|---|---|
| MIC50 | MIC90 | |
| All isolates (499) | 2 | 4 |
| Susceptible isolates (252)a | 2 | 4 |
| Mutidrug-resistant isolates (143)b | 2 | 4 |
Isolates were susceptible to amoxicillin, amoxicillin-clavulanate, cefuroxime, third-generation cephalosporins (cefixime and cefpodoxime), ciprofloxacin, cotrimoxazole, and fosfomycin.
Isolates were resistant to at least three of the seven antibacterial drugs/drug subclasses.
Although nitroxoline has been on the market for decades, resistance to nitroxoline in E. coli seems still to be very rare in Germany. This finding might be explained by the moderate consumption of nitroxoline in our country. In 2011, outpatient nitroxoline use was 1.0 million defined daily doses (DDD) (corresponding to 0.034 DDD per 1,000 inhabitants per day) (16).
Nitroxoline showed 100% in vitro activity against E. coli urine isolates, irrespective of their resistance profile. In a previous study, nitroxoline demonstrated the same in vitro activity against sulfonamide-susceptible and sulfonamide-resistant E. coli strains (5). In conclusion, nitroxoline should be equally effective against susceptible and multidrug-resistant E. coli bacteria causing acute or recurrent uncomplicated UTI.
ACKNOWLEDGMENTS
We are grateful to Rosen Pharma GmbH for funding the study by a grant.
We are grateful to the members of the Working Party “Antimicrobial Resistance” of the Paul-Ehrlich-Society for providing the clinical isolates.
Footnotes
Published ahead of print 2 September 2014
REFERENCES
- 1.Kahlmeter G, Poulsen HO. 2012. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO·SENS study revisited. Int. J. Antimicrob. Agents 39:45–51. 10.1016/j.ijantimicag.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Lee DS, Choe HS, Shim BS, Kim CS, Kim ME, Cho YH. 2011. Antimicrobial resistance in community-acquired urinary tract infections: results from the Korean Antimicrobial Resistance Monitoring System. J. Infect. Chemother. 17:440–446. 10.1007/s10156-010-0178-x. [DOI] [PubMed] [Google Scholar]
- 3.Maraki S, Mantadakis E, Michailidis L, Samonis G. 2013. Changing antibiotic susceptibilities of community-acquired uropathogens in Greece, 2005–2010. J. Microbiol. Immunol. Infect. 46:202–209. 10.1016/j.jmii.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Rosen Pharma GmbH. 2012. Fachinformation Nitroxolin forte 2012. Rosen Pharma GmbH, Blieskastel, Germany. [Google Scholar]
- 5.Jacobs MR, Robinson RG, Koorndorf HJ. 1978. Antibacterial activity of nitroxoline and sulphamethizole alone and in combination in urinary tract infections. S. Afr. Med. J. 54:959–962. [PubMed] [Google Scholar]
- 6.Fraser RS, Creanor J. 1974. Rapid and selective inhibition of RNA synthesis in yeast by 8-hydroxyquinoline. Eur. J. Biochem. 46:67–73. [DOI] [PubMed] [Google Scholar]
- 7.Wagenlehner FM, Münch F, Pilatz A, Bärmann B, Weidner W, Wagenlehner CM, Straubinger M, Blenk H, Pfister W, Kresken M, Naber KG. 2014. Urinary concentrations and antibacterial activities of nitroxoline at 250 milligrams versus trimethoprim at 200 milligrams against uropathogens in healthy volunteers. Antimicrob. Agents Chemother. 58:713–721. 10.1128/AAC.02147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergogne-Berezin E, Berthelot G, Muller-Serieys C. 1987. Present status of nitroxoline. Pathol. Biol. (Paris) 35:873–878 (In French.) [PubMed] [Google Scholar]
- 9.Bourlioux P, Botto H, Karam D, Amgar A, Camey M. 1989. Inhibition of bacterial adherence by nitroxoline on cellular adhesion and on urinary catheter surfaces. Pathol. Biol. (Paris) 37:451–454 (In French.) [PubMed] [Google Scholar]
- 10.Karam D, Amgar A, Bourlioux P. 1988. Inhibition of bacterial adhesion of uropathogenic Escherichia coli strains by the urine of patients treated with nitroxoline. Pathol. Biol. (Paris) 36:452–455 (In French.) [PubMed] [Google Scholar]
- 11.Wolf KM. 2013. Antagonism and adhesion of the probiotic E. coli strain Nissle 1917. Master's thesis Julius Maximilian University of Wuerzburg, Wuerzburg, Germany: (In German.) [Google Scholar]
- 12.Naber KG, Niggemann H, Stein G. 2014Nitroxoline for treatment of uncomplicated UTI. IPD meta analysis of four controlled clinical studies. Int. J. Infect. Dis. 21(Suppl 1):S200–S201. 10.1016/j.ijid.2014.03.840. [DOI] [Google Scholar]
- 13.Kresken M, Pfeifer Y, Hafner D, Wresch R, Körber-Irrgang B, Working Party “Antimicrobial Resistance” of the Paul-Ehrlich-Society for Chemotherapy 16 July 2014. Occurrence of multidrug resistance to oral antibiotics among Escherichia coli urine isolates from outpatient departments in Germany: extended-spectrum β-lactamases and the role of fosfomycin. Int. J. Antimicrob. Agents 10.1016/j.ijantimicag.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 14.ISO. 2006. Clinical laboratory testing and in vitro diagnostic test systems. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1: reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. ISO 20776-1:2006 International Organization for Standardization (ISO), Geneva, Switzerland. [Google Scholar]
- 15.Nationales Antibiotika-Sensitivitätstest-Komitee. 2014. Grenzwerte für Grenzwerte Nitroxolin bei Escherichia coli. http://www.nak-deutschland.org/tl_files/nak-deutschland/Nitroxolin-GW_V_1.0_31012014.pdf.
- 16.Kern WV. 2012. Antibiotika und chemotherapeutika. In Schwabe U, Paffrath D. (ed), Arzneiverordnungs report. Springer Medizinverlag, Berlin, Germany. [Google Scholar]