Abstract
Posaconazole oral suspension is widely used for antifungal prophylaxis and treatment in immunocompromised patients, with highly variable pharmacokinetics reported in patients due to inconsistent oral absorption. This study aimed to characterize the pharmacokinetics of posaconazole in adults and investigate factors that influence posaconazole pharmacokinetics byusing a population pharmacokinetic approach. Nonlinear mixed-effects modeling was undertaken for two posaconazole studies in patients and healthy volunteers. The influences of demographic and clinical characteristics, such as mucositis, diarrhea, and drug-drug interactions, on posaconazole pharmacokinetics were investigated using a stepwise forward inclusion/backwards deletion procedure. A total of 905 posaconazole concentration measurements from 102 participants were analyzed. A one-compartment pharmacokinetic model with first-order oral absorption with lag time and first-order elimination best described posaconazole pharmacokinetics. Posaconazole relative bioavailability was 55% lower in patients who received posaconazole than in healthy volunteers. Coadministration of proton pump inhibitors (PPIs) or metoclopramide, as well as the occurrence of mucositis or diarrhea, reduced posaconazole relative bioavailability by 45%, 35%, 58%, and 45%, respectively, whereas concomitant ingestion of a nutritional supplement significantly increased bioavailability (129% relative increase). Coadministration of rifampin or phenytoin increased apparent posaconazole clearance by more than 600%, with a smaller increase observed with fosamprenavir (34%). Participant age, weight, or sex did not significantly affect posaconazole pharmacokinetics. Posaconazole absorption was reduced by a range of commonly coadministered medicines and clinical complications, such as mucositis and diarrhea. Avoidance of PPIs and metoclopramide and administration with food or a nutritional supplement are effective strategies to increase posaconazole absorption.
INTRODUCTION
Posaconazole is a triazole antifungal agent with a broad antifungal spectrum and is widely used for antifungal prophylaxis and treatment in immunocompromised patients (1, 2). Significant pharmacokinetic variability has been reported in patients administered posaconazole, with dose-dependent, saturable oral absorption and a large food effect contributing to this variability (3, 4). In addition, a range of drug-drug interactions and conditions affecting the gastrointestinal tract are known to influence posaconazole absorption (5–8), adding to the inconsistent bioavailability often associated with posaconazole (9).
Despite these pharmacokinetic challenges, which may have been expected to compromise efficacy, posaconazole prophylaxis was associated with few cases of breakthrough invasive fungal infections (IFIs) in two pivotal randomized, controlled trials (1, 10). Exposure-response relationships with posaconazole and the value of therapeutic drug monitoring (TDM) have been widely debated (9, 11, 12), although increasing evidence supports the role of TDM in managing pharmacokinetic variability and optimizing posaconazole treatment (6, 9, 11, 13–15). Although an absolute target concentration has not yet been clearly defined for the prevention of breakthrough fungal infection, recent reports suggest a target posaconazole concentration of >0.7 mg/liter (9, 11). A number of studies have reported that <50% of patients achieve posaconazole exposure above this target (6, 7, 16–19). Knowledge on the factors that cause this pharmacokinetic variability is of the greatest importance to prevent suboptimal exposure and subsequent failure of prophylaxis.
Using pharmacokinetic studies undertaken in healthy volunteers and patients, this study aimed to characterize the pharmacokinetics of posaconazole oral suspension in adults and evaluate the influence of demographic and clinical covariates on posaconazole exposure by using a population pharmacokinetic approach to inform the optimal use of posaconazole in clinical practice. A tablet formulation of posaconazole with improved oral bioavailability has recently been approved in the United States (20); this analysis focuses on the oral suspension formulation of posaconazole, which is widely used and remains available worldwide.
MATERIALS AND METHODS
Pharmacokinetic data and participants.
Pharmacokinetic, demographic, and relevant clinical data from two posaconazole studies in healthy volunteers and in patients receiving a posaconazole oral suspension for the prophylaxis or treatment of fungal infections were available for analysis (6, 21). Information on study design, population, pharmacokinetic data, and participant demographics is included in Table 1. Further details on these studies have been described previously (6, 21).
TABLE 1.
Posaconazole pharmacokinetic data and participant demographics
| Parameter | Study 1a | Study 2b |
|---|---|---|
| Study type | Controlled pharmacokinetic study of posaconazole-fosamprenavir interaction | Observational study of posaconazole TDM |
| Study population | Healthy volunteers | Patients treated with posaconazole |
| N | 20 | 82 |
| Posaconazole dosing | Day 1, 200 mg; day 2, 200 mg twice a day; days 3–10, 400 mg twice a day | Multiple dosing: 160–1,200 mg total daily dose |
| No. of samples/dose intervalc | 11 | 1 |
| Median age (range), in yrs | 38 (18–54) | 50 (18–79) |
| Median wt (range), in kg | 74 (44–104) | 71 (38–122) |
| Sex [no. (%)] | ||
| Female | 9 (45) | 35 (43) |
| Male | 11 (55) | 47 (57) |
Population pharmacokinetic modeling.
The pharmacokinetic data were analyzed using nonlinear mixed-effects modeling with NONMEM 7.2 (Globomax LLC, Hanover, MD). The gfortran compiler (version 4.6) was used with NONMEM within Pirana (version 2.8; Pirana Software) and Perl-Speaks-NONMEM (version 3.5.3; http://psn.sourceforge.net/) was used for model execution. Data visualization was performed with R (version 2.15.2; http://www.r-project.org/), Xpose (version 4.4; http://xpose.sourceforge.net/), and Microsoft Excel 2010. The first-order conditional estimation method with interaction was used throughout model development.
Structural model and variability.
The structural pharmacokinetic model was developed using a stepwise approach. The base pharmacokinetic model was initially developed using the richly sampled concentration-time data (study 1, healthy volunteer data [21]), with sparsely sampled data (study 2, patient data [6]), and then incorporated into the model with appropriate goodness-of-fit testing.
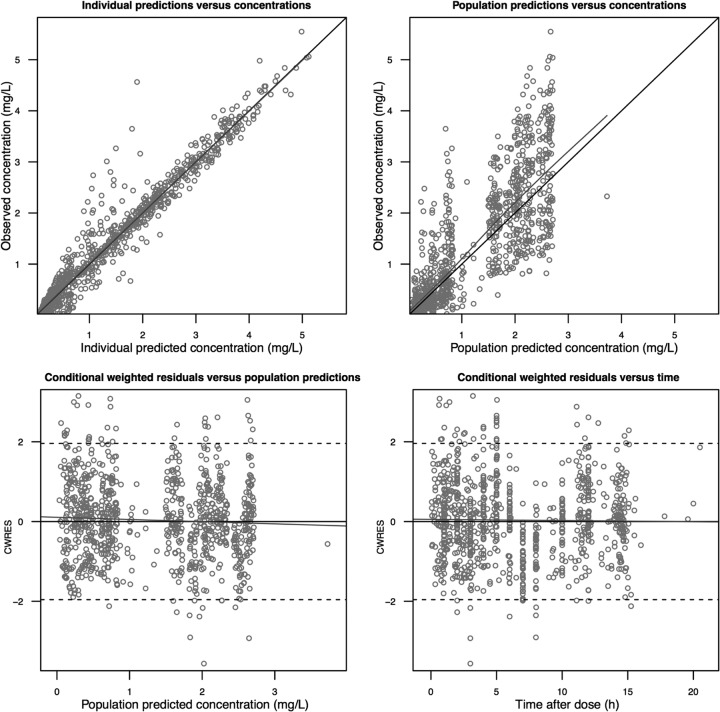
Discrimination between nested models was made by comparison of the objective function value (OFV). A significance level of P < 0.05, corresponding to a decrease of 3.84 in the OFV, was considered statistically significant. In addition, the following plots were used for diagnostic purposes: (i) observed versus individually predicted values; (ii) observed versus population-predicted values; (iii) time versus weighted residuals; (iv) population predictions versus weighted residuals.
Pharmacokinetic models incorporating either one or two compartments with linear elimination were investigated. Due to the erratic and incomplete absorption associated with the posaconazole suspension (5, 9), a range of approaches were investigated to describe posaconazole absorption. These included first-order absorption (with and without absorption lag time), dose-dependent saturable absorption, and a multiple-dose transit compartment model (22). Interindividual variability (IIV) and interoccasion variability (IOV; also termed intraindividual variability) in posaconazole pharmacokinetic parameters were evaluated by using exponential error models. Proportional, additive, and combined additive and proportional residual error models were evaluated.
Covariate model.
Participant-specific covariates, such as body weight, age, and sex, and clinical covariates, such as mucositis or diarrhea on the day of posaconazole concentration sampling and administration of posaconazole suspension via a nasogastric (NG) tube, and potential drug-drug and food-drug interactions, including concomitant proton pump inhibitors (PPIs), metoclopramide, phenytoin, or rifampin, a nutritional supplement, ranitidine, and fosamprenavir, were investigated. Only biologically plausible parameter-covariate relationships were explored. Continuous covariates were examined in the first instance by using linear parameter-covariate relations, with the exception of the effect of body weight on apparent clearance (CL), which was also tested using allometric scaling with an exponent of 0.75. Categorical covariates were parameterized as a multiplicative effect on the associated structural parameter.
Covariate testing was undertaken using a stepwise forward inclusion and backwards deletion procedure. During forward inclusion, all parameter-covariate relations were initially investigated, with the covariate causing the largest reduction in the NONMEM-derived OFV (i.e., −2-log likelihood) retained in the model for the next step, until no significant parameter-covariate relationships remained. During backwards deletion, all significant parameter-covariate relationships were reevaluated in the model obtained from the forward inclusion procedure, with covariates not meeting significance criteria being removed from the model in a stepwise manner. A P value of <0.005 (decrease in OFV of >7.88) was used to evaluate covariates during forward inclusion, while the backward deletion procedure used a stricter criterion (increase in OFV of >10.83 upon deletion; P < 0.001).
Model validation.
Prediction- and variability-corrected visual predictive checks (pvcVPCs) were used for model validation (23). Similar to visual predictive checks, pvcVPCs allow a graphical assessment of the predictive performance of a model by comparing model simulations and observed data in terms of central trend and variability but differ in that both model simulations and observed data are normalized to correct for differences arising from independent variables (e.g., differences in dose, time, or covariates) as well as the typical population variability in each bin (23). A total of 1,000 simulated data sets of individuals from the original data set were compared with prediction- and variability-corrected observed concentrations. In addition, a nonparametric bootstrap procedure (200 data sets) was used to determine the uncertainty of parameter estimates in the final model.
Effects of covariates on posaconazole exposure.
Simulations from the final pharmacokinetic model were performed to quantitate the influence of significant covariates on posaconazole exposure. For each significant covariate effect, 1,000 patient simulations were performed to predict steady-state posaconazole trough concentrations on day 10 of treatment following the recommended posaconazole oral suspension dosing regimen for antifungal prophylaxis (posaconazole suspension of 200 mg, three times daily). Predicted trough posaconazole concentrations with and without each covariate were compared with the recommended posaconazole target concentration for antifungal prophylaxis (>0.7 mg/liter) (9).
RESULTS
Model development and validation.
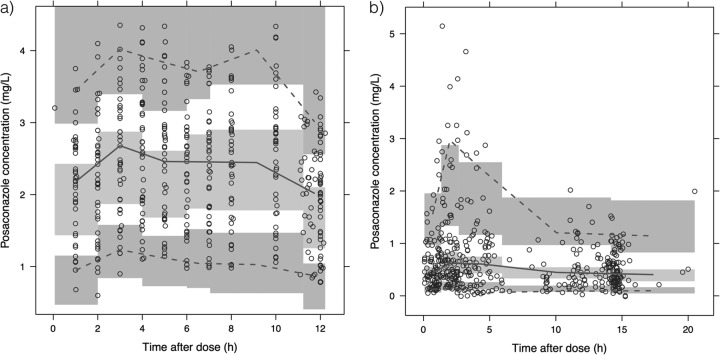
A total of 905 posaconazole concentration measurements (study 1, 440 measurements; study 2, 465 measurements) from a total of 102 participants were included in this analysis. A one-compartment pharmacokinetic model with first-order oral absorption with an absorption lag time and first-order elimination best described the pharmacokinetic data set. Models incorporating dose-dependent saturable absorption or a multiple-dose transit compartment model were not selected, because they did not significantly improve the goodness of fit of the structural pharmacokinetic model. Interoccasion variability in relative posaconazole bioavailability was associated with significantly improved goodness of fit and was retained in the final model. A proportional residual error model, stratified by study, was used. Goodness-of-fit plots and pvcVPCs used throughout model development indicated an acceptable model fit (Fig. 1). The pvcVPCs of the final model (Fig. 2) indicated good predictive performance, with acceptable agreement between prediction- and variability-corrected observed data and model-simulated confidence intervals for the median and 5th and 95th percentiles.
FIG 1.
Goodness-of-fit plots for the final posaconazole pharmacokinetic model.
FIG 2.
Prediction- and variability-corrected visual predictive checks of the final model, stratified by study: study 1 (a) and study 2 (b). Prediction- and variability-corrected observed concentrations are shown as open circles, with the solid and lower and upper dashed lines showing the median and 5th and 95th percentiles of the observed data, respectively. The shaded areas represent 95% confidence intervals for the model-predicted median and 5th and 95th percentiles constructed from 1,000 simulated data sets of individuals from the original data set.
Parameter estimates for the structural model, interindividual, interoccasion, and residual variability from the base and final models, and also bootstrap estimates and associated standard errors of the final model, are shown in Table 2. Posaconazole relative bioavailability after administration as a suspension was found to be significantly higher in healthy volunteers (study 1) than in patients receiving posaconazole (study 2). The extent of this effect was characterized as a relative difference in bioavailability based on the use of a categorical covariate.
TABLE 2.
Pharmacokinetic parameter estimates of the base model and final model and bootstrap estimates of the final model
| Parametera | Population estimate |
Bootstrap estimateb | Precision (RSE)d | |
|---|---|---|---|---|
| Base model | Final model | |||
| Structural model | ||||
| CL/F (liters/h) | 50.8 | 30.2 | 30.6 | 9.9 |
| V/F (liters) | 2,090 | 1,100 | 1,110 | 12.8 |
| ka (h−1) | 1.33 | 1.26 | 1.29 | 28.4 |
| Absorption lag time (h) | 1.79 | 1.79 | 1.81 | 9.2 |
| IIV and IOV | ||||
| IIV CL/F (% CV) | 58.1 | 46.4 | 45.8 | 9.17 |
| IIV V/F (% CV) | 73.8 | 30.2 | 29.6 | 32.3 |
| IIV ka (% CV) | 46.8 | 53.4 | 57 | 33.4 |
| IOV F (% CV) | 28.6 | 23.6 | 23.7 | 13.4 |
| F, volunteers vs patientsc | 257% | 123% | 126% | 26.5 |
| Residual variability | ||||
| Proportional error, study 1 (% CV) | 6.97 | 6.76 | 6.77 | 4.37 |
| Proportional error, study 2 (% CV) | 62.7 | 53.8 | 52.8 | 8.34 |
ka, first-order absorption rate constant; CL/F, apparent clearance; V/F, apparent volume of distribution; F, bioavailability.
Data obtained from nonparametric bootstrap analysis (200 data sets).
Difference in bioavailability in healthy volunteers versus patients.
RSE, relative standard error, expressed as a percentage.
One participant from study 2 was initially included in the modeling analysis but was found to have an outlying estimate for posaconazole volume of distribution. A sensitivity analysis was performed, including reestimation of the base pharmacokinetic model and stepwise covariate testing procedure, both with and without this participant included in the model. As inclusion of this participant was found to affect the selection of covariates during forward inclusion and backwards deletion, this participant was excluded from the analysis.
Effects of covariates on posaconazole pharmacokinetics.
Significant covariates selected from the stepwise covariate modeling procedure and included in the final pharmacokinetic model are shown in Table 3. Concurrent administration of proton pump inhibitors or metoclopramide was associated with significantly reduced posaconazole bioavailability, as was mucositis or diarrhea on the day of posaconazole concentration sampling, whereas a concurrent nutritional supplement significantly increased bioavailability. Concurrent administration of phenytoin, rifampin, or fosamprenavir was associated with significantly increased apparent posaconazole clearance. Covariate effects were estimated with good precision (range of relative standard error, 5.6 to 25.8%). The effects of these covariates on predicted posaconazole trough concentrations at steady state are shown in Fig. 3.
TABLE 3.
Significant covariate effects included in the final model
| Covariate | Effect on structural parametera | Bootstrap estimate of effect | Precision (RSE)b |
|---|---|---|---|
| Effect on relative F | |||
| Concurrent proton pump inhibitor | −45.1 | −43.8 | 11.7 |
| Concurrent metoclopramide | −34.5 | −33.5 | 21.3 |
| Concurrent nutritional supplement | 129 | 121 | 16.6 |
| Mucositis | −57.7 | −57.7 | 5.61 |
| Diarrhea | −45.1 | −38.6 | 15.5 |
| Effect on CL/F | |||
| Concurrent phenytoin/rifampin | 621 | 640 | 25.8 |
| Concurrent fosamprenavir | 34.2 | 34.3 | 23.4 |
The percent increase or decrease (positive and negative values, respectively); data are the result of a nonparametric bootstrap analysis (200 data sets).
RSE, relative standard error, expressed as a percentage.
FIG 3.
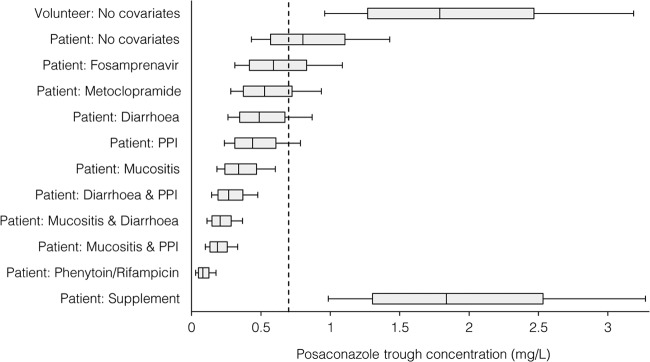
Effects of significant covariates on the predicted posaconazole trough concentration on day 10 of therapy following posaconazole at 200 mg three times daily; data are presented as an adjusted box plot. For each scenario, 1,000 patients or volunteers were simulated with or without the specified covariate(s). The central box line represents the median trough concentration, the lower and upper box ends represent the 25th and 75th percentiles, and the bars extend to the 10th and 90th percentiles. The dashed line represents the proposed minimum cutoff concentration for antifungal prophylaxis with posaconazole (0.7 mg/liter) (9).
Parameter-covariate relationships that were not associated with a significant effect on posaconazole pharmacokinetics included body weight on apparent posaconazole clearance or volume of distribution, participant age on apparent posaconazole clearance, volume of distribution or bioavailability, sex on apparent posaconazole clearance, volume of distribution or bioavailability, ranitidine coadministration on bioavailability, and NG administration of posaconazole on bioavailability.
DISCUSSION
This study reports an integrated analysis of the population pharmacokinetics of posaconazole in healthy volunteers and patients and identifies and quantifies the effects of commonly coadministered medicines and clinical factors on posaconazole exposure. A strength of this study is the ability to quantify the significance of multiple clinical factors that have been reported to influence posaconazole pharmacokinetics, using real world data collected from patients receiving the drug in the clinic.
A one-compartment pharmacokinetic model with first-order elimination adequately described posaconazole pharmacokinetics in this study, a finding that was consistent with previous population pharmacokinetic studies of posaconazole (24–26). Posaconazole exhibits dose-dependent, saturable absorption, with more-frequent dosing regimens resulting in higher systemic exposures than do less-frequent dosing regimens, despite an equivalent total daily dose (3, 4). Krishna et al. reported 2.5-fold higher posaconazole exposure among healthy volunteers receiving a posaconazole suspension at 200 mg four times daily, compared to those receiving 400 mg twice daily (4). This phenomenon led to the multiple daily dose regimens recommended with the current formulation of posaconazole (3). A saturable absorption model was investigated in this analysis but did not provide an improvement in model fit, an apparent limitation of this model that is shared with previous population pharmacokinetic models of posaconazole (24–26). In this analysis, a single posaconazole dosing regimen was used in study 1, and while a range of dosing regimens was used in study 2, concentration sampling was sparse; these factors are likely to have contributed to the lack of improvement observed with a saturable absorption model. This relationship has been described in the context of short-term administration of the drug to fasting healthy volunteers (3), and detailed pharmacokinetic data in patients at a range of posaconazole doses and dose frequencies would be required to further characterize the relationship between dose frequency and concentration with posaconazole.
Significantly higher bioavailability was observed in healthy volunteers in this study than in the patients receiving posaconazole (Table 2; Fig. 3), in agreement with previous reports of higher exposure in healthy volunteers (27). Healthy volunteers included in this analysis were administered posaconazole with food (21), whereas the timing of food consumption in relation to dose was not known for patients included in this analysis. As food is known to increase posaconazole exposure by up to 4-fold (4), it is likely that consistent and concomitant food intake at the time of dosing at least partially explains the higher bioavailability observed in healthy volunteers. Furthermore, the significant size of the food effect observed with posaconazole also explains the increased posaconazole bioavailability observed among patients coadministered a nutritional supplement in this study, supporting the use of nutritional supplements as an effective strategy to boost posaconazole systemic absorption (9, 28).
Coadministration of a proton pump inhibitor and metoclopramide was associated with significantly lower posaconazole bioavailability in this analysis, confirming the results of previous crossover studies (4, 5) and clinical observations (19, 29). PPIs reduce posaconazole solubility as a result of increased pH, with the dissolved posaconazole concentration in the gastrointestinal lumen dictating the extent of posaconazole absorption and, thus, systemic exposure (5). The prokinetic activity of metoclopramide is believed to explain its negative effect on posaconazole absorption (4).
Mucositis, and to a smaller extent diarrhea, were both associated with reduced posaconazole bioavailability (Table 3; Fig. 3). Gastrointestinal disorders are common among patients at risk of IFIs and may be related to chemotherapy, neutropenic enterocolitis, or as a symptom of graft-versus-host disease in allogeneic hematopoietic stem cell transplant recipients (29–31). A relationship between diarrhea on the day of posaconazole blood sampling and low posaconazole exposure has previously been reported among two large patient cohorts (29, 30), with a similar relationship described for mucositis (32). As many patients suffering from mucositis may be unable to eat, it is probable that a reduction in food intake with posaconazole contributes to the reduced bioavailability observed in this study.
Coadministration of fosamprenavir, phenytoin, or rifampin was associated with an increased apparent clearance of posaconazole, with phenytoin or rifampin causing far larger increases in clearance that were reflected in the very low posaconazole exposure in model simulations with this covariate (Fig. 3). Both phenytoin and rifabutin have been associated with a reduction in the posaconazole area under the concentration-time curve of approximately 50% in studies of healthy volunteers (33, 34), with a case study demonstrating a similar interaction with rifampin (35). Approximately 20 to 30% of a posaconazole dose is known to be glucuronidated via the phase 2 enzyme UDP-glucuronosyltransferase 1A4 (UGT1A4) in the liver (36, 37). Both phenytoin and rifampin are known UGT inducers (38, 39), suggesting that induction of this pathway is the likely mechanism for the increased posaconazole elimination when these medicines are coadministered. Taken together, these results suggest that coadministration of phenytoin or rifampin with posaconazole should be avoided; frequent monitoring of posaconazole concentration appears prudent if coadministration is deemed necessary.
No relationship between demographic covariates, such as body weight, age, or sex, and posaconazole pharmacokinetics was observed in this analysis, in agreement with other analyses of posaconazole (24, 27). In contrast with these findings, Kohl et al. identified an inverse relationship between patient age and the apparent volume of distribution of posaconazole in their analysis of prophylactic posaconazole use in patients undergoing allogeneic hematopoietic stem cell transplantation (25), and a relationship between patient weight and a larger apparent volume of distribution was observed in a recent population pharmacokinetic analysis of posaconazole (26). As the effects of changes in patient age or weight on posaconazole exposure were relatively small in these studies (25, 26), it is possible that differences in covariate testing methodology and significance criteria between those used in these studies and those in the present analysis may explain the conflicting results for these covariates.
Coadministration of the H2 receptor antagonist ranitidine did not significantly affect posaconazole pharmacokinetics. We previously reported a reduction in posaconazole exposure upon coadministration of ranitidine in a multiple regression analysis, although this effect was relatively minor compared to proton pump inhibitor coadministration (regression coefficient, −178 ng/ml versus −589 ng/ml for PPIs) (6). Vehreschild et al. identified a significant effect of ranitidine coadministration on the apparent clearance of posaconazole when ranitidine was included in the model as a single covariate, but ranitidine was not retained in the final pharmacokinetic model (26). These results suggest that concomitant ranitidine has little or no impact on posaconazole pharmacokinetics, although a controlled crossover interaction study would be needed to definitively elucidate the extent of this potential drug interaction.
In addition, NG administration of posaconazole did not significantly affect posaconazole pharmacokinetics in this analysis. A study in healthy volunteers demonstrated a 24% reduction in posaconazole area under the plasma concentration-time curve when administered via NG tube (40), with clinical studies in patients also confirming a significant reduction in posaconazole exposure (7, 41). The lack of significance of this covariate in the present study is likely due to the small number of patients who received posaconazole via NG tube in this analysis (n = 8).
In summary, this study provides an integrated population pharmacokinetic analysis of posaconazole oral suspension, demonstrating the impact of a range of drug interactions and clinical factors that influence posaconazole exposure in patients. These findings may assist clinicians in identifying factors that predispose a patient to a suboptimal posaconazole concentration. Furthermore, these findings support the need for therapeutic drug monitoring with posaconazole oral suspension to ensure adequate systemic exposure, a strategy that is widely recommended for other azole antifungal agents, such as voriconazole (42, 43). While several studies have confirmed that dose escalation with the posaconazole suspension above a cumulative daily dose of 800 mg does not increase, and may slightly decrease, posaconazole exposure (31, 44), avoidance of commonly coadministered medicines, such as PPIs and metoclopramide, as well as administration of posaconazole with food or a nutritional supplement are effective strategies to boost posaconazole absorption.
ACKNOWLEDGMENTS
This study was supported by internal funding from the Faculty of Pharmacy at the University of Sydney. M.J.D. was supported by an Australian Postgraduate Award.
R.J.M.B. has served as a consultant to and has received research grants from Gilead Sciences, Merck, Astellas, and Pfizer. A.J.M. has received in kind research support (placebo and pregabalin) from Pfizer for a clinical trial in sciatica. M.J.D. and D.M.B. declare no conflict of interest.
Footnotes
Published ahead of print 8 September 2014
REFERENCES
- 1.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh Y-T, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359. 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 2.Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik J-AH, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2–12. 10.1086/508774. [DOI] [PubMed] [Google Scholar]
- 3.Ezzet F, Wexler D, Courtney R, Krishna G, Lim J, Laughlin M. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211–220. 10.2165/00003088-200544020-00006. [DOI] [PubMed] [Google Scholar]
- 4.Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958–966. 10.1128/AAC.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walravens J, Brouwers J, Spriet I, Tack J, Annaert P, Augustijns P. 2011. Effect of pH and comedication on gastrointestinal absorption of posaconazole. Clin. Pharmacokinet. 50:725–734. 10.2165/11592630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Dolton MJ, Ray JE, Chen SCA, Ng K, Pont L, McLachlan AJ. 2012. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob. Agents Chemother. 56:5503–5510. 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray J, Campbell L, Rudham S, Nguyen Q, Marriott D. 2011. Posaconazole plasma concentrations in critically ill patients. Ther. Drug Monit. 33:387–392. 10.1097/FTD.0b013e31821fb197. [DOI] [PubMed] [Google Scholar]
- 8.Brüggemann RJM, Alffenaar J-WC, Blijlevens NMA, Billaud EM, Kosterink JGW, Verweij PE, Burger DM, Saravolatz LD. 2009. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin. Infect. Dis. 48:1441–1458. 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 9.Dolton MJ, Ray JE, Marriott D, Mclachlan AJ. 2012. Posaconazole exposure-response relationship: evaluating the utility of therapeutic drug monitoring. Antimicrob. Agents Chemother. 56:2806–2813. 10.1128/AAC.05900-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335–347. 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 11.Jang SH, Colangelo PM, Gobburu JVS. 2010. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin. Pharmacol. Ther. 88:115–119. 10.1038/clpt.2010.64. [DOI] [PubMed] [Google Scholar]
- 12.Cornely OA, Ullmann AJ. 2011. Lack of evidence for exposure-response relationship in the use of posaconazole as prophylaxis against invasive fungal infections. Clin. Pharmacol. Ther. 89:351–352. 10.1038/clpt.2010.261. [DOI] [PubMed] [Google Scholar]
- 13.Seyedmousavi S, Mouton JW, Verweij PE, Brüggemann RJ. 2013. Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert Rev. Anti Infect. Ther. 11:931–941. 10.1586/14787210.2013.826989. [DOI] [PubMed] [Google Scholar]
- 14.Crombag M-RBS, Huisman C, Kemper EM, Brüggemann RJM, Bijleveld YA. 2012. Posaconazole treatment in hematology patients: a pilot study of therapeutic drug monitoring. Ther. Drug Monit. 34:320–325. 10.1097/FTD.0b013e31824d135c. [DOI] [PubMed] [Google Scholar]
- 15.Mavridou E, Bruggemann RJM, Melchers WJG, Mouton JW, Verweij PE. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860–865. 10.1128/AAC.00931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eiden C, Meniane JC, Peyrière H, Eymard-Duvernay S, Le Falher G, Ceballos P, Fegueux N, Cociglio M, Reynes J, Hillaire-Buys D. 2011. Therapeutic drug monitoring of posaconazole in hematology adults under posaconazole prophylaxis: influence of food intake. Eur. J. Clin. Microbiol. Infect. Dis. 31:161–167. 10.1007/s10096-011-1288-9. [DOI] [PubMed] [Google Scholar]
- 17.Hoenigl M, Raggam RB, Salzer HJF, Valentin T, Valentin A, Zollner-Schwetz I, Strohmeier AT, Seeber K, Wölfler A, Sill H, Krause R. 2012. Posaconazole plasma concentrations and invasive mould infections in patients with haematological malignancies. Int. J. Antimicrob. Agents 39:510–513. 10.1016/j.ijantimicag.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Thompson GR, Rinaldi MG, Pennick G, Dorsey SA, Patterson TF, Lewis JS. 2009. Posaconazole therapeutic drug monitoring: a reference laboratory experience. Antimicrob. Agents Chemother. 53:2223–2224. 10.1128/AAC.00240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryant AM, Slain D, Cumpston A, Craig M. 2011. A post-marketing evaluation of posaconazole plasma concentrations in neutropenic patients with haematological malignancy receiving posaconazole prophylaxis. Int. J. Antimicrob. Agents 37:266–269. 10.1016/j.ijantimicag.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Merck Inc. 2014. Noxafil prescribing information, revised 11/2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/205053s000lbl.pdf Accessed 3 March 2014.
- 21.Brüggemann RJM, Van Luin M, Colbers EPH, van den Dungen MW, Pharo C, Schouwenberg BJJW, Burger DM. 2010. Effect of posaconazole on the pharmacokinetics of fosamprenavir and vice versa in healthy volunteers. J. Antimicrob. Chemother. 65:2188–2194. 10.1093/jac/dkq280. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Boeckmann A, Vick A. 2012. Implementation of dose superimposition to introduce multiple doses for a mathematical absorption model (transit compartment model). J. Pharmacokinet. Pharmacodyn. 39:251–262. 10.1007/s10928-012-9247-3. [DOI] [PubMed] [Google Scholar]
- 23.Bergstrand M, Hooker A, Wallin J, Karlsson M. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 13:143–151. 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.AbuTarif MA, Krishna G, Statkevich P. 2010. Population pharmacokinetics of posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Curr. Med. Res. Opin. 26:397–405. 10.1185/03007990903485056. [DOI] [PubMed] [Google Scholar]
- 25.Kohl V, Müller C, Cornely OA, Abduljalil K, Fuhr U, Vehreschild JJ, Scheid C, Hallek M, Rüping MJGT. 2010. Factors influencing pharmacokinetics of prophylactic posaconazole in patients undergoing allogeneic stem cell transplantation. Antimicrob. Agents Chemother. 54:207–212. 10.1128/AAC.01027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vehreschild JJ, Müller C, Farowski F, Vehreschild MJ, Cornely OA, Fuhr U, Kreuzer KA, Hallek M, Kohl V. 2012. Factors influencing the pharmacokinetics of prophylactic posaconazole oral suspension in patients with acute myeloid leukemia or myelodysplastic syndrome. Eur. J. Clin. Pharmacol. 68:987–995. 10.1007/s00228-012-1212-y. [DOI] [PubMed] [Google Scholar]
- 27.European Medicines Agency. 2005. Noxafil: EPAR—scientific discussion. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000610/WC500037781.pdf Accessed 1 November 2013. [Google Scholar]
- 28.Krishna G, Ma L, Vickery D, Yu X, Wu I, Power E, Beresford E, Komjathy S. 2009. Effect of varying amounts of a liquid nutritional supplement on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 53:4749–4752. 10.1128/AAC.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishna G, AbuTarif M, Xuan F, Martinho M, Angulo D, Cornely OA. 2008. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28:1223–1232. 10.1592/phco.28.10.1223. [DOI] [PubMed] [Google Scholar]
- 30.Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627–1636. 10.1592/phco.27.12.1627. [DOI] [PubMed] [Google Scholar]
- 31.Cornely OA, Helfgott D, Langston A, Heinz W, Vehreschild JJ, Vehreschild MJGT, Krishna G, Ma L, Huyck S, McCarthy MC. 2012. Pharmacokinetics of different dosing strategies of oral posaconazole in patients with compromised gastrointestinal function and who are at high risk for invasive fungal infection. Antimicrob. Agents Chemother. 56:2652–2658. 10.1128/AAC.05937-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebeaux D, Lanternier F, Elie C, Suarez F, Buzyn A, Viard J-P, Bougnoux M-E, Lecuit M, Jullien V, Lortholary O. 2009. Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob. Agents Chemother. 53:5224–5229. 10.1128/AAC.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishna G, Sansone-Parsons A, Kantesaria B. 2007. Drug interaction assessment following concomitant administration of posaconazole and phenytoin in healthy men. Curr. Med. Res. Opin. 23:1415–1422. 10.1185/030079907X187937. [DOI] [PubMed] [Google Scholar]
- 34.Krishna G, Parsons A, Kantesaria B, Mant T. 2007. Evaluation of the pharmacokinetics of posaconazole and rifabutin following co-administration to healthy men. Curr. Med. Res. Opin. 23:545–552. 10.1185/030079906X167507. [DOI] [PubMed] [Google Scholar]
- 35.Hohmann C, Kang EM, Jancel T. 2010. Rifampin and posaconazole coadministration leads to decreased serum posaconazole concentrations. Clin. Infect. Dis. 50:939–940. 10.1086/650740. [DOI] [PubMed] [Google Scholar]
- 36.Lipp H-P. 2010. Clinical pharmacodynamics and pharmacokinetics of the antifungal extended-spectrum triazole posaconazole: an overview. Br. J. Clin. Pharmacol. 70:471–480. 10.1111/j.1365-2125.2010.03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosal A, Hapangama N, Yuan Y, Achanfuo-Yeboah J, Iannucci R, Chowdhury S, Alton K, Patrick JE, Zbaida S. 2004. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil). Drug Metab. Dispos. 32:267–271. 10.1124/dmd.32.2.267. [DOI] [PubMed] [Google Scholar]
- 38.Anderson GD. 2004. Pharmacogenetics and enzyme induction/inhibition properties of antiepileptic drugs. Neurology 63(10 Suppl 4):S3–S8. 10.1212/WNL.63.10_suppl_4.S3. [DOI] [PubMed] [Google Scholar]
- 39.Oesch F, Arand M, Benedetti MS, Castelli MG, Dostert P. 1996. Inducing properties of rifampicin and rifabutin for selected enzyme activities of the cytochrome P-450 and UDP-glucuronosyltransferase superfamilies in female rat liver. J. Antimicrob. Chemother. 37:1111–1119. 10.1093/jac/37.6.1111. [DOI] [PubMed] [Google Scholar]
- 40.Dodds Ashley ES, Varkey JB, Krishna G, Vickery D, Ma L, Yu X, Malavade D, Goodwin M, Perfect JR, Power E. 2009. Pharmacokinetics of posaconazole administered orally or by nasogastric tube in healthy volunteers. Antimicrob. Agents Chemother. 53:2960–2964. 10.1128/AAC.01178-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Störzinger D, Borghorst S, Hofer S, Busch CJ, Lichtenstern C, Hempel G, Weigand MA, Hoppe-Tichy T. 2012. Plasma concentrations of posaconazole administered via nasogastric tube in patients of a surgical intensive care unit. Antimicrob. Agents Chemother. 56:4468–4470. 10.1128/AAC.06167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. 2014. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 69:1162–1176. 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolton MJ, Mikus G, Weiss J, Ray JE, McLachlan AJ. 2014. Understanding variability with voriconazole using a population pharmacokinetic approach: implications for optimal dosing. J. Antimicrob. Chemother. 69:1633–1641. 10.1093/jac/dku031. [DOI] [PubMed] [Google Scholar]
- 44.Ullmann AJ, Cornely OA, Burchardt A, Hachem R, Kontoyiannis DP, Töpelt K, Courtney R, Wexler D, Krishna G, Martinho M, Corcoran G, Raad I. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658–666. 10.1128/AAC.50.2.658-666.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]