Abstract
During a Spanish surveillance study, two natural variants of DHA β-lactamases, DHA-6 and DHA-7, were found, with the replacements Ala226Thr and Phe322Ser, respectively, with respect to DHA-1. The DHA-6 and DHA-7 enzymes were isolated from Escherichia coli and Enterobacter cloacae clinical isolates, respectively. The aim of this study was to genetically, microbiologically, and biochemically characterize the DHA-6 and DHA-7 β-lactamases. The blaDHA-6 and blaDHA-7 genes were located in the I1 and HI2 incompatibility group plasmids of 87.3 and 310.4 kb, respectively. The genetic contexts of blaDHA-6 and blaDHA-7 were similar to that already described for the blaDHA-1 gene and included the qnrB4 and aadA genes. The MICs for cephalothin, aztreonam, cefotaxime, and ceftazidime were 8- to 32-fold lower for DHA-6 than for DHA-1 or DHA-7 expressed in the same isogenic E. coli TG1 strain. Interestingly, the MIC for cefoxitin was higher in the DHA-6-expressing transformant than in DHA-1 or DHA-7. Biochemical studies with pure β-lactamases revealed slightly lower catalytic efficiencies of DHA-6 against cephalothin, ceftazidime, and cefotaxime than those of DHA-1 and DHA-7. To understand this behavior, stability experiments were carried out and showed that the DHA-6 protein displayed significantly higher stability than the DHA-1 and DHA-7 enzymes. The proximity of Thr226 to the N terminus in the tertiary protein structure in DHA-6 may promote this stabilization and, consequently, may induce a slight reduction in the dynamic of this enzyme that primarily affects the hydrolysis of some of the bulkiest antibiotics.
INTRODUCTION
Plasmid AmpC β-lactamases are clinically important cephalosporinases, particularly in Enterobacteriaceae, and the transmission of plasmids carrying AmpC genes has been detected in bacteria such as Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis (1, 2). At present, none of the commercially available β-lactamase inhibitors (clavulanic acid, sulbactam, and tazobactam) inactivate high-level class C producers (3), although tazobactam has shown some inhibitory activities in certain species, such as Morganella morganii (4, 5). Boronic acids and other compounds are promising new candidates as AmpC β-lactamase inhibitors (6, 7).
New extended-spectrum class C enzymes that are capable of hydrolyzing imipenem and cephalosporins with large side chains are emerging (2, 8, 9). These enzymes differ from typical AmpC β-lactamases as a result of amino acid insertions, deletions, and substitutions (2, 8). The three regions involved in these modifications are the omega loop, the R2 loop, and the helix H2 (2, 8).
The DHA enzymes are plasmid AmpC β-lactamases that were first described in 1997. Although at least 11 variants have been identified (see http://www.lahey.org/studies), complete kinetic and structural data are not yet available for these enzymes. A comparative study of the carbapenem-hydrolyzing activities of five plasmid-borne AmpC β-lactamases produced some kinetic data on the DHA-1 enzyme for cephaloridine and imipenem only (9), which were not tested in the present work.
The genetic contexts of the DHA-1 β-lactamase gene in different strains of M. morganii and K. pneumoniae were characterized and found to be similar to each other and included the qnrB4 (quinolone resistance) and aadA (streptomycin and spectinomycin resistance) genes (10, 11, 12).
In this study, we compared the genetic contexts of blaDHA-1, blaDHA-6, and blaDHA-7 in Enterobacteriaceae clinical isolates from Spain and obtained results that were highly consistent with those of previous work. We also provide here the first kinetic characterization of the DHA-1, DHA-6, and DHA-7 proteins, which differ from each other in two amino acids. By modeling, we concluded that the Thr226 mutation may affect the hydrolysis of some cephalosporins, including extended-spectrum cephalosporins, in DHA-type β-lactamases.
MATERIALS AND METHODS
Antibiotics and other chemicals.
Ampicillin, cephalothin, cefoxitin, ceftazidime, cefotaxime, aztreonam, clavulanic acid, sulbactam, and tazobactam were purchased from Sigma Chemical Co. (St. Louis, MO). Cefepime was obtained from Bristol-Myers Squibb (New York, NY). Imipenem and clavulanic acid were gifts from Merck (Whitehouse Station, NJ) and GlaxoSmithKline (Brentford, London, United Kingdom), respectively. Tazobactam was obtained from Wyeth (Pearl River, NY, USA). Sulbactam was a gift from Pfizer (Illertissen, Germany).
Nitrocefin was obtained from Unipath Oxoid (Basingstoke, Hants, United Kingdom), and isopropyl-β-d-thiogalactopyranoside (IPTG) was purchased from Roche (Basel, Switzerland).
Bacterial strains.
Klebsiella oxytoca 33/002 was used for cloning the blaDHA-1 gene and for MIC analysis. It was isolated in the Hospital Universitari Joan XXIII de Tarragona, Tarragona, Spain. Escherichia coli 30/021 was used for cloning the blaDHA-6 gene and for MIC analysis. It was isolated in the Vall d'Hebron Hospital, Barcelona, Spain. Enterobacter cloacae 30/034 was used for cloning the blaDHA-7 gene and for MIC analysis. It was isolated in the Vall d'Hebron Hospital, Barcelona, Spain. Escherichia coli TG1 {supE hsdΔ5 thi Δ(lac-proAB) F′[traD36 proAB+ lacIq ΔM15]} was used as the recipient in cloning experiments and for MIC analysis. Escherichia coli BL21 (hsdS gal [λcIts857 ind1 Sam7 nin5 lac UV5-T7 gene 1]) was used for expression experiments.
In vitro susceptibility testing.
All the antibiotic MICs were determined by microdilution according to CLSI methodology (13) and confirmed by Etest (bioMérieux, Marcy l'Etoile, France), and the results were interpreted according to the manufacturer's recommendations. For inhibition studies, ampicillin was tested when indicated with fixed concentrations of clavulanic acid, sulbactam, and tazobactam (4 mg/liter).
Genetic contexts of the blaDHA genes in Enterobacteriaceae isolates.
Pulsed-field gel electrophoresis (PFGE) with S1 nuclease digestion of whole-genome DNA (S1-PFGE) and PCR-based replicon typing (PBRT) were used to characterize the plasmids, as previously described (14). PFGE with I-CeuI digestion of whole-genome DNA, as described by Liu et al. (15), was used to determine whether the blaDHA genes were located in the chromosome.
The nucleotide sequences surrounding the blaDHA genes were determined by PCR and were compared with previously described structures (12). Sequencing reactions were performed with the BigDye Terminator kit (PE Applied Biosystems, Foster City, CA), and sequences were analyzed in an ABI Prism 3100 DNA sequencer (PE Applied Biosystems). The resulting sequences were then compared with those available in the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Cloning and DNA analysis.
PCR techniques were used to obtain the blaDHA-1, blaDHA-6, and blaDHA-7 genes from K. oxytoca 33/002, E. coli 30/021, and E. cloacae 30/034, respectively. The genes were then cloned into plasmid pBGS18, which harbored an external promoter, pBGS18-Pctx (16). For cloning the DHA genes, we used the primers DHA-pBGS18 fw (5′-AAAAGGTACCATGAAAAAATCGTTATCTGCAACAC, forward) and DHA-pBGS18 rv (5′-AAAAGAATTCTTATTCCAGTGCACTCAAAATAGC, reverse), which introduced the restriction sites KpnI and EcoRI, respectively. For microbiological analysis, all constructions were transformed in E. coli TG1.
Cellular fraction isolation.
Supernatant, periplasm, and cytoplasmic fractions were obtained from cultures of isogenic E. coli TG1 strains expressing the three DHA-type enzymes according to pET system manual instructions (Novagen, Darmstadt, Germany).
The QuantiChrom glucose-6-phosphate dehydrogenase kit from BioAssay Systems (Hayward, CA, USA) was used to rule out cellular contamination in the periplasmic and supernatant fractions.
Purification of DHA enzymes.
To purify the DHA-1, DHA-6, and DHA-7 proteins, the three corresponding genes were cloned in the pGEX-6P-1 vector with the primers DHA-pGEX fw (5′-AAGAATTCGCTGATAATGTCGCGG, forward) and DHA-pGEX rv (5′-AACTCGAGTTATTCCAGTGCACTC, reverse), which generated the restriction sites EcoRI and XhoI, respectively. The constructs were transformed in E. coli BL21 to produce a fusion between glutathione S-transferase (GST) and the DHA enzymes without their signal peptides. The β-lactamases were purified to homogeneity and the GST was removed from the DHA enzymes according to the manufacturer's instructions for the GST gene fusion system (Amersham Pharmacia Biotech Europe GmbH, Germany).
Determination of kinetic parameters.
In order to monitor the hydrolysis of antibiotics by DHA β-lactamases, the variation in absorbance that resulted from the opening of the β-lactam ring was recorded under the following conditions. The antibiotic extinction coefficients for nitrocefin, ampicillin, cefotaxime, ceftazidime, cefoxitin, and cephalothin were 15,000, −820, −7,500, −9,000, −7,700, and −6,500 M−1cm−1, respectively, at wavelengths of 260 nm for cefotaxime, ceftazidime, cefoxitin, and cephalothin, 482 nm for nitrocefin, and 235 nm for ampicillin. The antibiotics were dissolved in phosphate-buffered saline (PBS) supplemented with 20 μg bovine serum albumin (BSA)/ml, and the tests were repeated three times at 25°C. The kinetic parameters for nitrocefin were determined by measuring the initial hydrolysis rates and using the Hanes-Woolf linearization of the Henri-Michaelis-Menten equation. For the other antibiotics, the Km value was measured as the Ki in a competition experiment with nitrocefin as the reporter substrate. The kcat values were obtained by monitoring the hydrolysis of the antibiotic at a concentration of ≫10 times the Km. The 50% inhibitory concentration (IC50) studies with clavulanic acid, sulbactam, and tazobactam were performed according to a previously described method and by using nitrocefin as the competitor substrate (17).
Stability experiments.
Pure DHA-type enzymes were incubated at 50°C. The residual activity against nitrocefin was then measured at 10-min intervals for 40 min (16). Triplicate experiments were performed, and reported data are the mean values from three independent assays.
RESULTS AND DISCUSSION
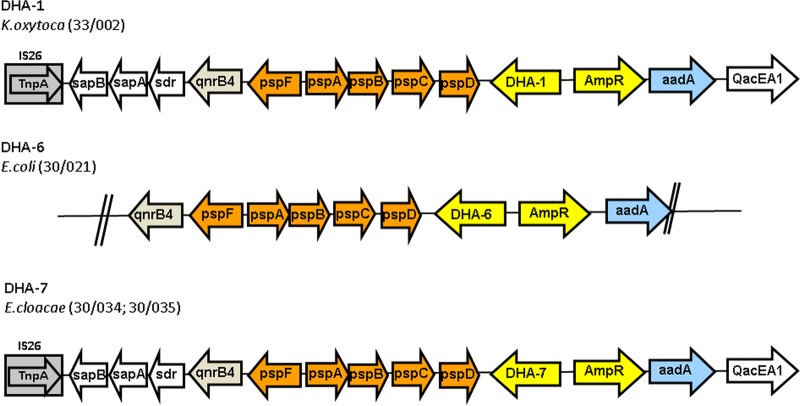
During a national multicenter survey of AmpC β-lactamases, the blaDHA-1, blaDHA-6, and blaDHA-7 genes were located in three different strains of Enterobacteriaceae, K. oxytoca, E. coli, and E. cloacae, respectively (18). Plasmid characterization by PFGE (see Materials and Methods) revealed that the DHA-6 β-lactamase is encoded by a plasmid of 87.3 kb and the incompatibility group I1. The DHA-7 β-lactamase gene hybridized with a 310.4-kb plasmid of incompatibility group HI2. The genetic contexts of blaDHA-6 and blaDHA-7 were compared with those of 19 blaDHA-1-producing isolates that were recovered during the same multicenter study (19). Although a certain degree of variability was detected, as reported in the relevant literature (10, 11, 12), the genetic structures that harbored the new blaDHA genes were conserved relative to those of blaDHA-1 (Fig. 1).
FIG 1.
Representation of the genetic contexts of blaDHA-6 and blaDHA-7 and one of the blaDHA-1 structures detected in the same multicenter study.
blaDHA-6 and blaDHA-7 are the first novel DHA β-lactamases from Spain to be described in detail. However, this is not the first description of a DHA β-lactamase in this country. An outbreak of K. pneumoniae carrying DHA-1 was described as recently as 2013 (20). Nevertheless, the genetic context of blaDHA-1 was not reported in that study. The description of a blaDHA gene in E. coli is not novel, as this gene was first described in Europe in 2006 (21). However, our description of blaDHA-7 represents the first report of a blaDHA gene in an E. cloacae strain. This discovery highlights the rapid spread of blaDHA genes among Enterobacteriaceae. The mobilization factor for blaDHA genes may be a transposable element. This conclusion is supported by the data showing that the genetic context is almost the same for the blaDHA genes described in this paper.
For comparative microbiological analysis, the three blaDHA genes were transformed into E. coli to yield an isogenic background. The MIC values of a large number of antibiotics were calculated for all the bacterial strains included in this study, including the original bacterial clinical isolates (Table 1).
TABLE 1.
MICs of several antibiotics for DHA-1, DHA-6, and DHA-7 β-lactamases in different bacterial strains
| Antibiotic | MIC (mg/liter) fora: |
||||||
|---|---|---|---|---|---|---|---|
| E. coli TG1 pBGS18-pCTX | K. oxytoca 33/002 (DHA-1) | E. coli 30/021 (DHA-6) | E. cloacae 30/034 (DHA-7) | E. coli TG1 pBGS18-pCTXb (DHA-1) | E. coli TG1 pBGS18-pCTX (DHA-6) | E. coli TG1 pBGS18-pCTX (DHA-7) | |
| Ampicillin | 2 | 2,056 | 512 | >2,056 | 1,028 | 256 | 2,056 |
| Ampicillin-clavulanic acidc | 2 | 2,056 | 512 | >2,056 | 1,028 | 256 | 2,056 |
| Ampicillin-sulbactamc | 2 | 128 | 128 | 2,056 | 128 | 128 | 2,056 |
| Ampicillin-tazobactamc | 2 | 32 | 64 | >2,056 | 64 | 128 | 2,056 |
| Cephalothin | 4 | 512 | 512 | >1,028 | 1,028 | 256 | 1,028 |
| Cefoxitin | 1 | 128 | 32 | 64 | 32 | 128 | 64 |
| Ceftazidime | 0.06 | 4 | 16 | 128 | 32 | 4 | 128 |
| Cefotaxime | 0.06 | 4 | 4 | 32 | 8 | 1 | 32 |
| Cefepime | <0.12 | 0.12 | 0.12 | 8 | 0.5 | 0.12 | 0.12 |
| Aztreonam | 0.12 | 16 | 2 | 128 | 8 | 2 | 32 |
| Imipenem | <0.03 | 0.25 | 0.5 | 4 | 0.12 | 0.06 | 0.12 |
Clinical isolates that harbor the β-lactamase gene are shown in parentheses.
pBGS18, pCTX plasmid expressing the indicated β-lactamases under the control of an external promoter.
Ampicillin was tested when indicated with fixed concentrations of clavulanic acid, sulbactam, or tazobactam (4 mg/liter).
Our results reveal slight differences between the isogenic bacterial isolates expressing DHA-type proteins, except for cefotaxime and ceftazidime. The DHA-1 and DHA-7 transformants were resistant to ceftazidime and cefotaxime, unlike DHA-6; the MICs were decreased 8- to 32-fold in DHA-6 relative to those in DHA-1 and DHA-7 (Table 1). There was also a 16-fold reduction in the MIC of aztreonam in DHA-6 relative to that in DHA-7. The differences were greatest between DHA-7 and DHA-6. Also, the cephalothin and ampicillin MICs were slightly lower for DHA-6. Interestingly, the cefoxitin MIC was higher for DHA-6 than for DHA-1 or DHA-7, and the cefepime MICs remained stable among the three different DHA-type enzymes. Microbiological analysis of the efficiencies of classical inhibitors showed that tazobactam and sulbactam exert some inhibitory activity against DHA-1 and DHA-6, as revealed by combination with ampicillin (Table 1).
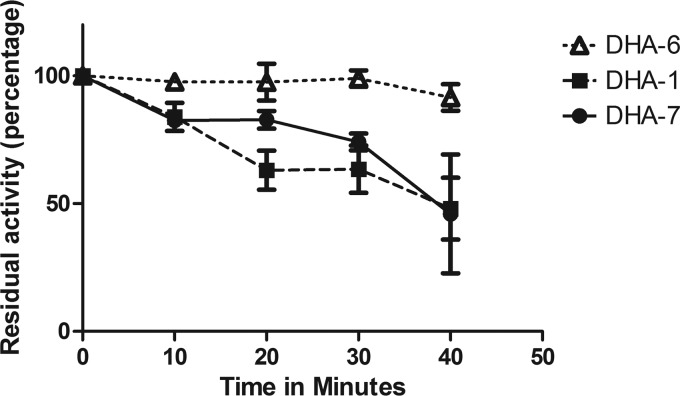
The kinetic parameters of the purified DHA β-lactamases were determined for nitrocefin, ampicillin, cephalothin, cefotaxime, ceftazidime, and cefoxitin (Table 2). In general, the results revealed only small differences among the three proteins. The catalytic efficiency against nitrocefin is 2-fold higher in DHA-1 than in DHA-6 and DHA-7. The catalytic efficiencies for nitrocefin, ampicillin, cefotaxime, and especially cephalothin were good for the three enzymes, but there was a slight reduction in the efficiencies for cephalothin, cefotaxime, and ceftazidime of DHA-6 compared to those of DHA-1 and DHA-7. The IC50 constants showed slightly better inhibition for clavulanic acid and sulbactam in DHA-6 than in either DHA-1 or DHA-7 β-lactamase (Table 2). The stability of these DHA enzymes was assessed with temperature inactivation studies. These results show that the DHA-6 protein displayed a significantly higher stability than did DHA-1 or DHA-7. After 40 min of incubation at 50°C, DHA-6 retained 90% of residual activity on nitrocefin, compared to 48% and 45% for DHA-1 and DHA-7, respectively (Fig. 2).
TABLE 2.
Kinetic data for the pure DHA-1, DHA-6, and DHA-7 β-lactamases
| Antibiotic and kinetic parameter | Data (mean± SD) for: |
||
|---|---|---|---|
| DHA-1 | DHA-6 | DHA-7 | |
| Nitrocefin | |||
| Km (μM) | 84 ± 8 | 279 ± 17 | 88 ± 11 |
| kcat (s−1) | 1,537 ± 120 | 2,319 ± 420 | 797 ± 67 |
| kcat/Km (μM−1s−1) | 18.297 | 8.311 | 9.056 |
| Ampicillin | |||
| Km (μM) | 0.877 ± 0.342 | 0.925 ± 0.088 | 1.466 ± 0.397 |
| kcat (s−1) | 1.346 ± 0.226 | 1.9 ± 0.26 | 1.475 ± 0.095 |
| kcat/Km (μM−1s−1) | 1.534 | 2.05 | 1.006 |
| Cephalothin | |||
| Km (μM) | 39.21 ± 16.33 | 66.39 ± 23.23 | 35.586 ± 15.66 |
| kcat (s−1) | 179 ± 23 | 198 ± 7 | 222 ± 2 |
| kcat/Km (μM−1s−1) | 4.565 | 2.98 | 6.23 |
| Cefotaxime | |||
| Km (μM) | 0.0824 ± 0.005 | 0.153 ± 0.021 | 0.0927 ± 0.002 |
| kcat (s−1) | 0.0907 ± 0.01 | 0.125 ± 0.02 | 0.177 ± 0.024 |
| kcat/Km (μM−1s−1) | 1.1 | 0.816 | 1.9 |
| Ceftazidime | |||
| Km (μM) | 207.07 ± 54 | 106.74 ± 22.74 | 240 ± 72 |
| kcat (s−1) | 1.748 ± 0.192 | 0.708 ± 0.115 | 2.003 ± 0.22 |
| kcat/Km (μM−1s−1) | 0.0084 | 0.0063 | 0.0083 |
| Cefoxitin | |||
| Km (μM) | 0.265 ± 0.137 | 0.256 ± 0.066 | 0.25 ± 0.127 |
| kcat (s−1) | 0.04349 ± 0.007 | 0.0486 ± 0.008 | 0.0412 ± 0.007 |
| kcat/Km (μM−1s−1) | 0.163 | 0.189 | 0.1648 |
| Clavulanic acid (IC50 [μM]) | 806 ± 394 | 361 ± 25 | 483 ± 279 |
| Sulbactam (IC50 [μM]) | 3.17 ± 0.23 | 2.99 ± 0.32 | 3.97 ± 0.21 |
| Tazobactam (IC50 [μM]) | 0.143 ± 0.007 | 0.124 ± 0.007 | 0.267 ± 0.044 |
FIG 2.
Percentage of residual activity of pure DHA-type enzymes after denaturation experiments at 50°C.
As the kinetic data do not completely clarify the microbiological differences observed between DHA-6 and DHA-1 or DHA-7, the cellular fractions of the cultures of isogenic E. coli that expressed three DHA-type enzymes were obtained, and the specific activities against nitrocefin were calculated. The putative contamination of periplasmic components or supernatant with cytoplasmic components was ruled out by assaying glucose-6-phosphate dehydrogenase activity. The results revealed that periplasmic fractions of DHA-1 and DHA-7 were four to two times more specifically active against nitrocefin. Also, a clear remaining β-lactamase activity was observed in DHA-7 supernatants, which was contrary to absent activity in DHA-1 and DHA-6 supernatants. Accordingly, these data showed less DHA-6 enzyme in the periplasmic fractions than DHA-1 and DHA-7, which may indicate slight differences in the secretion of the different DHA-type enzymes. These differences may contribute, together with the different kinetic properties, to the higher MICs observed in DHA-1- and DHA-7-expressing E. coli strains than those in DHA-6-expressing E. coli strains in most of the β-lactams tested.
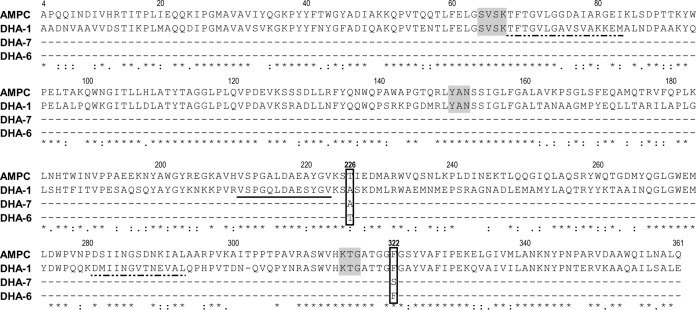
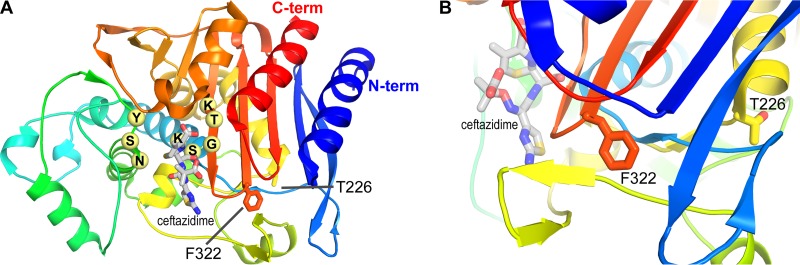
The three enzymes had mutations at only two positions. The Ala226 present in DHA-1 and DHA-7 was replaced by a threonine in DHA-6, and the Phe322 found in DHA-1 and DHA-6 was mutated into a serine in DHA-7 (Fig. 3 and 4). AmpC from E. coli is the closest class C β-lactamase (61% amino acid identity) for which a three-dimensional structure is available. The two positions with modifications in the DHA studied (residues 226 and 322) are located in well-conserved areas and, respectively, corresponded to amino acids 226 and 322 in this structure (PDB code 2BLS) (22). Position 226 lies at the C-terminal end of an α helix in the α/β structure. In AmpC, this amino acid is a threonine, as in DHA-6. Position 322 lies 5 amino acids after the third conserved motif characteristic of the β-lactamases (KTG). It is a phenylalanine part of an internal hydrophobic cluster in AmpC that is also conserved in DHA-1 and DHA-6 (Fig. 3 and 4).There is no direct involvement of the mutated amino acid in substrate recognition or hydrolysis, and the stability studies showed that this effect is not caused by a loss of stability of the DHA-6 protein. In contrast, this protein is significantly more stable. According to the structural analysis of the AmpC structure, the Ala226Thr mutation characterizing DHA-6 is located at a junction with the N terminus of the polypeptide chain. The side chain of the threonine, therefore, may provide a better, complementary shape to stabilize the N-terminal part of the enzyme (Fig. 4, blue coloration) and better resist heat denaturation. This stabilization may induce a slight reduction of the dynamic of this enzyme and primarily affect the hydrolysis of some of the bulkiest antibiotics.
FIG 3.
Amino acid sequence alignment of the DHA β-lactamase family (DHA-1, GenBank accession no. Y16410; DHA-6, HQ322612; DHA-7, HQ456945) with AmpC from Escherichia coli. Asterisks indicate strictly conserved amino acids, two dots indicate residues that are very similar, and one dot indicates residues that are more or less similar. Ala226Thr and Phe322Ser mutation positions are 0. The three classical conserved motifs are shaded in gray, the omega loop is indicated by a solid black line, and the H2 and H10 helices are represented by dashed lines. The alignment was created with the CLUSTAL W program of the EMBL-EBL.
FIG 4.
(A) AmpC structure in complex with ceftazidime represented as a cartoon with a rainbow coloring scheme (blue N terminus to red C terminus). Important residues that constitute the 3 conserved motifs appear as pale yellow spheres; ceftazidime appears as gray sticks. The 2 residues characteristic of the differences among DHA-1, DHA-6, and DHA-7 are also displayed as sticks. (B) Close-up view of the 2 residues of interest, with a 90° shift in orientation.
In summary, this work is the first detailed description of DHA-6 and DHA-7 β-lactamases and highlights the first isolation of a blaDHA gene in E. cloacae. It is also the first detailed kinetic characterization of three highly similar DHA proteins. This study identified the new position 226 in DHA-type enzymes as relevant for the hydrolysis of certain cephalosporins, such as cephalothin, ceftazidime, and cefotaxime.
ACKNOWLEDGMENTS
This study was funded by grant 278232 (MagicBullet) from the European Community, FP7; grant REIPI RD12/0015/014 from the Plan Nacional de I+D+I 2008 to 2011 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases, cofinanced by the European Development Regional Fund (EDRF), A Way to Achieve Europe; and grants PI09/1702 (to J.J.G.-L.) and PI12/00552 (to G.B.) from the Fondo de Investigación Sanitaria.
F.K. is an FRS-FNRS (Brussels, Belgium) research associate.
Footnotes
Published ahead of print 18 August 2014
REFERENCES
- 1.Beceiro A, Bou G. 2004. Class C β-lactamases: an increasing problem worldwide. Rev. Med. Microbiol. 15:141–152. 10.1097/00013542-200410000-00003. [DOI] [Google Scholar]
- 2.Jacoby GA. 2009. AmpC β-lactamases. Clin. Microbiol. Rev. 22:161–182. 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pérez-Llarena FJ, Bou G. 2009. Beta lactamase inhibitors: the story so far. Curr. Med. Chem. 16:3740–3765. 10.2174/092986709789104957. [DOI] [PubMed] [Google Scholar]
- 4.Akova M, Yang Y, Livermore DM. 1990. Interactions of tazobactam and clavulanate with inducible and constitutively expressed class I β-lactamases. J. Antimicrob. Chemother. 25:199–208. 10.1093/jac/25.2.199. [DOI] [PubMed] [Google Scholar]
- 5.Bush K, Macalintal C, Rasmussen BA, Lee VJ, Yang I. 1993. Kinetic interaction of tazobactam with beta-lactamases from all major structural classes. Antimicrob. Agents Chemother. 37:851–858. 10.1128/AAC.37.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morandi F, Caselli E, Morandi S, Focia PJ, Blázquez J, Shoichet BJ, Prati F. 2003. Nanomolar inhibitors of AmpC beta-lactamase. J. Am. Chem. Soc. 125:685–695. 10.1021/ja0288338. [DOI] [PubMed] [Google Scholar]
- 7.Buynak JD. 2013. β-Lactamase inhibitors: a review of the patent literature (2010-2013). Expert Opin. Ther. Pat. 23:1469–1481. 10.1517/13543776.2013.831071. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann P, Mammeri H. 2007. Extended-spectrum cephalosporinases: structure, detection and epidemiology. Future Microbiol. 2:297–307. 10.2217/17460913.2.3.297. [DOI] [PubMed] [Google Scholar]
- 9.Mammeri H, Guillon H, Eb F, Nordmann P. 2010. Phenotypic and biochemical comparison of the carbapenem-hydrolyzing activities of five plasmid-borne AmpC β-lactamases. Antimicrob. Agents Chemother. 54:4556–4560. 10.1128/AAC.01762-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdet C, Arlet G, Barnaud G, Lagrange PH, Philippon A. 2000. A novel integron in Salmonella enterica serovar Enteritidis, carrying the bla(DHA-1) gene and its regulator gene ampR, originated from Morganella morganii. Antimicrob. Agents Chemother. 44:222–225. 10.1128/AAC.44.1.222-225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verdet C, Benzerara Y, Gautier V, Adam O, Ould-Hocine Z, Arlet G. 2006. Emergence of DHA-1-producing Klebsiella spp. in the Parisian region: genetic organization of the ampC and ampR genes originating from Morganella morganii. Antimicrob. Agents Chemother. 50:607–617. 10.1128/AAC.50.2.607-617.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Y, Yu D, Wei Z, Shen P, Zhou Z, Yu Y. 2010. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 54:3967–3969. 10.1128/AAC.00137-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2012. Methods for dilution on antimicrobial susceptibility tests for bacteria that grow aerobically, vol 32, 9th ed, no 2, p 20 Approved standard M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.García A, Navarro F, Miró E, Villa L, Mirelis B, Coll P, Carattoli A. 2007. Acquisition and diffusion of bla CTX-M-9 gene by R478-IncHI2 derivative plasmids. FEMS Microbiol. Lett. 271:71–77. 10.1111/j.1574-6968.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu SL, Hessel A, Sanderson KE. 1993. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. U. S. A. 90:6874–6878. 10.1073/pnas.90.14.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pérez-Llarena FJ, Fernández A, Zamorano L, Kerff F, Beceiro A, Aracil B, Cercenado E, Miró E, Oliver A, Oteo J, Navarro F, Bou G. 2012. Characterization of a novel IMP-28 metallo-β-lactamase from a Spanish Klebsiella oxytoca isolate. Antimicrob. Agents Chemother. 56:4540–4543. 10.1128/AAC.00776-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirot D, Recule C, Chaibi EB, Bret L, Croize J, Chanal-Claris C, Labia R, Sirot J. 1997. A complex mutant of TEM-1 beta lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 41:1322–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miró E, Aguero J, Larrosa MN, Fernández A, Conejo MC, Bou G, González-López JJ, Lara N, Martínez-Martínez L, Oliver A, Aracil B, Oteo J, Pascual A, Rodríguez-Baño J, Zamorano L, Navarro F. 2013. Prevalence and molecular epidemiology of acquired AmpC beta-lactamases and carbapenemases in Enterobacteriaceae isolates from 35 hospitals in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 32:253–259. 10.1007/s10096-012-1737-0. [DOI] [PubMed] [Google Scholar]
- 19.Zamorano L, Miró E, Juan C, Gómez L, Bou G, González JJ, Aguero J, Aracil B, Conejo C, Oliver A, Navarro F, members of REIPI 2013. Plasmid typing and genetic context of transferable cephamycinases and carbapenemases in Enterobacteriaceae: findings from 35 Spanish hospitals, abstr P2410 Abstr. 23rd Eur. Congr. Clin. Microbiol. Infect. Dis, Berlin, Germany. [Google Scholar]
- 20.López-Camacho E, Gómez-Gil E, Tobes R, Manrique M, Lorenzo M, Galván B, Salvarelli E, Moatassim Y, Salanueva IJ, Pareja E, Codoñer FM, Alvarez-Tjado M, Garcillán-Barcia MP, De la Cruz F, Mingorance J. 2014. Genomic analysis of the emergence and evolution of multidrug resistance during a Klebsiella pneumoniae outbreak, including carbapenem and colistin resistance. J. Antimicrob. Chemother. 69:632–636. 10.1093/jac/dkt419. [DOI] [PubMed] [Google Scholar]
- 21.Giakkoupi P, Tambic-Andrasevic A, Vourli S, Skrlin J, Sestan-Crnek S, Tzouvelekis LS, Vatopoulos AC. 2006. Transferable DHA-1 cephalosporinase in Escherichia coli. Int. J. Antimicrob. Agents 27:77–80. 10.1016/j.ijantimicag.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Usher KC, Blaszczak LC, Weston GS, Shoichet BK, Remington SJ. 1998. Three-dimensional structure of AmpC beta-lactamase from Escherichia coli bound to a transition-state analogue: possible implications for the oxyanion hypothesis and for inhibitor design. Biochemistry 37:16082–16092. 10.1021/bi981210f. [DOI] [PubMed] [Google Scholar]