Abstract
Apramycin is a unique aminoglycoside with a dissociation of antibacterial activity and ototoxicity. We assessed the antibacterial efficacy of apramycin in two murine models of infection, Mycobacterium tuberculosis aerosol infection and Staphylococcus aureus septicemia. In both infection models, the efficacy of apramycin was comparable to that of amikacin. These results suggest that apramycin has the potential to become a clinically useful agent against drug-resistant pathogens and support further development of this promising unique aminoglycoside.
TEXT
Emerging resistance globally threatens the efficacy of antibiotics (1), arguably one of the most significant achievements in human medicine. The prospects for novel classes of antibiotics are scarce (2), and there is an urgent need to improve available antibiotic compounds (3).
The first aminoglycoside, streptomycin, was introduced almost 70 years ago, and since then, numerous aminoglycosides have been isolated, and new derivatives have been synthesized (4). Aminoglycosides have broad-spectrum activity and are used for treatment of serious life-threatening infections, including tuberculosis (5). As with all antibiotics, resistance to aminoglycosides has evolved in the past several decades and significantly impairs the clinical utility of these compounds (6). Resistance to aminoglycosides is conferred primarily by aminoglycoside-modifying enzymes, which inactivate these compounds by various modifications of their NH2 or OH substituents (7). In addition, methyltransferases that modify bacterial rRNA, the molecular target of aminoglycosides, confer high-level resistance to all clinically used aminoglycosides (8; for a review, see reference 9), including the most recently developed aminoglycoside, plazomicin, a semisynthetic aminoglycoside that has completed phase II clinical trials for complicated urinary tract infections (10, 11).
Apramycin is a structurally unique aminoglycoside, characterized by a bicyclic sugar moiety and a 4-monosubstituted 2-deoxystreptamine ring (12). In vitro, apramycin shows broad-range antibacterial activity against a wide range of Gram-positive and Gram-negative bacteria, including Pseudomonas aeruginosa (13, 14). As a result of its unique structure, apramycin is not inactivated by most of the known aminoglycoside-modifying enzymes (14), including N-acetlytransferases (AAC), O-nucleotidyltransferases (ANT), and O-phosphotransferases (APH). In addition, apramycin has been found to be the only aminoglycoside active against producers of rRNA methylases (11). Apramycin also shows high antimycobacterial activity in vitro (14), including activity against multidrug-resistant (MDR) strains of Mycobacterium tuberculosis and rapidly growing nontuberculous mycobacteria, such as Mycobacterium abscessus, M. massiliense, and M. bolletii, which are difficult-to-treat, obnoxious lung pathogens in patients with cystic fibrosis or bronchiectasis (15). Of note, the antimycobacterial activity of apramycin in vitro matches that of amikacin, an established second-line antituberculous agent and reference aminoglycoside used for treatment of MDR tuberculosis and for treatment of lung infections with M. abscessus (15, 16).
The clinical use of aminoglycosides is limited by their toxicity, in particular ototoxicity. The ability of these compounds to cause irreversible hearing damage affects approximately 20% of patients following brief courses of treatment and up to 90% of patients after long-term regimens (17, 18). Aminoglycoside ototoxicity is linked to the destruction of the sensory cells of the inner ear and has consistently been associated with both natural and semisynthetic aminoglycosides (17). However, we have recently made the surprising observation that apramycin shows a unique dissociation of antibacterial activity and ototoxicity, conferring upon it a low ototoxic potential (14). The low ototoxicity of apramycin, together with its promising in vitro antibacterial activity, prompted us to investigate the in vivo efficacy of this unique aminoglycoside for treatment of infectious diseases.
The in vivo efficacy of apramycin as an antituberculous agent was tested in a low-dose aerosol infection model in female gamma interferon (IFN-γ) knockout mice (Jackson Laboratories, Bar Harbor, ME), as described previously (19, 20). Evaluation of the antituberculous activity of apramycin in vivo was performed in an acute M. tuberculosis infection model to provide an assessment of drug efficacy against replicating bacteria. The animal efficacy studies were performed according to the guidelines of the Colorado State University Institutional Animal Care and Use Committee and approved under protocol number 13-4263A. Mice (5 or 6 per group) were challenged with an aerosol treatment of M. tuberculosis Erdman (TMC 107; ATCC 35801) via a GlasCol aerosol chamber in a certified animal biosafety level 3 (ABSL-3) laboratory according to guidelines of the Institutional Animal Care and Use Committee. Mice were infected in a single aerosol run (target dose, 100 CFU per moue). Control mice were sacrificed the day after aerosol challenge to enumerate the bacteria instilled in the lungs after infection, approximately 140 CFU. The MIC of M. tuberculosis Erdman is 1.5 mg/liter for both apramycin and the comparator amikacin. Starting 13 days after challenge, mice received the drug (200 mg/kg of body weight subcutaneously) once daily for 9 consecutive days. For endpoint analysis, mice were euthanized and lungs collected at day 22 following challenge. The left lung lobe was homogenized for enumeration of CFU by plating dilutions of the organ homogenates on 7H11 agar plates. The CFU numbers were converted to logarithms, which were then evaluated by a one-way analysis of variance (ANOVA), followed by a multiple comparison analysis of variance using the Tukey test (SAS software program, Research Triangle Park, NC). Differences were considered significant at the 95% level of confidence. Figure 1 shows the numbers of CFU in the lungs of mice following experimental infection. Apramycin demonstrated significant in vivo efficacy in reducing bacterial burden in lungs in comparison to the level for saline controls (P < 0.001), showing a 2.4-log10 CFU reduction after 9 consecutive days of treatment. Apramycin's antituberculous activity compared well to that of amikacin (1.8-log10 reduction; P > 0.05), an aminoglycoside and a well-established second-line agent for treatment of multidrug-resistant tuberculosis.
FIG 1.
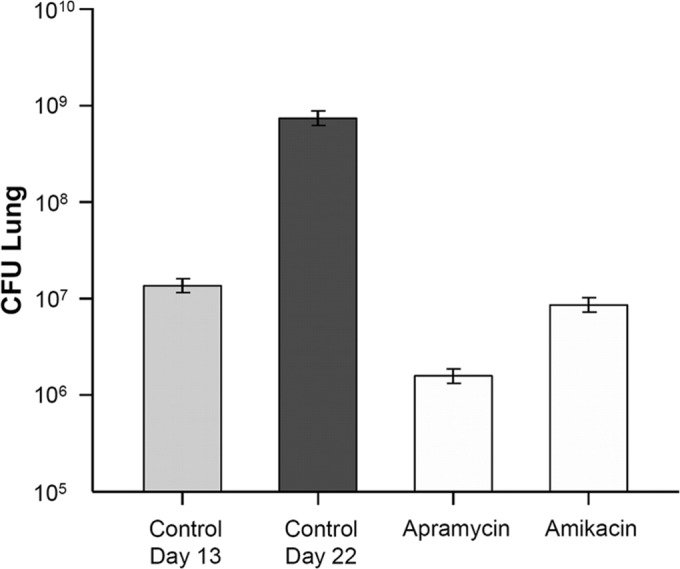
In vivo antituberculous activity of apramycin and the comparator amikacin in a murine model of acute, low-dose aerosol infection. Log10 numbers of CFU in the lungs of M. tuberculosis-infected mice are given. At day 13 following infection, mice were drug treated for 9 days, and lungs were collected for CFU determinations at day 22.
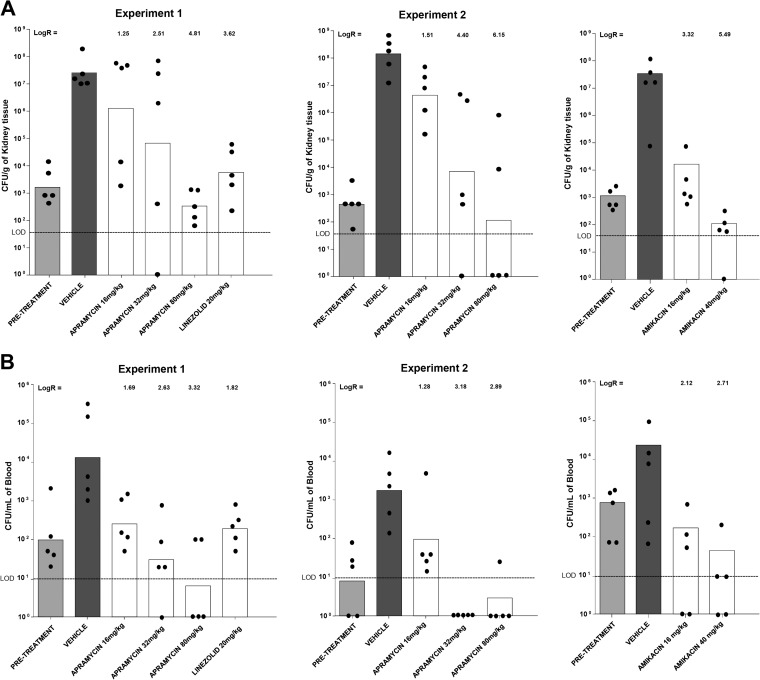
The in vivo efficacy of apramycin as a broad-range antibacterial agent was tested in a neutropenic model of Staphylococcus aureus septicemia (21). The animal experiments were carried out at Euprotec Limited, Manchester, United Kingdom, under United Kingdom Home Office Licenses, with ethical approval from the University of Manchester Ethics committee. Outbred male mice (strain Hsd:ICR CD-1) were supplied by Charles River UK. Mice were rendered temporarily neutropenic by immunosuppression with cyclophosphamide at 200 mg/kg 4 days before infection and 150 mg/kg 1 day before infection by intraperitoneal injection. The immunosuppression regimes led to neutropenia starting 24 h after administration, which continued throughout the study. For in vivo infection, a methicillin-resistant Staphylococcus aureus (MRSA) strain, clinical isolate AG041, was used. The in vitro activities of antibiotics against the MRSA strain AG041 were as follows (MIC [mg/liter]): for apramycin, 4.0 to 8.0; for amikacin, 4.0; for kanamycin, 2.0 to 4.0; for paromomycin, 2.0 to 4.0; for ciprofloxacin, 0.5; for oxacillin, 8.0; for cefoxitin, 12.0; and for linezolid, 1.0. Twenty-four hours after the second round of immunosuppression, mice were infected with S. aureus AG041 by intravenous injection into the lateral tail vein by use of ∼1 × 107 CFU/mouse. Apramycin was administered at 16, 32, and 80 mg/kg/dose (equivalent to 2 times, 4 times, and 10 times the MIC value in mg/kg). Linezolid (20 mg/kg/dose) was used as a positive control. Antibacterial treatment was initiated at 1 h postinfection and delivered subcutaneously at 10 ml/kg (linezolid was given by intravenous bolus injection). All drugs were administered at 1, 9, and 17 h postinfection. At 1 h (pretreatment group) or 24 h postinfection, blood samples were collected, and at 24 h postinfection, kidneys were removed for CFU determination. The data for apramycin were generated in two different and independent experiments, both of which are represented in Fig. 2. Figure 2 shows the numbers of CFU in mice with experimental septicemia. In animals infected with methicillin-resistant S. aureus, treatment with apramycin reduced the bacterial burden both in blood and in kidney. Compared to the level for the vehicle-treated mice, drug treatment reduced bacterial burden in the kidneys between 2 and 5 log10 and in blood between 2 and 3 log10 in a dose-dependent manner.
FIG 2.
In vivo activity of apramycin and comparators in a neutropenic murine model of S. aureus septicemia. (A) Bacterial burden in kidney (CFU/g tissue). (B) Bacterial burden in blood (CFU/ml). Given are the geometric mean and the data points for single animals. Log reductions are indicated. The pretreatment bar indicates the bacterial load at the initiation of treatment. LOD, limit of detection. Amikacin data were taken from reference 14 and are included for comparison (amikacin at 16 and 40 mg/kg is equivalent to 4 and 10 times the MIC value in mg/kg).
Our data show that the excellent antibacterial in vitro activity of apramycin reported previously for a wide range of infectious pathogens (14), including strains resistant to multiple antibiotics, extends to in vivo activity in two different standard models of infection, experimental S. aureus septicemia and M. tuberculosis pneumonia. While apramycin's unique structure precludes its inactivation by most of the known aminoglycoside-modifying enzymes, mutational alterations of 16S rRNA which confer resistance to amikacin and kanamycin in M. tuberculosis and M. abscessus (22–25) also confer resistance to apramycin (14). The in vivo efficacy of apramycin was comparable to that of the comparator aminoglycoside amikacin. As with all animal models, the results presented here do not ensure the efficacy of apramycin in treatment of human infectious diseases. However, the demonstration of efficacy in mouse infection models illustrates the potential clinical use of this unique aminoglycoside against serious infections. The mouse aerosol infection and septicemia models used here are established models for preclinical measurement of the efficacy of antibiotics (19–21).
The need for new antibiotics active against antibacterial-resistant microbes is undisputed. Emerging and high-level antibiotic resistance to various pathogens is observed in all areas of the world. The emergence and spread of carbapenem-resistant Enterobacteriaceae endowed with rRNA methylases conferring resistance to all clinically used aminoglycosides (11, 26) have added further urgency to the situation, given that carbapenem and aminoglycoside antibiotics have been the last resort for treating infections by multidrug-resistant Enterobacteriaceae (27). Our results on the in vivo efficacy of apramycin in established murine models of infection testify to the potent antibacterial activity of this compound and, together with its promising biocompatibility (14), support the further evaluation of the safety and efficacy of this unique aminoglycoside for further development.
ACKNOWLEDGMENTS
We thank Swapna Vaddi and Pia Thommes for help with the S. aureus infection experiments, Lisa Woolhiser for help with the M. tuberculosis infection experiments, and Susanna Salas for typing the manuscript.
The study was supported by the University of Zurich and by NIAID IDIQ Contract Task Order MHSN27220100000091/01.
The University of Zurich has filed a patent for the use of apramycin in treatment of human infectious diseases.
Footnotes
Published ahead of print 18 August 2014
REFERENCES
- 1.French GL. 2010. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents 36(Suppl 3):S3–S7. 10.1016/S0924-8579(10)70003-0. [DOI] [PubMed] [Google Scholar]
- 2.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6:29–40. 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 3.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12. 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 4.Davies J. 2007. In the beginning there was streptomycin, p 1–13 In Arya DP. (ed), Aminoglycoside antibiotics: from chemical biology to drug discovery. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- 5.Chambers HF. 1996. Chemotherapy of microbial diseases, p 1103–1121 In Hardman JG, Limbird LE. (ed), Goodman & Gilman's The pharmaceutical basis of therapeutics, 10th ed. McGraw-Hill, New York, NY. [Google Scholar]
- 6.Houghton JL, Green KD, Chen W, Garneau-Tsodikova S. 2010. The future of aminoglycosides: the end or renaissance? Chembiochem 11:880–902. 10.1002/cbic.200900779. [DOI] [PubMed] [Google Scholar]
- 7.Wright GD, Berghuis AM, Mobashery S. 1998. Aminoglycoside antibiotics: structure, function and resistance, p 27–69 In Rosen BP, Mobashery S. (ed), Resolving the antibiotic paradox. Springer, New York, NY. [PubMed] [Google Scholar]
- 8.Galimand M, Courvalin P, Lambert T. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 47:2565–2571. 10.1128/AAC.47.8.2565-2571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doi Y, Arakawa Y. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45:88-94. 10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 10.Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S, Gomez M, Wlasichuk KB, Diokno R, Miller GH, Moser HE. 2010. Synthesis and spectrum of the neoglycoside ACHN-490. Antimicrob. Agents Chemother. 54:4636–4642. 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. 2011. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J. Antimicrob. Chemother. 66:48–53. 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor S, Lam LK, Jones ND, Chaney MO. 1976. Apramycin, a unique aminocyclitol antibiotic. J. Org. Chem. 41:2087–2092. 10.1021/jo00874a003. [DOI] [PubMed] [Google Scholar]
- 13.Ryden R, Moore BJ. 1977. The in vitro activity of apramycin, a new aminocyclitol antibiotic. J. Antimicrob. Chemother. 3:609–613. 10.1093/jac/3.6.609. [DOI] [PubMed] [Google Scholar]
- 14.Matt T, Ng CL, Lang K, Sha SH, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, Bottger EC. 2012. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proc. Natl. Acad. Sci. U. S. A. 109:10984–10989. 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416. 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 16.Sturdy A, Goodman A, Jose RJ, Loyse A, O'Donoghue M, Kon OM, Dedicoat MJ, Harrison TS, John L, Lipman M, Cooke GS. 2011. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J. Antimicrob. Chemother. 66:1815–1820. 10.1093/jac/dkr221. [DOI] [PubMed] [Google Scholar]
- 17.Forge A, Schacht J. 2000. Aminoglycoside antibiotics. Audiol. Neurootol. 5:3–22. 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- 18.Duggal P, Sarkar M. 2007. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC Ear Nose Throat Disord. 7:5. 10.1186/1472-6815-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenaerts AJ, Gruppo V, Brooks JV, Orme IM. 2003. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 47:783–785. 10.1128/AAC.47.2.783-785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzblau SG, DeGroote MA, Cho SH, Andries K, Nuermberger E, Orme IM, Mdluli K, Angulo-Barturen I, Dick T, Dartois V, Lenaerts AJ. 2012. Comprehensive analysis of methods used for the evaluation of compounds against Mycobacterium tuberculosis. Tuberculosis (Edinb.) 92:453–488. 10.1016/j.tube.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Fernandez D, Shcherbakov D, Matt T, Leong NC, Kudyba I, Duscha S, Boukari H, Patak R, Dubbaka SR, Lang K, Meyer M, Akbergenov R, Freihofer P, Vaddi S, Thommes P, Ramakrishnan V, Vasella A, Bottger EC. 2014. 4′-O-substitutions determine selectivity of aminoglycoside antibiotics. Nat. Commun. 5:3112. 10.1038/ncomms4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sander P, Prammananan T, Böttger EC. 1996. Introducing mutations into a chromosomal rRNA gene using a genetically modified eubacterial host with a single rRNA operon. Mol. Microbiol. 22:841–848. 10.1046/j.1365-2958.1996.01532.x. [DOI] [PubMed] [Google Scholar]
- 23.Shcherbakov D, Akbergenov R, Matt T, Sander P, Andersson DI, Böttger EC. 2010. Directed mutagenesis of Mycobacterium smegmatis 16S rRNA to reconstruct the in-vivo evolution of aminoglycoside resistance in Mycobacterium tuberculosis. Mol. Microbiol. 77:830–840. 10.1111/j.1365-2958.2010.07218.x. [DOI] [PubMed] [Google Scholar]
- 24.Prammananan T, Sander P, Brown BA, Frischkorn K, Onyi GO, Zhang Y, Böttger EC, Wallace RJ., Jr 1998. A single 16S ribosomal RNA substitution is responsible for resistance to amikacin and other 2-deoxystreptamine aminoglycosides in Mycobacterium abscessus and Mycobacterium chelonae. J. Infect. Dis. 177:1573–1581. 10.1086/515328. [DOI] [PubMed] [Google Scholar]
- 25.Prammananan T, Sander P, Springer B, Böttger EC. 1999. RecA-mediated gene conversion and aminoglycoside resistance in strains heterozygous for rRNA. Antimicrob. Agents Chemother. 43:447–453. 10.1093/jac/43.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602. 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bratu S, Landman D, Haag R, Recco R, Eramo A, Alam M, Quale J. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165:1430–1435. 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]