Abstract
The emergence of multidrug-resistant (MDR) strains of hepatitis B virus (HBV) is a major concern. This study aimed to investigate the efficacy and safety of combination therapy with entecavir (ETV) plus tenofovir disoproxil fumarate (TDF) against MDR HBV. To adjust for differences in baseline characteristics, inverse probability weighting (IPW) using propensity scores for the entire cohort and weighted Cox proportional hazards models were applied. Ninety-three consecutive patients who were treated with ETV-TDF combination therapy for >6 months were included; at baseline, 45 were infected with HBV strains with genotypic resistance to lamivudine (LAM) and ETV (the LAM/ETV-R group), 28 with strains resistant to LAM and adefovir (ADV) (the LAM/ADV-R group), and 20 with strains resistant to LAM, ETV, and ADV (the LAM/ETV/ADV-R group). The median duration of rescue therapy was 13.0 (range, 6.7 to 31.7) months. Seventy-four of 93 patients (79.6%) achieved complete virologic suppression, after a median of 4.5 (95% confidence interval, 3.0 to 6.0) months. The cumulative probability of complete virologic suppression at month 6 was 63.6% (55.7%, 75.0%, and 65.0% in the LAM/ETV-R, LAM/ADV-R, and LAM/ETV/ADV-R groups, respectively). During the treatment period, these probabilities were not significantly different across the resistance profiles before and after IPW (P = 0.072 and P = 0.510, respectively). In multivariate analysis, a lower baseline HBV DNA level, but not resistance profiles, was an independent predictor of complete virologic suppression. Renal dysfunction was not observed during the treatment period. In conclusion, rescue therapy with ETV-TDF combination is efficient and safe in patients infected with MDR HBV strains regardless of the antiviral drug resistance profiles.
INTRODUCTION
The goal of chronic hepatitis B (CHB) treatment is to achieve early and sustained suppression of hepatitis B virus (HBV) replication, which is demonstrated to prevent progression of liver disease to cirrhosis and development of hepatocellular carcinoma (1, 2). The availability of nucleos(t)ide analogues such as tenofovir disoproxil fumarate (TDF) and entecavir (ETV), which are more potent than other antiviral drugs, has significantly improved treatment of CHB (3–5). However, many patients were treated with less potent antiviral drugs (i.e., lamivudine [LAM], telbivudine [LdT], and adefovir [ADV]) as first-line therapy and then with sequential monotherapies, which contributed to the development of multidrug resistance (MDR) (6, 7). The emergence of drug-resistant viral strains results in increased viral loads, followed by increases in serum alanine aminotransferase (ALT) levels and subsequent progression of liver disease (8–10). In the absence of treatment intervention with appropriate rescue therapy based on cross-resistance profiles, patients are at significant risk of hepatic decompensation (11). Previous antiviral treatment history may impair the antiviral efficacy of rescue therapy to induce viral suppression; therefore, the choice of optimal treatment in patients with MDR HBV strains is critical to avoid subsequent treatment failure (6).
To date, clinical data on the efficacy of the rescue therapies in patients infected with MDR HBV strains are limited. Therefore, recent international guidelines, which recommend rescue therapeutic regimens in these patients, lack solid clinical evidences (12–14). Combination therapy with a nucleoside and a nucleotide is recommended by the current European Association for the Study of the Liver clinical practice guideline based on in vitro cross-resistance data and insufficient clinical data (4). We previously reported that rescue therapy with combinations of ADV plus nucleoside analogues has limited efficacy in CHB patients with LAM and ETV resistance (15). A European study showed that combination therapy with ETV plus TDF is efficient and safe in patients with viral resistance patterns or with only partial antiviral responses to prior antiviral therapies. However, only 21 of 57 patients included in that study were determined to be infected with MDR HBV strains; moreover, only 1 patient showed an amino acid substitution profile conferring triple resistance to LAM, ETV, and ADV (12).
Therefore, we aimed to investigate the antiviral efficacy and safety of combination therapy with ETV and TDF, which are the most potent nucleoside and nucleotide analogues, respectively, in CHB patients who had developed MDR after antiviral treatment and to compare the efficacy according to genotypic resistance profiles.
MATERIALS AND METHODS
Study population.
This retrospective cohort study included CHB patients who had developed MDR after sequential treatment with multiple antivirals and who were treated with ETV (1.0 mg once daily) plus TDF (300 mg once daily) as rescue therapy for at least 6 months. MDR was defined as the presence of genotypic resistance to 2 or more groups of nucleos(t)ide analogues (l-nucleoside [LAM or LdT], acyclic phosphonate [ADV], and d-cyclopentane [ETV]) (6). Ninety-three consecutive patients who started ETV-TDF combination therapy from August 2011 to August 2013 at a tertiary hospital (Seoul National University Hospital, Seoul, Republic of Korea) were included. Patients were excluded if they had following conditions: prior exposure to TDF, coinfection with hepatitis C virus or human immunodeficiency virus (HIV), or a history of cytotoxic chemotherapy or organ transplantation.
This study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Seoul National University Hospital. We were exempt from the need for written informed consent because the data were analyzed anonymously.
Study measurements.
Laboratory measurements were assessed for all patients, including serum levels of ALT, creatinine, and HBV DNA and hepatitis B e antigen (HBeAg) and antibody (anti-HBe Ab) every 2 to 3 months. At baseline and at each follow-up visit, serum HBV DNA levels were quantified using the Cobas AmpliPrep/Cobas TaqMan version 2.0 assay (Roche Molecular System, Branchburg, NJ), which has a dynamic range of quantification of 20 to 1.7 × 108 IU/ml (1.3 to 8 log10 IU/ml) (16). HBeAg and anti-HBe Ab were determined using a radioimmunoassay (RIA Elisa Rapid kit; Shin Jin Medics, Seoul, Republic of Korea). Genotypic resistance, defined as the detection of HBV strains with amino acid substitutions conferring drug resistance, was evaluated in all study patients at baseline and in patients who developed virologic breakthrough during the rescue therapy. The amino acid substitutions conferring resistance to LAM (i.e., rtL180M and rtM204V/I/S), ADV (rtA181T/V and rtN236T), and ETV (rtL180M + rtM204V/I ± rtI169T ± rtV173L ± rtM250V/I/L/M ± rtT184S/A/I/L/G/C/M ± rtS202I/G) were analyzed (4, 6). Direct PCR-based DNA sequencing using the BigDye Terminator version 3.1 Ready Reaction cycle sequencing kit (Applied Biosystems, Foster City, CA) and the ABI Prism 3730 genetic analyzer (Perkin-Elmer, Foster City, CA) was performed to identify genotypic resistance as previously described (17).
Definitions and study endpoints.
The primary endpoint was complete virologic suppression, defined as undetectable HBV DNA by quantitative PCR assay. Secondary endpoints were the change in serum HBV DNA level from baseline during the rescue therapy, normalization of serum ALT (biochemical response), and virologic breakthrough. A virologic breakthrough was defined as an increase in the serum HBV DNA level of >1 log10 IU/ml above the nadir level achieved during the treatment period (3–5).
Statistical analysis.
For between-group comparisons, the Kruskal-Wallis test was performed for continuous variables, and either the χ2 test or Fisher's exact test was used for categorical variables. Cumulative probabilities and times to events were estimated using the Kaplan-Meier method and compared using the log rank test. For patients who were lost to follow-up, the length of follow-up was censored at the date of last visit. To identify independent predictors of complete virologic suppression, the Cox proportional hazards models were used. Subgroup analyses were also performed according to the baseline status of HBeAg and resistance-associated substitutions.
To adjust for differences in baseline characteristics and compare the antiviral efficacy of ETV-TDF combination therapy according to the resistance profiles, inverse probability weighting (IPW) based on the propensity score was used (18, 19). For each patient, a propensity score was calculated using a logistic regression model that included the baseline characteristics. This propensity score model yielded a c-statistic of 0.817. The three groups were balanced using an inverse probability weight for each patient, which was generated based on the propensity score. After IPW, the balance of baseline characteristics among the groups was assessed, and thereafter weighted Cox proportional hazards models were fitted. All tests were conducted as two-sided tests and a P value of <0.05 was considered significant. All statistical analyses were performed using SAS software version 9.3 (SAS Inc., Cary, NC) and PASW statistical software version 18.0 (IBM, Chicago, IL).
RESULTS
Characteristics of the study population.
The baseline clinical and demographic characteristics of the 93 patients are summarized in Table 1. All 93 patients were infected with HBV genotype C. At baseline, 45 were infected with HBV strains with amino acid substitutions conferring resistance to LAM and ETV (the LAM/ETV-R group), 28 with strains resistant to LAM and ADV (the LAM/ADV-R group), and 20 with strains resistant to LAM, ETV, and ADV (the LAM/ETV/ADV-R group). At the start of the rescue therapy with the ETV-TDF combination, 37 of the 93 patients (39.8%) experienced virologic breakthrough. Among these patients, 29 patients developed biochemical breakthrough following virologic breakthrough. The median duration of ETV-TDF combination therapy was 13.0 (range, 6.7 to 31.7) months. Three of the study patients (one patient of each group) were lost to follow-up after 9.3, 14.6, and 25.0 months of ETV-TDF combination therapy. At baseline, the three groups differed significantly in the two variables describing previous treatment history: the number of lines of prior antiviral treatment and the duration of previous treatment (P < 0.001 and P = 0.028, respectively). The LAM/ETV/ADV-R group had received significantly more lines of antiviral treatment prior to ETV-TDF combination therapy than either the LAM/ETV-R group (P < 0.001) or the LAM/ADV-R group (P < 0.001). The duration of previous treatment in the LAM/ETV-R group was longer than that in the LAM/ETV/ADV-R group (P = 0.010), whereas it was not significantly different from that in the LAM/ADV-R group (P = 0.058). In all of the study patients, except for one patient, antivirals were directly switched to ETV-TDF combination therapy without interruption of antiviral treatment.
TABLE 1.
Baseline characteristics by genotypic resistance profile
| Characteristic | Value for groupa |
P valueb | ||
|---|---|---|---|---|
| LAM/ETV-R (n = 45) | LAM/ADV-R (n = 28) | LAM/ETV/ADV-R (n = 20) | ||
| Age (yr)c | 56 (32–71) | 50.5 (23–68) | 54 (29–67) | 0.499 |
| Male gender | 31 (68.9) | 22 (78.6) | 14 (70.0) | 0.652 |
| Serum HBV DNA (log10 IU/ml)c | 3.66 (0.87–7.37) | 2.95 (1.88–6.71) | 2.60 (2.02–8.23) | 0.116 |
| Serum ALT (IU/liter)c | 30 (9–275) | 27.5 (11–843) | 26.5 (14–57) | 0.636 |
| Serum creatinine (mg/dl)c | 0.86 (0.53–1.26) | 0.88 (0.53–1.31) | 0.86 (0.54–1.14) | 0.869 |
| HBeAg positive | 31 (68.9) | 18 (64.3) | 14 (70.0) | 0.893 |
| Liver cirrhosisd | 16 (35.6) | 8 (28.6) | 4 (20.0) | 0.469 |
| Lines of prior antiviral treatment | 2 (1–5) | 3 (2–5) | 5 (3–5) | <0.001 |
| Duration of previous treatment (mo)c | 28.2 (2.7–78.7) | 17.5 (2.7–85.7) | 17.5 (1.4–40.2) | 0.028 |
| Time point of rescue therapy | ||||
| Virologic breakthrough | 21 (46.7) | 11 (39.3) | 5 (25.0) | 0.257 |
| Biochemical breakthrough | 17 (37.8) | 6 (21.4) | 6 (30.0) | 0.339 |
Unless otherwise indicated, data are number of patients, with percentages in parentheses. LAM, lamivudine; ETV, entecavir; ADV, adefovir; HBV, hepatitis B virus; ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen.
The Kruskal-Wallis test and χ2 test (or Fisher's exact test) were used to analyze the differences among the groups.
Data are medians, with ranges in parentheses.
Liver cirrhosis was diagnosed when the platelet count was below 100,000/mm3 and associated splenomegaly or esophageal-gastric varices were detected.
Virologic responses.
The overall mean changes in HBV DNA level induced by ETV-TDF combination therapy at months 3, 6, and 12 were −2.42 log10 IU/ml, −2.85 log10 IU/ml, and −3.19 log10 IU/ml, respectively. In the LAM/ETV-R group, the decline in serum HBV DNA levels from baseline to month 3 was significantly greater than that in the LAM/ETV/ADV-R group (P = 0.002), whereas the changes in these levels at months 6 and 12 were not significantly different among the three groups (P = 0.719 and P = 0.377, respectively) (Fig. 1 and Table 2). Overall, 74 of 93 patients (79.6%) achieved complete virologic suppression during the entire treatment period: 32 of 45 (71.1%) in the LAM/ETV-R group, 25 of 28 (89.3%) in the LAM/ADV-R group, and 17 of 20 (85.0%) in the LAM/ETV/ADV-R group. The median time required to reach an undetectable HBV DNA level was 4.5 (95% confidence interval [CI], 3.0 to 6.0) months in all patients: 4.7 (95% CI, 2.3 to 7.1) months in the LAM/ETV-R group, 3.0 (95% CI, 1.1 to 4.9) months in the LAM/ADV-R group, and 5.2 (95% CI, 3.6 to 6.7) months in the LAM/ETV/ADV-R group. The cumulative proportion of complete virologic suppression at month 6 was 63.6% overall: 55.7% in the LAM/ETV-R group, 75.0% in the LAM/ADV-R group, and 65.0% in the LAM/ETV/ADV-R group (Table 2). During the treatment period, there was no significant difference among the groups (P = 0.072) (Fig. 2). In multivariate Cox regression analysis, a lower baseline HBV DNA level was independently associated with complete virologic suppression (hazard ratio [HR], 0.565; 95% CI, 0.461 to 0.692; P < 0.001) (Table 3). In the entire cohort, the probabilities of complete virologic suppression were significantly influenced by the baseline HBV DNA levels at the beginning of the rescue therapy with the ETV-TDF combination (P < 0.001). Patients with HBV DNA levels of less than 104 IU/ml had a significantly higher probability of achieving complete virologic suppression (HR, 8.482; 95% CI, 4.286 to 16.786; P < 0.001) (see Fig. S1 in the supplemental material).
FIG 1.
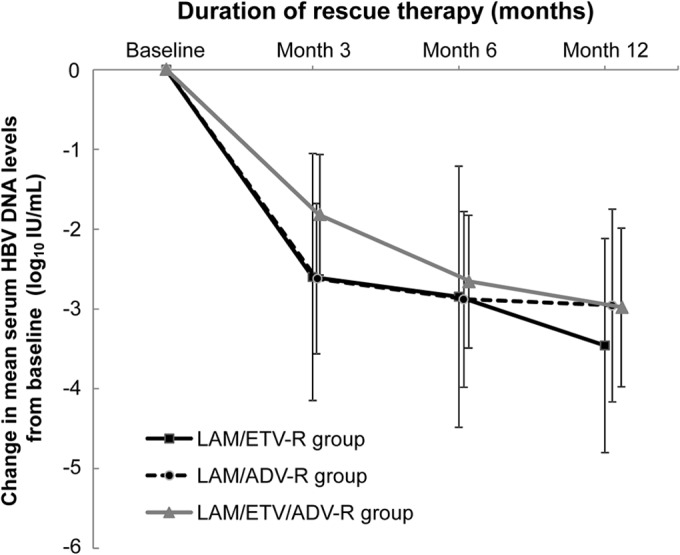
Changes in HBV viral loads during the rescue therapy. The changes in serum HBV DNA levels from baseline during the treatment period, plotted as mean log10 change from baseline values, are shown for each group. The data represent the means for 45 patients in the LAM/ETV-R group, 28 patients in the LAM/ADV-R group, and 20 patients in the LAM/ETV/ADV-R group at months 3, 6, and 12. Among the three groups, the declines in serum HBV DNA levels differed significantly at month 3 (P = 0.008) but not at months 6 and 12 (P = 0.719 and P = 0.377, respectively). The error bars represent the standard deviations. HBV, hepatitis B virus; LAM, lamivudine; ETV, entecavir; ADV, adefovir.
TABLE 2.
Virologic and biochemical response during rescue therapy by genotypic resistance profile
| Outcome and time point | Value for group |
P value | ||
|---|---|---|---|---|
| LAM/ETV-R (n = 45) | LAM/ADV-R (n = 28) | LAM/ETV/ADV-R (n = 20) | ||
| Virologic | ||||
| Change in HBV DNA (log10 IU/ml)a | ||||
| 3 mo | −2.86 (−4.83 to 2.90) | −2.30 (−4.83 to −0.67) | −2.10 (−3.50 to −0.05) | 0.008 |
| 6 mo | −3.02 (−5.82 to 1.73) | −2.52 (−5.84 to −1.04) | −2.48 (−4.66 to −1.02) | 0.719 |
| 12 mo | −3.19 (−6.43 to −1.72) | −2.48 (−5.84 to −1.14) | −2.51 (−4.73 to −1.18) | 0.377 |
| Complete virologic suppression (undetectable HBV DNA)b | 0.072c | |||
| 3 mo | 31.1 (45) | 50.0 (28) | 30.0 (20) | |
| 6 mo | 55.7 (45) | 75.0 (28) | 65.0 (20) | |
| 9 mo | 58.1 (32) | 86.6 (23) | 88.3 (18) | |
| 12 mo | 67.8 (18) | 93.3 (19) | 88.3 (16) | |
| 24 mo | 82.8 (2) | NAd | 88.3 (2) | |
| Biochemical (normalization of serum ALT)b | 0.003c | |||
| 3 mo | 5.9 (17) | 50.0 (6) | 50.0 (6) | |
| 6 mo | 23.5 (17) | 50.0 (6) | 50.0 (6) | |
| 9 mo | 30.5 (15) | 83.3 (3) | 50.0 (6) | |
| 12 mo | 44.5 (11) | 100.0 (2) | 83.3 (6) | |
Data are medians, with ranges in parentheses.
Data are cumulative probabilities of the response at the indicated time points, based on the Kaplan-Meier method, with the number of patients under observation in parentheses.
The log rank test was used to compare the hazard rates among the groups.
NA, not applicable.
FIG 2.
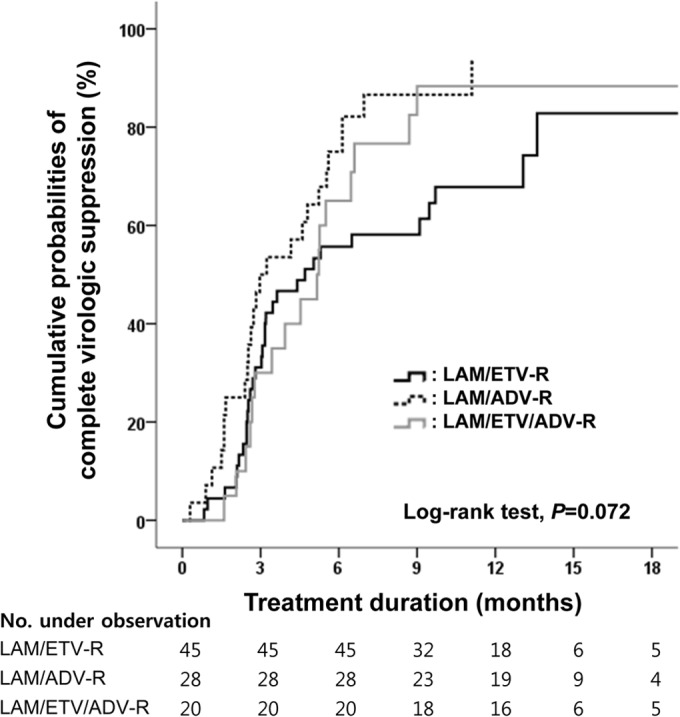
Cumulative probability of complete virologic suppression during the rescue therapy. Cumulative probabilities of complete virologic suppression, i.e., undetectable levels of HBV DNA according to PCR assays, during the treatment period are shown for each group. LAM, lamivudine; ETV, entecavir; ADV, adefovir.
TABLE 3.
Univariate and multivariate analysis of the clinical factors predictive of complete virologic suppression during rescue therapy
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Hazard ratioa | P valueb | Adjusted hazard ratio | P value | |
| Age (per yr) | 1.003 (0.978–1.028) | 0.843 | ||
| Baseline serum HBV DNA (per 1 log10 IU/ml) | 0.584 (0.489–0.697) | <0.001 | 0.565 (0.461–0.692) | <0.001 |
| Baseline serum ALT (per IU/liter) | 0.998 (0.994–1.002) | 0.262 | ||
| HBeAg (positive vs negative) | 0.700 (0.431–1.137) | 0.150 | ||
| Liver cirrhosis (positive vs negative)c | 1.082 (0.655–1.787) | 0.760 | ||
| Time point of rescue therapy | ||||
| Virologic breakthrough (yes vs no) | 0.747 (0.459–1.215) | 0.240 | ||
| Biochemical breakthrough (yes vs no) | 0.512 (0.298–0.879) | 0.015 | 1.238 (0.664–2.309) | 0.501 |
| Drug resistance | 0.078 | |||
| LAM/ETV-R vs LAM/ADV-R | 0.541 (0.316–0.924) | 0.024 | ||
| LAM/ETV-R vs LAM/ETV/ADV-R | 0.751 (0.414–1.362) | 0.345 | ||
| LAM/ADV-R vs LAM/ETV/ADV-R | 1.393 (0.751–2.584) | 0.294 | ||
Data in parentheses are 95% CIs.
P values were determined using Cox proportional hazards regression models. A P value of <0.05 indicates a significant difference.
Liver cirrhosis was diagnosed when the platelet count was below 100,000/mm3 and associated splenomegaly or esophageal-gastric varices were detected.
The cumulative probabilities of complete virologic suppression were comparable among the three groups in the subgroups of both HBeAg-positive and HBeAg-negative patients (P = 0.224 and P = 0.226, respectively) (see Fig. S2 in the supplemental material). Ten of 48 patients with ADV resistance (the LAM/ADV-R group and the LAM/ETV/ADV-R group) had the double substitution rtA181T/V + rtN236T at baseline and had rates of complete virologic suppression comparable to those for patients with a single substitution, rtA181T/V or rtN236T (P = 0.361) (see Fig. S3 in the supplemental material).
Biochemical responses.
During the treatment period, a biochemical response was achieved in 19 of 29 patients (65.5%) who had developed a biochemical breakthrough prior to ETV-TDF combination therapy. The cumulative probabilities of biochemical response at month 6 were 23.1% in the LAM/ETV-R group, 50.0% in the LAM/ADV-R group, and 50.0% in the LAM/ETV/ADV-R group (Table 2). The LAM/ETV-R group showed a significantly lower probability of biochemical response than either the LAM/ADV-R group (HR, 0.158; 95% CI, 0.048 to 0.525; P = 0.003) or the LAM/ETV/ADV-R group (HR, 0.301; 95% CI, 0.096 to 0.944; P = 0.039).
Virologic breakthrough.
Two patients experienced virologic breakthrough during the treatment period: one patient in the LAM/ETV-R group and one patient in the LAM/ADV-R group. At the time of the breakthrough, no additional amino acid substitution, other than substitutions detected at baseline, was detected. None of these patients developed a biochemical breakthrough.
Treatment response analysis using IPW.
After the study population was adjusted using IPW, the baseline characteristics, including the number of lines of prior antiviral treatment and duration of previous treatment, became more balanced among the groups (see Table S1 in the supplemental material).
The weighted cumulative probabilities of complete virologic suppression at month 6 were 65.9% in the LAM/ETV-R group, 84.6% in the LAM/ADV-R group, and 54.8% in the LAM/ETV/ADV-R group (Fig. 3). Weighted probabilities of complete virologic suppression were still comparable among the three groups (P = 0.510). In multivariate weighted Cox regression analysis, a lower baseline HBV DNA level remained an independent predictor for complete virologic suppression (HR, 0.676; 95% CI, 0.557 to 0.820; P < 0.001) (see Table S2 in the supplemental material). In the 29 patients with elevated serum ALT levels at baseline, weighted cumulative probabilities of biochemical response among the groups became comparable (P = 0.116). The LAM/ETV-R group showed weighted probabilities of biochemical response similar to those in the LAM/ADV-R group (HR, 0.388; 95% CI, 0.138 to 1.095; P = 0.074) and the LAM/ETV/ADV-R group (HR, 0.275; 95% CI, 0.063 to 1.196; P = 0.085).
FIG 3.
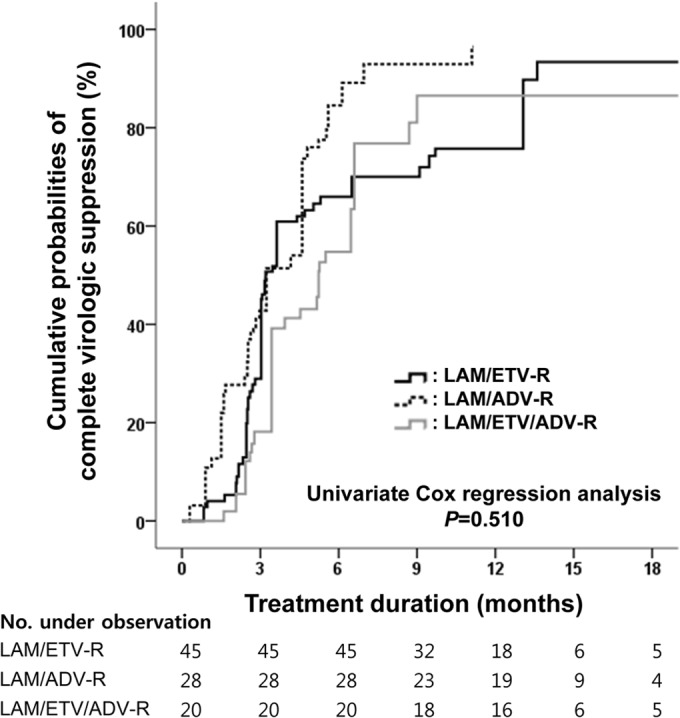
Weighted cumulative probability of complete virologic suppression during the rescue therapy. Weighted cumulative probabilities of complete virologic suppression during the treatment period are shown for each group. LAM, lamivudine; ETV, entecavir; ADV, adefovir.
Adverse events.
None of study patients experienced deterioration of renal function during the ETV-TDF combination therapy. The overall median changes in serum creatinine level from baseline to months 3, 6, and 12 were 0.03, 0.05, and 0.08 mg/dl, respectively (ranges, −0.19 to 0.42, −0.27 to 0.44, and −0.17 to 0.27, respectively).
DISCUSSION
In this study, we evaluated the antiviral efficacy of ETV-TDF combination therapy in patients infected with MDR HBV strains as rescue therapy and whether efficacy differed according to drug resistance profiles using IPW. In 74 of 93 patients (79.6%), serum HBV DNA levels declined to undetectable levels during this rescue therapy, and the overall probability of complete virologic suppression at month 6 exceeded 60%. The probabilities of complete virologic suppression were similar across the genotypic resistance profiles before and after adjustment for differences among the groups using IPW. Moreover, ETV-TDF combination therapy was well tolerated without serious adverse events, including renal dysfunction.
This is the largest of the studies that demonstrated the antiviral efficacy of ETV-TDF rescue therapy in CHB patients with genotypic resistance to multiple antivirals and the first study to investigate the efficacy of treatment in patients with substitution profiles conferring triple resistance to LAM, ETV, and ADV compared to other patients with MDR. Consequently, this study revealed several novel findings. First, the current study showed that ETV-TDF treatment is effective in patients with MDR and produces a relatively high rate of complete virologic suppression at an early time point, even in patients with triple resistance to LAM, ETV, and ADV. A prior study that evaluated the efficacy of ETV-TDF combination therapy in CHB patients pretreated with antiviral drugs showed that 51 of 57 patients (89.5%) achieved undetectable HBV DNA during the rescue therapy (12). However, that study included only 21 patients with MDR and defined undetectable HBV DNA by a quantitative PCR assay using a lower limit of 80 IU/ml, higher than the limit of 20 IU/ml of our study; therefore, it may have overestimated the efficacy of the ETV-TDF combination for MDR CHB patients. Second, our study suggested that ETV-TDF combination therapy may be more effective than TDF monotherapy in patients harboring HBV strains with substitutions associated with ADV resistance. In a previous study that assessed the efficacy of TDF monotherapy after prior treatment failure with nucleos(t)ide analogues, the cumulative probability of achieving undetectable HBV DNA at month 12 was 33% in patients with initial resistance against ADV, which was much lower than the result in our study (90.5%) (data not shown). Furthermore, that study defined undetectable HBV DNA using a lower limit of 400 copies/ml (60 IU/ml). If that study had used a more sensitive PCR assay, the antiviral efficacy of TDF monotherapy would probably have been worse (13). Because the follow-up period of our study was relatively short, additional long-term studies are clearly needed to evaluate the efficacy of the rescue treatment regimens in patients resistant to multiple antivirals. However, the results of studies conducted so far suggest that an appropriate combination of the most potent drugs with high genetic barriers and compensatory cross-resistance profiles, such as ETV and TDF, is necessary for these difficult-to-treat patients.
We acknowledge some limitations resulting from the nature of retrospective study design of this study. Therefore, we aimed to reduce the bias in patient selection and to describe the efficacy of treatment according to the resistance profiles by employment of IPW. Although the weighted probability of complete virologic suppression at month 6 in the LAM/ETV/ADV-R group was lower than that in either the LAM/ETV-R group or the LAM/ADV-R group, the genotypic resistance profiles did not affect the antiviral efficacy of ETV-TDF combination therapy. We also found that a lower baseline HBV DNA level independently predicted a favorable virologic response, and the virologic response achieved by the ETV-TDF combination was impaired in patients with high baseline HBV DNA levels. In contrast, liver cirrhosis was not associated with complete virologic suppression, which indicated that the antiviral efficacy of treatment with ETV-TDF combination is not affected by the presence of advanced liver disease.
Subgroup analysis of patients with substitutions associated with ADV resistance revealed that all 10 patients with the double amino acid substitution rtA181T/V + rtN236T at baseline achieved complete virologic suppression during the rescue therapy, and the probabilities of complete virologic suppression were comparable to those in patients with a single substitution, rtA181T/V or rtN236T. A previous in vitro study showed that the double substitution rtA181T/V + rtN236T decreases susceptibility to TDF by 1.2-to 6.8-fold but not that to ETV (20). Furthermore, in a previous clinical study which analyzed the antiviral efficacy of TDF monotherapy or TDF-LAM combination therapy in patients with prior failure with both LAM and ADV, HBV strains with the double substitution rtA181T/V + rtN236T were refractory to TDF monotherapy or TDF-LAM combination therapy, with persistent HBV replication during the treatment (14). Therefore, ETV-TDF combination therapy should be considered the treatment of choice in such patients to achieve early and sustained viral suppression. Adding ADV therapy in patients infected with LAM-resistant HBV strains was demonstrated to reduce the risk of developing resistance to ADV and hepatitis flare following virologic breakthrough compared to switching to ADV therapy (21–23). Similarly, treating patients infected with HBV strains resistant to ETV with combination therapy with ETV plus TDF can have an additional benefit over TDF monotherapy, especially with regard to a reduced risk of developing subsequent resistance. However, considering limited health budget resources, especially in Asian countries, and cost-effectiveness, TDF monotherapy may be an alternative therapeutic option in selected patients with MDR, such as patients with low HBV DNA levels at baseline and without amino acid substitution profiles conferring cross-resistance to TDF. Recently, TDF monotherapy was shown to be highly effective for treatment of CHB patients with LAM-resistant HBV strains and not to be associated with resistance development (24, 25). Further studies to evaluate the antiviral efficacy of TDF monotherapy against MDR HBV are warranted.
Virologic breakthrough was observed in 2 patients during the treatment period, and no additional amino acid substitution, other than substitutions detected at baseline, was newly detected by genotypic testing. After development of virologic breakthrough, ETV-TDF combination therapy was continued in both patients, and thereafter, substantial viral suppression was induced. The emergence of additional substitutions cannot be excluded, because a particular strain can be detected by direct PCR-based DNA sequencing only if present in ≥20% of the entire quasispecies pool (6, 26). Moreover, host factors, such as impaired drug transport or phosphorylation, which is required to convert TDF to the active form, might be involved in the failure to suppress viral replication (6). However, poor adherence to the rescue therapy was thought to be the principal factor causing virologic rebound in these patients. Adherence to antiviral drugs should be emphasized to maximize HBV viral suppression and minimize the risk of subsequent treatment failure, particularly in patients who have MDR HBV strains (27).
TDF is cleared principally by the kidneys, and TDF-associated nephrotoxicity in HIV-infected patients was previously reported, whereas it has been reported rarely in CHB patients (28–30). In our study, deterioration of renal function was not observed in any patient during the entire treatment period.
In summary, the results of our study indicate that this ETV-TDF combination is an efficient and safe rescue therapy for CHB patients infected with HBV strains resistant to multiple antiviral drugs regardless of the genotypic resistance profiles. Further studies would be necessary to evaluate whether TDF monotherapy has effect comparable to that of ETV-TDF combination therapy in CHB patients infected with MDR HBV strains.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by the Seoul National University Hospital Research Fund (grant no. 30-2014-0070).
Footnotes
Published ahead of print 25 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03845-14.
REFERENCES
- 1.Liaw YF. 2006. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir. Ther. 11:669–679. [PubMed] [Google Scholar]
- 2.Papatheodoridis GV, Dimou E, Dimakopoulos K, Manolakopoulos S, Rapti I, Kitis G, Tzourmakliotis D, Manesis E, Hadziyannis SJ. 2005. Outcome of hepatitis B e antigen-negative chronic hepatitis B on long-term nucleos(t)ide analog therapy starting with lamivudine. Hepatology 42:121–129. 10.1002/hep.20760. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M, Kuiken C. 2007. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology 46:254–265. 10.1002/hep.21698. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. 2012. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J. Hepatol. 57:167–185. 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Lok AS, McMahon BJ. 2009. Chronic hepatitis B: update 2009. Hepatology 50:661–662. 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 6.Zoulim F, Locarnini S. 2012. Management of treatment failure in chronic hepatitis B. J. Hepatol 56(Suppl 1):S112–S122. 10.1016/S0168-8278(12)60012-9. [DOI] [PubMed] [Google Scholar]
- 7.Yim HJ, Hussain M, Liu Y, Wong SN, Fung SK, Lok AS. 2006. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology 44:703–712. 10.1002/hep.21290. [DOI] [PubMed] [Google Scholar]
- 8.Nafa S, Ahmed S, Tavan D, Pichoud C, Berby F, Stuyver L, Johnson M, Merle P, Abidi H, Trepo C, Zoulim F. 2000. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology 32:1078–1088. 10.1053/jhep.2000.19619. [DOI] [PubMed] [Google Scholar]
- 9.Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. 2001. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology 34:785–791. 10.1053/jhep.2001.27563. [DOI] [PubMed] [Google Scholar]
- 10.Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. 2003. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. 36:687–696. 10.1086/368083. [DOI] [PubMed] [Google Scholar]
- 11.Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, Dienstag JL, Heathcote EJ, Little NR, Griffiths DA, Gardner SD, Castiglia M. 2003. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 125:1714–1722. 10.1053/j.gastro.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Petersen J, Ratziu V, Buti M, Janssen HL, Brown A, Lampertico P, Schollmeyer J, Zoulim F, Wedemeyer H, Sterneck M, Berg T, Sarrazin C, Lutgehetmann M, Buggisch P. 2012. Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: an international multicenter cohort study. J. Hepatol. 56:520–526. 10.1016/j.jhep.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 13.van Bommel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Huppe D, Stein K, Trojan J, Sarrazin C, Bocher WO, Spengler U, Wasmuth HE, Reinders JG, Moller B, Rhode P, Feucht HH, Wiedenmann B, Berg T. 2010. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology 51:73–80. 10.1002/hep.23246. [DOI] [PubMed] [Google Scholar]
- 14.Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, Desmond PV, Roberts SK, Locarnini S, Bowden S, Angus PW. 2011. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut 60:247–254. 10.1136/gut.2010.223206. [DOI] [PubMed] [Google Scholar]
- 15.Lee YB, Lee JH, Choi WM, Cho YY, Yoo JJ, Lee M, Lee DH, Cho Y, Yu SJ, Kim YJ, Yoon JH, Kim CY, Lee HS. 2013. Efficacy of adefovir-based combination therapy for patients with lamivudine- and entecavir-resistant chronic hepatitis B virus infection. Antimicrob. Agents Chemother. 57:6325–6332. 10.1128/AAC.01742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chevaliez S, Bouvier-Alias M, Laperche S, Hezode C, Pawlotsky JM. 2010. Performance of version 2.0 of the Cobas AmpliPrep/Cobas TaqMan real-time PCR assay for hepatitis B virus DNA quantification. J. Clin. Microbiol. 48:3641–3647. 10.1128/JCM.01306-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idilman R, Kaymakoglu S, Oguz Onder F, Ahishali E, Bektas M, Cinar K, Pinarbasi B, Karayalcin S, Badur S, Cakaloglu Y, Mithat Bozdayi A, Bozkaya H, Okten A, Yurdaydin C. 2009. A short course of add-on adefovir dipivoxil treatment in lamivudine-resistant chronic hepatitis B patients. J. Viral Hepat. 16:279–285. 10.1111/j.1365-2893.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. 1983. The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55. 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 19.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. 2007. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med. Care 45:S103–S107. 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 20.Villet S, Pichoud C, Billioud G, Barraud L, Durantel S, Trepo C, Zoulim F. 2008. Impact of hepatitis B virus rtA181V/T mutants on hepatitis B treatment failure. J. Hepatol. 48:747–755. 10.1016/j.jhep.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, Hussain M, Lok AS. 2006. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J. Hepatol. 44:283–290. 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Lampertico P, Vigano M, Manenti E, Iavarone M, Sablon E, Colombo M. 2007. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 133:1445–1451. 10.1053/j.gastro.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 23.Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. 2007. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 45:307–313. 10.1002/hep.21534. [DOI] [PubMed] [Google Scholar]
- 24.Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, Gurel S, Caruntu FA, Flaherty JF, Massetto B, Dinh P, Corsa A, Subramanian GM, McHutchison JG, Husa P, Gane E. 2014. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 146:980–988. 10.1053/j.gastro.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Corsa AC, Liu Y, Flaherty JF, Mitchell B, Fung SK, Gane E, Miller MD, Kitrinos KM. No resistance to tenofovir disoproxil fumarate through 96 weeks of treatment in patients with lamivudine-resistant chronic hepatitis B. Clin. Gastroenterol. Hepatol., in press. [DOI] [PubMed] [Google Scholar]
- 26.Shaw T, Bartholomeusz A, Locarnini S. 2006. HBV drug resistance: mechanisms, detection and interpretation. J. Hepatol. 44:593–606. 10.1016/j.jhep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Zoulim F. 2011. Hepatitis: treatment failure in chronic hepatitis B. Nat. Rev. Gastroenterol. Hepatol. 8:366–367. 10.1038/nrgastro.2011.104. [DOI] [PubMed] [Google Scholar]
- 28.Verhelst D, Monge M, Meynard JL, Fouqueray B, Mougenot B, Girard PM, Ronco P, Rossert J. 2002. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am. J. Kidney Dis. 40:1331–1333. 10.1053/ajkd.2002.36924. [DOI] [PubMed] [Google Scholar]
- 29.Woodward CL, Hall AM, Williams IG, Madge S, Copas A, Nair D, Edwards SG, Johnson MA, Connolly JO. 2009. Tenofovir-associated renal and bone toxicity. HIV Med. 10:482–487. 10.1111/j.1468-1293.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 30.Gracey DM, Snelling P, McKenzie P, Strasser SI. 2013. Tenofovir-associated Fanconi syndrome in patients with chronic hepatitis B monoinfection. Antivir. Ther. 18:945–948. 10.3851/IMP2649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


