Abstract
Dolutegravir (DTG) is an HIV integrase inhibitor (INI) with demonstrated activity in INI-naive and INI-resistant patients. The objective of this open-label, 2-period, single-sequence study was to evaluate the effect of fosamprenavir-ritonavir (FPV-RTV) on the steady-state plasma pharmacokinetics of DTG. Twelve healthy subjects received 50 mg DTG once daily for 5 days (period 1), followed by 10 days of 50 mg DTG once daily in combination with 700/100 mg FPV-RTV every 12 h (period 2). All doses were administered in the fasting state. Serial pharmacokinetic samples for DTG and amprenavir and safety assessments were obtained throughout the study. Noncompartmental pharmacokinetic analysis was performed, and geometric least-squares mean ratios and 90% confidence intervals were generated for within-subject treatment comparison. Fosamprenavir-ritonavir decreased the DTG area under the concentration-time curve, maximum concentration in plasma, and concentration in plasma at the end of the dosing interval by 35%, 24%, and 49%, respectively. Both DTG and DTG with FPV-RTV were well tolerated; no subject withdrew because of adverse events. The most frequently reported drug-related adverse events were rash, abnormal dreams, and nasopharyngitis. The modest decrease in DTG exposure when it was coadministered with FPV-RTV is not considered clinically significant, and DTG dose adjustment is not required with coadministration of FPV-RTV in INI-naive patient populations on the basis of established “no-effect” boundaries of DTG. In the INI-resistant population, as a cautionary measure, alternative combinations that do not include FPV-RTV should be considered. (This study has been registered at ClinicalTrials.gov under identifier NCT01209065.)
INTRODUCTION
The integrase inhibitor (INI) class of antiretroviral drugs has proven to be a valuable addition to treatment strategies for the management of HIV. Good tolerability and lack of cross-resistance to other classes of antiretroviral therapy have contributed to the use of INIs in the antiretroviral regimen for both treatment-experienced and treatment-naive patients (1). Dolutegravir (DTG) is an unboosted, once-daily [QD] INI that can be differentiated from previous INIs (e.g., raltegravir [RAL] and elvitegravir) by its resistance profile and predictable pharmacokinetics (PK) with low to moderate intersubject variability (2, 3). Drug-drug interaction studies between ritonavir (RTV)-boosted protease inhibitors (i.e., tipranavir [TPV], darunavir, lopinavir, and atazanavir) and DTG have been conducted previously to evaluate changes in the plasma exposure to DTG. These studies have reported modest changes in DTG PK, and dosage adjustments are not required when administered with most commonly used RTV-boosted protease inhibitors, except for TPV-RTV (4–6).
Dolutegravir is metabolized primarily via uridine 5′-diphospho-glucuronosyltransferase-1A1 (UGT1A1), although cytochrome P450 3A4 (CYP3A4) has a minor role (10% to 15%) in the process, and it is a substrate of the transport protein P-glycoprotein (7, 8). Therefore, drugs that induce or inhibit these metabolic pathways may affect the plasma exposure of DTG. Dolutegravir does not induce or inhibit CYP3A4, as determined in a clinical study using midazolam as a CYP3A4 probe, and would not be expected to alter the PK of drugs metabolized by CYP3A4 (e.g., RTV-boosted protease inhibitors) (3). This study evaluated the effect of fosamprenavir (FPV)-RTV on DTG and was not designed as a 2-way evaluation given the lack of impact of DTG on coadministered protease inhibitors in healthy-volunteer studies (4–6).
Ritonavir is commonly coadministered with protease inhibitors as a boosting agent. Ritonavir is an inducer and inhibitor of CYP3A4, is an inducer of UGT1A1, and has a time-dependent inhibition effect followed by an induction effect on P-glycoprotein (9). Fosamprenavir, the prodrug for amprenavir (APV), has been demonstrated to induce enzymatic activity of UGT1A1 and to induce or inhibit CYP3A4 enzymes (10, 11). Thus, a study to evaluate its impact on DTG exposure was warranted. This study was conducted to evaluate the effect of FPV-RTV on the steady-state PK parameters of DTG. Amprenavir PK parameters were compared to historical data as a secondary endpoint.
On the basis of accumulated data on the PK-pharmacodynamic relationship of DTG in INI-naive patient populations, the “no-effect” boundaries of alterations in DTG exposure for the need for dose adjustment have been defined (12, 13). The lower bound of the no-effect boundaries is defined as either 0.3 μg/ml (equivalent to 25% of the plasma DTG concentration at the end of the dosing interval [Cτ] at 50 mg QD) or 75% reduction in the DTG Cτ. The lower bound of 0.3 μg/ml is approximately 3-fold greater than the value of the in vitro protein-adjusted 90% inhibitory concentration against wild-type viruses (0.064 μg/ml) (3). The upper bound is currently not defined, as dose-limiting toxicity has not been observed with DTG in phase II/III studies at doses up to 50 mg twice daily (BID). The clinical significance of the effect of FPV-RTV observed in the current study was judged against the established no-effect boundary.
MATERIALS AND METHODS
Study design.
This open-label study (ClinicalTrials.gov identifier NCT01209065) was conducted with 12 healthy subjects at a single center, where the subjects were inpatients for the duration of the trial. Both adult men and women were enrolled from September 2010 to November 2010. Women of childbearing potential were sexually inactive by abstinence or required to use contraceptive methods with a failure rate of <1%. Oral hormonal contraceptives were not allowed in the study. Participants were excluded if there was laboratory evidence of chronic hepatitis B virus, hepatitis C virus, and/or HIV infection. Participants were asked to abstain from taking prescription and nonprescription drugs, including vitamins and herbal products, within 7 days of the first dose of study medication until completion of the follow-up visit. Use of antacids, vitamins, and iron supplements was strictly prohibited because coadministration of divalent cations with DTG has been shown to reduce the oral bioavailability of DTG (14). Participants had a screening visit within 30 days prior to the first dose of the study drug, 2 treatment periods, and a follow-up visit 7 to 14 days after the last dose of the study drug.
All participants in the first treatment period (period 1) received 50 mg DTG (Tivicay; ViiV Healthcare, Research Triangle Park, NC) every 24 h for 5 days. This dose of DTG was chosen because it was the clinical dose in phase III trials in INI-naive subjects (i.e., treatment-naïve as well as treatment-experienced subjects). During the second treatment period (period 2), all participants received 50 mg DTG every 24 h in combination with 700/100 mg FPV-RTV every 12 h for 10 days. On PK sampling days, all morning doses of the study drug were administered after a 10-hour overnight fast. A washout was not used between treatment periods. Safety evaluations (i.e., clinical chemistry and hematology, vital signs, and electrocardiograms) were performed throughout the study.
Serial blood samples for determining the plasma DTG concentration were collected at the following times: predose (within 15 min prior to the study dose) and 1, 2, 3, 4, 8, 12, and 24 h postdose on day 5 in period 1 and on day 10 in period 2. Predose plasma samples for DTG were also collected on days 8 and 9 in period 2. Plasma APV samples were collected predose (within 15 min prior to the study dose) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, and 12 h after the FPV-RTV morning dose on day 10 in period 2. All subjects were asked about adverse effects and the use of concomitant medications on a frequent basis. Written informed consent was obtained from all participants, and the protocol was approved by the institutional review board IntegReview in Austin, TX.
Bioanalytical methods.
Plasma samples were analyzed for DTG concentrations using a validated analytical method based on protein precipitation followed by high-performance liquid chromatography–tandem mass spectrometry (3). Dolutegravir was analyzed using [2H7,15N]GSK1349572 as an internal standard. The lower limit of quantification for DTG was 20 ng/ml using a 25-μl aliquot of human plasma, with a higher limit of quantification of 20,000 ng/ml. Three different concentrations of quality control samples were stored and analyzed with each batch. For sample analysis to be acceptable, not more than one-third of the total quality control results and not more than one-half of the results from each concentration level could deviate from the nominal concentration by more than 15%. Human plasma samples were analyzed for APV by Advion (Ithaca, NY) using a validated analytical method based on a solid-phase extraction process, followed by high-performance liquid chromatography–tandem mass spectrometry analysis. The lower limit of quantification for APV was 10 ng/ml using 50-μl aliquots of human plasma, with a higher limit of quantification of 10,000 ng/ml. Four quality control samples were prepared and examined; the samples had within ±15% bias and therefore were determined to be acceptable.
Pharmacokinetic analysis.
Noncompartmental PK analysis was performed using concentration in plasma-time data for DTG and APV (Model 200 WinNonlin Professional Edition, version 5.3; Pharsight Corporation, St. Louis, MO). Plasma PK parameters, which were calculated using the actual elapsed time from dosing, included the following: the area under the concentration-time curve from when the dose is administered until the end of the dosing interval (AUC0–τ), the maximum concentration in plasma (Cmax), the Cτ, the apparent oral clearance (CL/F), and the apparent terminal half-life (t1/2).
Statistical analysis.
The sample size of 12 subjects to obtain 10 evaluable subjects was chosen on the basis of the within-subject PK variability of DTG and the feasibility of addressing the objectives of the study.
The study was designed primarily to estimate the drug-drug interaction effect of FPV-RTV on DTG PK parameters. For DTG, an analysis of variance (15) was performed on the log-transformed PK parameters AUC0–τ, Cτ, Cmax, CL/F, and t1/2, with subject as the random effect and treatment as the fixed effect in the model, using the SAS (Cary, NC) Mixed Linear Models procedure (version 9 or higher). The ratio of geometric least-squares (GLS) means and the associated 90% confidence interval (CI) were estimated for the PK parameters of interest. Dolutegravir given alone was considered the reference treatment, and DTG coadministered with FPV-RTV was considered the test treatment.
Amprenavir PK parameters were compared with historical data without formal statistical comparison.
RESULTS
Twelve subjects were enrolled in the study, and all completed it as planned. The majority of subjects (58%) were male, and the mean age was 33.4 years. Fifty-eight percent of the subjects were of white/Caucasian/European heritage, with 25% of Asian descent and 17% of African-American descent.
Dolutegravir alone and in combination with FPV-RTV was well tolerated. No deaths or serious adverse events (AEs) occurred, and no subject was withdrawn during the study. The drug-related AEs are shown in Table 1. Rash and abnormal dreams were the most frequently reported drug-related AEs. Rash was reported by 2 subjects (17%) who were in the study group receiving both DTG and FPV-RTV. Abnormal dreams were reported by 2 subjects, 1 in each study period. Overall, more subjects reported AEs during coadministration of DTG combined with FPV-RTV (42%) than with DTG alone (17%). All AEs were mild in intensity (grade 1). There were no clinically significant trends in laboratory values, vital signs, or electrocardiograms.
TABLE 1.
Summary of drug-related AEs
| AE | Occurrence [n (%)] |
|
|---|---|---|
| 50 mg DTG (n = 12) | 50 mg DTG + FPV-RTV (n = 12) | |
| Any | 2 (17) | 5 (42) |
| Rash | 0 | 2 (17) |
| Abnormal dreams | 1 (8) | 1 (8) |
| Headache | 0 | 1 (8) |
| Insomnia | 1 (8) | 0 |
| Nausea | 0 | 1 (8) |
| Paraesthesia oral | 0 | 1 (8) |
The steady-state plasma exposure of DTG was reduced when concomitantly administered with FPV-RTV. Summaries of DTG PK parameters and statistical comparisons are presented in Tables 2 and 3. Figure 1 shows the mean plasma DTG concentration versus time profiles when DTG was administered alone and when DTG was coadministered with FPV-RTV. Following coadministration with FPV-RTV, the DTG AUC0–τ, Cmax, and Cτ were reduced by 35% (90% CI, 22% to 46%), 24% (90% CI, 8% to 37%), and 49% (90% CI, 37% to 59%), respectively. The dolutegravir CL/F increased by 53% (90% CI, 28% to 84%), and the t1/2 was reduced by 21% (90% CI, 15% to 26%), from 12.1 h (DTG alone) to 9.5 h (DTG coadministered with FPV-RTV).
TABLE 2.
Summary of selected plasma DTG pharmacokinetic parameters following repeat dose administrationa
| Treatment | n | Cmax (μg/ml) | Tmaxb (h) | AUC0–τ (μg · h/ml) | Cτ (μg/ml) | t1/2 (h) | CL/F (liters/h) |
|---|---|---|---|---|---|---|---|
| 50 mg DTG q24h | 12 | 2.53 ± 0.967 | 2.00 (1.0–4.0) | 32.9 ± 13.9 | 0.662 ± 0.316 | 12.3 ± 2.48 | 1.73 ± 0.587 |
| 50 mg DTG q24h + 700/100 mg FPV-RTV q12h | 12 | 2.07 ± 1.19 | 2.00 (1.0–4.0) | 22.6 ± 11.8 | 0.358 ± 0.189 | 9.86 ± 2.99 | 2.85 ± 1.55 |
AUC0–τ, area under the concentration-time curve from when the dose is administered until the end of the dosing interval; CL/F, apparent oral clearance; Cmax, maximum plasma concentration; Cτ, plasma concentration at the end of the dosing interval; q12h, every 12 h; q24h, every 24 h; t1/2, apparent terminal half-life; Tmax, time to Cmax. The values are shown as means ± standard deviations unless otherwise indicated.
Median (range).
TABLE 3.
Summary of DTG comparisons with and without FPV-RTV
| Plasma DTG PK parametera | DTG + FPV-RTV vs DTG alone [GLS mean ratio (90% CI)] (n = 12) |
|---|---|
| AUC0–τ | 0.651 (0.542–0.782) |
| Cmax | 0.763 (0.632–0.921) |
| Cτ | 0.510 (0.413–0.629) |
| CL/F | 1.534 (1.276–1.843) |
| t1/2 | 0.791 (0.735–0.851) |
AUC0–τ, area under the concentration-time curve from when the dose is administered until the end of the dosing interval; CL/F, apparent oral clearance; Cmax, maximum plasma concentration; Cτ, plasma concentration at the end of the dosing interval; t1/2, apparent terminal half-life.
FIG 1.
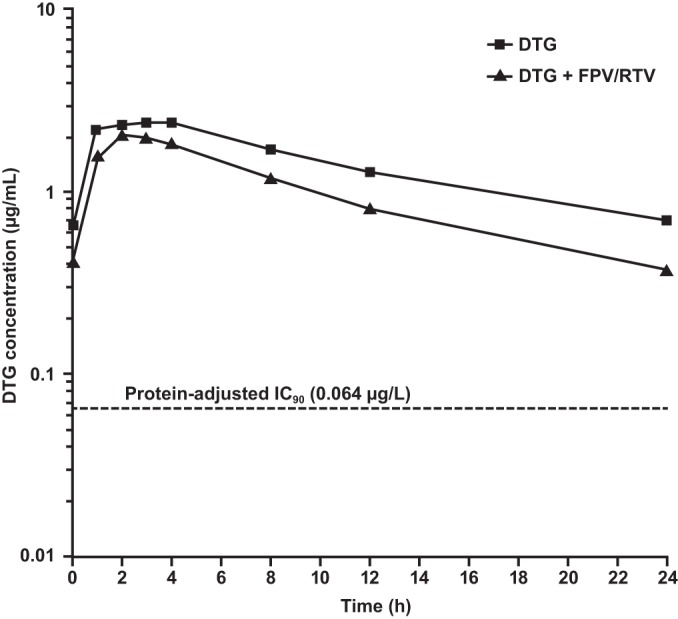
Mean DTG pharmacokinetic profile (n = 12) with and without FPV-RTV coadministration. IC90, in vitro protein-binding-adjusted 90% inhibitory concentration.
Table 4 lists the mean steady-state plasma PK of APV following coadministration of DTG and FPV-RTV. Amprenavir exposure following coadministration of DTG and FPV-RTV was similar to historical data, suggesting that DTG does not affect APV exposure (16, 17).
TABLE 4.
Summary of select plasma amprenavir pharmacokinetic parametersa
| Treatment | n | Cmax (μg/ml) | AUC0–τ (μg · h/ml) | Cτ (μg/ml) | C0 (μg/ml) | t1/2 (h) | Tmax (h)b |
|---|---|---|---|---|---|---|---|
| DTG + FPV-RTV | 12 | 6.57 ± 1.85 | 43.4 ± 16.4 | 2.18 ± 1.16 | 2.94 ± 1.29 | 9.16 ± 4.43 | 1.00 (0.50–2.00) |
| Historical FPV-RTV (16)d | 95 | 5.38 (5.06–5.73) | 34.8 (32.6–37.2) | 1.97 (1.83–2.72) | NAc | NA | |
| Historical FPV-RTV (17)d | 15 | 5.36 (4.57–6.30) | 35.9 (30.8–41.7) | 2.12 (1.75–2.56) | NA | NA |
Means ± standard deviations. AUC0–τ, area under the concentration-time curve from when the dose is administered until the end of the dosing interval; C0, plasma concentration immediately before dosing; Cmax, maximum plasma concentration; Cτ, plasma concentration at the end of the dosing interval; t1/2, apparent terminal half-life; Tmax, time to Cmax.
Median (range).
NA, not assessed.
Geometric mean (95% confidence interval).
DISCUSSION
Integrase inhibitors are a significant addition to the armamentarium of treatment options for people with HIV infection. Three INIs (DTG, RAL, and elvitegravir) are currently approved for the treatment of HIV-1-infected subjects in the United States and are valuable treatment options for HIV-infected patients. Dolutegravir possesses the advantages of not requiring RTV boosting and having a resistance profile that is different from those of other INIs (2). In phase III trials, DTG has demonstrated potency in treatment-experienced subjects, including those with resistance to other INIs (18, 19). Such patients might require concomitant FPV-RTV in the construction of a background regimen.
The results of this study showed that coadministration of DTG with FPV-RTV modestly reduced the steady-state plasma exposure of DTG. In addition, a cross-study comparison showed that DTG has no significant impact on APV exposure. The combination was well tolerated, with a low incidence of AEs. Two rashes were reported during coadministration of DTG and FPV-RTV. Because FPV-RTV is associated with a 19% incidence of rash, FPV-RTV is the likely etiology, although DTG cannot be excluded as a possible contributor (20). All AEs were mild in intensity, and all subjects completed the study.
The decrease in plasma DTG exposure by FPV-RTV was due to an increase in DTG clearance and a reduction in the t1/2. These results were expected, given the induction potential of both APV and RTV on UGT1A1 and CYP3A4, the metabolic pathway of DTG (7, 8). The effect of FPV-RTV observed in this study is consistent with the findings for another INI, RAL, which is also primarily metabolized by UGT1A1 and whose plasma exposure was reduced by FPV-RTV to a degree similar to that for DTG (11).
The decrease in DTG exposure by coadministration with FPV-RTV is not considered clinically significant, and DTG dose adjustment is not required on the basis of established no-effect boundaries (12). The dolutegravir Cτ, with an average of 0.358 μg/ml (Table 2) achieved when coadministered with FPV-RTV, is higher than the established lower bound of 0.3 μg/ml, and the GLS mean ratio (90% CI) of the DTG Cτ, 0.510 (0.413 to 0.629), is completely above 0.25. The clinical insignificance of the effect of FPV-RTV on DTG exposure is further supported by clinical efficacy data. In the phase III SAILING study in treatment-experienced and INI-naive subjects, 10 subjects received 50 mg DTG QD in combination with FPV-RTV, and 9 out of these 10 subjects demonstrated viral suppression (<50 copies/ml) at week 48 (12). Such data, although limited, were important in demonstrating that the antiviral activity of DTG is not compromised with modestly reduced exposure when coadministered with FPV-RTV. These data supported the recommendation by the European Medicines Agency not to adjust the dose of DTG when coadministered with FPV-RTV (21). In the United States, a more conservative approach is taken by the Food and Drug Administration because of the limited size of the clinical data set, and DTG is currently recommended to be given at 50 mg BID when coadministered with FPV-RTV (22). In the INI-resistant population, as a cautionary measure, alternative combinations that do not include FPV-RTV should be considered (22).
The 50-mg QD dose of DTG was chosen for this study because this dose is indicated for use in INI-naive subjects, representing the majority of the patient population to be treated with DTG. Dolutegravir is recommended to be dosed at 50 mg BID in subjects who are resistant to INIs. The effect of FPV-RTV on DTG exposure (AUC) from 50 mg BID is expected to be similar to that observed at 50 mg QD, because no evidence of enzyme saturation has been observed in DTG metabolism, and DTG showed linear PK from 50 mg QD to 50 mg BID (3, 21, 22). The effect of FPV-RTV on the DTG Cτ is expected to be smaller when DTG is given BID versus QD.
The 700/100-mg BID regimen of FPV-RTV was evaluated because this combination would most commonly be used with DTG in treatment-experienced patients. However, it is likely that other FPV doses would demonstrate similar or fewer effects. In a drug-drug interaction study with RAL (11), the 700/100-mg FPV-RTV BID dose decreased the RAL AUC by 54%, whereas the 1,400-mg BID and 1,400/100-mg QD doses decreased the RAL AUC by 29% and 30%, respectively. Thus, all FPV regimens would be expected to decrease the exposure of DTG, and the FPV-RTV BID dosing regimen would likely have the greatest effect on DTG exposure.
Dolutegravir was not expected to alter the plasma exposure of APV because DTG is not an inhibitor or an inducer of the P-glycoprotein, CYP3A4, or UGT1A1 metabolic pathways (7). Previous drug-drug interaction studies between DTG and protease inhibitors have reported no clinically significant alteration in the PK of TPV, atazanavir, darunavir, and lopinavir (4–6). The PK parameters of APV observed in the current study were similar to those presented in previously published data (16, 17). Therefore, the results from the current study are consistent with the lack of a significant effect of DTG on the CYP450 enzymes involved in the metabolism of protease inhibitors.
In conclusion, FPV-RTV modestly reduced DTG exposure when coadministered, as a result of net enzyme induction. On the basis of the established no-effect boundaries of DTG and the outcome of the SAILING study, DTG at 50 mg QD was an effective dose with FPV-RTV in INI-naive patients. In the INI-resistant population, as a cautionary measure, alternative combinations that do not include FPV-RTV should be considered.
ACKNOWLEDGMENTS
Funding for this work was provided by ViiV Healthcare.
Ivy Song, Julie Borland, Shuguang Chen, Amanda Peppercorn, and Stephen C. Piscitelli are employees of and own stock in GlaxoSmithKline. Toshihiro Wajima is an employee of Shionogi & Co., Inc.
We express our gratitude to John Fuchs for his assistance with the manuscript. We acknowledge Gina Uhlenbrauck and Clint Smith for editorial assistance during the development of the manuscript.
Footnotes
Published ahead of print 25 August 2014
REFERENCES
- 1.Arribas JR, Eron J. 2013. Advances in antiretroviral therapy. Curr. Opin. HIV AIDS 8:341–349. 10.1097/COH.0b013e328361fabd. [DOI] [PubMed] [Google Scholar]
- 2.Underwood MR, Johns BA, Sato A, Martin JN, Deeks SG, Fujiwara T. 2012. The activity of the integrase inhibitor dolutegravir against HIV-1 variants isolated from raltegravir-treated adults. J. Acquir. Immune Defic. Syndr. 61:297–301. 10.1097/QAI.0b013e31826bfd02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min S, Song I, Borland J, Chen S, Lou Y, Fujiwara T, Piscitelli SC. 2010. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob. Agents Chemother. 54:254–258. 10.1128/AAC.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song I, Borland J, Lou Y, Chen S, Patel P, Guta P, Wajima T, Peppercorn A, Piscitelli S. 2011. Effects of enzyme inducers, tipranavir and efavirenz, on the pharmacokinetics of the integrase inhibitor, dolutegravir (S/GSK1349572), abstr O_04. Abstr. 12th Int. Workshop Clin. Pharmacol. HIV Ther., Miami, FL. [Google Scholar]
- 5.Song I, Min SS, Borland J, Lou Y, Chen S, Patel P, Ishibashi T, Piscitelli SC. 2011. The effect of lopinavir/ritonavir and darunavir/ritonavir on the HIV integrase inhibitor S/GSK1349572 in healthy participants. J. Clin. Pharmacol. 51:237–242. 10.1177/0091270010371113. [DOI] [PubMed] [Google Scholar]
- 6.Song I, Borland J, Chen S, Lou Y, Peppercorn A, Wajima T, Min S, Piscitelli SC. 2011. Effect of atazanavir and atazanavir/ritonavir on the pharmacokinetics of the next-generation HIV integrase inhibitor, S/GSK1349572. Br. J. Clin. Pharmacol. 72:103–108. 10.1111/j.1365-2125.2011.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, Webster LO, Harmon KA, Clarke JD, Polli JW. 2013. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab. Dispos. 41:353–361. 10.1124/dmd.112.048918. [DOI] [PubMed] [Google Scholar]
- 8.Castellino S, Moss L, Wagner D, Borland J, Song I, Chen S, Lou Y, Min SS, Goljer I, Culp A, Piscitelli SC, Savina PM. 2013. Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor, dolutegravir, in humans. Antimicrob. Agents Chemother. 57:3536–3546. 10.1128/AAC.00292-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foisy MM, Yakiwchuk EM, Hughes CA. 2008. Induction effects of ritonavir: implications for drug interactions. Ann. Pharmacother. 42:1048–1059. 10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- 10.Brüggemann RJ, van Luin M, Colbers EP, van den Dungen MW, Pharo C, Schouwenberg BJ, Burger DM. 2010. Effect of posaconazole on the pharmacokinetics of fosamprenavir and vice versa in healthy volunteers. J. Antimicrob. Chemother. 65:2188–2194. 10.1093/jac/dkq280. [DOI] [PubMed] [Google Scholar]
- 11.Luber A, Slowinski D, Acosta E, Pakes G, Pappa K, Condoluci D. 2009. Steady-state pharmacokinetics (PK) of fosamprenavir (FPV) and raltegravir (RAL) alone and combined with unboosted and ritonavir-boosted FPV, abstr A1-1297 Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA. [Google Scholar]
- 12.Song I, Chen S, Piscitelli S, Min S. 2003. Pharmacokinetics (PK) and PK-pharmacodynamic (PD) relationship of dolutegravir (DTG) in integrase inhibitor (INI)–naive subjects, abstr A-1573 Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother., Denver, CO. [Google Scholar]
- 13.European Medicines Agency (EMA). 2012. Guideline on the investigation of drug interactions. CPMP/EWP/560/95/Rev. 1. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf. Accessed 7 August 2014. [Google Scholar]
- 14.Patel P, Song I, Borland J, Patel A, Lou Y, Chen S, Wajima T, Peppercorn A, Min SS, Piscitelli SC. 2011. Pharmacokinetics of the HIV integrase inhibitor S/GSK1349572 co-administered with acid-reducing agents and multivitamins in healthy volunteers. J. Antimicrob. Chemother. 66:1567–1572. 10.1093/jac/dkr139. [DOI] [PubMed] [Google Scholar]
- 15.SAS Institute Inc. 2002. The NLP procedure, 2005, SAS OnlineDoc 9.1.3, SAS Institute Inc., Cary, NC: http://support.sas.com/onlinedoc/913/ Accessed 7 May 2014. [Google Scholar]
- 16.Ford SL, Chen Y-C, Lou Y, Borland J, Min SS, Yuen GJ, Shelton MJ. 2008. Pharmacokinetic interaction between fosamprenavir-ritonavir and rifabutin in healthy subjects. Antimicrob. Agents Chemother. 52:534–538. 10.1128/AAC.00724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wire MB, Ballow CH, Borland J, Shelton MJ, Lou Y, Yuen G, Lin J, Lewis EW. 2007. Fosamprenavir plus ritonavir increases plasma ketoconazole and ritonavir exposure, while amprenavir exposure remains unchanged. Antimicrob. Agents Chemother. 51:2982–2984. 10.1128/AAC.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, Richmond G, Buendia CB, Fourie J, Ramgopal M, Hagins D, Felizarta F, Madruga J, Reuter T, Newman T, Small CB, Lombaard J, Grinsztejn B, Dorey D, Underwood M, Griffith S, Min S, SAILING Study Team 2013. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 382:700–708. 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 19.Nichols G, Mills A, Grossberg R, Lazzarin A, Maggiolo F, Molina J-M, Pialoux G, Wright D, Ait-Khaled M, Huang J, Vavro C, Wynne B, Yeo J. 2012. Antiviral activity of dolutegravir in subjects with failure on an integrase inhibitor-based regimen: week 24 phase 3 results from VIKING-3. J. Int. AIDS Soc. 15(Suppl 4):18112. [Google Scholar]
- 20.ViiV Healthcare. 2013. Lexiva product information. ViiV Healthcare, Research Triangle Park, NC. [Google Scholar]
- 21.ViiV Healthcare. 2013. Tivicay summary of product characteristics (EU). ViiV Healthcare, Research Triangle Park, NC. [Google Scholar]
- 22.ViiV Healthcare. 2013. Tivicay prescribing information (US). ViiV Healthcare, Research Triangle Park, NC. [Google Scholar]


