Abstract
Benznidazole (BZN) is the main trypanocidal drug used to treat Chagas disease, and the evidence supporting the benefits of BZN use during the chronic phase of the disease will favor its use in millions of individuals. However, more than 30% of patients treated with BZN may suffer adverse drug reactions (ADRs), and the development of tools to identify those patients at risk is highly desirable. In the present study, we aimed to identify predictive factors for ADRs in Chagas disease patients treated with BZN. Among 195 patients included in the study, 48.7% experienced ADRs and 31.3% had ADRs that caused BZN treatment discontinuation. Overall ADRs and ADRs that caused BZN treatment discontinuation were more common among women and in those who graduated from elementary school. Overall ADRs were also less frequent among black individuals. Based on logistic regression analysis, female sex (odds ratio [OR], 2.9; 95% confidence interval [CI], 1.5 to 5.4), graduation from elementary school (OR, 2.0; 95% CI, 1.1 to 3.8), and white (OR, 5.0; 95% CI, 1.0 to 24.1) and mulatto (OR, 5.6; 95% CI, 1.1 to 28.7) races were considered to predict overall ADRs, and female sex (OR, 2.3; 95% CI, 1.2 to 4.3) was considered to predict ADRs that caused BZN treatment discontinuation. Graduation from elementary school also presented a tendency to predict ADRs that caused BZN treatment discontinuation (OR, 1.8; 95% CI, 0.9 to 3.6). The logistic regression (LR) models to predict ADRs to BZN described in this study may become important tools to minimize ADRs and improve patients' compliance and thus assist physicians treating patients with Chagas disease with BZN.
INTRODUCTION
Even 100 years after it was first reported (1), Chagas disease remains a serious public health problem in Latin America, with a high social and economic burden (2, 3). Moreover, the prevalence of Chagas disease is increasing, mainly due to migration, in countries where it is not endemic, such as the United States and European countries (4–7).
Benznidazole (BZN) is the main trypanocidal drug used to treat Chagas disease (8, 9), and the parasitological cure rate when patients are treated with BZN during the acute phase of the disease is almost 80% (10, 11). Although BZN effectiveness during the chronic phase of the disease remains uncertain (12–14), there is compelling evidence supporting the beneficial use of BZN even during the chronic phase of Chagas disease (13, 15), and some international guidelines are now advocating the use of BZN even among adults with chronic disease (16–18). However, adverse drug reactions (ADRs) occur in 30% to 87% of the patients treated with BZN (19–21), and 12% to 29% of patients fail to complete their full course of treatment (19–21). Therefore, the development of tools to identify patients with high probabilities of developing ADRs to BZN is highly desirable, as this strategy would improve compliance to BZN treatment and minimize complications (21, 22).
Logistic regression (LR) is a method often used to predict outcomes in health studies (23, 24), and it can be used to identify which variables are associated with the occurrence of ADRs to BZN. Therefore, the present study aimed to identify predictive factors for ADRs and ADRs that caused BZN treatment to be discontinued among patients with chronic Chagas disease treated with BZN.
MATERIALS AND METHODS
Patients and procedures.
The present work is a single-center retrospective cohort study based on data collected from medical records of patients monitored at the Evandro Chagas Clinical Research Institute (IPEC) in December 1986 and October 2011. IPEC is a national reference center for treatment and research in infectious diseases and tropical medicine in Brazil. This study was approved by the local Institutional Review Board under number 0016.0.009.000-07.
From a total of 1,815 patients with Chagas disease, diagnosed by two simultaneously positive serological tests (enzyme-linked immunosorbent assay and indirect immunofluorescence), 228 received BZN treatment and were included in the present study. Of these, 33 patients were excluded from analysis due to the concomitant use of other medications during BZN treatment. The final studied population consisted of 195 individuals whose medical records were reviewed using a specific research form. The variables collected were sex, age, schooling, race, daily dose of BZN, treatment duration, treatment interruption due to ADRs related to BZN, use of other drugs prior to BZN treatment, and causality of ADRs. Race was self-reported by the patient. All patients who received BZN treatment were monitored monthly at our outpatient service throughout the duration of the BZN treatment. All patients were also advised to seek medical attention immediately in case any symptoms appeared. As the current recommended dose for BZN was not standardized at the time most of the patients were treated, the BZN regimen used depended on the experience of the physician who was accepting new patients at our outpatient service. The most-used regimen was a fixed BZN dose of 200 mg/day. After the publication of the Brazilian Consensus on Chagas Disease in 2005 (16), the 5-mg/kg/day regimen was adopted by all attending physicians of our outpatient service.
The independent variables in the present study were categorized as follows: sex (male or female); age (13 to 20, 21 to 40, and >40 years); schooling (failed to graduate from elementary school/illiterate or graduated from elementary school); race (black, mulatto, or white); daily dose of BZN (50 to 200 mg/day or 250 to 500 mg/day), treatment duration (≤30, 30 to 60, or ≥60 days), treatment interruption due to ADRs related to BZN (yes or no), and use of other drug prior to treatment with BZN (yes or no). The assessment of causality of ADRs (dependent variable) was performed by the application of the Naranjo's algorithm (25), the results of which depended on the compatibility of the time elapsed between the drug use and the reaction onset, the nature of the event and the pharmacological characteristics, and medical or pharmacological plausibility. This algorithm is composed of 10 questions with three possible answers: positive (“yes”), negative (“no”), or unknown (“not sure”). Based on the scores yielded by the algorithm, ADRs were classified as absent (score of −3 to 0), possible (score of 1 to 4), probable (score of 5 to 8), and definitive (score of ≥9). The two outcomes of interest in this study were overall ADRs and ADRs that caused BZN treatment to be discontinued. The presence of ADRs was established when the Naranjo's score was >1 (22). The use of Naranjo's algorithm is limited by the difference in the interpretation of some questions by different investigators (26, 27). The WHO adverse reactions terminology (WHO-ART) was used to code and classify ADRs (28).
Data analysis.
A statistical analysis was performed by descriptive analysis, with numerical variables expressed as mean ± standard deviation (SD). The Pearson's chi-square test was used for exploratory analysis of each variable collected and ADRs.
The binary logistic regression was performed to estimate the probability of developing overall ADRs or ADRs that caused BZN treatment to be discontinued. All variables for which the P value in a likelihood ratio test was lower than 0.10 were included in a stepwise logistic regression model, with a subsequent P value smaller than 0.05 required for inclusion in the final model. The Hosmer and Lemeshow test was used to show how adequately the model fits the data (29). Odds ratios (ORs) and their respective 95% confidence intervals (CIs) were also estimated. The predictive ability of the LR models was assessed by the area under receiver operator characteristic (ROC) curves.
EpiData (30), Software R 2.14 (31), and the Statistical Package for the Social Sciences (SPSS) 16.0 software (SPSS Inc., Chicago, IL) were used for data entry and analysis, respectively. Differences were considered significant at P values of ≤0.05.
RESULTS
Patient characteristics.
The majority of the patients included in the study were male, with ages ranging between 20 and 40 years. Most of them self-reported their race as white and graduated from elementary school (Table 1).
TABLE 1.
Patient characteristics (n = 195) and incidence of overall ADRs by variable category
| Variable | Mean (SD) | Category | Frequency (% [n]) | ADR incidence (% [n]) | P value |
|---|---|---|---|---|---|
| Age (yr) | 32.2 (9.8) | 13-19 | 3.1 (6) | 33.3 (2) | 0.136 |
| 20-40 | 79.5 (155) | 52.2 (81) | |||
| >40 | 17.4 (34) | 35.3 (12) | |||
| Sex | Male | 57.9 (113) | 39.8 (45) | 0.004 | |
| Female | 42.1 (82) | 61 (50) | |||
| Race | Black | 7.7 (15) | 13.3 (2) | 0.016 | |
| White | 58.5 (114) | 50.9 (58) | |||
| Mulatto | 33.8 (66) | 53.0 (35) | |||
| Schooling | Failed to graduate from elementary school/illiterate | 40.5 (79) | 36.7 (29) | 0.006 | |
| Graduated from elementary school | 59.5 (116) | 56.9 (66) | |||
| Use of other drug prior to BZN treatment | Yes | 12.3 (24) | 54.2 (13) | 0.568 | |
| No | 87.7 (171) | 47.8 (82) | |||
| BZN dose (mg/day) | 223.1 (55.5) | 100 to 200 | 64.6 (127) | 49.6 (63) | 0.681 |
| 250 to 500 | 14.9 (29) | 41.4 (12) | |||
| NDa | 20.5 (40) | 50 (20) | |||
| Duration of treatment (days) | 58.5 (36.3) | ≤30 | 20.0 (39) | 87.2 (34) | <0.001 |
| >30 and <60 | 25.6 (50) | 42 (21) | |||
| ≥60 | 54.4 (106) | 37.7 (40) |
ND, not determined in the medical records.
Most patients used 200 mg/day of BNZ and achieved the proposed length of treatment (≥60 days). Ninety-five patients (48.7%) presented ADRs that were attributed to BZN treatment. Of those, 61 patients (31.3%) interrupted their treatment due to ADRs. Treatment was restarted in 18 patients who succeeded in completing their treatment. However, 43 patients (22%) did not complete their treatment due to ADRs. There was only one serious ADR event (Stevens-Johnson syndrome) and there was no death related to BZN treatment.
Comparison of population characteristics in patients with and without ADRs.
The frequency of overall ADRs was significantly higher among women and in those patients who graduated from elementary school. On the other hand, black individuals presented fewer overall ADRs related to BZN treatment. The daily dose of BZN or the use of other drugs before beginning BZN treatment did not increase the risk of ADRs related to BZN treatment (Table 1). The frequency of ADRs that caused BZN treatment to be discontinued was also higher among women and those who graduated from elementary school. There was also a tendency for ADRs that caused BZN treatment to be discontinued to occur less frequently among black individuals (Table 2).
TABLE 2.
Incidence by variable categories of ADRs that caused BZN treatment to be discontinued
| Variable | Category | ADRs causing BZN treatment interruptiona |
P value | |
|---|---|---|---|---|
| Yes (n = 61) | No (n = 134) | |||
| Age (yr) | 13–19 | 16.7 (1) | 83.7 (5) | 0.4247 |
| 20–40 | 33.5 (52) | 66.5 (103) | ||
| >40 | 23.5 (8) | 76.5 (26) | ||
| Sex | Male | 24.8 (28) | 75.2 (85) | 0.0321 |
| Female | 40.2 (33) | 59.8 (49) | ||
| Race | Black | 6.7 (1) | 9.3 (14) | 0.0958 |
| White | 34.2 (39) | 65.8 (75) | ||
| Mulatto | 31.8 (21) | 68.2 (45) | ||
| Schooling | Failed to graduate from elementary school/illiterate | 22.8 (18) | 77.2 (61) | 0.0411 |
| Graduated from elementary school | 37.1 (43) | 62.9 (73) | ||
| Use of other drugs prior to BZN treatment | Yes | 41.7 (10) | 58.3 (14) | 0.3489 |
| No | 29.8 (51) | 70.2 (120) | ||
| BZN dose (mg/day) | 50 to 200 mg | 29.4 (37) | 70.6 (89) | 0.7801 |
| 250 to 500 mg | 29.6 (8) | 70.4 (19) | ||
| NDb | 38.1 (16) | 61.9 (26) | ||
Data are presented as % (n).
ND, not determined in the medical records.
The most common ADRs in order of frequency were skin disorders, gastrointestinal complaints, and nervous system disorders (Table 3). Regarding the frequency of specific ADR categories among subgroups, cutaneous rash (P = 0.03) and gastrointestinal complains (P = 0.04) were more common among women than men, skin and appendage disorders tended to be less frequent in blacks than in individuals from other races (P = 0.07), and pruritus (P = 0.007) and general disorders (P = 0.04) were more common among individuals who graduated from elementary school than among other subgroups (Table 3).
TABLE 3.
Adverse drugs reactions according to the system/organ damage during BZN treatment by age, sex, race, and schooling
| WHO-ART classification | No. (%) of adverse drug reactions by patient category |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) |
Sex |
Race |
Schooling |
Total (n = 195) | |||||||
| <20 (n = 6) | 20–40 (n = 155) | >40 (n = 34) | Male (n = 113) | Female (n = 82) | Black (n = 15) | Mulatto (n = 66) | White (n = 114) | Incomplete elementary school/illiterate (n = 79) | Complete elementary school (n = 116) | ||
| Skin and appendage disorders | |||||||||||
| Pruritus | 1 (16.7) | 28 (18.1) | 6 (17.7) | 18 (15.9) | 17 (20.7) | 0 (0) | 12 (18.2) | 23 (20.2) | 7 (8.7) | 28 (24.1) | 35 (17.9) |
| Cutaneous rash | 1 (16.7) | 15 (9.7) | 0 (0) | 5 (4.4) | 11 (13.4) | 1 (6.7) | 6 (9.1) | 9 (7.9) | 6 (7.6) | 10 (8.6) | 16 (8.2) |
| Vision disorders | 0 (0) | 1 (0.7) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 0 (0) | 1 (0.9) | 1 (1.3) | 0 (0) | 1 (0.5) |
| White cell and reticuloendothelial system disorders | 0 (0) | 2 (1.3) | 0 (0) | 0 (0) | 2 (2.4) | 0 (0) | 0 (0) | 2 (1.8) | 0 (0) | 2 (1.7) | 2 (1.0) |
| Body as a whole general disorders | 0 (0) | 5 (3.2) | 1 (2.9) | 5 (4.4) | 1 (1.2) | 0 (0) | 2 (3.0) | 4 (3.5) | 0 (0) | 6 (5.2) | 6 (3.1) |
| Resistance mechanisms disorders | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) | 1 (1.2) | 0 (0) | 1 (1.5) | 0 (0) | 0 (0) | 1 (0.9) | 1 (0.5) |
| Gastrointestinal system disorders | 0 (0) | 14 (9.0) | 3 (8.8) | 6 (5.3) | 11 (13.4) | 0 (0) | 6 (9.1) | 11 (9.7) | 8 (10.1) | 9 (7.8) | 17 (8.7) |
| Autonomic nervous system disorders | 0 (0) | 1 (0.7) | 0 (0) | 1 (0.9) | 0 (0) | 0 (0) | 1 (1.5) | 0 (0) | 1 (1.3) | 0 (0) | 1 (0.5) |
| Central and peripheral nervous system disorders | 0 (0) | 12 (7.8) | 1 (2.9) | 9 (8.0) | 4 (4.9) | 1 (6.7) | 6 (9.1) | 6 (5.3) | 5 (6.3) | 8 (6.9) | 13 (6.7) |
| Special senses other disorders | 0 (0) | 2 (1.3) | 1 (2.9) | 0 (0) | 3 (3.7) | 0 (0) | 1 (1.5) | 2 (1.8) | 1 (1.3) | 2 (1.7) | 3 (1.5) |
Prediction of overall ADRs.
The variables associated with overall ADRs at P < 0.05 (sex, schooling, and race) and age were included in the LR model. These variables were retained in the stepwise regression model and were also included in the final LR model. A goodness-of-fit test (Hosmer and Lemeshow) demonstrated a good performance of the model (P = 0.87). Based on LR model results, the following variables were considered to predict overall ADRs: female sex (OR, 2.9; 95% CI, 1.5 to 5.4), graduation from elementary school (OR, 2.0; 95% CI 1.1 to 3.8), and white (OR, 5.0; 95% CI, 1.0 to 24.1) and mulatto (OR, 5.6; 95% CI, 1.1 to 28.7) races (Table 4). The area under the ROC curve, which assesses the ability of the LR model to predict overall ADRs, was 0.71 (95% CI, 0.64 to 0.78) (Fig. 1).
TABLE 4.
Logistic regression model of overall ADRs to BZN
| Variable | βa | Wald | P value | ORb | 95% CIc |
|---|---|---|---|---|---|
| Female sex | 1.05 | 10.56 | 0.001 | 2.86 | 1.52–5.40 |
| Graduated from elementary school | 0.71 | 4.72 | 0.030 | 2.03 | 1.07–3.84 |
| Age (yr) | |||||
| 20–40 | 0.90 | 0.93 | 0.336 | 2.45 | 0.40–15.11 |
| >40 | 0.35 | 0.00 | 0.97 | 1.04 | 0.15–7.11 |
| Race | |||||
| Mulatto | 1.73 | 4.35 | 0.037 | 5.65 | 1.11–28.72 |
| White | 1.60 | 3.94 | 0.047 | 4.96 | 1.02–24.09 |
Coefficient.
OR, odds ratio.
CI, confidence interval (the ORs and 95% CIs are derived from stepwise multivariate logistic regression).
FIG 1.
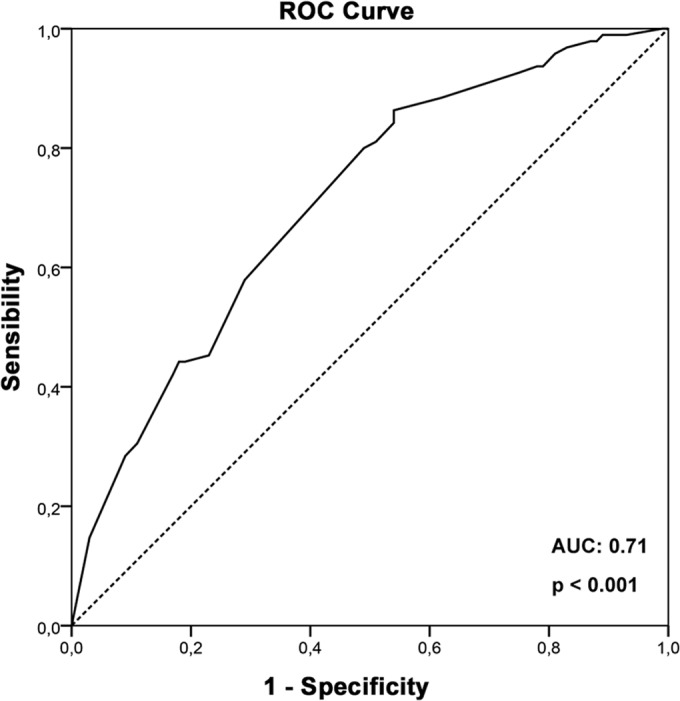
Receiver operator characteristic (ROC) curve based on the probability of development of overall ADRs calculated by the logistic regression model for each patient. The area under the ROC curve (AUC) assesses the ability of the LR model to predict overall ADRs.
Prediction of ADRs that caused BZN treatment to be discontinued.
A second LR model to predict ADRs that caused BZN treatment to be discontinued was built using the same variables used in the LR model to predict overall ADRs. A goodness-of-fit test (Hosmer and Lemeshow) demonstrated a good performance of the new model (P = 0.998). Based on LR model results, only female sex predicted ADRs that caused BZN treatment to be discontinued (OR, 2.3; 95% CI, 1,2 to 4.3). Graduation from elementary school also presented a tendency to predict ADRs that caused BZN treatment to be discontinued (OR, 1.8; CI 95% 0.9 to 3.6) (Table 5). The area under the ROC curve, which assesses the ability of the LR model to predict ADRs that caused BZN treatment to be discontinued, was 0.67 (95% CI, 0.59 to 0.75) (Fig. 2).
TABLE 5.
Logistic regression model of ADRs that caused BZN treatment to be discontinued
| Variable | βa | Wald | P value | ORb | 95% CIc |
|---|---|---|---|---|---|
| Female sex | 0.82 | 6.30 | 0.012 | 2.28 | 1.20–4.34 |
| Graduated from elementary school | 0.59 | 2.83 | 0.092 | 1.80 | 0.91–3.58 |
| Age (yr) | |||||
| 20–40 | 0.93 | 0.67 | 0.41 | 2.54 | 0.27–23.51 |
| >40 | 0.37 | 0.10 | 0.76 | 1.45 | 0.14–15.01 |
| Race | |||||
| Mulatto | 1.55 | 2.01 | 0.156 | 4.70 | 0.55–39.92 |
| White | 1.67 | 2.43 | 0.119 | 5.30 | 0.65–43.26 |
Coefficient.
OR, odds ratio.
CI, confidence interval (the ORs and 95% CIs are derived from stepwise multivariate logistic regression).
FIG 2.
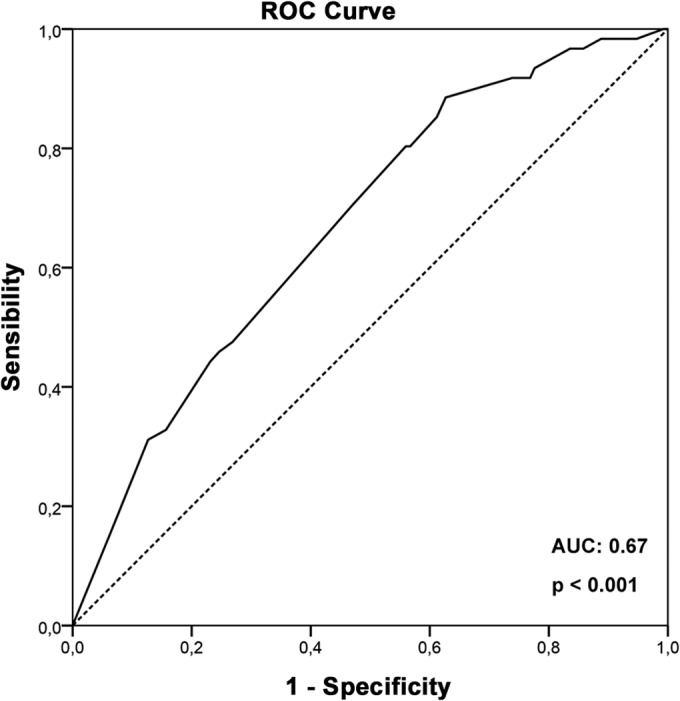
Receiver operator characteristic (ROC) curve based on the probability of development of ADRs that caused BZN treatment to be discontinued calculated by the logistic regression model for each patient. The area under the ROC curve (AUC) assesses the ability of the LR model to predict ADRs that caused BZN treatment to be discontinued.
DISCUSSION
Even today, the World Health Organization estimates that there are 10 million individuals living with chronic Chagas disease throughout the world (32). If the international guidelines that advocate the use of BZN among adults with chronic disease (16–18) are to be followed, strategies to improve compliance to BZN treatment and minimize ADRs related to BZN treatment should be developed. As more than 30% of patients treated with BZN present ADRs during their treatment (19, 20), the risk score presented in this paper is an important tool to predict the occurrence of ADRs and allow the establishment of strategies to minimize their consequences and improve compliance.
BZN is a 2-nitroimidazole which acts by a reductive stress mechanism that involves the covalent modification of macromolecules by nitroreductive intermediates (33) and by the synthesis inhibition and increased degradation of DNA, RNA, and proteins by interacting with topoisomerases (34). However, these same mechanisms are believed to be involved in BZN host toxicity (35, 36). Moreover, the exact mechanisms involved in the hypersensitivity related to BZN are difficult to identify and may be mediated either by IgE- or non-IgE-dependent pathways (37). Recently, the same complexity in understanding immunological mechanisms was reported for drug allergy; however, race and genetics are increasingly appearing to be important in the predisposition to certain types of drug allergies (38, 39).
In the present study, we used four different variables in LR models to predict the occurrence of overall ADRs and ADRs that caused BZN treatment to be discontinued. Schooling, sex, and race were included due to their association with the occurrence of overall ADRs, while age was included because of its clinical relevance (22). From these variables, race showed the greatest association, because black individuals presented a much lower incidence of overall ADRs than other individuals. Women and individuals with increased levels of education presented higher incidences of both overall ADRs and ADRs that caused BZN treatment to be discontinued than other individuals. Regarding specific ADRs, there was a strong tendency for skin disorders to be less frequent in black individuals than in individuals from other races. Therefore, genetic differences may play a role in the mechanisms involved in the hypersensitivity related to BZN. However, more studies are needed to investigate the physiological mechanisms involved in the lower percentage of ADRs related to BZN in black individuals. Women presented a higher incidence of ADRs than did men, supporting the findings of previous studies (19, 40, 41) and the inclusion of sex as one of the variables of our prediction model. We and others (42) reported that the specific ADR categories that were more common in women than men were cutaneous rash and gastrointestinal complaints. The higher prevalence of ADRs in women may be attributed to their higher compliance to treatment and metabolic alterations due to hormone levels (38). Regarding age, almost all ADRs occurred in the group of patients between 20 and 40 years old. This result is in accordance with recent findings presented by Lobo et al. (43), in which a greater incidence rate of ADRs was observed in adults than in children and older patients (61.0% versus 18.9% and 20.0%, respectively; P = 0.0001) in a cohort of hospitalized patients at a tertiary care hospital in Northern Brazil. However, previous studies have shown a higher incidence of ADRs related to BZN in elderly patients (14), while a low incidence of ADRs was described for children and adolescents (44–46). As our study did not include individuals older than 65 years, this could explain the higher incidence of ADRs found by us among adults. Finally, schooling was also an important variable related to the occurrence of ADRs that was included in our prediction model. Both pruritus and general disorders were more common among individuals who graduated from elementary school than among other individuals, and an increased educational level may increase the probability that the patient will report their ADRs to their physicians.
In this study, we developed two LR models that demonstrated good performance for prediction of the occurrence of overall ADRs due to BZN or ADRs that caused BZN treatment to be discontinued in patients with Chagas disease. The development of clinical prediction models may assist physicians and researchers to understand factors associated with ADRs, as well as their prediction and prevention (47, 48). The identification of patients with a high probability of development of ADRs due to BZN may affect decision making in regard to the prescription of BZN to patients with chronic Chagas disease and identification of patients who need additional advice regarding the risks of BZN treatment and instructions on how to manage side effects if they occur (49). In this setting, active pharmaceutical care can be offered for the clinical management of patients at a high risk for the development of ADRs, which may improve patient compliance with treatment and decrease BZN treatment interruption (50).
Limitations.
The retrospective design of this study restrained the quality of the results obtained. Another limitation was that the sample size of this study did not allow the validation of our logistic model. However, the variables associated with ADRs included in our prediction model were also found by others to be related to ADRs due to BZN (19, 45). The percentage of BZN treatment interruption described by us is higher than the percentage observed by Viotti et al. (19), but it is similar to the percentage of interruption described for other studies conducted in Brazil (21).
Another limitation of our study was that the BZN dose was not standardized at the time most of the patients were treated. In consequence, most patients used a fixed BZN dose of 200 mg/day, which is below the current recommended BZN dose of 5 mg/kg/day. However, we and others (42) found no difference in the incidence of ADRs between individuals using suboptimal doses (<5 mg/kg/day) and those using the current recommended doses (5 mg/kg/day) of BZN, reinforcing the finding that BZN dose does not have an effect on the incidence of ADRs.
Also, our results should be applied only to adult populations, as only 6 patients in this study were younger than 19 years old.
Conclusions.
The occurrence of both overall ADRs and ADRs that cause BZN treatment to be discontinued can be predicted by logistic regression models for patients with Chagas disease. Overall, ADRs were more common among women and in those who graduated from elementary school and less frequent among black individuals. ADRs that caused BZN treatment to be discontinued were more common among women and those who graduated from elementary school. There was also a tendency for ADRs that caused BZN treatment to be discontinued to be less frequent among black individuals. The two prediction models developed in the present study may become important tools to assist physicians in clinical practice to treat patients with Chagas disease with BZN. The identification of those patients with a high risk for the development of ADRs may allow the establishment of strategies to minimize ADR incidence and consequences and improve patients' compliance. This would result in a higher proportion of patients adequately treated with BZN, with a consequent improvement in the benefit generated by BZN treatment.
ACKNOWLEDGMENTS
This study was supported by the Evandro Chagas Clinical Research Institute of the Oswaldo Cruz Foundation.
We all meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
We declare no conflicts of interest.
Footnotes
Published ahead of print 11 August 2014
REFERENCES
- 1.Chagas C. 1909. Nova tripanozomiaze humana. Mem. Inst. Oswaldo Cruz 1:159–218. 10.1590/S0074-02761909000200008. [DOI] [Google Scholar]
- 2.Lannes-Vieira J, de Araujo-Jorge TC, Soeiro Mde N, Gadelha P, Correa-Oliveira R. 2010. The centennial of the discovery of Chagas disease: facing the current challenges. PLoS Negl. Trop. Dis. 4:e645. 10.1371/journal.pntd.0000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncayo A, Silveira AC. 2009. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem. Inst. Oswaldo Cruz 104(Suppl 1):S17–S30. 10.1590/S0074-02762009000900005. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Molina JA, Norman F, Lopez-Velez R. 2012. Chagas disease in non-endemic countries: epidemiology, clinical presentation and treatment. Curr. Infect. Dis. Rep. 14:263–274. 10.1007/s11908-012-0259-3. [DOI] [PubMed] [Google Scholar]
- 5.Aagaard-Hansen J, Nombela N, Alvar J. 2010. Population movement: a key factor in the epidemiology of neglected tropical diseases. Trop. Med. Int. Health 15:1281–1288. 10.1111/j.1365-3156.2010.02629.x. [DOI] [PubMed] [Google Scholar]
- 6.Gascon J, Bern C, Pinazo MJ. 2010. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 115:22–27. 10.1016/j.actatropica.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Schmunis GA, Yadon ZE. 2010. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 115:14–21. 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Yun O, Lima MA, Ellman T, Chambi W, Castillo S, Flevaud L, Roddy P, Parreno F, Albajar Vinas P, Palma PP. 2009. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10-year experience of Medecins Sans Frontieres. PLoS Negl. Trop. Dis. 3:e488. 10.1371/journal.pntd.0000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villar JC, Marin-Neto JA, Ebrahim S, Yusuf S. 2002. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst.Rev. 5:CD003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rassi A, Jr, Rassi A, Marcondes de Rezende J. 2012. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. North Am. 26:275–291. 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Rassi A, Jr, Rassi A, Marin-Neto JA. 2010. Chagas disease. Lancet 375:1388–1402. 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Molina JA, Perez-Ayala A, Moreno S, Fernandez-Gonzalez MC, Zamora J, Lopez-Velez R. 2009. Use of benznidazole to treat chronic Chagas' disease: a systematic review with a meta-analysis. J. Antimicrob. Chemother. 64:1139–1147. 10.1093/jac/dkp357. [DOI] [PubMed] [Google Scholar]
- 13.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, Postan M, Armenti A. 2006. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann. Intern. Med. 144:724–734. 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 14.Cancado JR. 2002. Long-term evaluation of etiological treatment of Chagas disease with benznidazole. Rev. Inst. Med. Trop. Sao Paulo 44:29–37. 10.1590/S0036-46652002000100006. [DOI] [PubMed] [Google Scholar]
- 15.Bern C. 2011. Antitrypanosomal therapy for chronic Chagas' disease. N. Engl. J. Med. 364:2527–2534. 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 16.Ministerio da Saude Secretaria de Vigilancia em Saúde. 2005. Brazilian consensus on Chagas disease. Rev. Soc. Bras. Med. Trop. 38(Suppl. 3):7–29 (In Portugese.) [PubMed] [Google Scholar]
- 17.Bern C, Montgomery SP, Herwaldt BL, Rassi A, Jr, Marin-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171–2181. 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 18.WHO. 2002. Control of Chagas disease: report of a WHO expert committee. World Health Organization. Technical report series, no. 905. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 19.Viotti R, Vigliano C, Lococo B, Alvarez MG, Petti M, Bertocchi G, Armenti A. 2009. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev. Anti Infect. Ther. 7:157–163. 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- 20.Pinazo MJ, Munoz J, Posada E, Lopez-Chejade P, Gallego M, Ayala E, del Cacho E, Soy D, Gascon J. 2010. Tolerance of benznidazole in treatment of Chagas' disease in adults. Antimicrob. Agents Chemother. 54:4896–4899. 10.1128/AAC.00537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Pontes VM, Souza Junior AS, Cruz FM, Coelho HL, Dias AT, Coelho IC, Oliveira Mde F. 2010. [Adverse reactions in Chagas disease patients treated with benznidazole, in the State of Ceara]. Rev. Soc. Bras. Med. Trop. 43:182–187 (In Portugese.) 10.1590/S0037-86822010000200015. [DOI] [PubMed] [Google Scholar]
- 22.Hasslocher-Moreno AM, do Brasil PE, de Sousa AS, Xavier SS, Chambela MC, Sperandio da Silva GM. 2012. Safety of benznidazole use in the treatment of chronic Chagas' disease. J. Antimicrob. Chemother. 67:1261–1266. 10.1093/jac/dks027. [DOI] [PubMed] [Google Scholar]
- 23.Dreiseitl S, Ohno-Machado L. 2002. Logistic regression and artificial neural network classification models: a methodology review. J. Biomed. Inform. 35:352–359. 10.1016/S1532-0464(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 24.Chang YJ, Yeh ML, Li YC, Hsu CY, Lin CC, Hsu MS, Chiu WT. 2011. Predicting hospital-acquired infections by scoring system with simple parameters. PLoS One 6:e23137. 10.1371/journal.pone.0023137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. 1981. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 30:239–245. 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi TK, Seger DL, Bates DW. 2000. Identifying drug safety issues: from research to practice. Int. J. Qual. Health Care 12:69–76. 10.1093/intqhc/12.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Son YM, Lee JR, Roh JY. 2011. Causality assessment of cutaneous adverse drug reactions. Ann. Dermatol. 23:432–438. 10.5021/ad.2011.23.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Collaborating Centre for International Drug Monitoring. 2005. The WHO adverse reaction terminology. Uppsala Monitoring Centre, Uppsala, Sweden. [Google Scholar]
- 29.Hosmer DW, Lemeshow S. 2000. Applied logistic regression, 2nd ed. Wiley & Sons, New York, NY. [Google Scholar]
- 30.Lauritsen JM, Bruus M. 2003. A comprehensive tool for validated entry and documentation of data, 3rd ed. The EpiData Association, Odense, Denmark. [Google Scholar]
- 31.R Development Core Team. 2013. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 32.WHO. 2010. First WHO report on neglected tropical diseases: working to overcome the global impact of neglected tropical diseases. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 33.Docampo R. 1990. Sensitivity of parasites to free radical damage by antiparasitic drugs. Chem. Biol. Interact. 73:1–27. 10.1016/0009-2797(90)90106-W. [DOI] [PubMed] [Google Scholar]
- 34.Stoppani AO. 1999. The chemotherapy of Chagas disease. Medicina (B Aires) 59(Suppl 2):S147–S165 (In Spanish.) [PubMed] [Google Scholar]
- 35.Castro JA, Meca MM, Bartel LC. 2006. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis). Hum. Exp. Toxicol. 25:471–479. 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- 36.Bartel LC, Montalto de Mecca M, de Castro CR, Bietto FM, Castro JA. 2010. Metabolization of nifurtimox and benznidazole in cellular fractions of rat mammary tissue. Hum. Exp. Toxicol. 29:813–822. 10.1177/0960327110361756. [DOI] [PubMed] [Google Scholar]
- 37.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. 2004. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 113:832–836. 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 38.Thong BY, Tan TC. 2011. Epidemiology and risk factors for drug allergy. Br. J. Clin. Pharmacol. 71:684–700. 10.1111/j.1365-2125.2010.03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang JJ, Burchard EG, Choudhry S, Johnson CC, Ownby DR, Favro D, Chen J, Akana M, Ha C, Kwok PY, Krajenta R, Havstad SL, Joseph CL, Seibold MA, Shriver MD, Williams LK. 2008. Differences in allergic sensitization by self-reported race and genetic ancestry. J. Allergy Clin. Immunol. 122:820–827 e829. 10.1016/j.jaci.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans RS, Lloyd JF, Stoddard GJ, Nebeker JR, Samore MH. 2005. Risk factors for adverse drug events: a 10-year analysis. Ann. Pharmacother. 39:1161–1168. 10.1345/aph.1E642. [DOI] [PubMed] [Google Scholar]
- 41.Riedl MA, Casillas AM. 2003. Adverse drug reactions: types and treatment options. Am. Fam. Physician 68:1781–1790. [PubMed] [Google Scholar]
- 42.Tornheim JA, Lozano Beltran DF, Gilman RH, Castellon M, Solano Mercado MA, Sullca W, Torrico F, Bern C. 2013. Improved completion rates and characterization of drug reactions with an intensive Chagas disease treatment program in rural Bolivia. PLoS Negl. Trop. Dis. 7:e2407. 10.1371/journal.pntd.0002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobo MG, Pinheiro SM, Castro JG, Momente VG, Pranchevicius MC. 2013. Adverse drug reaction monitoring: support for pharmacovigilance at a tertiary care hospital in Northern Brazil. BMC Pharmacol. Toxicol. 14:5. 10.1186/2050-6511-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Andrade AL, Zicker F, de Oliveira RM, Almeida Silva S, Luquetti A, Travassos LR, Almeida IC, de Andrade SS, de Andrade JG, Martelli CM. 1996. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348:1407–1413. 10.1016/S0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 45.Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H. 2011. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics 127:e212–e218. 10.1542/peds.2010-1172. [DOI] [PubMed] [Google Scholar]
- 46.Demoly P, Bousquet J. 2001. Epidemiology of drug allergy. Curr. Opin. Allergy Clin. Immunol. 1:305–310. [DOI] [PubMed] [Google Scholar]
- 47.Modayil RR, Harugeri A, Parthasarathi G, Ramesh M, Prasad R, Naik V, Giriyapura V. 2010. Adverse drug reactions to antiretroviral therapy (ART): an experience of spontaneous reporting and intensive monitoring from ART centre in India. Pharmacoepidemiol. Drug. Saf. 19:247–255. 10.1002/pds.1907. [DOI] [PubMed] [Google Scholar]
- 48.Onder G, Petrovic M, Tangiisuran B, Meinardi MC, Markito-Notenboom WP, Somers A, Rajkumar C, Bernabei R, van der Cammen TJ. 2010. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch. Intern. Med. 170:1142–1148. [DOI] [PubMed] [Google Scholar]
- 49.Carrilero B, Murcia L, Martinez-Lage L, Segovia M. 2011. Side effects of benznidazole treatment in a cohort of patients with Chagas disease in non-endemic country. Rev. Esp. Quimioter. 24:123–126. [PubMed] [Google Scholar]
- 50.Gallagher PF, O'Connor MN, O'Mahony D. 2011. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin. Pharmacol. Ther. 89:845–854. 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]


