Abstract
The emergence of the New Delhi metallo-β-lactamase (NDM) among Enterobacteriaceae has become a global concern because of its high levels of in vitro resistance to nearly all available antibiotics. However, recent in vivo studies demonstrated the efficacies of carbapenems against NDM-1-producing isolates despite high MICs. Herein, we report in vivo findings with ceftazidime and ceftazidime-avibactam against an isogenic pair (wild type and NDM-1) and four clinical NDM-producing isolates that demonstrate discordance between MICs measured in vitro and the in vivo activity of ceftazidime-avibactam against this resistant genotype.
TEXT
Over the past decade, Gram-negative bacteria that produce carbapenemases, enzymes that efficiently hydrolyze carbapenems and other β-lactams, have emerged throughout the United States and globally (1, 2). More recently, and increasingly troublesome, the carbapenemase New Delhi metallo-β-lactamase (NDM) has been a more common occurrence. This is a challenging genotype for clinicians to treat, as few antimicrobial agents maintain in vitro potency against it, and novel agents with activity against it have yet to become available (3, 4). Moreover, agents stable to NDM hydrolysis (i.e., aztreonam) are often hydrolyzed by other β-lactamases (i.e., CTX-M type, CMY type, etc.) that are frequently coproduced by NDM-producing strains (4). While the novel non-β-lactam β-lactamase inhibitor avibactam has been shown to restore the in vitro potency of ceftazidime against Ambler class A and C (and some class D) β-lactamases, the combination of ceftazidime and avibactam displays high MICs in vitro against Enterobacteriaceae isolates that produce metallo-β-lactamases, such as NDM (5–7). Of note, previous work conducted by our group with carbapenems raised a question about the potencies of these NDM enzymes in vivo (8, 9). Contrary to the observed in vitro resistance, human simulated doripenem and ertapenem regimens demonstrated in vivo efficacies against isogenic and clinical NDM-1-producing isolates in a murine thigh infection model. The aim of the current study was to further elucidate the impact of NDM-type metallo-β-lactamase production on the in vivo efficacies of humanized exposures of ceftazidime-avibactam and ceftazidime alone.
Commercially available ceftazidime for injection (Sandoz, Inc.) was used for all in vivo experimentation. Analysis-grade avibactam was supplied by AstraZeneca Pharmaceuticals. Drug-dosing solutions were diluted in 0.9% normal saline (NS), stored refrigerated until the time of use, and discarded after 24 h.
Six Enterobacteriaceae isolates (3 Escherichia coli and 3 Klebsiella pneumoniae) were used for the in vivo studies, including a wild-type K. pneumoniae strain (454), a derived isogenic strain harboring an NDM-1 plasmid (10, 11), and four other clinical NDM-producing strains. Ceftazidime and ceftazidime-avibactam MICs were determined in quintuplicate by broth microdilution in accordance with CLSI guidelines (12). Additionally, the MIC of avibactam alone was determined for each isolate.
The protocol was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. The well-described murine neutropenic thigh infection model employed by our group, based on early work by Harry Eagle (13, 14), was used to determine efficacy. Briefly, pathogen-free female ICR mice weighing approximately 20 to 22 g were rendered transiently neutropenic with 100- and 150-mg/kg of body weight intraperitoneal injections of cyclophosphamide given 1 and 4 days prior to inoculation, respectively. Three days prior to inoculation, the mice were given a single 5-mg/kg intraperitoneal injection of uranyl nitrate. To verify β-lactamase production, the zone diameter of inhibition was determined on a disc (BD Sensi-Disc) containing ceftazidime (30 μg) on a lawn growth of each isolate made from the same plate used for inoculum preparation.
The thigh of each mouse was inoculated with a 0.1-ml solution containing approximately 107 CFU/ml; 2 h later, groups of three mice were administered 2,000 mg of human simulated regimens of ceftazidime every 8 h (2-h infusion) or 2,000/500 mg of ceftazidime-avibactam every 8 h (2-h infusion) for 24 h as previously described by our group for the murine thigh infection model (13). Pharmacokinetic studies were performed and confirmed that the concentrations we obtained were similar to those previously reported (data not shown). Control animals were administered normal saline at the same volume, route, and frequency as those of the ceftazidime regimen. Untreated control mice were sacrificed just prior to the initiation of therapy (0 h), while the treatment and control mice were sacrificed 24 h after the initiation of therapy. Serial dilutions of thigh homogenate were plated for the determination of bacterial densities. Efficacy, defined as the change in bacterial density, was calculated as the change in log10 CFU/ml obtained for treated mice after 24 h compared with that obtained for the 0-h controls.
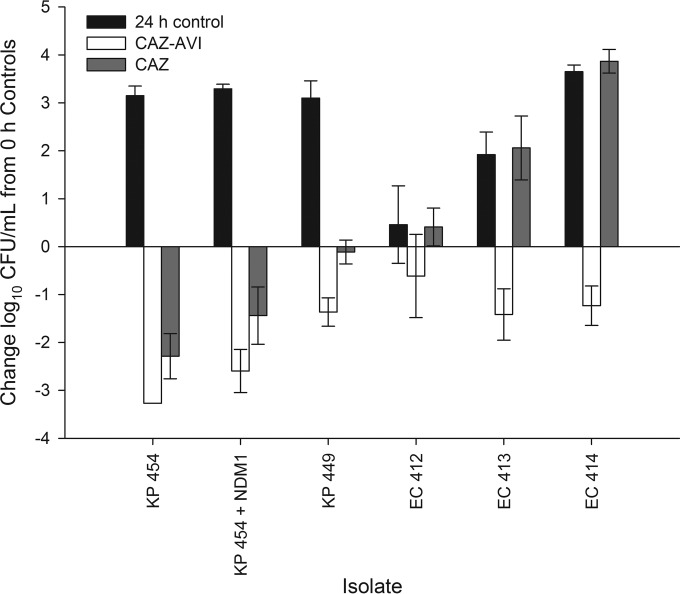
The phenotypic and genotypic profiles of the 6 Enterobacteriaceae isolates are listed in Table 1. The ceftazidime and ceftazidime-avibactam MICs for all of the NDM-producing isolates were >128 μg/ml. The mean (± standard deviation) bacterial density for the 0-h control mice at the start of dosing was 5.96 ± 0.20 log10 CFU/ml, and it increased to 8.49 ± 1.35 log10 CFU/ml after 24 h. As anticipated, the ceftazidime and ceftazidime-avibactam regimens resulted in a >2-log reduction against the wild-type strain (K. pneumoniae 454) (Fig. 1). Unexpectedly, CAZ and CAZ-AVI resulted in 1.4- and 2.6-log reductions against the isogenic strain with the NDM-1 plasmid (K. pneumoniae 454 plus NDM-1 plasmid), respectively. Against the four clinical NDM-producing isolates, ceftazidime-avibactam produced 0.61- to 1.42-log reductions in antibacterial activity, while ceftazidime alone failed to show activity against three isolates and showed modest activity (0.11-log reduction) against K. pneumoniae 449. To confirm these observations, ceftazidime-avibactam efficacy studies were repeated for the four clinical isolates, and the results were nearly identical (reported as combined data).
TABLE 1.
Phenotypic and genotypic profiles of the six Enterobacteriaceae isolates utilized in the in vivo efficacy studies
| Isolate | Known β-lactamase content (8,16) | AVI MIC (μg/ml)a | Modal MIC (μg/ml)b |
|
|---|---|---|---|---|
| CAZ-AVI | CAZ | |||
| K. pneumoniae 454 | SHV-1 | >64 | ≤0.125 | 0.25 |
| K. pneumoniae 454 and NDM-1 plasmid | SHV-1, NDM-1 | >64 | >128 | >128 |
| K. pneumoniae 449 | CTX-M-15, NDM type, OXA-1, TEM-1 | >64 | >128 | >128 |
| E. coli 412 | CTX-M-15, NDM-6 | 16 | >128 | >128 |
| E. coli 413 | NDM-1, SHV-12, TEM-1 | 16 | >128 | >128 |
| E. coli 414 | CTX-M-15, NDM type, OXA-1 | 16 | >128 | >128 |
AVI, avibactam.
CAZ-AVI, ceftazidime-avibactam; CAZ, ceftazidime.
FIG 1.
Comparative efficacies of human simulated regimens of ceftazidime-avibactam (CAZ-AVI) and ceftazidime (CAZ) alone against a collection of clinical NDM-producing Enterobacteriaceae and an isogenic NDM-1 pair in the neutropenic murine thigh infection model. Bars represent mean ± standard deviation of the results determined for 10 to 11 (CAZ-AVI) or 5 to 6 (CAZ) thighs per treatment group. The lower limit of detection (2.6 log10 CFU/ml) was observed for the CAZ-AVI treatment group against K. pneumoniae 454. KP, Klebsiella pneumoniae; EC, Escherichia coli.
Through the use of an isogenic pair and four genotypically characterized clinical isolates, we were able to ascertain the efficacy of the ceftazidime-avibactam regimen against NDM-producing Enterobacteriaceae in a murine thigh infection model. A moderate reduction in bacterial density was demonstrated for ceftazidime alone against the isogenic NDM strain despite an fT>MIC (percentage of the dosing interval during which free drug concentrations exceed the MIC) of 0%. Similar to our previous studies, these data suggest that the in vivo effectiveness of this enzyme in reducing the antibacterial activity of the compound is discordant with the observations derived from in vitro MIC testing (8, 9). However, against the four clinical NDM-producing strains, all of which were tested with in vitro ceftazidime MIC values of >128 μg/ml, ceftazidime failed to show activity, likely due to the coproduction of other β-lactamases (e.g., ESBLs, OXAs), which is consistent with previously published in vivo observations of other NDM-producing clinical isolates (4, 9). Conversely, the ceftazidime-avibactam regimen demonstrated activity against all five NDM-producing strains. While avibactam cannot inhibit NDM, the compound does inhibit class A and class C β-lactamases (5, 15); thus, it is hypothesized that this observed in vivo potency signifies that the lone presence of NDM does not in and of itself result in in vivo resistance to human simulated combined pharmacokinetics of ceftazidime and avibactam. Similarly, ertapenem and doripenem were each shown to produce bacterial reductions against other NDM-producing Enterobacteriaceae clinical isolates despite unfavorable in vitro MICs (8, 9). Taken collectively, these data suggest discordance between in vitro potency and in vivo efficacy for certain β-lactams against NDM-producing Enterobacteriaceae. While the exact mechanism for the unexpected in vivo activity of ceftazidime-avibactam against NDM-producing isolates remains uncertain, a better understanding of this phenomenon might well have implications for the treatment of organisms with NDM-mediated resistance. For ceftazidime-avibactam, while an avibactam control was not utilized, the direct antibacterial activity of avibactam (with MICs of ≥16 μg/ml) is unlikely to explain the apparently discordant efficacies, as concentrations of avibactam have never reached 8 μg/ml in mice (13). Future in vitro experimentation on the hydrolyzing capability of the NDM-producing strains or purified NDM enzyme on ceftazidime or ceftazidime-avibactam may provide insight into the mechanism responsible for these observations. While high-level β-lactam resistance is an interesting and potentially important observation in the era of limited therapies for NDM-mediated resistance, in the absence of genotypic profiling, it may be important to consider that it may be due to enzyme-mediated (i.e., solely to NDM, other enzymes, or a combination of the two) and non-enzyme-mediated resistance mechanisms. Further studies are required to determine the mechanistic explanation for these findings and whether they can be applied in the clinical arena.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius, Henry Christensen, Jennifer Hull, Jami Jain, Lucinda Lamb, Sara Robinson, Debora Santini, Wonhee So, Christina Sutherland, and Pamela Tessier for their assistance with the animal experimentation and in vitro testing. We also thank Patrice Nordmann (INSERM U914, K. Bicêtre, France) for providing the isogenic NDM isolate.
This study was supported by AstraZeneca Pharmaceuticals, Inc. (Waltham, MA, USA) and Forest-Cerexa (a subsidiary of Actavis PLC).
D.P.N. has received research grants and honoraria from and participates in the advisory board for AstraZeneca, and W.W.N. is an employee of AstraZeneca. S.H.M and J.L.C. have no conflicts to declare.
Footnotes
Published ahead of print 15 September 2014
REFERENCES
- 1.Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 20:440–458. 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798. 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson AP, Woodford N. 2013. Global spread of antibiotic resistance: the example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J. Med. Microbiol. 62:499–513. 10.1099/jmm.0.052555-0. [DOI] [PubMed] [Google Scholar]
- 4.Bush K. 2013. Carbapenemases: partners in crime. J. Glob. Antimicrob. Resist. 1:7–16. 10.1016/j.jgar.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J. Antimicrob. Chemother. 64:326–329. 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagacé-Wiens PR, Tailor F, Simner P, DeCorby M, Karlowsky JA, Walkty A, Hoban DJ, Zhanel GG. 2011. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob. Agents Chemother. 55:2434–2437. 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 55:390–394. 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2013. Efficacy of humanized carbapenem exposures against New Delhi metallo-β-lactamase (NDM-1)-producing enterobacteriaceae in a murine infection model. Antimicrob. Agents Chemother. 57:3936–3940. 10.1128/AAC.00708-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiskirchen DE, Nordmann P, Crandon JL, Nicolau DP. 2014. In vivo efficacy of human simulated regimens of carbapenems and comparator agents against NDM-1-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 58:1671–1677. 10.1128/AAC.01946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girlich D, Poirel L, Carattoli A, Kempf I, Lartigue MF, Bertini A, Nordmann P. 2007. Extended-spectrum β-lactamase CTX-M-1 in Escherichia coli isolates from healthy poultry in France. Appl. Environ. Microbiol. 73:4681–4685. 10.1128/AEM.02491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391–397. 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically, 9th ed. Approved standard M07-A9. Clinical Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:6137–6146. 10.1128/AAC.00851-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eagle H. 1950. Effect of schedule of administration on therapeutic efficacy of penicillin: importance of aggregate time penicillin remains at effectively bactericidal levels. Am. J. Med. 9:280–299. 10.1016/0002-9343(50)90425-6. [DOI] [PubMed] [Google Scholar]
- 15.Ehmann DE, Jahic H, Ross PL, Gu R-F, Hu J, Durand-Réville TF, Lahiri S, Thresher J, Livchak S, Gao N, Palmer T, Walkup GK, Fisher SL. 2013. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J. Biol. Chem. 288:27960–27971. 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging Gram-negative organisms, including metallo-β-lactamase producers. Antimicrob. Agents Chemother. 57:3299–3306. 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]