Abstract
The in vitro susceptibilities of 30 isolates of Plasmodium vivax to a number of antimalarials (chloroquine [CQ], mefloquine, amodiaquine, quinine, and artesunate [AS]) were evaluated. The isolates came from the region of Urabá in Colombia, in which malaria is endemic, and were evaluated by the schizont maturation test. The 50% inhibitory concentration (IC50) was 0.6 nM (95% confidence interval [CI], 0.3 to 1.0 nM) for artesunate, 8.5 nM (95% CI, 5.6 to 13.0 nM) for amodiaquine, 23.3 nM (95% CI, 12.4 to 44.1 nM) for chloroquine, 55.6 nM (95% CI, 36.8 to 84.1 nM) for mefloquine, and 115.3 nM (95% CI, 57.7 to 230.5 nM) for quinine. The isolates were classified according to whether the initial parasites were mature or immature trophozoites (Tfz). It was found that the IC50s for chloroquine and artesunate were significantly different in the two aforementioned groups (P < 0.001). The IC50s of CQ and AS were higher in the isolates from mature Tfz (CQ, 39.3 nM versus 17 nM; AS, 1.4 nM versus 0.3 nM), and 10% of the isolates showed lower susceptibilities to one of the antimalarial drugs, 13.3% to two antimalarial drugs, and 3.3% to more than three antimalarial drugs. It should be highlighted that despite the extensive use of chloroquine in Colombia, P. vivax continues to be susceptible to antimalarials. This is the first report, to our knowledge, showing in vitro susceptibilities of P. vivax isolates to antimalarials in Colombia.
INTRODUCTION
Plasmodium vivax is responsible for >50% of malaria cases worldwide and is prevalent in Southeast Asia, the Western Pacific, and Central and South America. P. vivax malaria is known for causing relapses (1). In recent years, complicated clinical conditions have been reported for this type of malaria that are similar to those found for Plasmodium falciparum. Indeed, the two species coexist in many parts of the world (2). In Colombia, 85% of the territory is suitable for the transmission of Plasmodium spp., and according to an epidemiological report from the National Health Institute of Colombia, 53,963 cases of malaria were reported in 2012, of which 40,314 (74.7%) corresponded to P. vivax malaria (3).
The therapeutic failure of chloroquine to treat P. vivax malaria has been reported in different regions around the world. The first case of therapeutic failure was reported in Papua New Guinea in 1989 (4, 5), and since then, cases have also been recorded in Asia, primarily in Indonesia, Malaysia, Myanmar, India, Philippines, Vietnam, South Korea, and Thailand. Cases have also been reported in Ethiopia in Africa (6–12) and in Guyana, Brazil, Venezuela, Peru, and Colombia in South America (13–17). In Colombia, whereas one study reported the therapeutic failure of chloroquine in 11% (3/27) of the patients evaluated (17), other studies reported 100% efficacy for this antimalarial (14, 18). Therefore, the in vitro susceptibility profile of P. vivax to antimalarials must be determined.
This work investigated the in vitro susceptibilities of fresh isolates of P. vivax from the region of Urabá (Antioquia, Colombia) to chloroquine, mefloquine, amodiaquine, quinine, and artesunate using the schizont maturation test.
MATERIALS AND METHODS
Predosing of plates with antimalarials.
Seven serial dilutions of the antimalarials chloroquine (CQ; Sigma R-6628), mefloquine (MQ; Sigma R-2319), amodiaquine (AQ; Sigma R-2799), quinine (Qn; Sigma R-0132), and artesunate (AS; Sigma R-3731) were predosed. Each antimalarial plate was predosed in triplicate, in the ranges 1.646 nM to 1.200 nM, 12.5 nM to 800 nM, 2.34 nM to 150 nM, 3.7 nM to 2.700 nM, and 0.05 nM to 40 nM for CQ, MQ, AQ, Qn, and AS, respectively (4, 19–22). The predosed plates were stored at 4°C until they were used. The quality control of the predosing stage was carried out with the NF54 strain of P. falciparum (sensitive to all known antimalarials) (23), and parasitemia was recorded through the radioisotope method (24).
P. vivax isolates.
Between September 2010 and November 2012 at the malaria diagnosis stations in the municipalities of Turbo and Apartadó (Urabá, Antioquia, Colombia), where malaria was endemic, patients who met the following inclusion criteria were included: patients of any age and gender, women who were not pregnant, patients with single P. vivax infection (according to thick smear test and confirmed by the rapid diagnostic test SD Bio Line malaria Ag Pf/Pan; Standard Diagnostics, Inc., 05FK66), and patients with a parasitemia level of >2,000 parasites/μl of blood. Additional inclusion criteria were patients with a diagnosis of uncomplicated malaria according to the WHO criteria adapted for Colombia (25), patients who took no antimalarials in the month before the test, and patients with no chloroquine in the urine (according to the Saker-Solomons method). Each patient who fulfilled the criteria and was willing to take part in the study signed an informed consent form previously approved by the ethics committee of the Universidad de Antioquia through Act 012 of 18 June 2009. In the case of minors, the consent form was signed by a parent or guardian who was >18 years of age.
Collecting and processing samples.
In order to evaluate the in vitro susceptibility of P. vivax to antimalarials, 10 ml of venous blood was taken from each patient and put in a Vacutainer tube with heparin. The samples were processed within 4 h after being collected from the patients. Additionally, 200 μl of blood was poured onto filter paper (Whatman 3) to confirm P. vivax monoinfection in the analyzed samples using PCR (26).
In the thick smear test, 200 asexual parasites were counted in all of the different parasitic stages, including rings or immature trophozoites (Tfz imm), amoeboid trophozoites (Tfz amoeb), mature trophozoites (Tfz mat), preschizonts (pre-sch), and mature schizonts (Sch mat) (27). According to the initial parasitic stages, the isolates were divided into one of two groups. Group 1 consisted of isolates with >133 immature Tfz (rings plus amoeboid Tfz) out of 200 asexual parasites, and group 2 consisted of isolates that did not fulfill the previous criterion and had >133 mature Tfz out of 200 asexual parasites (21, 27).
Assay of in vitro susceptibility of P. vivax to antimalarials using the schizont maturation method.
Before the susceptibility assay, the leukocytes in a 10-ml sample of heparinized blood were eliminated during two rounds of filtration using cellulose columns, in accordance with the method described by Russell et al. (22). The brand of cellulose used (S6288; Sigma-Aldrich) differed from that employed in the Russell et al. method, but it had similar characteristics.
The parasitized erythrocytes, recovered during the second filtration, were centrifuged at 1,500 rpm for 5 min. The supernatant liquid was then removed, and 800 μl of the parasitized erythrocytes was used to prepare a suspension at a hematocrit level of 2% in McCoy's 5A medium (M4892; Sigma-Aldrich), supplemented with 25% AB+ of human serum. A total of 200 μl of this suspension was poured into each well of the predosed plates, which were incubated at 37°C in an atmosphere of 5% CO2, 5% O2, and 90% N2 until 40% of the initial parasitic stages of each isolate matured into schizonts in the drug-free control wells. After incubation, the supernatant was removed from each well, and the thick smear test was performed (28). To obtain readings from the tests, 200 asexual parasites differentiated by their parasitic stages were counted as previously described (27).
Inhibition percentages and inhibitory concentrations.
The inhibition percentage of schizont maturation in a treatment group was calculated by relating the number of schizonts present in the controls (>40%) with the number of schizonts in the treatment groups. Calculations were performed to determine the means or medians, standard deviations, and coefficients of variation of the schizont inhibition percentages obtained in three replicants of the antimalarial concentrations evaluated. The 50% inhibitory concentrations (IC50s) were estimated using a dose-response regression model from the drug-free controls by the GraphPad Prism version 5.0 program. The IC50s that were within the concentration range evaluated for each antimalarial and that had an r2 value of >9.0 were classified as valid. The IC50 of each antimalarial was correlated with the antimalarial group to which it belonged (aminoquinolines and artemisinins) in order to find overlapping susceptibilities.
Statistical analysis.
The statistical analysis was carried out using the GraphPad Prism version 5.0 software. The Kruskal-Wallis test was applied to determine the nonparametric comparisons, and the Fisher test was employed to calculate statistical significance. The nonparametric correlation analysis was carried out using the Spearman correlation (rs) model.
RESULTS
Of the 68 P. vivax monoinfection isolates (confirmed by PCR) that fulfilled the inclusion criteria, 30 (44.1%) achieved a schizont maturation of >40%. The mean initial parasitemia level was 9,853.3 parasites/μl of blood (95% confidence interval [CI], 7,640.7 to 17,494.0 parasites/μl). The isolates took an average of 28.8 h (95% CI, 25.7 to 31.9 h) to reach schizont maturation, and each isolate reached an average maturation of 54% (95% CI, 46% to 100%). Of the evaluated isolates, 15 (50%) were classified as group 1, with >133 immature Tfz out of 200 asexual parasites and a mean initial parasitemia level of 12,605 parasites/μl of blood (95% CI, 8,985 to 16,226 parasites/μl). The other 15 (50%) were classified into group 2, with >133 mature Tfz out of 200 asexual parasites and a mean initial parasitemia level of 6,949 parasites/μl of blood (95% CI, 4,749 to 9,150 parasites/μl). The parasitemia levels were significantly different in the two groups (P < 0.05). In group 1, 66.7% (10/15) of the isolates achieved schizont maturation after 30 h, 20% (3/15) reached this stage after 40 h, and 13.3% (2/15) achieved maturation after 48 h; in group 2, 73.3% (11/15) achieved maturation after 30 h, and the rest reached this stage after 20 h. The majority of cultures in both groups had maturation times of 30 h. The maturation times were significantly higher in the isolates of group 1 than in those of group 2 (P < 0.0001) (Table 1).
TABLE 1.
Parasitic stages, maturation times, and percentages of schizonts obtained by the isolate groups
| Pre- and postculture isolate characteristic | Group 1a (n = 15) |
Group 2b (n = 15) |
F test P value | ||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| Immature Tfz (R + Ta)c | 172.2 | 161.1–183.3 | 18.33 | 0.0–38.5 | <0.0001 |
| Mature Tfzc | 23.3 | 13.8–32.9 | 174.7 | 151.4–198.1 | <0.0001 |
| Preschizontsc | 3.0 | 0.7–5.3 | 1.4 | 0.2–2.6 | 0.19 |
| Schizontsc | 1.5 | 0.2–2.8 | 5.5 | 0.0–14.1 | 0.32 |
| Maturation time (h) | 34.9 | 31.0–38.8 | 22.7 | 20.1–25.2 | <0.0001 |
| % schizonts postmaturation | 59.9 | 46.5–76.4 | 48 | 39.2–56.8 | 0.12 |
Group 1 included isolates with predominance of immature trophozoites.
Group 2 included isolates with predominance of mature trophozoites.
Data are counts of parasites at the beginning of the assay carried out with 200 asexual parasites. R, rings; Ta, amoeboid trophozoites (27).
In vitro susceptibility of P. vivax to antimalarials.
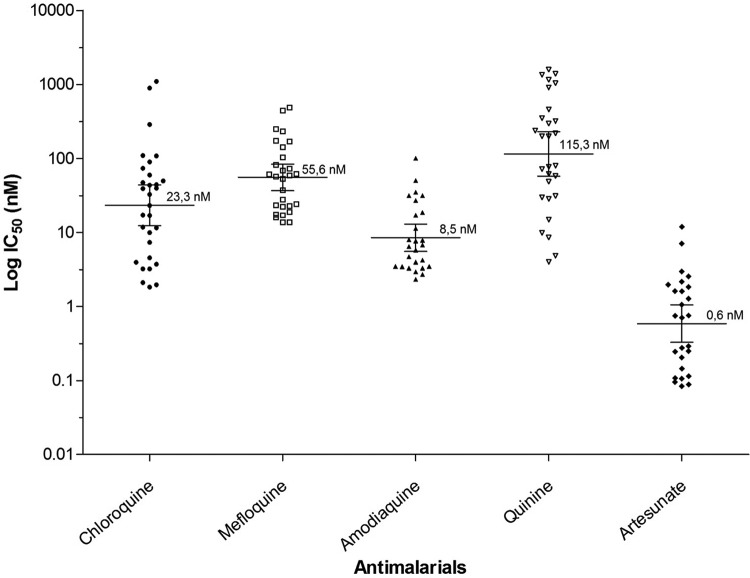
The number of valid tests and the IC50 found for each antimalarial can be seen in Table 2. Due to the fact that only 30 isolates matured successfully, the IC50 medians were analyzed based on the predominance of the initial parasitic stages, as shown in Table 3. The minimum and maximum values of the IC50s were found for CQ (2.5 nM to 1,109.0 nM), MQ (10.6 nM to 522.3 nM), AQ (2.7 nM to 105.2 nM), Qn (4.0 nM to 1,398.0 nM), and AS (0.1 nM to 10.7 nM) (Fig. 1).
TABLE 2.
IC50s of the antimalarials evaluated in the P. vivax isolates from Urabá, Colombia
| Antimalarial | No. (%) of valid assaysa | IC50b (95% CI) (nM) |
|---|---|---|
| Chloroquine | 30 (100) | 23.3 (12.4–44.1) |
| Mefloquine | 28 (93.3) | 55.6 (36.8–84.0) |
| Amodiaquine | 26 (86.7) | 8.5 (5.6–13.0) |
| Quinine | 28 (93.3) | 115.3 (57.7–230.5) |
| Artesunate | 26 (86.7) | 0.6 (0.3–1.0) |
Number of valid assays with IC50s over total number of assays (n = 30).
IC50, geometric mean of the inhibitory concentration; 95% CI, 95% confidence interval.
TABLE 3.
Comparison of median IC50 of each antimalarial based on initial parasitic stage in P. vivax isolates
| Antimalarial | Group 1a |
Group 2a |
F test P value | ||
|---|---|---|---|---|---|
| No. of isolates | IC50b (median [IQR]) (nM) | No. of isolates | IC50b (median [IQR]) (nM) | ||
| Chloroquine | 15 | 17.0 (3.2–59.8) | 15 | 39.3 (11.5–73.7) | <0.0001 |
| Mefloquine | 13 | 24.1 (17.3–58.8) | 15 | 72.2 (37.4–169.5) | 0.644 |
| Amodiaquine | 12 | 3.7 (3.0–31.6) | 14 | 7.2 (4.6–17.5) | 0.199 |
| Quinine | 14 | 65.0 (24.8–946.2) | 14 | 209.2 (53.5–378.3) | 0.301 |
| Artesunate | 12 | 0.3 (0.1–1.0) | 14 | 1.4 (0.2–2.0) | <0.0001 |
Isolates with valid IC50 for each antimalarial.
Analysis of the median of the concentration that inhibited 50% of the maturation of the schizonts. IQR, interquartile range (25% to 75%).
FIG 1.
Geometric means of the IC50s of antimalarials in P. vivax isolates in Urabá, Colombia.
Correlation of the IC50s of the different antimalarials for P. vivax from Colombia.
The overlapping susceptibilities of the 30 isolates of P. vivax to all of the antimalarials evaluated are shown in Table 4. The susceptibilities of the quinoline group antimalarials were compared to that of AS (a derivative of artemisinin). Positive correlations to AS were found for CQ and MQ (P < 0.001) (Table 4).
TABLE 4.
Overlapping susceptibilities of P. vivax among different antimalarials
| Antimalarial |
rs for indicated antimalariala |
|||
|---|---|---|---|---|
| Mefloquine | Amodiaquine | Quinine | Artesunate | |
| Chloroquine | 0.557** | 0.489* | 0.457* | 0.689*** |
| Mefloquine | 0.456* | 0.564** | 0.639*** | |
| Amodiaquine | 0.475* | 0.414* | ||
| Quinine | 0.483* | |||
Correlations are shown for the in vitro susceptibilities of the Colombian isolates of P. vivax to all of the antimalarials evaluated. Statistical significances are designated as follows: ***, P < 0.00; **, P < 0.01; *, P < 0.05. A positive correlation can be seen among the antimalarials evaluated.
DISCUSSION
This study analyzed the susceptibilities of 30 isolates of P. vivax to different antimalarials in Colombia. Chloroquine is a drug of special interest as it corresponds to a first-line treatment for P. vivax malaria in Colombia (25). Given the difficulty of maintaining P. vivax in continuous cultures (29–31), an in vitro short-term growth method was used which consisted of maintaining the parasites in culture for periods of <48 h until they reached schizont maturation (32). This method has helped to determine the in vitro susceptibility of P. vivax to antimalarials in different geographic regions of Africa and Asia (20–22, 33–35).
The IC50s observed for the isolates from the region of Urabá, which is an area with low malaria endemicity for South America but high endemicity for Colombia, were low for all of the antimalarials. However, there were isolates that presented IC50s for CQ that were 47.6 times higher than the mean IC50 (23.3 nM). Four (13.3%) out of 30 isolates presented IC50s of >100 nM, which is similar to what has been found in Asian countries such as Thailand, Indonesia, and Myanmar and in Papua New Guinea and Brazil (36) (Table 5).
TABLE 5.
Susceptibility profiles of the P. vivax isolates to antimalarials in different geographical regions
| Reference or source | Yr | Country | No. of successful assays | Geometric mean IC50 (nM) of antimalarials reported in the literature |
||||
|---|---|---|---|---|---|---|---|---|
| CQ | MQ | AQ | AS | Qn | ||||
| 10 | 1995 | Thailand | 57 | 360.5a | —d | — | — | — |
| 27 | 2002 | Thailand | 121 | 50.3b | — | — | — | — |
| 22 | 2003 | Thailand | 34 | 96.5 | — | — | — | — |
| 19 | 2004 | Thailand | 20 | 96.9 | 335.9 | 30.1 | 1.3 | 393.4 |
| 42 | 2007 | Thailand | 81 | 46.8 | — | — | — | — |
| Indonesia | 145 | 312a | — | — | — | — | ||
| 20 | 2007 | Myanmar | 2 | >100a | — | — | — | — |
| 15 | <100 | — | — | — | — | |||
| 36 | 2008 | Thailand | NDc | 12e | — | — | — | — |
| ND | 415f | — | — | — | — | |||
| 21 | 2008 | Indonesia | 216 | 295a | 12.7 | 15.4 | 1.31 | — |
| 55.2e | 7.9e | 16.6e | — | — | ||||
| 2,812f | 12.5f | 24.2f | — | — | ||||
| 38 | 2008 | Thailand | 65 | 36.7 | 111 | 34 | 8.3 | — |
| Indonesia | 85 | 114a | 9.87 | 13.7 | 1.4 | — | ||
| 33 | 2009 | Korea | 50 | 75.6 | 103.2 | — | 1.5 | 75.4 |
| Tailandia | 24 | 96.9 | 335.9 | — | 1.3 | 393.4 | ||
| 43 | 2011 | Thailand | 34 | 167.2a | 139.4 | — | 32.6 | — |
| 37 | 2011 | China | 42 | 12.6 | 11.2 | — | 0.3 | — |
| 36 | 2014 | Brazil | 32 | 32 | 57 | — | 21 | — |
| This study | 2014 | Colombia | 30 | 23.3 | 55.6 | 8.5 | 0.6 | 115.3 |
Susceptibility to chloroquine may be lost when the IC50 is >100 nM.
Thirty-hour incubation.
ND, no data.
—, not analyzed in the study.
Ring stage.
Trophozoite mature stage.
It is important to note that the schizont maturation method entails variables like the level of maturity of the parasitic stages at the beginning of the assay. These variables influence the maturation time of the schizonts and the reproducibility of the results. This is one of the possible reasons that the IC50s of CQ and AS were significantly different (P < 0.0001) in the two groups established according to initial parasitic stages, as has been reported previously in the literature. Despite the differences found, the mean IC50s for all of the antimalarials were observed to be within the ranges of susceptibility, suggesting that the Colombian isolates were susceptible even when they were cultured from mature trophozoites (21, 37).
Whereas the mature trophozoite in the case of P. falciparum is considered to be the target stage of CQ, this target is not recognized for P. vivax. It is believed that each species has characteristics that can explain the differences in susceptibility of each parasitic stage to chloroquine (21, 37). During studies that monitor the resistance of P. vivax to antimalarials, it is important to take into account the target parasite form. In this study, the cultures that began with a predominance of mature forms (group 2) presented significantly higher IC50s than those that began with immature forms (group 1).
When determining the susceptibility of P. vivax to antimalarials, the monitoring of schizont maturation is fundamental in order to avoid taking premature readings, which would cause a smaller percentage of schizonts to be recorded than expected. However, excess maturation time would cause the schizonts to rupture, and their numbers would be reduced (21).
The positive correlation observed between the susceptibilities of the isolates to the quinolines CQ, MQ, AQ, Qn, and AS (a derivative of artemisinins) suggests a common mode of action, as proposed by other authors (21, 27). Artesunate is a first-line drug for the treatment of P. falciparum malaria in Colombia (25). This study shows that in Colombia, AS is also effective against P. vivax, as an IC50 of 0.6 nM (95% CI, 0.3 to 1.0 nM) was determined. Studies carried out between 2004 and 2009 in different regions of Asia, including Thailand and Indonesia, reported an IC50 of <10 nM for the effect of artesunate against P. vivax. Such results suggest that derivatives of artemisinins have been effective against this type of parasite. However, an IC50 of 32.6 nM was found for artesunate in Thailand in 2011 and an IC50 of 21.0 nM was found for this antimalarial in a Brazilian region in 2014, which suggests a susceptibility loss of P. vivax to this antimalarial in two different geographic regions (Table 5) (9, 19, 21, 22, 36, 38). In this study, when the isolates were classified according to the initial parasitic stages, IC50s of 0.3 nM for AS in group 1 and 1.4 nM in group 2 were found (Table 3). Although these values are significantly different, they are below 10 nM, which indicates that this antimalarial is effective against P. vivax in Colombia. With respect to MQ, in this study, an IC50 of 55.6 nM (Table 2) was found, which differs from the values reported from 2008 to 2011 for Thailand, Indonesia, and China and from the values reported in 2004 and 2009 for Thailand and Korea (19, 33). In the case of AQ, an IC50 of 8.5 nM (Table 2) was recorded, which is similar to the values reported in different regions of Asia (IC50, <30 nM) between 2004 and 2008 (Table 5) (19, 22, 39). This suggests that AQ is effective against P. vivax in the various geographical regions studied in Asia and South America. Finally, Qn in this study had an IC50 of 115.3 nM, which was almost three times below the values reported in the literature (393.4 nM) (Table 5). However, it should be noted that there are very few reports of quinine being evaluated in vitro against P. vivax. More monitoring of this antimalarial in different geographical regions would help to establish its effectiveness.
Given the difficulty in maintaining an in vitro culture of P. vivax (29–31), resistance studies have been complemented by use of genetic markers. One example of a genetic marker is the pvmdr-1 gene that encodes the protein associated with multiple-drug resistance. The presence of mutations is associated with the loss of in vitro susceptibility of P. vivax to the antimalarials. Furthermore, the mutations in the pvdhfr and pvdhpr genes of P. vivax that encode the dihydrofolate reductase and dihydropteroate synthase proteins, respectively, have been shown to be associated with a loss of susceptibility to sulfadoxine-pyrimethamine (40).
In 2008, Suwanarusk et al. (39) evaluated how in vitro susceptibility is associated with the presence of mutations in the pvmdr-1 gene. They found a significantly high IC50 (78.6 nM) for mefloquine and an increase in the number of copies of the pvmdr-1 gene compared with isolates which had a low IC50 (38 nM) and a single copy of the gene. The presence of the Y976F mutation of the pvmdr-1 gene has been associated with a greater loss of susceptibility to CQ (IC50, 154 nM) compared to that of the isolates without mutation (IC50, 34 nM) but with greater susceptibilities to artesunate (IC50s, 1.8 nM [mutant] versus 9.5 nM [native]) and mefloquine (IC50s, 14 nM [mutant] versus 121 nM [wild type]).
In Central America, polymorphisms have been found in the pvmdr-1 and pvdhfr genes that may be related to the loss of susceptibility of P. vivax to antimalarials. However, there is no evidence from in vitro susceptibility studies to support this association (41). In South America, only isolated cases of the therapeutic failure to CQ for the treatment of P. vivax malaria have been documented, and no data on CQ drug levels to ensure therapeutic levels were included. Also, there are few reports of in vitro susceptibility of P. vivax to antimalarials. However, ongoing clinical efficacy monitoring would be important, in addition to integrated in vitro susceptibility and molecular marker studies. For example, in the Amazonian region of Brazil, in 2013, Chehuan et al. (42) reported that 12 (10.7%) out of 112 isolates with an IC50 of >100 nM were considered resistant to CQ, while 3 (6.4%) of 47 were considered resistant to MQ. The same study showed that Amazonian P. vivax strains with both CQ and MQ resistance may be common, and a nonsynonymous mutation at pvdhps codon 382 (S→C) was associated with in vitro susceptibility to CQ; thus, further studies should be done to confirm this observation. On the other hand, in 2014, Aguiar et al. (36) found IC50s similar to those reported for CQ and MQ in this region; however, the IC50s for AS observed in this work for Colombia were 20 times lower than those reported in Brazil (Table 5).
In conclusion, this study showed that the Colombian isolates of P. vivax continue to be susceptible to all of the antimalarials evaluated. It is important to note that this is the first report that has investigated the effect of the IC50s of antimalarials against P. vivax in Colombia. However, the presence of isolates with IC50s of >100 nM for CQ, MQ, and Qn suggests the need for periodically monitoring the therapeutic responses to antimalarials and for the evaluation of antimalarial drug levels in blood. Importantly, it will be associated with the IC50s in vitro of antimalarials in P. vivax and their tendency to increase toward an eventual therapeutic failure. It is also important to search for specific mutations in genetic markers as has been reported in Asia (10, 20, 21, 37, 39, 43–45).
ACKNOWLEDGMENTS
We thank Colciencias (111549326137, RC 488-2009) and the Universidad de Antioquia, Colombia (CODI-Sustainability strategy 2014–2015 UdeA).
We thank the Instituto Colombiano de Medicina Tropical (ICMT), Apartadó, for providing the conditions necessary to carry out the field work and the hospitals Antonio Roldán Betancur of the Municipality of Apartadó and Francisco Valderrama of the Municipality of Turbo for their support in acquiring the patients. We also thank Omar Triana for his advice on the methodology, Gonzalo Alvarez for his help with the statistics, microbiologist and bioanalyst Gabriel Vélez for his support with the experimental activities carried out in the laboratory at Apartadó and with the inclusion of patients, microbiologists and bioanalysts Claudia Patiño and Alexandra Ríos for their help with the inclusion of patients and their support with field activities in Turbo, bacteriologist Luz Yaned Usuga for acquiring patients in Apartadó, and investigative assistant Fernando Méndez for his support with field activities.
Footnotes
Published ahead of print 11 August 2014
REFERENCES
- 1.WHO. 2011. World malaria report 2011. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Price RN, Douglas NM, Anstey NM. 2009. New developments in Plasmodium vivax malaria: severe disease and the rise of chloroquine resistance. Curr. Opin. Infect. Dis. 22:430–435. 10.1097/QCO.0b013e32832f14c1. [DOI] [PubMed] [Google Scholar]
- 3.Instituto Nacional de Salud. 2012. Boletín epidemiológico semanal. Instituto Nacional de Salud, Bogota, Colombia. [Google Scholar]
- 4.Rieckmann KH, Davis DR, Hutton DC. 1989. Plasmodium vivax resistance to chloroquine? Lancet 334:1183–1184. 10.1016/S0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 5.Schuurkamp GJ, Spicer PE, Kereu RK, Bulungol PK, Rieckmann KH. 1992. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 86:121–122. 10.1016/0035-9203(92)90531-G. [DOI] [PubMed] [Google Scholar]
- 6.Fryauff DJ, Baird JK, Candradikusuma D, Masbar S, Sutamihardja MA, Leksana B, Tuti S, Marwoto H, Richie T, Romzan A. 1997. Survey of in vivo sensitivity to chloroquine by Plasmodium falciparum and P. vivax in Lombok, Indonesia. Am. J. Trop. Med. Hyg. 56:241–244. [DOI] [PubMed] [Google Scholar]
- 7.Fryauff DJ, Sumawinata I, Purnomo, Richie TL, Tjitra E, Bangs MJ, Kadir A, Ingkokusumo G. 1999. In vivo responses to antimalarials by Plasmodium falciparum and Plasmodium vivax from isolated Gag Island off northwest Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 60:542–546. [DOI] [PubMed] [Google Scholar]
- 8.Muhamad P, Ruengweerayut R, Chacharoenkul W, Rungsihirunrat K, Na-Bangchang K. 2011. Monitoring of clinical efficacy and in vitro sensitivity of Plasmodium vivax to chloroquine in area along Thai Myanmar border during 2009–2010. Malar. J. 10:44. 10.1186/1475-2875-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pukrittayakamee S, Chantra A, Simpson JA, Vanijanonta S, Clemens R, Looareesuwan S, White NJ. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680–1685. 10.1128/AAC.44.6.1680-1685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan-ariya P, Na-Bangchang K, Tin T, Limpaibul L, Brockelman CR, Karbwang J. 1995. Clinical response and susceptibility in vitro of Plasmodium vivax to the standard regimen of chloroquine in Thailand. Trans. R. Soc. Trop. Med. Hyg. 89:426–429. 10.1016/0035-9203(95)90039-X. [DOI] [PubMed] [Google Scholar]
- 11.Teka H, Petros B, Yamuah L, Tesfaye G, Elhassan I, Muchohi S, Kokwaro G, Aseffa A, Engers H. 2008. Chloroquine-resistant Plasmodium vivax malaria in Debre Zeit, Ethiopia. Malar. J. 7:220. 10.1186/1475-2875-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoda T, Rakue Y, Mizota T, Looareesuwan S. 2005. The efficacy of anti-malaria drugs against Plasmodium vivax on the Thailand-Myanmar border. J. Tokyo Med. Univ. 63:140–143. [Google Scholar]
- 13.Alecrim M, Das G, Alecrim W, Macêdo V. 1999. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev. Soc. Bras. Med. Trop 32:67–68. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez LG, Piñeros JG, Tobón A, Ríos AM, Maestre AE, Blair S, Carmona J. 2007. Eficacia de tres esquemas con cloroquina-primaquina para el tratamiento de la malaria por Plasmodium vivax en Colombia. CES Med. 21:51–60 (In Spanish.) [Google Scholar]
- 15.Osorio L, Perez LP, Gonzalez IJ. 2007. Evaluación de la eficacia de los medicamentos antimaláricos en Tarapacá, Amazonas Colombiano. Biomédica 27:133–140 10.7705/biomedica.v27i1.239. [DOI] [PubMed] [Google Scholar]
- 16.Ruebush TK, Zegarra J, Cairo J, Andersen EM, Green M, Pillai DR, Marquiño W, Huilca M, Arévalo E, Garcia C, Solary L, Kain KC. 2003. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am. J. Trop. Med. Hyg. 69:548–552. [PubMed] [Google Scholar]
- 17.Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeño N, Dunne M, Berman J. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 65:90–93. [DOI] [PubMed] [Google Scholar]
- 18.Rios A, Alvarez G, Blair S. 2013. Ten years of chloroquine efficacy for uncomplicated Plasmodium vivax malaria treatment, Turbo, Antioquia, 2002 and 2011. Biomedica 33:429–438 (In Spanish.) 10.7705/biomedica.v33i3.1631. [DOI] [PubMed] [Google Scholar]
- 19.Chotivanich K, Udomsangpetch R, Chierakul W, Newton PN, Ruangveerayuth R, Pukrittayakamee S, Looareesuwan S, White NJ. 2004. In vitro efficacy of antimalarial drugs against Plasmodium vivax on the western border of Thailand. Am. J. Trop. Med. Hyg. 70:395–397. [PubMed] [Google Scholar]
- 20.Druilhe P, Brasseur P, Blanc C, Makler M. 2007. Improved assessment of Plasmodium vivax response to antimalarial drugs by a colorimetric double-site Plasmodium lactate dehydrogenase antigen capture enzyme-linked immunosorbent assay. Antimicrob. Agents Chemother. 51:2112–2116. 10.1128/AAC.01385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell B, Chalfein F, Prasetyorini B, Kenangalem E, Piera K, Suwanarusk R, Brockman A, Prayoga P, Sugiarto P, Cheng Q, Tjitra E, Anstey NM, Price RN. 2008. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 52:1040–1045. 10.1128/AAC.01334-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell BM, Udomsangpetch R, Rieckmann KH, Kotecka BM, Coleman RE, Sattabongkot J. 2003. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 47:170–173. 10.1128/AAC.47.1.170-173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponnudurai T, Leeuwenberg AD, Meuwissen JH. 1981. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop. Geogr. Med. 33:50–54. [PubMed] [Google Scholar]
- 24.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710–718. 10.1128/AAC.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ministerio de la Protección Social. 2010. Guía para la atención clínica integral del paciente con malaria. Ministerio de la Protección Social, Bogota, Colombia. [Google Scholar]
- 26.Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. 1999. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am. J. Trop. Med. Hyg. 60:687–692. [DOI] [PubMed] [Google Scholar]
- 27.Tasanor O, Noedl H, Na-Bangchang K, Congpuong K, Sirichaisinthop J, Wernsdorfer WH. 2002. An in vitro system for assessing the sensitivity of Plasmodium vivax to chloroquine. Acta Trop. 83:49–61. 10.1016/S0001-706X(02)00056-6. [DOI] [PubMed] [Google Scholar]
- 28.Moody AH, Fleck SL. 1985. Versatile Field's strain. J. Clin. Pathol. 38:842–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golenda CF, Li J, Rosenberg R. 1997. Continuous in vitro propagation of the malaria parasite Plasmodium vivax. Proc. Natl. Acad. Sci. U. S. A. 94:6786–6791. 10.1073/pnas.94.13.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panichakul T, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Cui L, Udomsangpetch R. 2007. Production of erythropoietic cells in vitro for continuous culture of Plasmodium vivax. Int. J. Parasitol. 37:1551–1557. 10.1016/j.ijpara.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Udomsangpetch R, Somsri S, Panichakul T, Chotivanich K, Sirichaisinthop J, Yang Z, Cui L, Sattabongkot J. 2007. Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol. Int. 56:65–69. 10.1016/j.parint.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Rieckmann KH, Campbell GH, Sax LJ, Mrema JE. 1978. Drug sensitivity of Plasmodium falciparum: an in vitro microtechnique. Lancet 311:22–23. 10.1016/S0140-6736(78)90365-3. [DOI] [PubMed] [Google Scholar]
- 33.Chotivanich K, Sattabongkot J, Choi YK, Park JS, Sritabal J, Lim CS, Udomsangpetch R, White NJ, Lee WJ. 2009. Antimalarial drug susceptibility of Plasmodium vivax in the Republic of Korea. Am. J. Trop. Med. Hyg. 80:902–904. [PMC free article] [PubMed] [Google Scholar]
- 34.Kosaisavee V, Suwanarusk R, Nosten F, Kyle DE, Barrends M, Jones J, Price R, Russell B, Lek-Uthai U. 2006. Plasmodium vivax: isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isolates. Exp. Parasitol. 114:34–39. 10.1016/j.exppara.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Renapurkar DM, Pradhan VR, Sutar NK, Deshmukh RA, Pandit CH, Marathe SN. 1989. Micro test for assaying sensitivity of Plasmodium vivax in vitro. Chemotherapy 35:160–163. 10.1159/000238664. [DOI] [PubMed] [Google Scholar]
- 36.Aguiar AC, Pereira DB, Amaral NS, De Marco L, Krettli AU. 2014. Plasmodium vivax and Plasmodium falciparum ex vivo susceptibility to anti-malarials and gene characterization in Rondonia, West Amazon, Brazil. Malar. J. 13:73. 10.1186/1475-2875-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharrock WW, Suwanarusk R, Lek-Uthai U, Edstein MD, Kosaisavee V, Travers T, Jaidee A, Sriprawat K, Price RN, Nosten F, Russell B. 2008. Plasmodium vivax trophozoites insensitive to chloroquine. Malar. J. 7:94–94. 10.1186/1475-2875-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu F, Gao Q, Chotivanich K, Xia H, Cao J, Udomsangpetch R, Cui L, Sattabongkot J. 2011. In vitro anti-malarial drug susceptibility of temperate Plasmodium vivax from central China. Am. J. Trop. Med. Hyg. 85:197–201. 10.4269/ajtmh.2011.10-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suwanarusk R, Chavchich M, Russell B, Jaidee A, Chalfein F, Barends M, Prasetyorini B, Kenangalem E, Piera KA, Lek-Uthai U, Anstey NM, Tjitra E, Nosten F, Cheng Q, Price RN. 2008. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J. Infect. Dis. 198:1558–1564. 10.1086/592451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnadas C, Tichit M, Bouchier C, Ratsimbasoa A, Randrianasolo L, Raherinjafy R, Jahevitra M, Picot S, Ménard D. 2008. Plasmodium vivax dhfr and dhps mutations in isolates from Madagascar and therapeutic response to sulphadoxine-pyrimethamine. Malar. J. 7:35–35. 10.1186/1475-2875-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jovel IT, Mejía RE, Banegas E, Piedade R, Alger J, Fontecha G, Ferreira PE, Veiga MI, Enamorado IG, Bjorkman A, Ursing J. 2011. Drug resistance associated genetic polymorphisms in Plasmodium falciparum and Plasmodium vivax collected in Honduras, Central America. Malar. J. 10:376–376. 10.1186/1475-2875-10-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chehuan YF, Costa MR, Costa JS, Alecrim MG, Nogueira F, Silveira H, Brasil LW, Melo GC, Monteiro WM, Lacerda MV. 2013. In vitro chloroquine resistance for Plasmodium vivax isolates from the Western Brazilian Amazon. Malar. J. 12:226. 10.1186/1475-2875-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamedi Y, Safa O, Zare S, Tan-ariya P, Kojima S, Looareesuwan S. 2004. Therapeutic efficacy of artesunate in Plasmodium vivax malaria in Thailand. Southeast Asian J. Trop. Med. Public Health 35:570–574. [PubMed] [Google Scholar]
- 44.Suwanarusk R, Russell B, Chavchich M, Chalfein F, Kenangalem E, Kosaisavee V, Prasetyorini B, Piera KA, Barends M, Brockman A, Lek-Uthai U, Anstey NM, Tjitra E, Nosten F, Cheng Q, Price RN. 2007. Chloroquine resistant plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One 2:e1089. 10.1371/journal.pone.0001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Treiber M, Wernsdorfer G, Wiedermann U, Congpuong K, Sirichaisinthop J, Wernsdorfer WH. 2011. Sensitivity of Plasmodium vivax to chloroquine, mefloquine, artemisinin and atovaquone in north-western Thailand. Wien. Klin. Wochenschr. 123(Suppl 1):20–25. 10.1007/s00508-011-0044-6. [DOI] [PubMed] [Google Scholar]