Abstract
Adaptive antibiotic resistance is a newly described phenomenon by which Acinetobacter baumannii induces efflux pump activity in response to host-associated environmental cues that may, in part, account for antibiotic treatment failures against clinically defined susceptible strains. To that end, during adaptation to growth in human serum, the organism induces approximately 22 putative efflux-associated genes and displays efflux-mediated minocycline tolerance at antibiotic concentrations corresponding to patient serum levels. Here, we show that in addition to minocycline, growth in human serum elicits A. baumannii efflux-mediated tolerance to the antibiotics ciprofloxacin, meropenem, tetracycline, and tigecycline. Moreover, using a whole-cell high-throughput screen and secondary assays, we identified novel serum-associated antibiotic efflux inhibitors that potentiated the activities of antibiotics toward serum-grown A. baumannii. Two compounds, Acinetobacter baumannii efflux pump inhibitor 1 (ABEPI1) [(E)-4-((4-chlorobenzylidene)amino)benezenesulfonamide] and ABEPI2 [N-tert-butyl-2-(1-tert-butyltetrazol-5-yl)sulfanylacetamide], were shown to lead to minocycline accumulation within A. baumannii during serum growth and inhibit the efflux potential of the organism. While both compounds also inhibited the antibiotic efflux properties of the bacterial pathogen Pseudomonas aeruginosa, they did not display significant cytotoxicity toward human cells or mammalian Ca2+ channel inhibitory effects, suggesting that ABEPI1 and ABEPI2 represent promising structural scaffolds for the development of new classes of bacterial antibiotic efflux pump inhibitors that can be used to potentiate the activities of current and future antibiotics for the therapeutic intervention of Gram-negative bacterial infections.
INTRODUCTION
Acinetobacter baumannii has emerged as a major nosocomial pathogen that can cause ventilator-associated pneumonia (VAP) and bacteremia, with associated mortality rates as high as 60% among susceptible patient populations (1–6). The high rates of A. baumannii-associated morbidity and mortality have largely been attributed to the emergence of antibiotic resistance that has compromised the effectiveness of the currently available antibiotics. Indeed, the Centers for Disease Control and Prevention recently reported that 63% of all U.S. A. baumannii infections are caused by multidrug-resistant strains that are resistant to three or more classes of antibiotics, and strains that are resistant to all classes of antibiotics have recently been identified in the United States and elsewhere (7–10).
A. baumannii antibiotic resistance is thought to be mediated by an expansive repertoire of enzymatic determinants, such as β-lactamases, as well as efflux pumps that extrude toxic agents, including antibiotics, from the cell (3, 11, 12). With regard to the efflux pumps, the organism has been shown to harbor representatives of each of the five so-called bacterial drug efflux pump families: CraA and AmvA are major facilitator superfamily (MFS) pumps that are proposed to efflux chloramphenicol and erythromycin, respectively (13, 14), AbeM is a multidrug and toxic compound extrusion (MATE) family protein that effluxes aminoglycosides, quinolones, and chloramphenicol (15), AbeS is a small multidrug resistance (SMR) family pump that confers resistance to erythromycin and novobiocin, as well as low-level tolerance to aminoglycosides, quinolones, tetracycline, and trimethoprim (16), AdeABC, AdeFGH, and AdeIJK are resistance-nodulation-division (RND) family pumps that have been associated with resistance to aminoglycosides, β-lactams, fluoroquinolones, tetracyclines, tigecycline, macrolides, chloramphenicol, and trimethoprim (17–21). Furthermore, A. baumannii is known to harbor several horizontally acquired Tet efflux pumps belonging to the MFS that confer tetracycline resistance (12, 22). While the antimicrobial effects of these efflux pumps have been well documented, the mechanisms by which the organism regulates their expression are only beginning to be understood.
In addition to the aforementioned well-characterized efflux pumps, A. baumannii is reported to harbor an array of putative efflux pumps that may confer antibiotic resistance (23). For instance, the common laboratory strains A. baumannii AYE and ATCC 17978 contain 46 and 73 genes, respectively, that are annotated as putative drug efflux pumps. It remains to be seen if these factors do indeed modulate antibiotic tolerance or which endogenous or exogenous cues regulate their activity. Nonetheless, recent studies suggest that they are likely to have clinical significance. Indeed, Hood and colleagues (24) found that 18 previously uncharacterized putative drug efflux-associated factors were significantly upregulated and conferred resistance to levofloxacin and amikacin during A. baumannii growth under physiologically relevant salt conditions. Likewise, in another study, A. baumannii grown in human serum was found to induce the expression of 22 putative drug efflux-associated genes and efflux-mediated tolerance to minocycline at levels that are clinically relevant (25). Such regulated changes in efflux pump expression and activity in response to host-associated environmental cues are thought to temporarily increase the ability of a bacterium to survive antibiotic challenge and are hypothesized to allow otherwise clinically defined antibiotic susceptible strains to resist antibiotic insult; this phenomenon was recently termed adaptive efflux-mediated resistance by Fernández and Hancock (26).
The current study was designed to further our understanding of the adaptive antibiotic efflux potential of A. baumannii during growth in human serum and to identify the small-molecule inhibitors of these efflux properties. The results revealed that in addition to minocycline, serum-induced efflux pumps are associated with the ability of A. baumannii to tolerate ciprofloxacin, meropenem, tetracycline, and tigecycline. Further, using a high-throughput screening strategy and secondary assays, we identified two structurally distinct classes of novel efflux pump inhibitors that restore the antibiotic susceptibility of serum-grown A. baumannii and lack the inherent problems commonly associated with other classes of antibiotic efflux pump inhibitors, namely, mammalian cytotoxicity and calcium channel inhibition. These compounds may represent promising structural scaffolds for the development of new classes of bacterial antibiotic efflux pump inhibitors that can be used as adjunctive therapy to potentiate the activities of current and future antibiotics for the therapeutic intervention of A. baumannii infections.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A. baumannii strains 98-37-09, 01-12-05, 07-09-54, and 07-09-61 are clinical isolates obtained from the Centers for Disease Control and Prevention and were previously described (27). Pseudomonas aeruginosa strain PAO1 and Klebsiella pneumoniae strain CKP4 are prototypic laboratory strains that were generously provided by Barbara Iglewski (University of Rochester, Rochester, NY) and Thomas Russo (State University of New York, Buffalo, NY), respectively (28). All strains were grown in either Luria-Bertani (LB) medium (Becton Dickinson, Franklin Lakes, NJ) or 100% human serum (MP Biomedicals, Solon, OH). Where indicated, LB or serum was supplemented with the indicated concentration of minocycline (Sigma-Aldrich, St. Louis, MO), ciprofloxacin (Sigma-Aldrich), meropenem (LKT Laboratories, Minneapolis-St. Paul, MN), or tigecycline (Pfizer, Groton, CT).
Antibiotic susceptibility assays.
The antibiotic susceptibilities of A. baumannii and P. aeruginosa grown in either LB medium or 100% human serum were measured, as previously described (25). Briefly, the indicated bacterial species/strain was grown overnight in LB medium, diluted into fresh medium (1:100 dilution), and grown to mid-exponential phase (optical density at 600 nm [OD600], 0.4 to 0.5) at 37°C, with aeration. A total of 1 × 105 CFU were transferred to individual wells of a 96-well round-bottom plate containing 100 μl of LB or 100% human serum supplemented with 2-fold increasing concentrations (0 to 2 μg ml−1) of minocycline, amikacin, gentamicin, kanamycin, meropenem, ceftriaxone, erythromycin, colistin, polymyxin B, ciprofloxacin, levofloxacin, nalidixic acid, sulfamethoxazole, trimethoprim, tigecycline, or 0 to 16 μg ml−1 tetracycline and incubated at 37°C for 48 h. To quantify the antimicrobial effects of each antibiotic toward bacteria grown in LB or serum, the well constitutes were serially diluted in phosphate-buffered saline (PBS) and plated on LB agar to enumerate the CFU ml−1. Where indicated, antimicrobial susceptibility assays were also performed in the presence of 50 μg ml−1 efflux pump inhibitors, verapamil, reserpine, or phenylalanine arginine β-naphthylamide (PAβN), or with the indicated amount of the putative efflux inhibitors Acinetobacter baumannii efflux pump inhibitor 1 (ABEPI1) [(E)-4-((4-chlorobenzylidene)amino)benezenesulfonamide] and ABEPI2 [N-tert-butyl-2-(1-tert-butyltetrazol-5-yl)sulfanylacetamide].
High-throughput screen for A. baumannii serum-dependent antibiotic efflux pump inhibitors.
The TimTec ActiProbe-25K diversity set and Natural Products Library (29,900 compounds total; TimTec, Newark, DE) were initially screened for putative efflux pump inhibitors by identifying compounds that potentiated the antimicrobial property of a subinhibitory concentration of minocycline toward A. baumannii grown in human serum. To do so, A. baumannii strain 98-37-09 was grown for 16 h in LB medium at 37°C on a rotary shaker at 225 rpm. Approximately 1 × 105 CFU were then transferred to individual wells of a 96-well round-bottom plate (Corning Costar, Tewksbury, MA) containing 100 μl of human serum supplemented with minocycline (0.5 μg ml−1 [0.5× MIC in serum]) and individual members of the TimTec ActiProbe or Natural Products Library (50 μM). The plates were then incubated at 37°C for 48 h. Putative efflux pump inhibitors were identified as compounds that inhibited A. baumannii growth in human serum containing minocycline and were subsequently retested in triplicate, as indicated above. Untreated A. baumannii grown in serum supplemented with minocycline ± PAβN served as positive and negative controls, respectively.
To distinguish compounds with inherent antimicrobial properties from the putative efflux pump inhibitors, the compounds were directly assessed for antibacterial activity in human serum in the absence of minocycline. To do so, 1 × 105 CFU of A. baumannii strain 98-37-09 were inoculated into individual wells of a microtiter plate containing 100 μl of 100% human serum supplemented with increasing concentrations of the test compound (0 to 128 μg ml−1) and incubated at 37°C for 48 h. The compounds that displayed antimicrobial activity were archived, whereas those that did not exhibit direct antibacterial activity were considered putative efflux inhibitors, and the minimum effective concentration (MEC) at which they potentiated the antimicrobial activity of minocycline toward serum-grown A. baumannii was determined. For MEC determination, individual wells of 96-well round-bottom plates containing 100% human serum supplemented with 0.5× the MIC of minocycline (0.5 μg ml−1) and increasing concentrations of test compound (0 to 128 μg ml−1) were inoculated with approximately 1 × 105 CFU of A. baumannii strain 98-37-09 and incubated for 48 h at 37°C. The MEC was defined as the lowest concentration of test compound required to inhibit the growth of A. baumannii in serum in the presence of 0.5 μg ml−1 minocycline.
Cellular accumulation of minocycline.
High-pressure liquid chromatography and triple-quadrupole mass spectrometry were used to measure the intracellular levels of minocycline in A. baumannii during growth in human serum in the absence and presence of each putative efflux pump inhibitor. To do so, A. baumannii 98-37-09 was grown in 5 ml of 100% human serum supplemented with 0.5 μg ml−1 minocycline, in the absence and presence of 0.5× the MEC of each putative efflux pump inhibitor (test compound) or the known efflux pump inhibitor verapamil. The cultures were incubated for 48 h with shaking, at which point an aliquot was removed, serially diluted, and plated to determine the number of viable CFU per mixture. The remaining cells were pelleted by centrifugation at 900 × g at 4°C, washed twice in PBS, and mechanically lysed with a FastPrep cell disrupter (MP Biomedicals, Santa Ana, CA) for 20 s at 5 m s−1, and the cellular debris was pelleted via centrifugation at 900 × g at 4°C. The amount of minocycline present within the supernatant of the ruptured cells was measured, as previously described (29). Briefly, doxycycline (0.5 μg ml−1) was first added to each supernatant to serve as an internal control to account for sample-to-sample preparation variability. The supernatant was then combined with acetonitrile (ACN) at a 1:10 ratio and centrifuged at 16,000 × g at 4°C to collect minocycline and doxycycline. The supernatants were discarded, and the residual liquid was evaporated in a speed vacuum for 2 h at 8,000 × g. To quantify the amount of antibiotics retained, the sample materials were suspended in 50% acetonitrile, filtered through a 0.2-μm low-protein binding hydrophilic membrane (Millipore, Billerica, MA), and then separated on a Shimadzu high-performance liquid chromatography instrument (Fisher Scientific) using a BetaBasic C18 reverse-phase column (Thermo Scientific). Separation was carried out with two mobile-phase solutions consisting of solution A, which was water with 0.1% formic acid, and solution B, which was 100% ACN. The gradient profile of the chromatography runs was 0 to 0.1 min of 8% solution B, ramped up step-wise (37% to 60% solution B) from 0.1 to 6.5 min, and then holding for 1 min before ramping down from 60% to 10% from 7.5 to 8 min. This was followed by a hold at 10% solution B for 1 min. From 10 to 13 min, the gradient was ramped up to 100% solution B and held until 11.5 min, was reduced to 8% solution B, and was held under these conditions for an additional 4 min. The column was equilibrated in 8% solution B at 40°C, and the flow rate was set to 0.2 ml min−1. Mass spectrometry analysis of the fractions was carried out using a TSQ Quantum Ultra triple-quadrupole mass spectrometer (Thermo Fisher Scientific). The data were analyzed using the Xcalibur software (Thermo Scientific), and the following parameters were used to detect minocycline and the internal control doxycycline: 458.208 m/z→282.971 m/z (collision energy, 43; tube lens, 119) for minocycline and 445.144 m/z→266.900 m/z (collision energy, 39; tube lens, 127) for doxycycline. Analysis of the raw data was conducted by using area under the curve calculations with the Genesis algorithm to determine the concentration in each sample. The difference between the peak intensity and the y intercept of preestablished minocycline and doxycycline standard curves was divided by the slope of the standard curve to quantify the amount of minocycline within each sample. The total concentration of minocycline within each cell was calculated by normalization to the number of cells within each corresponding culture.
Cytotoxicity assay.
The human cytotoxicity of putative efflux pump inhibitors was measured alone and in combination with minocycline using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cell viability assays, according to the manufacturer's recommendations (American Type Culture Collection, Manassas, VA). Briefly, human HepG2 cells were grown to approximately 1 × 106 cells per well in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and then treated with 1× or 4× the MEC of the indicated compound alone or in combination with 0.5 μg ml−1 minocycline for 24 h. Cell viability was measured following the addition of the tetrazolium salt (MTT), as per the manufacturer's recommendations; the cells challenged with 50 μg ml−1 mitomycin C (Sigma-Aldrich) and the mock-treated cells served as positive and negative controls, respectively.
Ethidium bromide efflux assay.
Standard bacterial ethidium bromide efflux activity assays were used to measure the efflux inhibitory properties of the compounds of interest, as previously described (18, 30–33). For the assays, an overnight culture of A. baumannii 98-37-09 was diluted (1:100) into 100% human serum or fresh LB and grown to mid-exponential phase. The cell pellets were collected via centrifugation at 900 × g for 20 min, washed 3× with 20 mM sodium phosphate buffer, and resuspended to an OD600 of 0.2 in sodium phosphate buffer. Approximately 1 × 106 CFU were loaded into individual wells of 96-well white-bottom plates and mixed with 10 μg ml−1 ethidium bromide, and ethidium fluorescence (excitation, 530 nm; emission, 600 nm) was measured every 5 min for 90 min on a SPECTRAmax5 fluorometer (Molecular Devices, Sunnyvale, CA). To determine if the putative efflux pump inhibitors affected ethidium bromide efflux, the cells were treated with the indicated amount of the compound of interest or the efflux pump inhibitor PAβN at 2 min after fluorescence monitoring began. The mock-treated cells served as a negative control; plating confirmed that the test conditions used did not affect cell viability.
Mammalian calcium channel assays.
Fluo-4 Direct calcium channel assay kits were used, according to the manufacturer's recommendations (Life Technologies, Carlsbad, CA), to determine whether the compounds of interest affect human Ca2+ channel activity. Briefly, 5 × 104 human HEK 293T embryonic kidney cells were grown in individual wells of 96-well black-walled plates (Corning Costar). Next, 2× Fluo-4 dye supplemented with probenecid (5 mM) was added to each well and allowed to equilibrate for 1 h at 37°C. To determine whether ABEPI1 or ABEPI2 affects Ca2+ channel activity, Fluo-4 fluorescence measurements (excitation, 495 nm; emission, 516 nm) were taken at 1-s intervals for 15 s. At that time point, the cells were treated with either dimethyl sulfoxide (DMSO) (mock), 50 μg ml−1 the Ca2+ channel inhibitor verapamil (positive control), or 1× the MEC of ABEPI1 or ABEPI2, followed by the calcium channel stimulator carbamylcholine chloride (50 μg ml−1; Fisher Scientific) at 60 s, and fluorescence was measured for an additional 120 s on a FlexStation 3 benchtop multimode microplate reader (Molecular Devices).
RESULTS
Serum-induced efflux pumps extrude multiple antibiotics.
Antibiotic efflux pumps have been found to contribute to bacterial resistance to virtually every currently available antibiotic. Ten A. baumannii antibiotic efflux systems have been characterized to date, and a bioinformatics assessment of the publically available genomes suggests that the organism is likely to produce an expansive repertoire of additional efflux pumps (13–17, 19, 20, 23, 31–33). In that regard, we previously found that A. baumannii growth in human serum, a biologically relevant medium, induces the expression of 22 previously uncharacterized putative drug efflux pump-associated genes (see Table S1 in the supplemental material), and their expression corresponds to efflux-mediated tolerance to the antibiotic minocycline at levels correlating to patient serum levels during treatment (25).
As a means to evaluate this phenomenon further, we assessed whether A. baumannii growth in serum elicits drug efflux-mediated tolerance to other tetracyclines, quinolones (ciprofloxacin, levofloxacin, and nalidixic acid), aminoglycosides (amikacin, gentamicin, and kanamycin), a carbapenem (meropenem), a cephalosporin (ceftriaxone), a macrolide (erythromycin), polypeptides (colistin and polymyxin B), a sulfonamide (sulfamethoxazole), glycylcycline (tigecycline), and trimethoprim. To do so, A. baumannii 98-37-09 was cultured in LB or 100% human serum in the presence of increasing concentrations of each antibiotic, and the antimicrobial effects of each antibiotic were measured by plating for CFU.
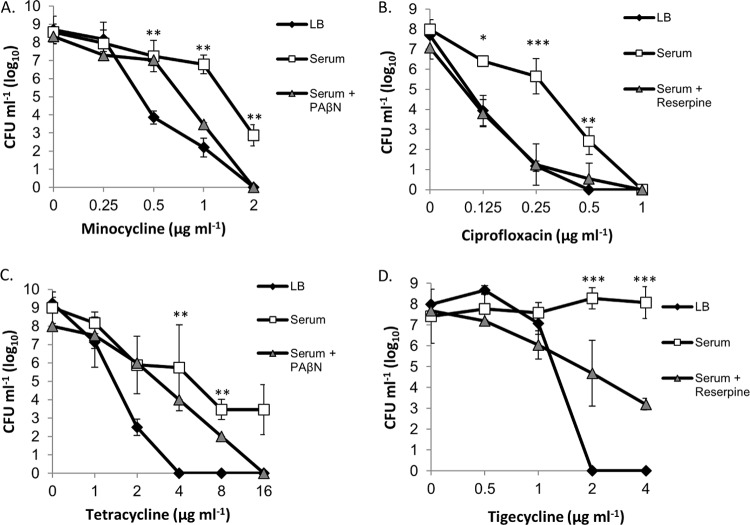
As expected based on our previous results (25), A. baumannii 98-37-09 cells grown in human serum were significantly (P < 0.01) less susceptible to minocycline at concentrations of ≥0.25 μg ml−1 than were the cells cultured in LB (Fig. 1A). Further, minocycline susceptibility was restored to the cells grown in serum supplemented with the known efflux pump inhibitor PAβN, suggesting that the observed serum-associated minocycline tolerance was efflux mediated as opposed to being caused by antibiotic inactivation by the serum components. Similar susceptibility assays using other antibiotics revealed that this serum-associated adaptive resistance phenomenon is not minocycline specific. Indeed, serum-grown 98-37-09 cells were less susceptible to the antibiotic ciprofloxacin at concentrations of ≤1 μg ml−1 than were LB-grown cells; ciprofloxacin susceptibility was restored to the cells grown in serum supplemented with the known efflux pump inhibitor reserpine (Fig. 1B). Likewise, A. baumannii grown in human serum displayed efflux-mediated tolerance to tetracycline at antibiotic concentrations of ≥2 μg ml−1 (Fig. 1C). More specifically, the treatment of LB-grown A. baumannii with 4 to 16 μg ml−1 tetracycline reduced cell viability to undetectable levels (<1 × 101 CFU), whereas the cells grown in serum displayed considerable antibiotic tolerance, between 1 × 106 and 1 × 104 CFU. Serum-grown A. baumannii tetracycline susceptibility was partially restored in the presence of the drug efflux pump inhibitor PAβN, suggesting that efflux pumps, in part, modulate the tetracycline tolerance of an organism during serum growth. Similar phenotypes were also observed for the representatives of three of 11 previously characterized A. baumannii lineages evaluated (27), indicating that serum-associated efflux pump-mediated minocycline and ciprofloxacin tolerance is a semiconserved A. baumannii response that is presumably dependent on the genetic composition of the organism evaluated (data not shown).
FIG 1.
Antimicrobial effects of minocycline, ciprofloxacin, tetracycline, and tigecycline toward LB- and serum-grown A. baumannii. (A) Plotted are the CFU of strain 98-37-09 following incubation in LB or serum (with/without the efflux pump inhibitor PAβN) supplemented with 0 to 2 μg ml−1 of minocycline. (B) CFU of strain 98-37-09 following incubation in LB or serum (with/without the efflux pump inhibitor reserpine) supplemented with 0 to 1 μg ml−1 of ciprofloxacin. (C) CFU of strain 98-37-09 following incubation in LB or serum (with/without the efflux pump inhibitor PAβN) supplemented with 0 to 16 μg ml−1 of tetracycline. (D) CFU of strain 01-12-05 after incubation in LB or serum (with/without the efflux pump inhibitor reserpine) supplemented with 0 to 4 ml−1 of tigecycline. The mean CFU ± standard deviations are plotted on the y axis, and antibiotic concentration is indicated on the x axis; the asterisks indicate statistically significant differences in CFU between growth in LB and serum (Student's t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
While we did not observe significant differences between the susceptibilities of serum- and LB-grown 98-37-09 cells and those of other classes of antibiotics tested, it was observed during the course of our investigations that strains representing seven of the 11 other lineages tested displayed various but significantly increased efflux-mediated tolerance to the antibiotic tigecycline during serum growth (representative results shown in Fig. 1D). As an example, tigecycline displayed clear antimicrobial activity toward A. baumannii 01-12-05 during growth in LB medium at concentrations of ≥1 μg ml−1, but the strain appeared to be highly resistant to the antibiotic during growth in serum. Susceptibility was partially restored by adding the efflux pump inhibitor reserpine, suggesting that serum-associated efflux pump activity contributes to the ability of a strain to tolerate tigecycline during growth in serum.
The observed serum-dependent efflux pump-mediated antibiotic tolerance may, in part, account for the clinical failure of antibiotics toward clinically defined susceptible A. baumannii strains; during adaptation to host-associated environmental conditions, such as serum, the organism may induce efflux pumps that allow clinically defined antibiotic-susceptible organisms to tolerate antibiotic challenge in vivo. Accordingly, adjunctive therapy with corresponding efflux pump inhibitors may provide a valuable strategy for limiting antibiotic tolerance within the host and, consequently, be an attractive therapeutic approach for both current and future antibiotics.
High-throughput screening for agents that potentiate antimicrobial activity of minocycline toward serum-grown A. baumannii.
As a means of identifying new chemical classes of A. baumannii antibiotic efflux inhibitors, we exploited the finding that during growth in human serum, A. baumannii expresses efflux pumps that mediate cellular tolerance to minocycline (25) (Fig. 1A). Accordingly, the 29,900-member TimTec ActiProbe small-molecule library and Natural Product Library were screened for agents that eliminate the tolerance of A. baumannii to minocycline during serum growth. To do so, approximately 1 × 105 A. baumannii 98-37-09 cells were inoculated into individual wells of a microtiter plate containing 100% human serum supplemented with 0.5 μg ml−1 minocycline (0.5× the serum MIC). A total of 50 μM each library member was added, the plates were incubated for 48 h at 37°C, and growth was measured as a function of turbidity. Most compounds (99.6% [29,806 compounds]) did not affect the growth of the organism, whereas 94 compounds (0.4%) inhibited the ability of the strain to grow in serum supplemented with minocycline. Repeat testing in which the well constituents were serially diluted and plated on LB agar plates verified that 85 compounds did indeed limit A. baumannii growth in serum supplemented with minocycline, resulting in a 2- to 6-log reduction in the number of viable CFU in comparison to the cells treated with minocycline alone.
To distinguish whether compounds of interest potentiate the activity of minocycline as opposed to displaying antibacterial properties on their own, each compound was subsequently evaluated for antimicrobial activity toward A. baumannii in serum or LB in the absence of minocycline. Twelve of the 85 compounds tested (12.7%) exhibited antimicrobial activity toward A. baumannii grown in serum and/or LB in the absence of minocycline and may represent novel antimicrobial agents (data not shown). The remaining 73 compounds did not display antimicrobial activity in the absence of minocycline, suggesting that a subset of these compounds may represent efflux pump inhibitors that potentiate the antimicrobial activity of minocycline toward serum-grown A. baumannii. Accordingly, their minimum effective concentration (MEC) was defined as a means both to rank the compounds based on their potency and also as a prerequisite for more extensive characterization, as described below. To define the MEC of each compound, A. baumannii 98-37-09 was inoculated into individual wells of a microtiter plate containing 100% human serum supplemented with 0.5 μg ml−1 minocycline and increasing concentrations (0 to 125 μg ml−1) of the test compound, and it was then incubated for 48 h. The smallest amount of compound required to potentiate the antimicrobial activity of minocycline, as defined by growth inhibition, was defined as the MEC. Plating confirmed that the addition of 1× the MEC of each compound elicited a ≥1.9-log reduction in A. baumannii cells grown in serum supplemented with minocycline alone (see Table S2 in the supplemental material).
A. baumannii minocycline accumulation.
As an initial means of determining whether the compounds of interest displayed characteristics expected of an A. baumannii efflux pump inhibitor, and simultaneously prioritizing compounds for further characterization, we considered that efflux pump inhibition would lead to intracellular antibiotic accumulation compared to that in the cells in which efflux was active. Accordingly, triple-quadrupole mass spectrometry was used to measure the cellular antibiotic concentration of A. baumannii cells grown in human serum supplemented with 0.5 μg ml−1 minocycline in the absence and presence of 0.5× the MEC test compound. These concentrations were used, and the plating of each culture validated that the conditions did not affect A. baumannii viability (data not shown).
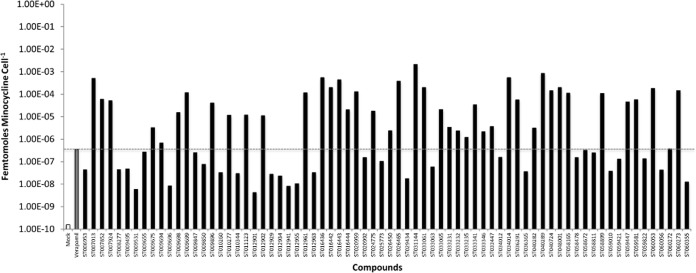
During growth in serum (efflux active conditions), the cellular minocycline concentration was determined to be 1.58 × 10−10 femtomoles (fmol) per bacterial cell, whereas the addition of the known efflux pump inhibitor verapamil increased the cellular concentration nearly 1,000-fold (3.56 × 10−7 fmol cell−1), indicating that the approach is appropriate for measuring efflux pump-dependent cellular antibiotic accumulation. As shown in Fig. 2, while virtually all of the compounds evaluated appeared to induce minocycline accumulation compared to that in mock-treated cells, 41 compounds stimulated minocycline accumulation within serum-grown A. baumannii cells to levels equaling or exceeding that of the known antibiotic efflux pump inhibitor verapamil, and these were considered to be highest priority agents that presumably include efflux inhibitors and compounds that lead to antibiotic accumulation via unappreciated means. Regardless of the mechanism, these 41 compounds were considered to be putatively clinically valuable agents that potentiate the antimicrobial activity and cellular accumulation of minocycline toward A. baumannii in serum and were carried forward for further characterization.
FIG 2.
A. baumannii intracellular minocycline concentration measures. Shown are the fmol of minocycline per cell following the growth of strain 98-37-09 in serum supplemented with 0.5 μg ml−1 minocycline (y axis) in the absence (white bar) or presence (gray bar) of the efflux pump inhibitor verapamil, or the presence of 0.5× the minimum effective concentration of the indicated putative efflux pump inhibitor (x axis). The dashed gray line represents the concentration of minocycline per cell grown in human serum supplemented with minocycline and verapamil for comparison.
Human cytotoxicity measures.
Given that our overarching goal was to identify therapeutically relevant novel compounds for future medicinal chemistry-based improvement and refinement, we considered that the most desirable compounds would display little or no human cytotoxicity. Indeed, while PAβN has proven to be a valuable bacterial drug efflux inhibitor tool/compound in the laboratory setting, the compound displays toxicity at concentrations required for antimicrobial efficacy in the host and, consequently, has limited therapeutic promise (34). Thus, to distinguish putatively nontoxic from cytotoxic compounds, conventional 3-(4,5-dimethythiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assays were performed for each compound of interest at 1× and 4× their MEC. As shown in Table S2 in the supplemental material, 19 (46.3%) of the compounds tested elicited significant toxicity toward HepG2 cells, which was defined as <75% cellular survival during 48-h treatment at 4× the MEC, and these compounds were deprioritized. Conversely, 22 (53.6%) compounds displayed ≥75% survival (75.1% to 100%) and were considered to exhibit either no or low-level human HepG2 cytotoxicity; the low-level cytotoxicity may be reduced by future medicinal chemistry campaigns. It should be noted that 75% human cell survival was used as a culling criterion, because it approximates the toxicity measures of the antibiotic minocycline when tested alone under these assay conditions (77.9% HepG2 survival at 2 μg ml−1). Thus, we expect that molecules displaying human cytotoxicity measures correlating with minocycline under these assay conditions represent the most promising scaffolds for future medicinal chemistry-based optimization.
Spectrum of activity.
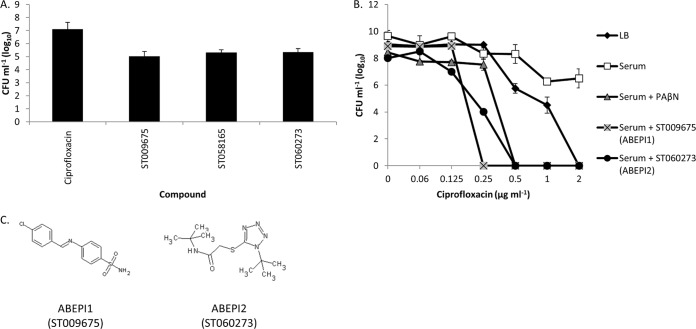
As a means to further prioritize nontoxic compounds of interest based on their therapeutic promise, we considered that broad-spectrum antimicrobial efflux pump inhibitors may be more clinically valuable than narrow-spectrum agents that potentiate the activity of only a limited number of antibiotics or that display activity toward a single bacterial species. As described above, in addition to minocycline, A. baumannii growth in human serum facilitates the efflux and the tolerance of ciprofloxacin in an organism (Fig. 1B). Consequently, we evaluated whether each compound potentiated the activity of ciprofloxacin toward serum-grown cells. To do so, 1 × 105 CFU A. baumannii 98-37-09 was inoculated into individual wells of microtiter plates containing 100% human serum supplemented with 0.125 μg ml−1 ciprofloxacin and 0×, 1×, or 2× the MEC of the compound, as defined by the lowest concentration needed to potentiate the antimicrobial effects of minocycline (above). The plates were incubated for 48 h, at which point the ability of each compound to potentiate ciprofloxacin was measured as the growth inhibition. Nineteen of the compounds evaluated did not affect the tolerance of the organism to ciprofloxacin under these conditions, suggesting that they are narrow-spectrum agents that limit minocycline efflux but not ciprofloxacin efflux, and these were deprioritized. Conversely, 3 compounds potentiated the antimicrobial activity of ciprofloxacin, suggesting that they represent broad-spectrum antibiotic drug efflux pump inhibitors. Plating confirmed that when administered in combination with ciprofloxacin, each compound reduced A. baumannii viability ≥1.5 log at 1× the MEC of the compound compared to that of the cells treated with ciprofloxacin alone (Fig. 3A; see also Table S2 in the supplemental material).
FIG 3.
Spectrum of activity. (A) CFU of strain 98-37-09 grown in human serum supplemented with 0.125 μg ml−1 ciprofloxacin in the absence or presence of 1× the MEC of the putative efflux pump inhibitor ST009675 (2 μg ml−1), ST058165 (32 μg ml−1), or ST060273 (32 μg ml−1); error bars indicate the standard deviations. (B) Mean CFU measures ± standard deviations for P. aeruginosa PAO1 following incubation in LB or serum with or without the efflux pump inhibitor PAβN, ST009675 (2 μg ml−1), or ST060273 (32 μg ml−1) supplemented with 0 to 2 μg ml−1 of ciprofloxacin. (C) Structures of ABEPI1 (ST009675) and ABEPI2 (ST060273).
During the course of our studies, we also investigated whether the Gram-negative pathogens K. pneumoniae and P. aeruginosa exhibit antibiotic tolerance to minocycline and/or ciprofloxacin during growth in human serum. While K. pneumoniae strain CKP4 did not, it was found that serum-grown P. aeruginosa PAO1 cells exhibit efflux-mediated tolerance to ciprofloxacin, as described below. Thus, as an additional means of evaluating the spectrum of activity and simultaneously identifying the highest priority compounds of interest that potentiate the activity of antibiotics across bacterial species, we evaluated whether the aforementioned three putative broad-spectrum A. baumannii efflux pump inhibitors also inhibited P. aeruginosa serum-dependent ciprofloxacin tolerance. To do so, PAO1 was inoculated into individual wells of a microtiter plate containing 100% human serum supplemented with 1× the MEC of test compound and increasing concentrations of ciprofloxacin (0 to 2 μg ml−1), and cell viability was measured. The results revealed that two of the three putative broad-spectrum efflux pump inhibitors also potentiated the activity of ciprofloxacin toward serum-grown P. aeruginosa (Fig. 3B), suggesting that these compounds, ABEPI1 and ABEPI1 (Fig. 3C), represent broad-spectrum agents that may potentiate the antimicrobial properties of antibiotics toward at least two bacterial species of immediate health care concern, A. baumannii and P. aeruginosa. PAβN alone (i.e., in the absence of ciprofloxacin) did not display antimicrobial properties toward serum-grown PAO1 cells under these assay conditions (data not shown).
ABEPI1 and ABEPI2 inhibit A. baumannii efflux properties.
To distinguish whether the antimicrobial potentiation of ABEPI1 and ABEPI2 correlates to the inhibition of the efflux properties of A. baumannii, conventional ethidium bromide efflux assays were performed in the presence and absence of each compound, as previously described (18, 30–33). The assay is predicated upon the fluorescent properties of ethidium bromide during intercalation into cellular nucleic acids, whereby efflux active cells display limited intracellular ethidium bromide accumulation and, consequently, low fluorescence. Conversely, efflux inhibition leads to increased cellular ethidium bromide levels and correspondingly high fluorescence relative to that of efflux-proficient cells.
As shown in Fig. 4, the mock-treated cells displayed a low-level ethidium bromide fluorescence signal that slowly increased during the course of the experiment, presumably reflecting the slow accumulation of dye over time despite efflux pump activity. Conversely, efflux-deficient PAβN-treated cells exhibited significantly increased cellular ethidium bromide accumulation compared to that in mock-treated cells, confirming that the assay conditions were appropriate for measuring the efflux properties of A. baumannii cells. Likewise, both ABEPI1 and ABEPI2 displayed a significantly increased signal compared to that of the mock-treated cells at all measured time points, indicating that they act as A. baumannii efflux pump inhibitors. More specifically, APEPI1 dramatically increased cellular fluorescence to levels exceeding that of PAβN within the first 20 min of treatment, at which point the potency of the compound appeared to level off. The APEPI2 treatment measures were essentially identical to those of PAβN until approximately 35 min posttreatment, at which point efflux inhibition appeared to drop below the PAβN levels, although the observed differences were not considered significantly different. Thus, ABEPI1 and ABEPI2 appear to represent novel A. baumannii drug efflux inhibitors.
FIG 4.
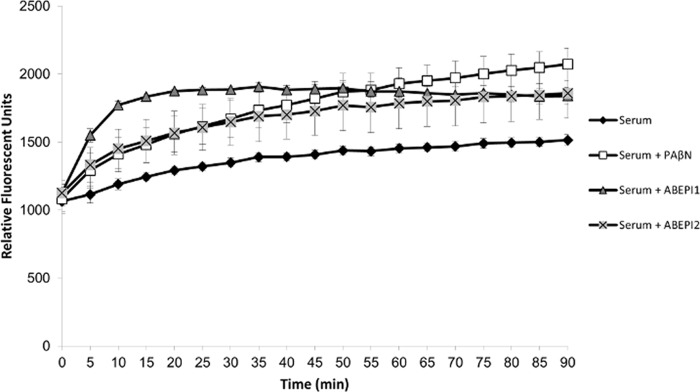
Ethidium bromide (EtBr) efflux assay. Shown are the mean (± standard deviation) EtBr fluorescence values for strain 98-37-09 following growth in human serum and treatment with PAβN (10 μg ml−1; white squares), ABEPI1 (ST009675; 2 μg ml−1; gray triangles), or ABEPI2 (32 μg ml−1; gray X). The mock-treated cells are also shown (black diamonds).
Mammalian Ca2+ channel blocking assays.
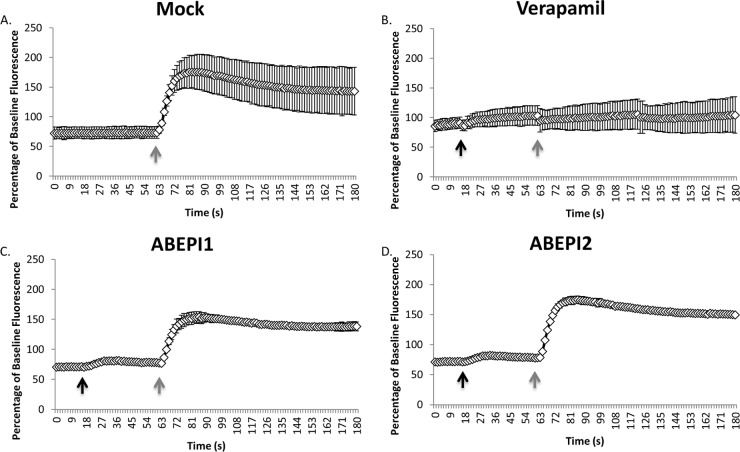
In considering whether ABEPI1 and/or ABEPI2 represent attractive antibiotic efflux pumps worthy of future medicinal chemistry-based improvement, we were cognizant of the fact that many laboratory bacterial efflux inhibitor tool compounds cannot be used in the clinical setting, because they limit mammalian ion channel activity. Verapamil is one such agent, which effectively limits bacterial antibiotic efflux pumps but also elicits human neurotoxicity due to the inhibition of host Ca2+ channels (35). Thus, we measured the effects of ABEPI1 and ABEPI2 on mammalian calcium channel functions using Fluo-4 Direct calcium channel assay kits, in which the dye Fluo-4 was used to measure changes in mammalian cytoplasmic Ca2+ levels in response to the calcium channel stimulator carbachol in the absence and presence of test compound. Figure 5A shows the profile of human embryonic kidney (HEK 293T) intracellular Ca2+ levels prior to and following the addition of carbachol, which stimulates endoplasmic calcium-channel activity and, consequently, the release of Ca2+ into the cytoplasm. As expected, carbachol treatment induced an approximately 2.3-fold increase in cytoplasmic Ca2+ levels. Conversely, the treatment of HEK 293T cells with the known calcium channel blocker verapamil virtually eliminated Ca2+ channel activity and cytoplasmic accumulation, indicating that the system was appropriate for measuring mammalian cytoplasmic channel activity and inhibition (Fig. 5B). As shown in Fig. 5C and D, HEK 293T treatment with 1× or 4× the MEC (not shown) of either ABEPI1 or ABEPI2 did not appear to significantly affect mammalian cell Ca2+ channel stimulation in response to carbachol.
FIG 5.
Mammalian calcium channel inhibition assays. (A) Cytoplasmic Ca2+ measures of mock-treated cells pre- and posttreatment with carbachol (60 s) (gray arrow). (B to D) Same as panel A except that cells were treated with verapamil (50 μg ml−1) (B) or 1× the MEC of ABEPI1 (ST009675; 2 μg ml−1) (C) or ABEPI2 (ST060273; 32 μg ml−1) (D) at 45 s (black arrow) pre-carbachol treatment.
Taken together, it appears as though ABEPI1 and ABEPI2 represent novel and structurally distinct molecules that potentiate the activities of antibiotics toward serum-grown bacterial cells by inhibiting the drug efflux properties of an organism, leading to cellular antibiotic accumulation and, consequently, antimicrobial effects. Moreover, the compounds did not display significant human cytotoxicity measures or Ca2+ channel blocking activity that has plagued the development of antibiotic drug efflux inhibitors. Such compounds may represent attractive starting scaffolds for medicinal chemistry-based improvement and refinement, with the ultimate goal of creating adjunctive efflux pump inhibitors to be used in combination with current antibiotics for improving the treatment of bacterial infections.
DISCUSSION
Antibiotic resistance is a general term used to describe the phenomenon whereby antibiotics used to treat a bacterial pathogen are rendered useless or less effective by the target organism. The so-called bacterial drug efflux pumps have been shown to contribute to antibiotic resistance by modulating the cellular concentration of a given antibiotic within bacterial cells and can generally be divided into five classes of transporters: small multidrug resistance (SMR) pumps of the drug/metabolite transporter superfamily, ATP-binding cassette (ABC) transporters, the major facilitator superfamily (MFS), the resistance-nodulation-division (RND) superfamily, and multidrug and toxic compound extrusion (MATE) transporters (see reference 26 for a recent review). While some members of these families selectively extrude specific antibiotics, the majority efflux a variety of antimicrobial agents. For instance, the A. baumannii MATE family transporter AbeM is believed to efflux fluoroquinolones, aminoglycosides, quinolones, chloramphenicol, and erythromycin (15). Thus, AbeM inhibitors may be valuable therapeutic agents that restore the utility of several antibiotic classes. However, such a strategy is complicated by the fact that many antibiotics are extruded by multiple efflux pumps. Thus, in considering the development of an antimicrobial efflux inhibitor program, clinically relevant inhibitors must limit the activities of multiple efflux pumps as a prerequisite for efficacy.
A. baumannii harbors representatives of all the major efflux families, and this is thought to contribute to the antimicrobial resistance of the organism, a phenotype that has, in part, led to its designation as one of the six bacterial ESKAPE (Enterococcus faecium, Staphylococcus aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter species) pathogens of immediate health care concern (36). While much has been learned about the substrates affected by previously characterized efflux pumps, studies regarding their expression and biological consequences are in their infancy. Likewise, bioinformatics analysis has revealed that the organism is likely to express a plethora of previously uncharacterized factors that may also contribute to clinical antibiotic failure.
Ventilator-associated pneumonia and bacteremia are the two most severe types of A. baumannii infection, both of which are thought to include dissemination of the organism to visceral organs via the circulatory system (1, 2, 6, 37). For that reason, much effort has been devoted toward defining the cellular components of the organism that modulate growth and persistence in the blood by using human serum as a convenient growth medium for such studies. In that regard, several A. baumannii virulence factors, including phospholipase D (PLD), penicillin binding protein 7/8, K1 capsule polysaccharide, and outer membrane protein A (OmpA), have been shown to augment the ability of the organism to survive in human serum and to contribute to its ability to cause disease in animal models of infection, validating serum as a biologically relevant medium for A. baumannii study (27, 38–41). More recently, a transcriptional profiling-based study revealed that during growth in human serum, A. baumannii differentially expresses several putative virulence factors. Perhaps most strikingly, that study also showed that serum growth induces high-level expression of ≥22 previously uncharacterized putative drug efflux-associated proteins and corresponds to the efflux-mediated resistance of the organism to the antibiotic minocycline in serum (25). Such a phenomenon was recently termed adaptive resistance, a connotation used to describe processes by which clinically defined susceptible bacterial species temporally alter gene expression in response to an environmental cue(s) in a manner that confers tolerance to a given antibacterial agent (26).
One of the goals of the immediate work was to expand our understanding of the adaptive efflux-mediated antibiotic resistance potential of A. baumannii during growth in human serum. The results presented here indicate that serum growth-associated antibiotic efflux is conserved across all genetic lineages that we have evaluated thus far, although strain-to-strain differences clearly do occur in terms of the specific antibiotics extruded. For instance, the A. baumannii clinical isolate 98-37-09, which was used for most of these studies, displays tolerance to minocycline, tetracycline, and ciprofloxacin during growth in serum. While serum growth-associated minocycline resistance has also been observed for a subset of the strains evaluated, it is not completely conserved across isolates. For instance, the serum growth of strain 07-09-54 does not exhibit resistance to minocycline but does display efflux-mediated high-level tolerance to meropenem (data not shown). Likewise, strain 98-37-09 does not exhibit serum-dependent tigecycline tolerance, but seven representatives of the other 11 lineages evaluated do. Taken together, these results suggest that during serum growth, A. baumannii has the capacity to express a multitude of antibiotic efflux pumps that confer antibiotic resistance, but tolerance is dependent on the genetic composition and, consequently, the efflux pump repertoire of the strain evaluated. Notably, the same phenomenon was observed for the prototypical P. aeruginosa laboratory strain PAO1, which displayed efflux-mediated tolerance to ciprofloxacin during serum growth comparison to that of the laboratory medium. It remains to be seen whether this correlates to other P. aeruginosa strains or other antibiotics. Likewise, it is not yet clear which serum-dependent factors activate antibiotic efflux. However, the efflux of antibiotics is presumably a by-product of the transporters themselves, which are likely functioning to aid in cellular adaptation to nutrient-limiting conditions. By extension, one would predict that other host-associated environments are likely to also elicit such a phenotype, upregulating either the efflux pumps orchestrating serum antibiotic tolerance and/or other so-called antibiotic efflux pumps. In support of that hypothesis, it has been shown that the growth of A. baumannii in physiologically relevant salt concentrations induces antibiotic efflux-mediated tolerance to the antibiotics amikacin and levofloxacin (24).
Given the biological importance of these findings, we reasoned that small-molecule inhibitors of serum-associated antibiotic efflux functions would represent valuable therapeutics to be used in combination with current and possibly future antibiotics. Such agents would potentiate the antimicrobial agents despite the efflux potential of A. baumannii in serum, thereby improving the potency of such antibiotics. Thus, a whole-cell high-throughput assay was performed to identify agents that potentiate the antimicrobial activity of a subinhibitory concentration of minocycline toward serum-grown cells, with the expectation that a subset of these compounds would represent serum-associated minocycline efflux pump inhibitors.
Our screening campaign and secondary assays identified two structurally distinct compounds, ABEPI1 and ABEPI2, which display the early characteristics of promising A. baumannii serum-associated efflux pump inhibitors. Both compounds restore the antimicrobial activities of minocycline and ciprofloxacin toward serum-grown A. baumannii but did not affect the antibiotic susceptibilities of the LB-grown cells, suggesting that they affect serum-associated efflux factors. Further, both compounds limit the efflux properties of the organism, as measured in standard ethidium bromide assays, and lead to minocycline accumulation within treated cells. Moreover, neither compound displayed cytotoxicity toward human cells or human Ca2+ channel inhibitory activities, two issues that have limited the development of other bacterial efflux pump inhibitors.
Sulfonamide is a convenient and widely used functional group in medicinal chemistry that improves the physicochemical properties, such as solubility, of small-molecule organic scaffolds, and it has been exploited in antibacterial, antiviral, anti-inflammatory, and anticancer agent development (reviewed in reference 42). In that regard, Acinetobacter baumannii efflux pump inhibitor 1 (ABEPI1) [(E)-4-((4-chlorobenzylidene)amino)benezenesulfonamide] is a sulfonamide derivative that does not display antimicrobial activity on its own but strongly potentiates the activities of minocycline and ciprofloxacin, leads to minocycline accumulation within bacterial cells, and has low cytotoxicity toward human cells. Twenty structurally similar analogs were found in our screening campaign to be putative efflux pump inhibitors (see Table S2 in the supplemental material), affording some insight into a preliminary structure-activity relationship for ABEPI1. Certain analogs showed that substitutions at the 4-position of the benzylidene influence the activity of the chemical series. The 4-methylbenzylidene analog increased the MEC 2-fold and displayed a 100,000-fold reduction in the accumulation of minocycline within A. baumannii cells. The 4-ethylbenzylidene analog exhibited reduced activity as well, as measured by an 8-fold increase in the MEC, and a roughly 30-fold reduction in minocycline accumulation in comparison to that with EPI1. The 4-bromobenzylidene analog has only about a 5-fold decrease in minocycline accumulation and has the same MEC as EPI1, indicating that certain halogens may have increased activity over hydrocarbons in this position. The 4-fluorobenzylidene, in contrast with the other two halogens, shows a 40-fold reduction in minocycline accumulation and a 32-fold increase in MEC. Other functional groups in this position also displayed measurable effects on activity: the 4-ethoxybenzylidene and 4-(dimethylamino)benzylidene analogs both had increased MEC values, decreased minocycline accumulation, and increased toxicity. Clearly, additional analoging is required to develop a refined structure-activity relationship (SAR), but those tested thus far indicate the 4-substituted benzylidenes to be at least one path to improving upon the scaffold for a more effective ABEPI1-derived efflux pump inhibitor. In comparison to ABEPI1, ABEPI2 [N-tert-butyl-2-(1-tert-butyltetrazol-5-yl)sulfanylacetamide] appears to be less potent, with a relatively high MEC of 32 μg ml−1; however, it also displays promising activities, leading to an accumulation of 6.84 × 10−6 fmol of minocycline cell−1, potentiating the activity of ciprofloxacin toward both A. baumannii and P. aeruginosa, having low cytotoxicity toward human cells, and inhibiting efflux pump activity. Additionally, the compound offers a scaffold amenable to possible structural alterations that in future studies might lead to analogs that further improve the efficacy of the compound.
While ABEPI1 and ABEPI2 exhibit similar antibiotic potentiation profiles with regard to minocycline and ciprofloxacin, early extended-spectrum-of-activity studies indicate that they also display differing activities and consequently are both likely to target a common efflux pump (or set), but they also affect differing subsets of antibiotic efflux pumps and, consequently, display broad-spectrum activity. More specifically, ABEPI1 is capable of improving the activity of tigecycline toward the carbapenem-resistant A. baumannii strain 07-09-61 to levels equaling that of PAβN, whereas ABEPI2 does not improve the activity of tigecycline (Fig. 6A). Conversely, ABEPI2 was found to improve the activity of tobramycin toward P. aeruginosa PAO1 during growth in human serum to levels equaling that with PAβN (Fig. 6B), but ABEPI1 did not demonstrate tobramycin-potentiating activity (Fig. 6B).
FIG 6.
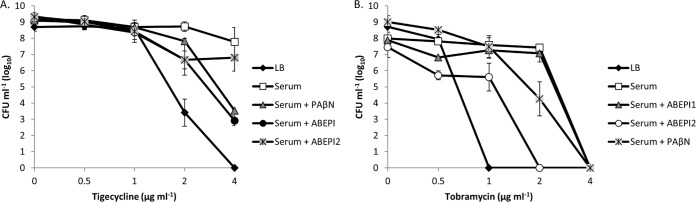
Differential antibiotic potentiation effects of ABEPI1 and ABEPI2. (A) CFU of A. baumannii strain 07-09-61 after incubation in LB or serum supplemented with 0 to 4 ml−1 tigecycline. Also shown are the tigecycline antimicrobial effects in serum-grown cells in the presence of PAβN (10 μg ml−1), ABEPI1 (ST009675; 2 μg ml−1), or ABEPI2 (ST060273; 32 μg ml−1). (B) CFU of P. aeruginosa strain PAO1 after incubation in LB or serum supplemented with 0 to 4 ml−1 tobramycin; the antimicrobial effects of tobramycin toward serum-grown cells in the presence of PAβN (10 μg ml−1), ABEPI1 (ST009675; 2 μg ml−1), or ABEPI2 (ST060273; 32 μg ml−1) are also shown. Values plotted are means ± standard deviations from at least four biological replicates.
Taken together, the results of these studies indicate that A. baumannii and presumably P. aeruginosa impart adaptive efflux resistance mechanisms during growth in human serum that may, in part, contribute to antibiotic treatment failure toward laboratory-defined susceptible strains. Further, ABEPI1 and ABEPI2 may represent attractive compounds for future medicinal chemistry-based campaigns designed to limit Gram-negative bacterial efflux.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by National Institute of Allergy and Infectious Diseases award R01AI103507 and University of Rochester Drug Discovery Pilot funds (to P.M.D.) and by Training Program in Oral Sciences T90FR021985 (to C.B.).
Footnotes
Published ahead of print 11 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03535-14.
REFERENCES
- 1.Garnacho-Montero J, Ortiz-Leyba C, Fernández-Hinojosa E, Aldabó-Pallás T, Cayuela A, Marquez-Vácaro JA, Garcia-Curiel A, Jiménez-Jiménez FJ. 2005. Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med. 31:649–655. 10.1007/s00134-005-2598-0. [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582. 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roca I, Espinal P, Vila-Farrés X, Vila J. 2012. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front. Microbiol. 3:148. 10.3389/fmicb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmonds A, Munoz J, Aguero-Rosenfeld M, Carbonaro C, Montecalvo M, Clones B, LaGamma EF. 2009. Outbreak of Acinetobacter infection in extremely low birth weight neonates. Pediatr. Infect. Dis. J. 28:210–214. 10.1097/INF.0b013e31818cb0aa. [DOI] [PubMed] [Google Scholar]
- 5.Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J, Cosgrove SE, Anderson A, Carnell J, Jernigan DB, Kleinbaum DG, Perl TM, Standiford HC, Srinivasan A. 2007. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg. Infect. Dis. 13:97–103. 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in U.S. hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317. 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 7.Doi Y, Husain S, Potoski BA, McCurry KR, Paterson DL. 2009. Extensively drug-resistant Acinetobacter baumannii. Emerg. Infect. Dis. 15:980-982. 10.3201/eid1506.081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosso F, Quinteira S, Peixe L. 2010. Emergence of an extreme-drug-resistant (XDR) Acinetobacter baumannii carrying blaOXA-23 in a patient with acute necrohaemorrhagic pancreatitis. J. Hosp. Infect. 75:82–83. 10.1016/j.jhin.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Hampton T. 2013. Report reveals scope of U.S. antibiotic resistance threat. JAMA 310:1661–1663. 10.1001/jama.2013.280695. [DOI] [PubMed] [Google Scholar]
- 10.Tan SY, Chua SL, Liu Y, Høiby N, Andersen LP, Givskov M, Song Z, Yang L. 2013. Comparative genomic analysis of rapid evolution of an extreme-drug-resistant Acinetobacter baumannii clone. Genome Biol. Evol. 5:807–818. 10.1093/gbe/evt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, Ecker DJ, Massire C, Eshoo MW, Sampath R, Thomson JM, Rather PN, Craft DW, Fishbain JT, Ewell AJ, Jacobs MR, Paterson DL, Bonomo RA. 2006. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 50:4114–4123. 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rumbo C, Gato E, López M, Ruiz de Alegría C, Fernández-Cuenca F, Martínez-Martínez L, Vila J, Pachón J, Cisneros JM, Rodríguez-Baño J, Pascual A, Bou G, Tomas M. 2013. Contribution of efflux pumps, porins, and β-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 57:5247–5257. 10.1128/AAC.00730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajamohan G, Srinivasan VB, Gebreyes WA. 2010. Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1919–1925. 10.1093/jac/dkq195. [DOI] [PubMed] [Google Scholar]
- 14.Roca I, Marti S, Espinal P, Martínez P, Gibert I, Vila J. 2009. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:4013–4014. 10.1128/AAC.00584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su XZ, Chen J, Mizushima T, Kuroda T, Tsuchiya T. 2005. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob. Agents Chemother. 49:4362–4364. 10.1128/AAC.49.10.4362-4364.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srinivasan VB, Rajamohan G, Gebreyes WA. 2009. Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:5312–5316. 10.1128/AAC.00748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortez-Cordova J, Kumar A. 2011. Activity of the efflux pump inhibitor phenylalanine-arginine β-naphthylamide against the AdeFGH pump of Acinetobacter baumannii. Int. J. Antimicrob. Agents 37:420–424. 10.1016/j.ijantimicag.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Coyne S, Rosenfeld N, Lambert T, Courvalin P, Périchon B. 2010. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:4389–4393. 10.1128/AAC.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damier-Piolle L, Magnet S, Brémont S, Lambert T, Courvalin P. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:557–562. 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornsey M, Ellington MJ, Doumith M, Thomas CP, Gordon NC, Wareham DW, Quinn J, Lolans K, Livermore DM, Woodford N. 2010. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1589–1593. 10.1093/jac/dkq218. [DOI] [PubMed] [Google Scholar]
- 21.Wieczorek P, Sacha P, Hauschild T, Zórawski M, Krawczyk M, Tryniszewska E. 2008. Multidrug-resistant Acinetobacter baumannii–the role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem. Cytobiol. 46:257–267. 10.2478/v10042-008-0056-x. [DOI] [PubMed] [Google Scholar]
- 22.Coyne S, Courvalin P, Périchon B. 2011. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 55:947–953. 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie JM. 2006. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2:e7. 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hood MI, Jacobs AC, Sayood K, Dunman PM, Skaar EP. 2010. Acinetobacter baumannii increases tolerance to antibiotics in response to monovalent cations. Antimicrob. Agents Chemother. 54:1029–1041. 10.1128/AAC.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs AC, Sayood K, Olmsted SB, Blanchard CE, Hinrichs S, Russell D, Dunman PM. 2012. Characterization of the Acinetobacter baumannii growth phase-dependent and serum responsive transcriptomes. FEMS Immunol. Med. Microbiol. 64:403–412. 10.1111/j.1574-695X.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 26.Fernández L, Hancock RE. 2012. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 25:661–681. 10.1128/CMR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs AC, Hood I, Boyd KL, Olson PD, Morrison JM, Carson S, Sayood K, Iwen PC, Skaar EP, Dunman PM. 2010. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect. Immun. 78:1952–1962. 10.1128/IAI.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holloway BW, Morgan AF. 1986. Genome organization in Pseudomonas. Annu. Rev. Microbiol. 40:79–105. 10.1146/annurev.mi.40.100186.000455. [DOI] [PubMed] [Google Scholar]
- 29.Cai H, Rose K, Liang LH, Dunham S, Stover C. 2009. Development of a liquid chromatography/mass spectrometry-based drug accumulation assay in Pseudomonas aeruginosa. Anal. Biochem. 385:321–325. 10.1016/j.ab.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 30.Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, Blais J, Cho D, Chamberland S, Renau T, Leger R, Hecker S, Watkins W, Hoshino K, Ishida H, Lee VJ. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105–116. 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnet S, Courvalin P, Lambert T. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375–3380. 10.1128/AAC.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchand I, Damier-Piolle L, Courvalin P, Lambert T. 2004. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 48:3298–3304. 10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peleg AY, Adams J, Paterson DL. 2007. Tigecycline efflux as a mechanism for nonsusceptibility in Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:2065–2069. 10.1128/AAC.01198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watkins WJ, Landaverry Y, Léger R, Litman R, Renau TE, Williams N, Yen R, Zhang JZ, Chamberland S, Madsen D, Griffith D, Tembe V, Huie K, Dudley MN. 2003. The relationship between physicochemical properties, in vitro activity and pharmacokinetic profiles of analogues of diamine-containing efflux pump inhibitors. Bioorg. Med. Chem. Lett. 13:4241–4244. 10.1016/j.bmcl.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Koh JY, Cotman CW. 1992. Programmed cell death: its possible contribution to neurotoxicity mediated by calcium channel antagonists. Brain Res. 587:233–240. 10.1016/0006-8993(92)91002-V. [DOI] [PubMed] [Google Scholar]
- 36.Rice LB. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 197:1079–1081. 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 37.Howard A, O'Donoghue M, Feeney A, Sleator RD. 2012. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3:243–250. 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi CH, Lee JS, Lee YC, Park TI, Lee JC. 2008. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 8:216. 10.1186/1471-2180-8-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SW, Choi CH, Moon DC, Jin JS, Lee JH, Shin JH, Kim JM, Lee YC, Seol SY, Cho DT, Lee JC. 2009. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol. Lett. 301:224–231. 10.1111/j.1574-6968.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- 40.Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, MacDonald U, Schultz LW, Umland TC, Campagnari AA. 2010. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 78:3993–4000. 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo TA, MacDonald U, Beanan JM, Olson R, MacDonald IJ, Sauberan SL, Luke NR, Schultz LW, Umland TC. 2009. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J. Infect. Dis. 199:513–521. 10.1086/596317. [DOI] [PubMed] [Google Scholar]
- 42.Winum JY, Scozzafava A, Montero JL, Supuran CT. 2006. Therapeutic potential of sulfamides as enzyme inhibitors. Med. Res. Rev. 26:767–792. 10.1002/med.20068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.