Abstract
We used two established neutropenic murine models of pulmonary aspergillosis and mucormycosis to explore the association between the posaconazole area under the concentration-time curve (AUC)-to-MIC ratio (AUC/MIC) and treatment outcome. Posaconazole serum pharmacokinetics were verified in infected mice to ensure that the studied doses reflected human exposures with the oral suspension, delayed-release tablet, and intravenous formulations of posaconazole. Sinopulmonary infections were then induced in groups of neutropenic mice with Aspergillus fumigatus strain 293 (posaconazole MIC, 0.5 mg/liter) or Rhizopus oryzae strain 969 (posaconazole MIC, 2 mg/liter) and treated with escalating daily dosages of oral posaconazole, which was designed to achieve AUCs ranging from 1.10 to 392 mg · h/liter. After 5 days of treatment, lung fungal burden was analyzed by quantitative real-time PCR. The relationships of the total drug AUC/MIC and the treatment response were similar in both models, with 90% effective concentrations (EC90s) corresponding to an AUC/MIC threshold of 76 (95% confidence interval [CI], 46 to 102) for strain 293 versus 87 (95% CI, 66 to 101) for strain 969. Using a provisional AUC/MIC target of >100, these exposures correlated with minimum serum posaconazole concentrations (Cmins) of 1.25 mg/liter for strain 293 and 4.0 mg/liter for strain 969. The addition of deferasirox, but not liposomal amphotericin or caspofungin, improved the activity of a suboptimal posaconazole regimen (AUC/MIC, 33) in animals with pulmonary mucormycosis. However, no combination was as effective as the high-dose posaconazole monotherapy regimen (AUC/MIC, 184). Our analysis suggests that posaconazole pharmacodynamics are similar for A. fumigatus and R. oryzae when indexed to pathogen MICs.
INTRODUCTION
Posaconazole is a broad-spectrum triazole antifungal approved for the prevention of invasive aspergillosis and candidiasis and for the treatment of oropharyngeal candidiasis. Posaconazole is frequently used off-label for the treatment of other documented mold infections, including invasive mucormycosis, when therapeutic options are limited. The enhanced potency of posaconazole against species of the order Mucorales relative to those of other triazoles may be explained, in part, by an increased affinity for fungal P450 demethylase (CYP51) and increased penetration or reduced efflux from the fungal cell membrane (1, 2). Consequently, posaconazole MICs for Mucor, Rhizopus, Absidia, and Cunninghamella spp. fall in the range of clinically achievable serum concentrations, although some isolates (3, 4), especially those among Rhizopus oryzae, exhibit higher MICs (>2 mg/liter) and are less responsive to treatment in animal models (5, 6). Consistent with these preclinical observations, uncontrolled retrospective case series have reported encouraging efficacy with salvage posaconazole treatment in invasive mucormycosis (7). In recently published guidelines from the European Society for Clinical Microbiology and Infectious Diseases and the European Confederation of Medical Mycology, posaconazole receives a moderate recommendation for use in the treatment of invasive mucormycosis (8).
Optimal dosing strategies for posaconazole for treating mucormycosis are not well defined. Inadequate data concerning posaconazole dosing for Mucorales infection may reflect, in part, the pharmacokinetic limitations of the oral suspension formulation; absorption is sometimes erratic and limited to 800 mg per day (9, 10). However, with the introduction of new tablet and intravenous (i.v.) formulations (11, 12), clinicians can now potentially achieve much higher posaconazole exposures in patients than previously possible. Moreover, an understanding of posaconazole pharmacokinetic/pharmacodynamic (PK/PD) properties against Mucorales infection will also be essential for establishing provisional susceptibility breakpoints, given the limited clinical data for these uncommon mycoses.
Recent posaconazole PK/PD studies in murine models of invasive aspergillosis provided a starting point for understanding what exposures may be required for optimal response in mucormycosis. Mavridou and colleagues (13) reported that total posaconazole area under the concentration-time curve (AUC)-to-MIC ratios (AUC/MIC) of 321 to 1,000 were required to improve the 14-day survival rate from 50% to 85% in nonimmunosuppressed mice challenged with a lethal intravenous inoculum of Aspergillus fumigatus. Using a neutropenic murine model of pulmonary aspergillosis, Howard and colleagues (14) found that a posaconazole AUC/MIC of 167 was associated with half-maximal activity assessed by reductions in serum galactomannan levels, with maximal suppression of galactomannan levels at a posaconazole dose of 20 mg/kg of body weight. Similarly, Lepak and colleagues (15) reported that a free AUC (fAUC)/MIC target of 1.09 (equivalent to an AUC/MIC of 109) was required to suppress lung fungal burden in animals infected with wild-type and Cyp51 mutant isolates of A. fumigatus. Other investigators have reported a correlation of posaconazole efficacy with average or trough posaconazole serum concentrations (5, 16) and MICs in experimental mucormycosis (17, 18), but data describing the relationship between posaconazole AUC/MICs and treatment effect in experimental mucormycosis are lacking.
Our laboratory has characterized the pharmacodynamics of echinocandins and lipid amphotericin B formulations in neutropenic murine infection models of aspergillosis and mucormycosis, using standardized immunosuppression strategies and a quantitative PCR (qPCR) as a dynamic and sensitive indicator of lung fungal burden and treatment response (19–22). Therefore, we performed a parallel analysis of posaconazole pharmacodynamics in neutropenic murine models of pulmonary aspergillosis and mucormycosis to examine if higher posaconazole serum AUC/MIC thresholds are required to reduce lung fungal burden in pulmonary mucormycosis. As a secondary endpoint, we examined if the addition of deferasirox, liposomal amphotericin B, or caspofungin to a suboptimal posaconazole regimen can improve mycological efficacy in vivo.
MATERIALS AND METHODS
Reagents.
Cyclophosphamide and cortisone acetate were obtained from Sigma-Aldrich (St. Louis, MO). The human clinical formulations of posaconazole suspension and caspofungin acetate (Merck & Co., Inc., Rahway, NJ), liposomal amphotericin B (Gilead, Inc., Foster City, CA), and deferasirox (Novartis, East Hanover, NJ) were obtained from the respective manufacturers, reconstituted, and diluted according to their recommendations as required.
Animals.
Eight-week-old female BALB/c mice (weight, 18 to 22 g; Harlan Laboratories) were used for all the experiments. The mice were housed in a HEPA-filtered cage system located in a sterile barrier isolation suite. The cages contained sterilized food and 5% dextrose water that was provided ad libitum. All the mice were cared for in accordance with institutional protocols for humane and ethical care following review of the protocol by The University of Texas M. D. Anderson Cancer Center Institutional Animal Care and Use Committees.
Inoculum preparation.
Two reference isolates, A. fumigatus strain 293 and R. oryzae strain 969, originally cultured from patients with invasive disease, were selected for testing based on previous testing in our animal models. Inocula were prepared fresh on the day of each experiment by cultivating isolates on yeast agar (YAG) (0.5% yeast extract, 1.0% dextrose, 0.2% vitamin mix, 0.1% trace elements, 1.5% agar, 1% MgSO4) plates for 4 days (strain 969) or 7 days (strain 293). The agar plates were then flooded with phosphate-buffered saline (PBS)–0.1% Tween 20. The suspension was then passed through a 40-μm-pore-size filter (BD Biosciences, San Jose, CA) to remove hyphal elements. The resulting spore suspension was concentrated to 3 × 107 spores/ml for strain 969 or 1.5 × 108 spores/ml for strain 293. Posaconazole MICs were confirmed using the NCCLS M39-A2 microdilution protocol (23).
Immunosuppression and infection.
The mice were immunosuppressed with 200-mg/kg and 150-mg/kg intraperitoneal (i.p.) injections of cyclophosphamide 4 days and 1 day, respectively, prior to infection to induce neutropenia. A single 300-mg/kg subcutaneous dose of cortisone acetate was administered 1 day before infection to suppress pulmonary macrophage function. Before inoculation, the mice were rendered unconscious with 5% isoflurane delivered via a small-animal anesthesia chamber. An inoculum of 5 × 106 strain 293 or 1 × 106 strain 969 spores was then administered (40 μl) into the nose of the mouse by micropipette, alternating droplets in each mouse between the right and left nares between breaths. The mice were held in an upright position and allowed to inhale the inoculum until normal breathing resumed and the animal regained consciousness. The administered inoculum was previously found to produce progressively fatal invasive pulmonary aspergillosis or mucormycosis by 5 to 7 days without the administration of antifungal therapy (19, 22).
After inoculation, posaconazole suspension was then administered by oral gavage (200 μl) starting 12 h after infection and then once daily thereafter for 5 days. Posaconazole doses of >40 mg/kg were divided into two separate administrations to reduce the dosing volumes. Each treatment group/dose consisted of at least 10 mice.
Lung fungal burden analysis.
The processing and quantification of lung fungal burden were performed as previously described (19, 24, 25). Briefly, after 5 days of posaconazole therapy, the mice were humanly euthanized by CO2 narcosis, and the lungs of each one were excised, weighed, and homogenized in 1 ml of sterile PBS using a 2-ml screw-cap cryovial containing acid-washed glass beads. Tissue was then homogenized in alternating 15-s bursts in a mini bead beater (Bio-Spec, Bartlesville, OK) followed by cooling in crushed ice. DNA was then isolated from an aliquot of the lung homogenate (80 μl) using the DNeasy kit (Qiagen, Valencia, CA) and analyzed in duplicate by real-time quantitative PCR (qPCR) using primers and dually labeled hybridization probes specific for A. fumigatus and R. oryzae 18S rRNA genes, respectively (24, 25). The cycle threshold of each sample was interpolated from a seven-point standard curve prepared by spiking uninfected mouse lungs with known concentrations of strain 293 or strain 969 (102 to 107 spores). A plasmid internal standard was amplified in separate reactions to correct for percent differences in DNA recovery (19).
Posaconazole pharmacokinetics.
Previous studies in murine models of candidiasis and aspergillosis reported linear pharmacokinetics with posaconazole doses ranging from 0.08 to 160 mg/kg (14, 15, 26). In pulmonary models of aspergillosis, posaconazole was effective against wild-type A. fumigatus isolates over a range of 10 to 40 mg/kg/day (14, 15). Therefore, we performed a limited single-dose posaconazole pharmacokinetic validation study at low (5 mg/kg/day), intermediate (15 mg/kg/day), and high (50 mg/kg/day) doses in infected animals at doses predicted to be efficacious against Mucorales infection. Immunosuppressed mice were inoculated with strain 969 and administered single doses of posaconazole suspension by oral gavage. At 0, 2, 4, 8, 12, and 24 h, two mice were humanely euthanized, and their blood was collected and allowed to clot on ice. Serum was separated by centrifugation and stored at −80°C until analysis by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC/MS) at the fungus testing laboratory at the University of Texas, San Antonio, Health Science Center (http://pathology.uthscsa.edu/strl/fungus/d_levels.shtml).
Posaconazole pharmacokinetic parameters were determined using a noncompartmental model for oral drug administration (Kinetica 5; Thermo Scientific, Waltham, MA). The elimination half-life was calculated using nonlinear least squares regression. The AUC was calculated using the trapezoidal rule. For dose levels not directly measured, the AUC and minimum concentration (Cmin) were estimated by interpolation. The eventual tested dose range of posaconazole was 0.1 to 80 mg/kg/day with a total drug AUC range of 1.10 to 392 mg · h/liter.
Pharmacodynamic analysis.
Based on previous investigations (13–15), we focused on the relationship between the posaconazole AUC/MIC index and treatment response for strains 293 and 969 in the infection models. Total drug concentrations were used for all calculations. Lung fungal burden qPCR data were modeled according to a 4-parameter variable-slope inhibitory sigmoidal Emax model: Y = Emin + (Emax − Emin)/(1 + 10[N(AUC/MIC − EC50]), where Emin represents fungal burden in untreated mice, Emax is the maximal fungal burden reduction, EC50 is the concentration needed to achieve a 50% response, N is the Hill slope, and Y is the log-transformed fungal burden determined by qPCR. The model fit was assessed visually and by the coefficient of determination (R2) and precision of the EC50 estimate, as well as the Akaike information criterion (AIC). AUC/MIC exposures required for stasis and EC50 and EC90 responses were predicted for strain 293- and strain 969-infected animals using the Emax models. All analyses were performed using JMP version 11 (SAS Institute, Cary, NC).
Combinations with suboptimal posaconazole exposures.
Based on the pharmacodynamic analysis described above, we examined the effect of 10 mg/kg/day deferoxamine by oral gavage, 1 mg/kg/day caspofungin by intraperitoneal injection, or 5 mg/kg/day liposomal amphotericin B by intravenous injection in groups of 10 mice infected with strain 969 treated with a suboptimal dose of posaconazole (10 mg/kg/day, which produced an approximately 1-log10 fungal burden reduction). Infected mice treated with a maximal dose of posaconazole (80 mg/kg/day) were also used as a reference group for the analysis. Treatment groups were compared using the Kruskal-Wallis test with Tukey's post hoc comparison.
Monte Carlo simulation.
Crystal Ball 11.1 (Oracle Corporation, Redwood City, CA) was used to perform 5,000-subject Monte Carlo simulations of the steady-state AUC from 0 to 24 h (AUC0–24)/MIC using pharmacokinetic data and percent coefficient of variation estimates published for the 200-mg four-times-daily posaconazole oral suspension (27, 33), the 600-mg delayed-release tablet on day 1 and then 300 mg daily (27, 33), and 600-mg intravenous posaconazole on day 1 and then 300 mg daily (27, 33). Subject weight was fixed to 70 kg for all simulations. Pharmacokinetic data were varied according to log-normal distributions, whereas protein binding was varied according to a uniform distribution ±5%. MICs were fixed at single values ranging from 0.015 to 2 mg/liter. The probabilities of attaining the posaconazole AUC/MIC target at the respective A. fumigatus and R. oryzae MICs were then compared using the EUCAST breakpoint AUC/MIC of >167 (28) versus the AUC/MIC target identified from our experimental infection models.
RESULTS
Isolate susceptibility.
MICs were determined in triplicate on different days using NCCLS M39-A2 microdilution methods (24). The mean MICs for posaconazole were 0.5 mg/liter for strain 293 and 2 mg/liter for strain 969. The mean amphotericin B MICs were 0.5 mg/liter for both isolates. The minimum effective concentrations of caspofungin were 0.25 mg/liter for strain 293 and >32 mg/liter for strain 969.
Pharmacokinetics.
The time course of serum posaconazole plasma concentrations after single 5-, 15-, and 50-mg/kg doses is shown in Fig. 1. Peak serum drug concentrations were achieved around 4 h after oral doses that ranged from 2.73 to 16.1 mg/liter. Trough (Cmin at 24 h) concentrations ranged from 1.2 to 4.7 mg/liter. The elimination half-life ranged from 11.65 to 16.87 h (mean, 13.2 ± 3.2 h). The AUC0–24 ranged from 38.22 to 225 mg · h/liter, with the lowest and highest posaconazole doses analyzed by UPLC/MS.
FIG 1.
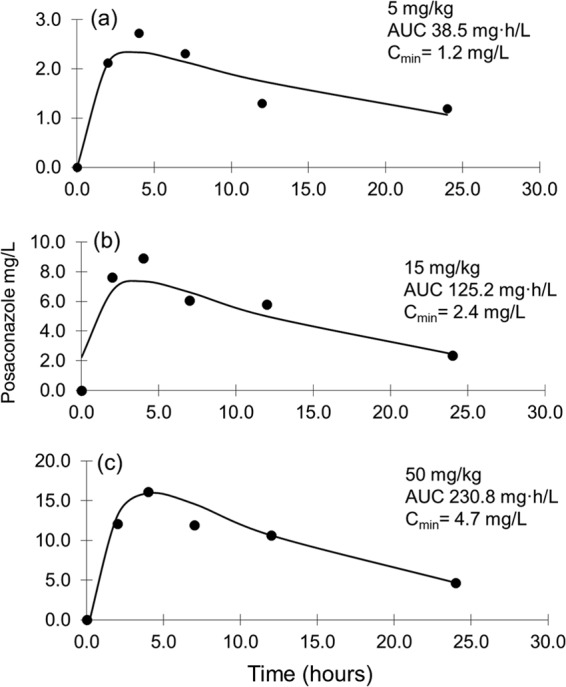
Single-dose posaconazole serum pharmacokinetics in infected neutropenic animals. Posaconazole doses of 5 mg/kg/day by oral gavage (a), 15 mg/kg/day by oral gavage (b), and 50 mg/kg/day by oral gavage (c). Each datum point represents the mean serum concentration determined in two infected neutropenic mice.
AUC/MIC relationship with fungal burden.
Untreated control animals infected with strain 293 or strain 969 displayed increases in day 5 lung fungal burden that were similar to those of the animals treated with the lowest posaconazole dose (1 mg/kg/day) (mean log10 conidial equivalent, 6.1 ± 0.76 for strain 293 versus 6.2 ± 0.58 for strain 969; P = 0.65). A sigmoid dose-response relationship was observed with increasing posaconazole serum exposures for each of the two isolates (Fig. 2). However, the daily posaconazole doses required to achieve maximal reductions in lung fungal burden were higher for strain 969 than those for strain 293 (10 mg/kg/day versus 50 mg/kg/day, respectively).
FIG 2.
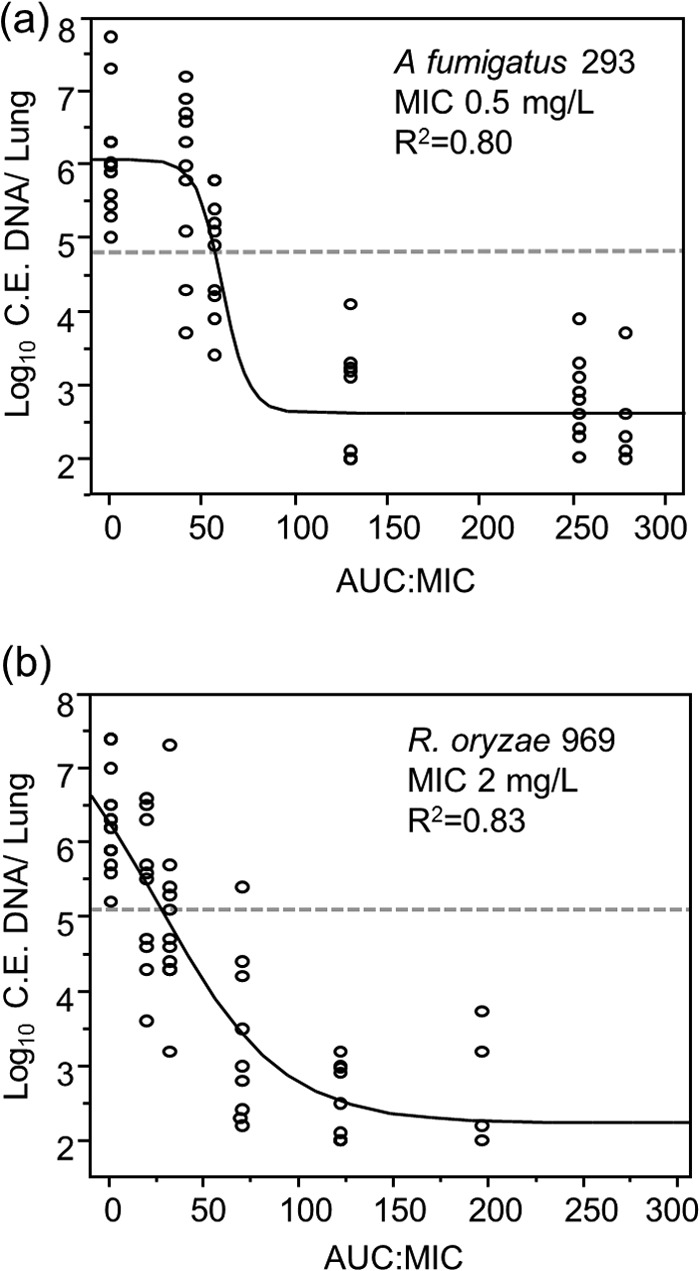
Relationship between total posaconazole serum AUC/MIC and day 5 lung fungal burden in neutropenic murine models of invasive pulmonary aspergillosis and mucormycosis. Each datum point represents the mean A. fumigatus strain 293 (a) or R. oryzae strain 969 (b) lung fungal burden of a single mouse, expressed as conidial equivalent (C.E.) DNA/lung. Lines represent the fit of a 4-parameter logistic regression model used to describe the relationship between AUC/MIC and fungal burden. Dotted lines indicate the initial lung fungal burden determined within 1 h after inoculation.
The relationship between the serum posaconazole AUC0–24, indexed to pathogen MICs, and strain 293 or 969 fungal burden, determined by qPCR, is presented in Fig. 2. The model-predicted posaconazole AUC/MIC associated with stasis was slightly lower for strain 969 (27; 95% confidence interval [CI], 19 to 36) than for strain 293 (57; 95% CI, 52 to 61), but the EC50s for A. fumigatus were similar (53 [95% CI, 43 to 63] and 63 [95% CI, 53 to 75], respectively). Similarly, the posaconazole EC90 thresholds corresponding with an approximately 2-log10 net reduction in fungal burden for strains 293 and 969 were similar for the two isolates (76 [95% CI, 46 to 102] and 87 [95% CI, 66 to 101], respectively). Based on the isolates tested in our models, we estimated an AUC/MIC threshold target of >100 by interpolation at posaconazole Cmins of 1.25 mg/ml for strain 293 and 4.0 mg/ml for strain 969.
Combinations with suboptimal posaconazole.
Combination therapy is often recommended in situations where optimal pharmacokinetic exposures are unobtainable with a single drug. Therefore, we examined the activities of a suboptimal exposure of posaconazole (10 mg/kg/day; AUC/MIC, 33; posaconazole Cmin, 1.25 mg/liter) in experimental sinopulmonary mucormycosis combined with “standard” doses of liposomal amphotericin B or caspofungin used for aspergillosis or the iron-chelating agent deferasirox. The highest-dose posaconazole regimen tested in the model (80 mg/kg/day) was included for comparison. We observed significantly greater reductions in lung fungal burden when 10 mg/kg/day posaconazole was combined with 10 mg/kg/day oral deferasirox (1.4-log10 improvement, P < 0.05) but not with liposomal amphotericin B (0.8-log10 improvement, P > 0.05) or caspofungin (0.72-log10 improvement, P > 0.05). However, none of the combination regimens was as effective as the monotherapy high-dose (80-mg/kg) posaconazole regimen (AUC/MIC, 184; 2.5-log10 improvement, P < 0.01) (Fig. 3).
FIG 3.
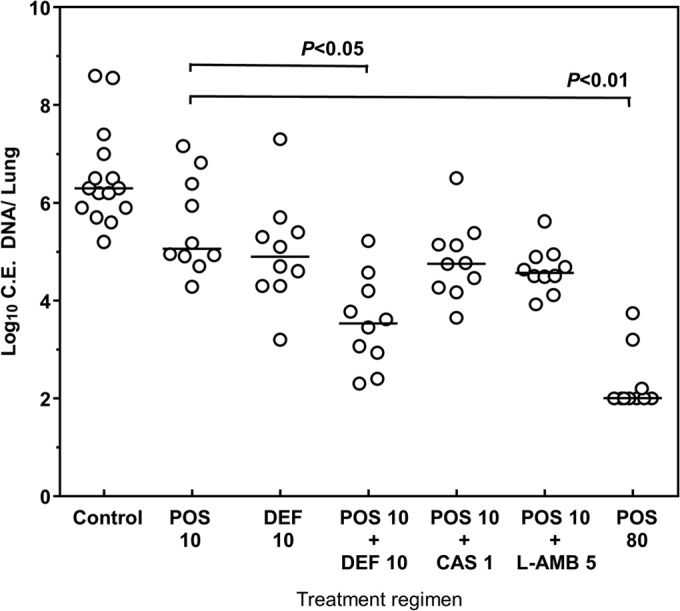
Activities of various antifungal combinations in the setting of suboptimal posaconazole exposures in experimental pulmonary mucormycosis. Each datum point represents the log10 conidial equivalent (C.E.) lung fungal DNA. Each line represents median concentration of the treatment group. POS 10, 10 mg/kg/daily posaconazole by oral gavage (AUC/MIC, 33); DEF 10, 10 mg/kg/day deferoxamine by oral gavage; CAS 1, 1 mg/kg/day caspofungin by intraperitoneal injection; L-AMB 5, 5 mg/kg/day liposomal amphotericin B by intravenous injection; POS 80, 80 mg/kg/daily posaconazole by oral gavage (AUC/MIC, 196). Groups were analyzed using the Kruskal-Wallis test with Tukey's post hoc comparison.
Monte Carlo simulations.
In agreement with prior EUCAST simulations (28), we found that the probability of achieving posaconazole AUC/MICs of >100 diminished as the pathogen MIC increased to >0.06 mg/liter, which is below the current wild-type epidemiological cutoff (ECOFF) value of 0.25 mg/liter (Table 1). However, the delayed-release tablet and intravenous formulations reliably achieved AUC/MICs of >100 in simulated patients (95% to 97%) up to an MIC of 0.125 mg/liter.
TABLE 1.
Probability of posaconazole serum AUC/MIC target attainment at respective MICs in 5,000 simulated patients
| MIC (mg/liter) | Probability of posaconazole serum AUC/MIC target attainment (%) using: |
|||
|---|---|---|---|---|
| Suspension, 800 mg/day, EUCAST simulationsa | Suspension, 800 mg/day, current studyb | Tablets, 300 mg/dayc | Intravenous, 300 mg/dayd | |
| 0.015 | 99.9 | 99.0 | 99.9 | 99.9 |
| 0.03 | 96 | 97.0 | 99.9 | 99.9 |
| 0.06 | 68 | 83 | 99 | 99 |
| 0.125 | 15.3 | 64 | 95 | 97 |
| 0.25e | 0.6 | 18 | 80 | 81 |
| 0.5 | 0 | 0 | 24 | 15 |
| 1.0 | 0 | 0 | 0 | 0 |
| 2.0 | 0 | 0 | 0 | 0 |
DISCUSSION
Limited data regarding the relationship between posaconazole exposures or serum levels and treatment response in an in vivo model of invasive pulmonary mucormycosis are available. We found that when indexed to the MIC, the serum posaconazole exposures required for maximal suppression of lung fungal burden (as measured by quantitative PCR) in neutropenic mice infected with A. fumigatus strain 293 or R. oryzae strain 969 were similar. Our data suggest that a total drug serum AUC/MIC target of >100 may be an appropriate benchmark for future PD studies and explorations of breakpoints in Mucorales species. Using a posaconazole protein-binding estimate of 99% derived from bioassay data by Andes et al. (26), an AUC/MIC target of >100 is equivalent to an fAUC/MIC target of >1.
The exposure-response relationship for aspergillosis observed in this study is consistent with those of previous work that identified posaconazole AUC/MICs of 167 to 176 as a provisional PK/PD threshold for dosing (14, 15). Among these studies, the one that is most comparable to the current study was performed by Lepak and colleagues (15), who examined posaconazole PD targets in wild-type and CYP51 mutants of A. fumigatus in neutropenic mice using a similar qPCR assay. Although the investigators used a different mouse strain, a higher infecting inoculum, and a longer duration of therapy (7 days), the posaconazole AUC/MIC target reported for strain 293 associated with stasis in their model was 44.3 versus 57 (95% CI, 52 to 61) in our model. The median AUC/MIC needed to achieve stasis against all 10 A. fumigatus isolates tested in their study was 87.5, with an additional 1-log10 decrease occurring at a median AUC/MIC of 192.6, consistent with a recommended target AUC/MIC of >100. Our work also confirms previous work suggesting that MICs are a useful predictor of posaconazole efficacy in vivo (5, 15–18).
PK/PD analysis is critical for establishing clinically relevant susceptibility breakpoints, especially for less commonly culture-documented pathogens such as Aspergillus spp. and R. oryzae. In the 2012 EUCAST rationale document for posaconazole clinical breakpoints against Aspergillus spp., an epidemiological cutoff (ECOFF) value for A. fumigatus was defined at 0.25 μg/ml (28), which is lower than the MIC of strain 293 used in the present study. Interestingly, PK/PD simulations in the EUCAST document suggested that 200 mg of posaconazole suspension administered four times daily would not achieve an AUC/MIC of ≥167 in most patients, even at a wild-type MIC of 0.06 μg/ml. Using a slightly lower PK/PD target identified in the current study (AUC/MIC of >100) yielded similar results for the oral suspension. However, it appears from our simulations that the delayed-release tablet and intravenous formulations will consistently achieve the AUC/MIC exposures needed to cover wild-type A. fumigatus isolates up to an MIC of 0.125 mg/liter. Our work suggests that a similar breakpoint of 0.125 mg/liter may be valid, pending further testing with additional isolates and delineation of the population ECOFF values for R. oryzae.
Given the higher posaconazole MICs of many Mucorales species, combination therapy may still be an appealing strategy, at least during the initial treatment phases of this aggressive disease (27). However, very limited in vivo preclinical data exist to suggest which combination therapy would be most effective or which antifungal should be added for a patient who may have less than optimal concentrations (29). Among the options we examined, which have been reported to be additive or synergistic in vivo (29, 30), deferasirox was the only agent that significantly improved the activity of suboptimal posaconazole exposures in neutropenic mice infected with strain 969. This observation is consistent with that of a large body of preclinical work supporting a possible role of concomitant deferasirox in the treatment of mucormycosis, even though a pilot clinical trial of this strategy yielded disappointing results (31), possibly due to imbalances in the patient populations that were enrolled (32). None of the combinations was as effective in improving posaconazole exposures, suggesting that dose escalation with the intravenous (i.v.) or delayed-release tablet formulations, if proven to have an acceptable safety margin, may ultimately yield greater benefits for refractory mucormycosis than combination therapy with current antifungals. This area clearly needs more detailed study.
Our study has some limitations, namely, the fact that the pharmacodynamic analysis was performed with only one well-characterized A. fumigatus isolate and one well-characterized R. oryzae isolate in our infection models. Another possible limitation of our study is that we did not verify posaconazole pharmacokinetics at the highest dose tested in the model (80 mg/kg/day), which was selected after completion of our pharmacokinetic verification studies to confirm that the plateau portion of the posaconazole dose-response curve was in fact reached at 50 mg/kg/day against the more resistant R. oryzae isolate. Nevertheless, Lepak and colleagues using a similar model, reported that posaconazole AUCs were linear with respect to daily dosages up to 160 mg/kg/day, allowing the AUC to be estimated by linear extrapolation or interpolation (15). Therefore, we believe that our estimation of the posaconazole exposure at 80 mg/kg is justified for the current analysis.
In summary, we found that posaconazole AUC/MIC targets associated with near-maximal early antifungal effects were similar in neutropenic sinopulmonary murine models of A. fumigatus aspergillosis and R. oryzae mucormycosis. Using a provisional AUC/MIC target exposure of >100 may achieve sufficient exposures to effectively treat A. fumigatus or R. oryzae infection in patients receiving the delayed-release tablet or i.v. formulation of posaconazole for MICs until the MIC reaches 0.25 mg/liter, which is in agreement with the current ECOFF thresholds proposed by EUCAST for A. fumigatus. Finally, in the setting of suboptimal posaconazole AUC/MICs, the addition of deferasirox was associated with significantly improved antifungal effects, although the greatest improvement in antifungal activity observed in the model occurred with higher posaconazole exposures.
ACKNOWLEDGMENTS
Funding for this study was provided by Merck & Co, Inc. (to D.P.K.) through the investigator-initiated sponsored research program. D.P.K. also acknowledges his support from the Francis King Black Endowed Professorship. D.P.K. received research support and honoraria from Merck, Astellas, and Pfizer. R.E.L. has received research support from Pfizer, Inc., and honoraria from Gilead, Inc.
Footnotes
Published ahead of print 2 September 2014
REFERENCES
- 1.Xiao L, Madison V, Chau AS, Loebenberg D, Palermo RE, Mcnicholas PM. 2004. Three-dimensional models of wild-type and mutated forms of cytochrome p450 14 alpha-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob. Agents Chemother. 48:568–574. 10.1128/AAC.48.2.568-574.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chau AS, Chen G, Mcnicholas PM, Mann PA. 2006. Molecular basis for enhanced activity of posaconazole against Absidia corymbifera and Rhizopus oryzae. Antimicrob. Agents Chemother. 50:3917–3919. 10.1128/AAC.00747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun QN, Najvar LK, Bocanegra R, Loebenberg D, Graybill JR. 2002. In vivo activity of posaconazole against Mucor spp. in an immunosuppressed-mouse model. Antimicrob. Agents Chemother. 46:2310–2312. 10.1128/AAC.46.7.2310-2312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dannaoui E, Meletiadis J, Mouton JW, Meis J, Verweij PE. 2003. In vitro susceptibilities of zygomycetes to conventional and new antifungals. J. Antimicrob. Chemother. 51:45–52. 10.1093/jac/dkg020. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez MM, Pastor FJ, Calvo E, Salas V, Sutton DA, Guarro J. 2009. Correlation of in vitro activity, serum levels, and in vivo efficacy of posaconazole against Rhizopus microsporus in a murine disseminated infection. Antimicrob. Agents Chemother. 53:5022–5025. 10.1128/AAC.01026-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salas V, Pastor FJ, Rodriguez MM, Calvo E, Mayayo E, Guarro J. 2011. In vitro activity and in vivo efficacy of posaconazole in treatment of murine infections by different isolates of the Aspergillus terreus complex. Antimicrob. Agents Chemother. 55:676–679. 10.1128/AAC.00736-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:e61–e65. 10.1086/500212. [DOI] [PubMed] [Google Scholar]
- 8.Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, De Hoog S, Hope W, Johnson E, Kathuria S, Lackner M, Lass-Florl C, Lortholary O, Meis JF, Meletiadis J, Munoz P, Richardson M, Roilides E, Tortorano AM, Ullmann AJ, Van Diepeningen A, Verweij P, Petrikkos G. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 20(Suppl):5–26. 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 9.Ullmann AJ, Cornely OA, Burchardt A, Hachem R, Kontoyiannis DP, Topelt K, Courtney R, Wexler D, Krishna G, Martinho M, Corcoran G, Raad I. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658–666. 10.1128/AAC.50.2.658-666.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezzet F, Wexler D, Courtney R, Krishna G, Lim J, Laughlin M. 2005. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin. Pharmacokinet. 44:211–220. 10.2165/00003088-200544020-00006. [DOI] [PubMed] [Google Scholar]
- 11.Maertens J, Cornely OA, Ullmann AJ, Heinz WJ, Krishna G, Patino H, Caceres M, Kartsonis N, Waskin H, Robertson MN. 2014. Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease. Antimicrob. Agents Chemother. 58:3610–3617. 10.1128/AAC.02686-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna G, Ma L, Martinho M, Preston RA, O'Mara E. 2012. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J. Antimicrob. Chemother. 67:2725–2730. 10.1093/jac/dks268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavridou E, Bruggemann RJ, Melchers WJ, Mouton JW, Verweij PE. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860–865. 10.1128/AAC.00931-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard SJ, Lestner JM, Sharp A, Gregson L, Goodwin J, Slater J, Majithiya JB, Warn PA, Hope WW. 2011. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J. Infect. Dis. 203:1324–1332. 10.1093/infdis/jir023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepak AJ, Marchillo K, Vanhecker J, Andes DR. 2013. Posaconazole pharmacodynamic target determination against wild-type and cyp51 mutant isolates of Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 57:579–585. 10.1128/AAC.01279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo G, Gebremariam T, Lee H, French SW, Wiederhold NP, Patterson TF, Filler SG, Ibrahim AS. 2013. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob. Agents Chemother. 57:3340–3347. 10.1128/AAC.00313-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spreghini E, Orlando F, Giannini D, Barchiesi F. 2010. In vitro and in vivo activities of posaconazole against zygomycetes with various degrees of susceptibility. J. Antimicrob. Chemother. 65:2158–2163. 10.1093/jac/dkq276. [DOI] [PubMed] [Google Scholar]
- 18.Dannaoui E, Meis JF, Loebenberg D, Verweij PE. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647–3650. 10.1128/AAC.47.11.3647-3650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiederhold NP, Tam VH, Chi JD, Prince RA, Kontoyiannis DP, Lewis RE. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:469–473. 10.1128/AAC.50.2.469-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis RE, Leventakos K, Liao G, Kontoyiannis DP. 2011. Efficacy of caspofungin in neutropenic and corticosteroid-immunosuppressed murine models of invasive pulmonary mucormycosis. Antimicrob. Agents Chemother. 55:3584–3587. 10.1128/AAC.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis RE, Liao G, Hou J, Chamilos G, Prince RA, Kontoyiannis DP. 2007. Comparative analysis of amphotericin B lipid complex and liposomal amphotericin B kinetics of lung accumulation and fungal clearance in a murine model of acute invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 51:1253–1258. 10.1128/AAC.01449-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis RE, Albert ND, Liao G, Hou J, Prince RA, Kontoyiannis DP. 2010. Comparative pharmacodynamics of amphotericin B lipid complex and liposomal amphotericin B in a murine model of pulmonary mucormycosis. Antimicrob. Agents Chemother. 54:1298–1304. 10.1128/AAC.01222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M39-A2. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 24.Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464–1471. 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim AS, Bowman JC, Avanessian V, Brown K, Spellberg B, Edwards JE, Jr, Douglas CM. 2005. Caspofungin inhibits rhizopus oryzae 1,3-beta-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 49:721–727. 10.1128/AAC.49.2.721-727.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andes D, Marchillo K, Conklin R, Krishna G, Ezzet F, Cacciapuoti A, Loebenberg D. 2004. Pharmacodynamics of a new triazole, posaconazole, in a murine model of disseminated candidiasis. Antimicrob. Agents Chemother. 48:137–142. 10.1128/AAC.48.1.137-142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, Kontoyiannis DP, Walsh TJ. 2012. Combination therapy for mucormycosis: why, what, and how? Clin. Infect. Dis. 54(Suppl. 1):S73–S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Committee on Antimicrobial Susceptibility Testing. 2012. Posaconazole and Aspergillus spp.: rationale for the EUCAST clinical breakpoints v 1.0. http://www.eucast.org/clinical_breakpoints/.
- 29.Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, Schwartz J, Skory CD, Edwards JE, Jr, Spellberg BJ. 2007. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J. Clin. Invest. 117:2649–2657. 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim AS, Spellberg B, Edwards J., Jr 2008. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr. Opin. Infect. Dis. 21:620–625. 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spellberg B, Ibrahim AS, Chin-Hong PV, Kontoyiannis DP, Morris MI, Perfect JR, Fredricks D, Brass EP. 2012. The deferasirox-Ambisome therapy for mucormycosis (defeat mucor) study: a randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 67:715–722. 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnelly JP, Lahav M. 2012. Deferasirox as adjunctive therapy for mucormycosis. J. Antimicrob. Chemother. 67:519–520. 10.1093/jac/dkr540. [DOI] [PubMed] [Google Scholar]
- 33.Merck & Co, Inc. 2014. Noxafil prescribing information (suspension, delayed release tablet, and intravenous formulation). Merck & Co, Inc., Whitehouse Station, NJ. [Google Scholar]


