Abstract
We characterized 30 community-associated extended-spectrum-β-lactamase-producing Escherichia coli isolates collected from five hospitals in the United States. Nineteen sequence types were identified. All sequence type 131 (ST131) isolates had the fimH30 allele. IncFII-FIA-FIB was the most common replicon type among the blaCTX-M-carrying plasmids, followed by IncFII-FIA and IncA/C. Restriction analysis of the IncFII-FIA-FIB and IncFII-FIA plasmids yielded related profiles for plasmids originating from different hospitals. The plasmids containing blaCTX-M or blaSHV were stably maintained after serial passages.
TEXT
Since its emergence in the 1980s, extended-spectrum-β-lactamase (ESBL)-producing Escherichia coli has continued to spread worldwide (1). Besides the resistance to penicillins and cephalosporins conferred by the production of ESBL, these organisms are frequently resistant to other classes of agents, including aminoglycosides, trimethoprim-sulfamethoxazole, and fluoroquinolones, which limits the therapeutic options for infections caused by ESBL-producing organisms (2). In addition to the steadily rising overall prevalence of ESBL-producing E. coli strains, the last decade has observed a major epidemiological shift in these organisms, as they have escaped the health care environment to cause community-associated infections in individuals with minimal or no recent exposure to the health care system (3, 4). This change has coincided with the expansion of a single clonal lineage of E. coli, defined as sequence type 131 (ST131) by multilocus sequence typing (MLST), which often produces CTX-M-type ESBLs. ST131 accounted for an estimated 28% of E. coli isolates in one study (5), with an even higher prevalence among ESBL-producing isolates in another (6).
We recently conducted a multicenter cohort study to document the occurrence of community-associated ESBL-producing E. coli infections in the United States (7). In this study, we identified 107 cases of community-associated ESBL-producing E. coli infections from five health care systems in a 1-year period. Fifty-four percent of them were due to E. coli ST131, and 91% produced CTX-M-type ESBLs. The findings confirmed the predominance of CTX-M-producing ST131 isolates among community-associated ESBL-producing E. coli infections in the United States, but it also indicated that nearly half of the infections were not due to ST131. In addition, little is known about the nature of plasmids carrying genes encoding CTX-M-type or other ESBLs in ST131 and other clonal lineages associated with their spread in the community.
The objective of this follow-up study was to conduct further molecular analysis of representative community-associated ESBL-producing E. coli isolates and identify clones and plasmids involved in this emerging phenomenon in the United States. Thirty community-associated ESBL-producing E. coli isolates collected at hospitals and their affiliated clinics in 5 locations in the United States (New York, Pennsylvania, Michigan, Texas, and Iowa) between 2009 and 2010 were included. They were selected from the 107 community-associated ESBL-producing E. coli isolates reported earlier (8), in order to represent different locations (5 to 7 isolates per location), ESBLs (19 isolates with CTX-M-15, 5 isolates with CTX-M-14, and 6 isolates with SHV-type ESBL), and ST131 status (10 ST131 isolates and 20 non-ST131 isolates). Non-ST131 isolates were overrepresented, since the molecular epidemiology of E. coli ST131 isolates in the United States has been investigated in detail (5, 9, 10), whereas less is known about ESBL-producing non-ST131 isolates circulating in the community.
Antimicrobial susceptibility testing was performed by the broth microdilution method using Sensititre GN4F (Trek Diagnostics, Oakwood Village, OH) and interpreted according to the latest Clinical and Laboratory Standards Institute (CLSI) breakpoints (7, 11). For tigecycline, the breakpoint recommended by the U.S. Food and Drug Administration was used. Of the 30 isolates tested, 93.3% were resistant to ceftriaxone, while 60% and 63.3% were susceptible to ceftazidime and cefepime, respectively (Table 1). The rates of resistance to ciprofloxacin and levofloxacin were 80% and 83.3%, respectively. A total of 36.7%, 46.7%, and 3.3% of the isolates were resistant to gentamicin, tobramycin, and amikacin, respectively. Among other classes of agents, 66.7% were resistant to trimethoprim-sulfamethoxazole, 6.7% were resistant to minocycline, and 70% were resistant to tetracycline. All isolates were susceptible to carbapenems, piperacillin-tazobactam, nitrofurantoin, and tigecycline.
TABLE 1.
Antimicrobial susceptibilities of community-associated ESBL-producing E. coli isolatesa
| Agent(s) | Resistance profile (%) |
MIC (μg/ml) |
||||
|---|---|---|---|---|---|---|
| Susceptible | Intermediate | Resistant | 50% | 90% | Range | |
| Ampicillin-sulbactam | 33.3 | 60 | 6.7 | 16/8 | 16/8 | ≤4/2 to >16/8 |
| Piperacillin-tazobactam | 100 | 0 | 0 | ≤8/4 | ≤8/4 | ≤8/4 |
| Ticarcillin-clavulanic acid | 53.3 | 43.3 | 3.3 | 16/2 | 64/2 | ≤8/2 to >64/2 |
| Ampicillin | 0 | 0 | 100 | >16 | >16 | >16 |
| Piperacillin | 6.7 | 20 | 73.3 | >64 | >64 | ≤16 to >64 |
| Cefazolin | 0 | 0 | 100 | >16 | >16 | >16 |
| Ceftriaxone | 3.3 | 3.3 | 93.3 | >32 | >32 | ≤0.5 to >32 |
| Ceftazidime | 60 | 16.7 | 23.3 | 4 | >16 | ≤1 to >16 |
| Cefepime | 63.3 | 10b | 26.7 | ≤4 | >32 | ≤4 to >32 |
| Aztreonam | 33.3 | 13.3 | 53.3 | 16 | >16 | ≤1 to >16 |
| Doripenem | 100 | 0 | 0 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ertapenem | 100 | 0 | 0 | ≤0.25 | ≤0.25 | ≤0.25 |
| Imipenem | 100 | 0 | 0 | ≤0.5 | ≤0.5 | ≤0.5 |
| Meropenem | 100 | 0 | 0 | ≤0.5 | ≤0.5 | ≤0.5 |
| Amikacin | 93.3 | 3.3 | 3.3 | ≤8 | 16 | ≤8 to >32 |
| Gentamicin | 60.0 | 3.3 | 36.7 | ≤2 | >8 | ≤2 to >8 |
| Tobramycin | 43.3 | 10 | 46.7 | 8 | >8 | ≤2 to>8 |
| Ciprofloxacin | 16.7 | 3.3 | 80 | >2 | >2 | ≤0.5 to >2 |
| Levofloxacin | 16.7 | 0 | 83.3 | >8 | >8 | ≤1 to >8 |
| Trimethoprim-sulfamethoxazole | 33.3 | 0 | 66.7 | >4/76 | >4/76 | ≤2/38 to >4/76 |
| Nitrofurantoin | 100 | 0 | 0 | ≤32 | ≤32 | ≤32 |
| Minocycline | 76.7 | 16.7 | 6.7 | 2 | 8 | ≤1 to >8 |
| Tetracycline | 30 | 0 | 70 | >8 | >8 | ≤4 to >8 |
| Tigecycline | 100 | 0 | 0 | ≤1 | ≤1 | ≤1 |
n = 30.
Dose-dependent susceptibility.
Multilocus sequence typing (MLST) was performed as previously described (12). New STs were registered through the E. coli MLST website (see http://mlst.warwick.ac.uk/mlst/dbs/Ecoli). The ST131 isolates were further subtyped using the fimH sequence (13). MLST of the 30 ESBL-producing isolates identified 19 different STs (Table 2). As expected from the inclusion criteria (10 presumptive ST131 isolates based on PCR screening [7, 14]), 10 isolates belonged to ST131. Seven of them were associated with blaCTX-M-15, while 2 isolates and 1 isolate were associated with blaCTX-M-14 and blaSHV-5, respectively. Two isolates each had ST38 and ST44 associated with blaCTX-M-15 or blaCTX-M-14, originating from hospitals in different states (ST38 from Michigan and Iowa and ST44 from Iowa and Texas). ST38 has been reported in CTX-M-producing E. coli worldwide, including isolates from community-associated infections, whereas less is known about ST44 (15–18). The remaining 16 STs only occurred in 1 isolate each. Twenty-four of the isolates were positive for fimH. All 10 ST131 isolates had fimH30, which assigned them to the highly virulent H30 subclone of ST131 (19). The high degree of clonal diversity of the non-ST131 isolates suggests that although the success of ST131 is clearly a major factor in the emerging epidemic of community-associated ESBL-producing E. coli isolates, nonmicrobiological factors may also be responsible for the spread of ESBL-producing non-ST131 isolates into the community.
TABLE 2.
Molecular characteristics of community-associated ESBL-producing E. coli isolates
| Isolate | Location | Source | β-Lactamase(s) | fimHa | ST | Replicon type of the plasmid carrying ESBL-encoding genes | Plasmid-mediated coresistanced |
|---|---|---|---|---|---|---|---|
| CA01 | Pennsylvania | Urine | CTX-M-15, CMY-2 | − | 617 | NTb | None |
| CA02 | Pennsylvania | Urine | SHV-2 | + | 906 | NAc | NA |
| CA03 | Pennsylvania | Urine | CTX-M-15 | + | 131 | N | None |
| CA04 | Pennsylvania | Blood | SHV-7 | + | 101 | L/M | None |
| CA05 | Pennsylvania | Urine | CTX-M-14 | + | 131 | FII | None |
| CA06 | Pennsylvania | Urine | CTX-M-14 | − | 127 | FII | None |
| CA07 | Michigan | Blood | CTX-M-15 | + | 131 | NA | NA |
| CA08 | Michigan | Urine | CTX-M-14 | + | 131 | FII-FIA-FIB | SXT, TET |
| CA09 | Michigan | Urine | CTX-M-15 | + | 90 | FII-FIA-FIB | GEN, TET |
| CA10 | Michigan | Urine | SHV-5 | + | 12 | A/C | None |
| CA11 | Michigan | Urine | CTX-M-15 | − | 38 | Y | None |
| CA12 | Michigan | Abscess | CTX-M-15 | + | 131 | FII-FIA | SXT |
| CA13 | Michigan | Urine | CTX-M-15 | + | 448 | NT | None |
| CA14 | Texas | Urine | CTX-M-15 | + | 131 | FII-FIA-FIB | SXT, TET |
| CA15 | Texas | Urine | CTX-M-15 | + | 1280 | NT | None |
| CA16 | Texas | Blood | CTX-M-14 | + | 1193 | NT | SXT, TET |
| CA17 | Texas | Urine | CTX-M-15 | + | 410 | FII-FIA-FIB | TET |
| CA18 | Texas | Urine | CTX-M-15 | + | 44 | FII-FIA-FIB | GEN, SXT, TET |
| CA19 | New York | Ascites | CTX-M-15 | + | 131 | FII-FIA | SXT |
| CA20 | New York | Urine | CTX-M-14 | + | 405 | FII-FIB | SXT, TET |
| CA21 | New York | Urine | SHV-5 | − | 648 | NT | GEN, SXT |
| CA22 | New York | Urine | SHV-5 | + | 744 | A/C | SXT, TET |
| CA23 | New York | Urine | CTX-M-15 | − | 167 | FII-FIA-FIB | GEN, SXT |
| CA24 | New York | Urine | CTX-M-15 | − | 3554 | FIA-FIB | SXT, TET |
| CA25 | Iowa | Urine | CTX-M-14 | + | 38 | NA | NA |
| CA26 | Iowa | Urine | CTX-M-15 | + | 131 | NA | NA |
| CA27 | Iowa | Urine | SHV-5 | + | 131 | FIA-FIB | None |
| CA28 | Iowa | Urine | CTX-M-15 | + | 44 | FII-FIA-FIB | GEN, SXT, TET |
| CA29 | Iowa | Urine | CTX-M-15 | + | 205 | NA | NA |
| CA30 | Iowa | Urine | CTX-M-15 | + | 131 | FII-FIA | SXT, TET |
+, present; −, not present.
NT, nontypeable.
NA, no transformant available.
SXT, trimethoprim-sulfamethoxazole; TET, tetracycline; GEN, gentamicin.
Plasmid DNA was extracted from the clinical isolates using the standard alkaline lysis method (20). E. coli strain DH10B competent cells were transformed with the plasmid DNA by electroporation, and β-lactamase-producing transformants were selected on lysogenic agar plates containing 50 μg/ml ampicillin. Transfer of the plasmids carrying ESBL-encoding genes was confirmed by PCR of blaCTX-M or blaSHV and reduced susceptibility to cefotaxime and/or ceftazidime. The plasmid replicons were determined using DNA extracted from the E. coli DH10B transformants according to the protocol by Carattoli et al. (21). The E. coli DH10B transformants with plasmids carrying ESBL-encoding genes were obtained for 25 isolates. The frequently occurring plasmid replicons included IncFII-FIA-FIB (7 isolates), IncFII-FIA (3 isolates), and IncA/C (2 isolates) (Table 2). IncFII-FIA-FIB was identified in various STs, including ST131, ST44, ST90, ST167, and ST410, whereas IncFII-FIA was identified in ST131 only. Coresistance to the non-β-lactam agents gentamicin, trimethoprim-sulfamethoxazole, or tetracycline was observed for 15 of the 25 transformants using the disk diffusion method (Table 2). Notably, all IncFII-FIA-FIB and IncFII-FIA plasmids conferred resistance to at least one non-β-lactam agent (trimethoprim-sulfamethoxazole, gentamicin, and/or tetracycline). Two IncFII-FIA-FIB plasmids conferred resistance to trimethoprim-sulfamethoxazole, tetracycline, and gentamicin. These multidrug resistance traits of IncFII-FIA-FIB and IncFII-FIA plasmids may provide them with a selective advantage. Furthermore, multireplicon IncF plasmids are considered to possess an ability to evolve the regulatory sequences for these replicons, and this versatility has been associated with the abrupt worldwide emergence of IncF plasmids carrying blaCTX-M (22). In particular, IncFII plasmids carrying blaCTX-M have been widely reported (23–25). The IncFII-FIA-FIB and IncFII-FIA plasmids were found across various STs, including ST131 isolates, suggesting that they may be serving as the vehicles for the spread of blaCTX-M between ST131 isolates and non-ST131 isolates in the community.
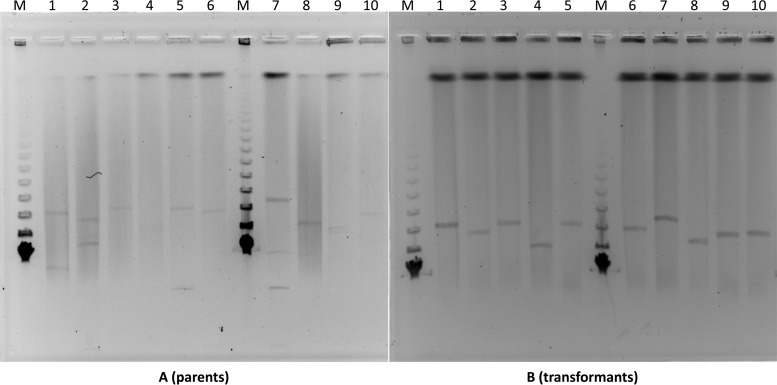
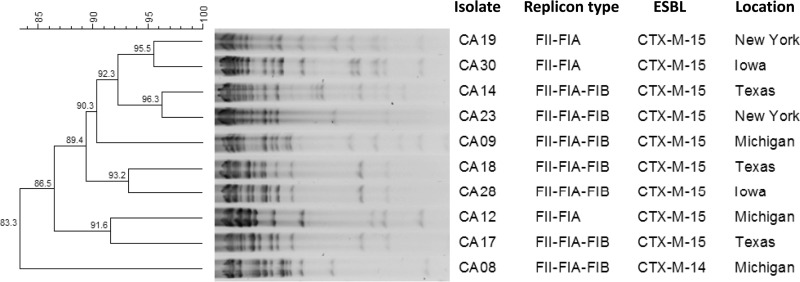
For the 10 IncFII-FIA-FIB and IncFII-FIA plasmids, their sizes were estimated by S1 pulsed-field gel electrophoresis (PFGE) for the clinical isolates and their transformants (26). All of the tested clinical isolates possessed >1 plasmid, with some possessing up to 3 (Fig. 1). The sizes of the IncFII-FIA-FIB and IncFII-FIA plasmids varied, ranging from approximately 100 to 200 kb. They were then extracted from the transformants, digested with EcoRI, and profiled by an overnight electrophoresis on a 0.7% agarose gel. Dendrograms representing the similarities of the restriction patterns were constructed according to the unweighted-pair group method using average linkages (UPGMA) using BioNumerics version 6.01 (Applied Maths, Austin, TX). The plasmid restriction analysis yielded 4 related profiles using a similarity cutoff of 90%, with each profile containing 2 isolates from hospitals in different states (Fig. 2).
FIG 1.
Plasmid analysis of IncFII plasmids carrying genes encoding CTX-M-type ESBLs in community-associated E. coli isolates and their transformants. IncFII plasmids represent the largest plasmids in the parents ranging approximately between 100 and 200 kb. Lane 1, CA08; lane 2, CA09; lane 3, CA14; lane 4, CA17; lane 5, CA18; lane 6, CA23; lane 7, CA28; lane 8, CA12; lane 9, CA19; lane 10, CA30; lane M, molecular marker.
FIG 2.
EcoRI plasmid restriction profiles of IncFII plasmids extracted from E. coli transformants.
To determine the stability of the plasmids carrying ESBL-encoding genes, 15 representative transformants containing blaCTX-M (n = 12) or blaSHV (n = 3) with plasmids of various replicon types were grown separately in lysogenic broth free of antimicrobial agents at 37°C with shaking. After overnight growth, the cells were diluted into fresh lysogenic broth to a concentration of approximately 103 CFU/ml and incubated further for 8 successive days. The colonies were isolated on lysogenic agar plates, and 10 random colonies were subjected to PCR to determine the presence or absence of the ESBL genes (blaCTX-M or blaSHV, as appropriate) from each culture on each day (a total of 80 colonies for each transformant). One transformant completely lost its IncN plasmid by day 5, and another partially lost its IncFII-FIA-FIB plasmid on day 8 (Table 3). Otherwise, none of the remaining 13 transformants lost blaCTX-M or blaSHV during the 8 days of serial broth culture. Therefore, plasmids containing blaCTX-M or blaSHV were stably maintained in the majority of the isolates, even in the absence of selective pressure, suggesting that the cost of maintaining these plasmids carrying ESBL-encoding genes may be minimal for the host E. coli.
TABLE 3.
Stability of plasmids carrying ESBL-encoding genes in E. coli DH10 transformants
| Isolate | β-Lactamase | Replicon type | No. in which blaCTX-M or blaSHV was detected by PCR (10 colonies/strain) by daya: |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| CA03TF | CTX-M-15 | N | 8 (+), 2 (−) | 9 (+), 1 (−) | 6 (+), 4 (−) | 9 (+), 1 (−) | 10 (−) | 10 (−) | 10 (−) | 10 (−) |
| CA04TF | SHV-7 | L/M | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA05TF | CTX-M-14 | FII | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA06TF | CTX-M-14 | FII | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA08TF | CTX-M-14 | FII-FIA-FIB | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA09TF | CTX-M-15 | FII-FIA-FIB | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA10TF | SHV-5 | A/C | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA11TF | CTX-M-15 | Y | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA12TF | CTX-M-15 | FII-FIA | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA18TF | CTX-M-15 | FII-FIA-FIB | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA20TF | CTX-M-14 | FII-FIB | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA22TF | SHV-5 | A/C | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA24TF | CTX-M-15 | FIA-FIB | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
| CA28TF | CTX-M-15 | FII-FIA-FIB | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 6 (+), 4 (−) |
| CA30TF | CTX-M-15 | FII-FIA | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) | 10 (+) |
+, positive; −, negative.
The emergence of community-associated ESBL-producing E. coli infections in the United States is driven by the epidemic ST131 clone and diverse non-ST131 clones. The most common IncFII-FIA-FIB and IncFII-FIA plasmids occur in ST131, as well as non-ST131 isolates in distant states, suggesting the potential role of plasmid-mediated dissemination of ESBL-producing E. coli into and within the community. Further genetic characterization of these key groups of plasmids is ongoing.
ACKNOWLEDGMENTS
We thank the participating sites for collecting the community-associated E. coli isolates used in the study.
Y.D. was supported by research grants from the National Institutes of Health (R21AI107302 and R01AI104895).
Footnotes
Published ahead of print 18 August 2014
REFERENCES
- 1.Kang CI, Cheong HS, Chung DR, Peck KR, Song JH, Oh MD, Choe KW. 2008. Clinical features and outcome of community-onset bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 27:85–88. 10.1007/s10096-007-0401-6. [DOI] [PubMed] [Google Scholar]
- 2.Minarini LA, Gales AC, Palazzo IC, Darini AL. 2007. Prevalence of community-occurring extended-spectrum beta-lactamase-producing Enterobacteriaceae in Brazil. Curr. Microbiol. 54:335–341. 10.1007/s00284-006-0307-z. [DOI] [PubMed] [Google Scholar]
- 3.Rupp ME, Fey PD. 2003. Extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: considerations for diagnosis, prevention and drug treatment. Drugs 63:353–365. 10.2165/00003495-200363040-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, Tórtola T, Mirelis B, Navarro G, Cuenca M, Esteve M, Peña C, Llanos AC, Cantón R, Pascual A. 2008. Community infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Arch. Intern. Med. 168:1897–1902. 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 5.Colpan A, Johnston B, Porter S, Clabots C, Anway R, Thao L, Kuskowski MA, Tchesnokova V, Sokurenko EV, Johnson JR, VICTORY (Veterans Influence of Clonal Types on Resistance: Year 2011) Investigators 2013. Escherichia coli sequence type 131 (ST131) subclone H30 as an emergent multidrug-resistant pathogen among U.S. veterans. Clin. Infect. Dis. 57:1256–1265. 10.1093/cid/cit503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson JR, Urban C, Weissman SJ, Jorgensen JH, Lewis JS, Jr, Hansen G, Edelstein PH, Robicsek A, Cleary T, Adachi J, Paterson D, Quinn J, Hanson ND, Johnston BD, Clabots C, Kuskowski MA, AMERECUS Investigators 2012. Molecular epidemiological analysis of Escherichia coli sequence type ST131 (O25:H4) and blaCTX-M-15 among extended-spectrum-β-lactamase-producing E. coli from the United States, 2000 to 2009. Antimicrob. Agents Chemother. 56:2364–2370. 10.1128/AAC.05824-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doi Y, Park YS, Rivera JI, Adams-Haduch JM, Hingwe A, Sordillo EM, Lewis JS, Jr, Howard WJ, Johnson LE, Polsky B, Jorgensen JH, Richter SS, Shutt KA, Paterson DL. 2013. Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clin. Infect. Dis. 56:641-648. 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YA, Qureshi ZA, Adams-Haduch JM, Park YS, Shutt KA, Doi Y. 2012. Features of infections due to Klebsiella pneumoniae carbapenemase-producing Escherichia coli: emergence of sequence type 131. Clin. Infect. Dis. 55:224-231. 10.1093/cid/cis387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JR, Nicolas-Chanoine MH, DebRoy C, Castanheira M, Robicsek A, Hansen G, Weissman S, Urban C, Platell J, Trott D, Zhanel G, Clabots C, Johnston BD, Kuskowski MA, MASTER Investigators 2012. Comparison of Escherichia coli ST131 pulsotypes, by epidemiologic traits, 1967–2009. Emerg. Infect. Dis. 18:598–607. 10.3201/eid1804.111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JR, Tchesnokova V, Johnston B, Clabots C, Roberts PL, Billig M, Riddell K, Rogers P, Qin X, Butler-Wu S, Price LB, Aziz M, Nicolas-Chanoine MH, Debroy C, Robicsek A, Hansen G, Urban C, Platell J, Trott DJ, Zhanel G, Weissman SJ, Cookson BT, Fang FC, Limaye AP, Scholes D, Chattopadhyay S, Hooper DC, Sokurenko EV. 2013. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J. Infect. Dis. 207:919–928. 10.1093/infdis/jis933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151. 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weissman SJ, Johnson JR, Tchesnokova V, Billig M, Dykhuizen D, Riddell K, Rogers P, Qin X, Butler-Wu S, Cookson BT, Fang FC, Scholes D, Chattopadhyay S, Sokurenko E. 2012. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl. Environ. Microbiol. 78:1353–1360. 10.1128/AEM.06663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clermont O, Dhanji H, Upton M, Gibreel T, Fox A, Boyd D, Mulvey MR, Nordmann P, Ruppé E, Sarthou JL, Frank T, Vimont S, Arlet G, Branger C, Woodford N, Denamur E. 2009. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J. Antimicrob. Chemother. 64:274–277. 10.1093/jac/dkp194. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Shibata N, Yamane K, Wachino J, Ito K, Arakawa Y. 2009. Change in the prevalence of extended-spectrum-beta-lactamase-producing Escherichia coli in Japan by clonal spread. J. Antimicrob. Chemother. 63:72–79. 10.1093/jac/dkn463. [DOI] [PubMed] [Google Scholar]
- 16.van der Bij AK, Peirano G, Goessens WH, van der Vorm ER, van Westreenen M, Pitout JDD. 2011. Clinical and molecular characteristics of extended-spectrum-β-lactamase-producing Escherichia coli causing bacteremia in the Rotterdam area, Netherlands. Antimicrob. Agents Chemother. 55:3576–3578. 10.1128/AAC.00074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peirano G, van der Bij AK, Gregson DB, Pitout JD. 2012. Molecular epidemiology over an 11-year period (2000 to 2010) of extended-spectrum β-lactamase-producing Escherichia coli causing bacteremia in a centralized Canadian region. J. Clin. Microbiol. 50:294–299. 10.1128/JCM.06025-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SH, Byun JH, Choi SM, Lee DG, Kim SH, Kwon JC, Park C, Choi JH, Yoo JH. 2012. Molecular epidemiology of extended-spectrum β-lactamase-producing Escherichia coli in the community and hospital in Korea: emergence of ST131 producing CTX-M-15. BMC Infect. Dis. 12:149. 10.1186/1471-2334-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 4(6):e00377-13. 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Russell D. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 21.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228. 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Villa L, García-Fernández A, Fortini D, Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 65:2518–2529. 10.1093/jac/dkq347. [DOI] [PubMed] [Google Scholar]
- 23.Peirano G, Costello M, Pitout JD. 2010. Molecular characteristics of extended-spectrum beta-lactamase-producing Escherichia coli from the Chicago area: high prevalence of ST131 producing CTX-M-15 in community hospitals. Int. J. Antimicrob. Agents 36:19–23. 10.1016/j.ijantimicag.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Naseer U, Haldorsen B, Tofteland S, Hegstad K, Scheutz F, Simonsen GS, Sundsfjord A, Norwegian ESBL Study Group 2009. Molecular characterization of CTX-M-15-producing clinical isolates of Escherichia coli reveals the spread of multidrug-resistant ST131 (O25:H4) and ST964 (O102:H6) strains in Norway. APMIS 117:526–536. 10.1111/j.1600-0463.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- 25.Seiffert SN, Hilty M, Kronenberg A, Droz S, Perreten V, Endimiani A. 2013. Extended-spectrum cephalosporin-resistant Escherichia coli in community, specialized outpatient clinic and hospital settings in Switzerland. J. Antimicrob. Chemother. 68:2249–2254. 10.1093/jac/dkt208. [DOI] [PubMed] [Google Scholar]
- 26.Bueno MF, Francisco GR, O'Hara JA, de Oliveira Garcia D, Doi Y. 2013. Coproduction of 16S rRNA methyltransferase RmtD or RmtG with KPC-2 and CTX-M group extended-spectrum β-lactamases in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 57:2397-2400. 10.1128/AAC.02108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]