Abstract
Food is now recognized as a natural resource of novel antimicrobial agents, including those that target the virulence mechanisms of bacterial pathogens. Iberin, an isothiocyanate compound from horseradish, was recently identified as a quorum-sensing inhibitor (QSI) of the bacterial pathogen Pseudomonas aeruginosa. In this study, we used a comparative systems biology approach to unravel the molecular mechanisms of the effects of iberin on QS and virulence factor expression of P. aeruginosa. Our study shows that the two systems biology methods used (i.e., RNA sequencing and proteomics) complement each other and provide a thorough overview of the impact of iberin on P. aeruginosa. RNA sequencing-based transcriptomics showed that iberin inhibits the expression of the GacA-dependent small regulatory RNAs RsmY and RsmZ; this was verified by using gfp-based transcriptional reporter fusions with the rsmY or rsmZ promoter regions. Isobaric tags for relative and absolute quantitation (iTRAQ) proteomics showed that iberin reduces the abundance of the LadS protein, an activator of GacS. Taken together, the findings suggest that the mode of QS inhibition in iberin is through downregulation of the Gac/Rsm QS network, which in turn leads to the repression of QS-regulated virulence factors, such as pyoverdine, chitinase, and protease IV. Lastly, as expected from the observed repression of small regulatory RNA synthesis, we also show that iberin effectively reduces biofilm formation. This suggests that small regulatory RNAs might serve as potential targets in the future development of therapies against pathogens that use QS for controlling virulence factor expression and assume the biofilm mode of growth in the process of causing disease.
INTRODUCTION
Quorum sensing (QS) is a cell-to-cell communication system widely distributed among bacteria in which small diffusible signal molecules are employed to regulate gene expression in a dose-dependent manner (1). After reaching a threshold concentration, the QS signal molecules will bind to and activate their receptors, which results in a coordinated population expression of QS-regulated genes. These genes include those that upregulate the synthesis of QS signal molecules (autoinduction) but, more importantly, they also include genes that encode virulence factors required for bacterial infections (2). Thus, QS inhibitors (QSIs) have been proposed as antipathogenic agents and have been shown to attenuate the capability of pathogens to cause infections (3, 4). QSIs possess different modes of action, including interfering with the synthesis of quorum-sensing signaling molecules (5) or competitively binding to the QS signal receptors (6). The regulation of the bacterial QS systems is complex, and this further expands the targets for the design of novel QSIs (7, 8).
Isothiocyanates (ITCs) are biologically active compounds found in cruciferous vegetables and have gained research interest as cancer chemopreventive agents (9). Sulforaphane (SFN) (10), allyl isothiocyanate (AITC) (11), and phenethyl isothiocyanate (PEITC) (12) are examples of ITCs with such cancer-preventing activities. In addition to their cancer-preventing activities, ITCs are also known for their antimicrobial activity (13, 14).
Recently, Jakobsen et al. (15) described several ITC-containing compounds (iberin, cheirolin, iberverin, and alyssin) found in various foods; a few of the tested food extracts were found to actively inhibit QS in Pseudomonas aeruginosa, particularly the las and rhl system. Among these ITCs, iberin (Fig. 1), which the authors identified as the QSI component of horseradish, had the greatest QS-inhibiting effect. Using a DNA microarray approach, iberin was found to inhibit 49 QS-controlled genes, including lasB, rhlAB, chiC, lecA, pivA, and phz. This study also suggested that iberin inhibits the rhl QS system (but not the las QS system) by blocking the interaction of N-butanoyl homoserine lactone (C4-HSL) with its cognate receptor RhlR. The mode of inhibition in iberin was speculated to occur through competition with acylhomoserine lactone (AHL) molecules for their receptor; however, iberin bears weak structural similarity to these molecules.
FIG 1.
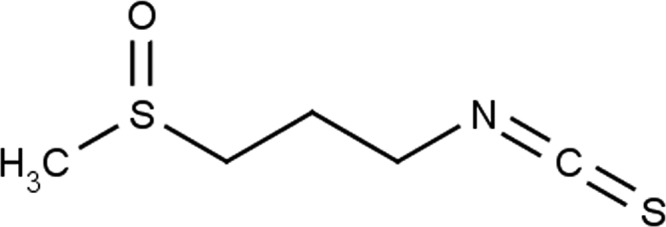
Chemical structure of the isothiocyanate compound 1-isothiocyanato-3-(methyl-sulfinyl) propane, otherwise known as iberin.
Therefore, we decided to elucidate the underlying mechanisms of the inhibition of QS by iberin in P. aeruginosa. With cRNA sequencing (RNA sequencing [RNA-Seq])-based transcriptomics and isobaric tags for relative and absolute quantitation (iTRAQ)-based proteomics approaches, we investigated the various genes and proteins affected by iberin treatment. We also show that iberin inhibits the expression of the small regulatory RNAs rsmY and rsmZ, which in turn regulate the levels of pyoverdine production (16). Hence, we propose that the mode of inhibition by iberin is through targeting the Gac/Rsm network in P. aeruginosa. Lastly, we show that iberin effectively inhibits P. aeruginosa biofilm formation. As such, iberin and ITCs therefore are an interesting class of QSIs with a novel mode of action, and the use of systems biology analyses provides insight for the development of dual functioning antivirulence and antibiofilm drugs.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. P. aeruginosa strain PAO1 (17) was used for all experiments. For marker selection in P. aeruginosa, 30 μg gentamicin (GEN) ml−1, 50 μg tetracycline (TET) ml−1, and 200 μg carbenicillin (CAR) ml−1 were used, as appropriate. Batch cultivation of P. aeruginosa was carried out at 37°C in ABT minimal medium (18) supplemented with 0.25% (wt/vol) glucose and 0.25% (wt/vol) Casamino Acids (ABTGC medium). P. aeruginosa cells were harvested at late-log phase for both the RNA-Seq and iTRAQ proteomic analyses.
TABLE 1.
Characteristics of the bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference |
|---|---|---|
| PAO1 | Prototypic wild-type strain | 17 |
| ΔpvdA | Isogenic pvdA mutant of PAO1 | 33 |
| PAO1 miniTn7-gfp | PAO1 carrying the miniTn7-gfp that constitutively expresses gfp | 33 |
| PAO1 lasB-gfp | PAO1 carrying the lasB-gfp fusion reporter | 39 |
| PAO1 rsmZ-gfp | PAO1 carrying the rsmZ-gfp fusion reporter | 30 |
| PAO1 rsmY-gfp | PAO1 carrying the rsmY-gfp fusion reporter | 30 |
| PAO1 pvdA-gfp | PAO1 carrying the pvdA-gfp fusion reporter | 33 |
A PAO1 suspension (optical density at 600 nm [OD600], 0.01) was added to a 24-well plate with or without 500 μM iberin. The ABTGC medium was used for culturing bacteria, and each well had a final volume of 1 ml. The plate was incubated at 200 rpm and 37°C. After reaching an OD600 of 0.5 (measured using an Infinite 200 Pro Series plate reader [Tecan], approximately after 3.5 h of incubation), the cultures were mixed immediately with 2 volumes of RNAprotect bacterial reagent (Qiagen). After 5 min of incubation at room temperature, the samples were centrifuged at 7,000 × g for 5 min at 4°C, the supernatant was removed, and the pellets were stored at −80°C.
RNA preparation.
Total RNA was extracted with the RNeasy Protect bacterial minikit with on-column DNase digestion, according to the manufacturer's instructions (Qiagen). The integrity of total RNA and DNA contamination was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies) and Qubit 2.0 fluorometer (Invitrogen). The 16S, 23S, and 5S rRNAs were removed using the Ribo-Zero magnetic kit (bacteria) (Epicentre).
RNA sequencing and data analysis.
Gene expression analysis (two biological replicates) was conducted by Illumina RNA sequencing (RNA-Seq technology). The rRNA-depleted RNA was fragmented to 200- to 300-bp fragments, and then first- and second-strand cDNA were synthesized, followed by end repair and adapter ligation. After 12 cycles of PCR enrichment, the quality of the libraries was assessed using the Bioanalyzer (Agilent Technologies, USA). The libraries were sequenced using the Illumina HiSeq 2000 platform with a paired-end protocol and read lengths of 100 nucleotides.
The sequence reads were assembled and analyzed in the RNA-Seq and expression analysis application of CLC Genomics Workbench 6.0 (CLC bio, Aarhus, Denmark). The PAO1 genome (http://www.ncbi.nlm.nih.gov/nuccore/110645304) was utilized as the reference genome for the assembly. The following criteria were used to filter the unique sequence reads: minimum length fraction of 0.9, minimum similarity fraction of 0.8, and maximum number of two mismatches. The data were normalized by calculating the reads per kb per million mapped reads (total reads/mapped reads in millions × gene length in kb) for each gene and annotated with PseudoCAP (see http://www.geneontology.org/GO.current.annotations.shtml). A t test was performed on the transformed data (0.5 was added to each number to deal with zero counts) to identify the genes with significant changes in expression (P < 0.05; fold change, >2.0 or less than −2.0) between the control and iberin-treated samples.
iTRAQ proteomics analyses.
The iTRAQ proteomics experiment was performed at the Proteomic Core Facility of the Biological Research Center, School of Biological Sciences, Nanyang Technological University, Singapore, and carried out as per our previous study (19).
Protein preparation and digestion.
P. aeruginosa PAO1 was grown in ABTG medium (AB minimal medium with 2.5 μg/ml thiamine and 0.5% glucose) with or without 500 μM iberin to late-log phase at 37°C, with shaking, after which the cells were harvested. After harvesting, the cell pellet was washed with 1× phosphate-buffered saline (PBS) and resuspended in 2 ml of lysis buffer containing 0.5 M triethyl ammonium bicarbonate (TEAB), 0.1 M SDS, and protease inhibitor cocktails (Sigma-Aldrich). The cells were ruptured by sonication, and the cell debris was removed by centrifugation at 16,000 × g and 4°C for 15 min. Three biological replicates for each growth condition were pooled, and 200 μg of proteins from each growth condition were dissolved in an equal volume of sample buffer (Invitrogen) supplemented with 0.5% 2-mercaptoethanol and denatured by boiling at 95°C for 5 min. One-dimensional (1D) gel electrophoresis was carried out using 10% SDS-PAGE for in-gel digestion.
The proteins were first reduced in 5 mM Tris-(2-carboxyethyl)phosphine (TCEP) for 1 h at 60°C, followed by blocking cysteine residues in 10 mM methyl methanethiosulfate (MMTS) for 30 min at room temperature in the dark. Trypsin was added at a ratio of 1:50 (trypsin to sample). It was then incubated at 37°C overnight. The tryptic peptides were extracted by 50% acetonitrile (ACN)–5% acetic acid from gel three times and were desalted using Sep-Pak C18 cartridges (Waters, Milford, MA) and dried in a SpeedVac (Thermo Electron, Waltham, MA). All chemicals were purchased from Sigma-Aldrich, unless stated otherwise.
iTRAQ labeling.
The iTRAQ labeling of the tryptic peptides was performed using a 4-plex iTRAQ reagent kit (Applied Biosystems, Foster City, CA), according to the manufacturer's protocol. Two hundred micrograms of peptides from each condition were individually labeled with respective isobaric tags (control sample, 114; iberin-treated sample, 115), followed by 2 h of incubation, quenching by water, desalting using C18 solid-phase extraction cartridge, and then vacuum centrifugation to dryness. The iTRAQ-labeled peptides were reconstituted in buffer A (10 mM ammonium acetate, 85% acetonitrile, 0.1% formic acid) and fractionated using an electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) column (200 by 4.6 mm, 5-μm particle size, 200-Å pore size) by a high-performance liquid chromatography (HPLC) system (Shimadzu, Japan) at flow rate of 1.0 ml/min, using our previously optimized protocol (20). The HP liquid chromatograms were recorded at 280 nm, and fractions were collected online using an automated fraction collector. Twenty fractions were collected and concentrated using a vacuum centrifuge and reconstituted in 3% ACN with 0.1% formic acid for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
LC-MS/MS.
The peptides were separated and analyzed on a home-packed nanopore C18 column (15 cm by 75 μm; ReproSil-Pur C18-AQ, 3 μm; Dr. Maisch, Germany) with a PicoFrit nanospray tip (New Objective, Woburn, MA, USA) on a Tempo nano-multidimensional LC (MDLC) system coupled with a QSTAR Elite hybrid LC-MS/MS system (Applied Biosystems). Peptides from each fraction were analyzed in triplicate by LC-MS/MS over a gradient of 90 min. The flow rate of the LC system was set to a constant 300 nl/min. Data acquisition in QSTAR Elite was set to positive ion mode using the Analyst QS 2.0 software (Applied Biosystems). MS data were acquired in positive ion mode with a mass range of 300 to 1,600 m/z. Peptides with +2 to +4 charge states were selected for MS/MS. For each MS spectrum, the three most abundant peptides above a five-count threshold were selected for MS/MS and dynamically excluded for 30 s, with a mass tolerance of 0.03 Da. Smart information-dependent acquisition was activated with automatic collision energy and automatic MS/MS accumulation. The fragment intensity multiplier was set to 20, and the maximum accumulation time was 2 s.
Data analysis.
The spectra acquired from the three technical replicates were submitted to ProteinPilot (version 3.0.0.0; Applied Biosystems) for peak-list generation, protein identification, and quantification. The user-defined parameters of the Paragon algorithm in the ProteinPilot software were configured as follows: (i) sample type, iTRAQ 2-plex (peptide labeled); (ii) cysteine alkylation, MMTS; (iii) digestion, trypsin; (iv) instrument, QSTAR Elite electrospray ionization (ESI); (v) special factors, urea denaturation; (vi) species, none; (vii) specify processing, quantitation and bias correction; (viii) identification (ID) focus, biological modifications and amino acid substitutions; (ix) database, P. aeruginosa PAO1 PAO1-UW; (x) search effort, thorough ID; and (xi) result quality, unused ProtScore (conf) of >0.05 (10.0%). The default precursor and MS/MS tolerance for the QSTAR ESI MS instrument were adopted automatically by the software. For iTRAQ analysis, the peptide for quantification was automatically selected by the Pro Group algorithm to calculate the reporter peak area, error factor (EF), and P value. The resulting data were auto-bias corrected by a built-in ProteinPilot algorithm to eliminate any variations imparted due to the unequal mixing during the combination of different labeled samples. During bias correction, the software identifies the median average protein ratio and corrects it to unity and then applies this factor to all quantitation results.
The following cutoffs were used for protein identification: an unused protein score of ≥2 (i.e., 99% confidence of identification) and having >1 peptide identified; in this way, a total of 1,948 proteins were identified. Using a P value cutoff of 0.05 and a fold change of at least +2.0 (for upregulation) and −2.0 (for downregulation), the abundances of 119 proteins were found to be significantly affected by iberin; the abundance of 27 proteins was upregulated, while the abundances of 92 proteins were downregulated (shown in Tables 2 and 3, respectively).
TABLE 2.
Genes/proteins found to be significantly upregulated upon iberin treatmenta
| Strain no. | Gene | QSb | Description of productc | RNA-seq (500 μM) |
iTRAQ proteomics (500 μM) |
||
|---|---|---|---|---|---|---|---|
| Fold changed | P value | Fold changed | P value | ||||
| PA0140 | ahpF | Alkyl hydroperoxide reductase subunit F | 4.85 | <0.01 | |||
| PA0182 | Probable short-chain dehydrogenase | 7.6 | 0.04 | ||||
| PA0185 | Probable permease of ABC transporter | 8.9 | 0.04 | ||||
| PA0201 | Hypothetical protein | 25.9 | 0.01 | ||||
| PA0202 | Probable amidase | 20.7 | 0.03 | ||||
| PA0283 | sbp | Sulfate-binding protein precursor | 26.0 | 0.01 | |||
| PA0284 | Hypothetical protein | 32.9 | 0.04 | ||||
| PA0565 | Conserved hypothetical protein | 2.91 | <0.01 | ||||
| PA0849 | trxB2 | Thioredoxin reductase 2 | 2.94 | 0.01 | |||
| PA0865 | hpd | 4-Hydroxyphenylpyruvate dioxygenase | 42.2 | 0.04 | 2.75 | <0.01 | |
| PA0878 | Hypothetical protein | 7.9 | 0.04 | ||||
| PA1240 | Probable enoyl-CoA hydratase/isomerase | 3.45 | <0.01 | ||||
| PA1260 | Amino acid ABC transporter periplasmic-binding protein | 4.49 | <0.01 | ||||
| PA1285 | Probable transcriptional regulator | 5.2 | 0.05 | ||||
| PA1310 | phnW | 2-Aminoethylphosphonate–pyruvate aminotransferase | 19.3 | 0.05 | |||
| PA1332 | Hypothetical protein | 17.7 | 0.05 | ||||
| PA1334 | Probable oxidoreductase | 74.7 | 0.04 | 5.74 | <0.01 | ||
| PA1493 | cysP | Sulfate-binding protein of ABC transporter | 8.2 | <0.01 | |||
| PA1999 | dhcA | DhcA, dehydrocarnitine CoA transferase, subunit A | 9.1 | 0.01 | |||
| PA2000 | dhcB | DhcB, dehydrocarnitine CoA transferase, subunit B | 9.5 | <0.01 | |||
| PA2008 | fahA | Fumarylacetoacetase | 54.8 | 0.03 | |||
| PA2009 | hmgA | Homogentisate 1,2-dioxygenase | 62.0 | 0.02 | |||
| PA2197 | Conserved hypothetical protein | 2.07 | <0.01 | ||||
| PA2247 | bkdA1 | 2-Oxoisovalerate dehydrogenase (alpha subunit) | 2.71 | <0.01 | |||
| PA2248 | bkdA2 | 2-Oxoisovalerate dehydrogenase (beta subunit) | 2.98 | <0.01 | |||
| PA2249 | bkdB | Branched-chain alpha-keto acid dehydrogenase (lipoamide component) | 2.64 | <0.01 | |||
| PA2277 | arsR | ArsR protein | 15.3 | 0.05 | |||
| PA2278 | arsB | ArsB protein | 9.6 | 0.02 | |||
| PA2310 | Hypothetical protein | 20.5 | 0.03 | ||||
| PA2311 | Hypothetical protein | 51.8 | 0.05 | ||||
| PA2312 | Probable transcriptional regulator | 29.6 | <0.01 | ||||
| PA2327 | X | Probable permease of ABC transporter | 6.6 | 0.05 | |||
| PA2359 | Probable transcriptional regulator | 34.5 | 0.03 | ||||
| PA2444 | glyA2 | Serine hydroxymethyltransferase | 37.4 | 0.05 | |||
| PA2445 | gcvP2 | Glycine cleavage system protein P2 | 3.67 | <0.01 | |||
| PA2483 | Conserved hypothetical protein | 3.30 | <0.01 | ||||
| PA2490 | Conserved hypothetical protein | 16.4 | 0.05 | ||||
| PA2491 | mexS | MexS | 34.1 | 0.03 | 5.08 | 0.01 | |
| PA2493 | mexE | RND multidrug efflux membrane fusion protein MexE precursor | 5.69 | <0.01 | |||
| PA2494 | mexF | RND multidrug efflux transporter MexF | 129.4 | 0.04 | 5.69 | <0.01 | |
| PA2495 | oprN | Multidrug efflux outer membrane protein OprN precursor | 102.3 | 0.03 | 5.34 | <0.01 | |
| PA2535 | Probable oxidoreductase | 3.02 | <0.01 | ||||
| PA2575 | Hypothetical protein | 28.5 | 0.02 | 4.01 | <0.01 | ||
| PA2579 | kynA | l-Tryptophan:oxygen 2,3-oxidoreductase (decyclizing) KynA | 5.3 | 0.05 | |||
| PA2580 | Conserved hypothetical protein | 26.1 | 0.01 | 4.58 | 0.01 | ||
| PA2594 | X | Conserved hypothetical protein | 9.0 | 0.02 | |||
| PA2599 | Conserved hypothetical protein | 12.1 | 0.05 | ||||
| PA2600 | Hypothetical protein | 21.0 | <0.01 | ||||
| PA2610 | Conserved hypothetical protein | 5.2 | 0.03 | ||||
| PA2758 | Probable transcriptional regulator | 63.7 | 0.02 | ||||
| PA2759 | Hypothetical protein | 179.3 | 0.01 | ||||
| PA2767 | Probable enoyl-CoA hydratase/isomerase | 5.8 | 0.03 | ||||
| PA2786 | Hypothetical protein | 8.6 | 0.05 | ||||
| PA2812 | Probable ATP-binding component of ABC transporter | 20.4 | 0.03 | ||||
| PA2813 | Probable glutathione S-transferase | 32.7 | 0.04 | ||||
| PA2844 | Conserved hypothetical protein | 8.3 | 0.04 | ||||
| PA2845 | Hypothetical protein | 91.2 | 0.04 | ||||
| PA2931 | cifR | CifR | 10.4 | 0.03 | |||
| PA2932 | morB | Morphinone reductase | 45.7 | 0.03 | |||
| PA3035 | Probable glutathione S-transferase | 8.0 | <0.01 | ||||
| PA3126 | ibpA | Heat shock protein IbpA | 4.37 | <0.01 | |||
| PA3222 | Hypothetical protein | 5.4 | 0.04 | ||||
| PA3229 | Hypothetical protein | 311.5 | 0.04 | ||||
| PA3230 | Conserved hypothetical protein | 69.5 | 0.01 | ||||
| PA3938 | Probable periplasmic taurine-binding protein precursor | 24.5 | 0.05 | ||||
| PA3446 | Conserved hypothetical protein | 63.5 | 0.05 | ||||
| PA3450 | Probable antioxidant protein | 39.4 | 0.01 | ||||
| PA3931 | Conserved hypothetical protein | 44.7 | 0.05 | ||||
| PA4166 | Probable acetyltransferase | 77.6 | 0.02 | ||||
| PA4167 | dkgB | Probable oxidoreductase | 112.0 | <0.01 | 4.93 | <0.01 | |
| PA4173 | Conserved hypothetical protein | 12.4 | 0.01 | ||||
| PA4236 | katA | Catalase | 4.01 | <0.01 | |||
| PA4354 | Conserved hypothetical protein | ||||||
| PA4355 | pyeM | PyeM | 37.3 | <0.01 | |||
| PA4356 | xenB | Xenobiotic reductase | 4.28 | <0.01 | |||
| PA4385 | groEL | GroEL protein | 6.4 | 0.04 | 2.48 | <0.01 | |
| PA4386 | groES | GroES protein | 6.4 | 0.03 | |||
| PA4387 | Conserved hypothetical protein | 8.0 | 0.05 | ||||
| PA4613 | katB | Catalase | 5.10 | <0.01 | |||
| PA4623 | Hypothetical protein | 727.4 | 0.01 | ||||
| PA4973 | thiC | Thiamine biosynthesis protein ThiC | 2.58 | <0.01 | |||
Upregulation was defined as a >5-fold increase in the RNA-Seq result and a >2-fold increase in the iTRAQ proteomics result. Only genes/proteins showing a significant difference in expression from the control (i.e., P value < 0.05) were selected.
An “X” indicates that the gene/protein is regulated by quorum sensing (4), in reference to the QS-regulated genes/proteins, as determined previously.
CoA, coenzyme A; RND, resistance-nodulation-cell division.
Empty lines indicate no significant change in expression levels.
TABLE 3.
Genes/proteins found to be significantly downregulated upon iberin treatmenta
| Strain no. | Gene | QSb | Description of productc | RNA-seq (500 μM) |
iTRAQ proteomics (500 μM) |
||
|---|---|---|---|---|---|---|---|
| Fold changed | P value | Fold changed | P value | ||||
| PA0059 | osmC | X | Osmotically inducible protein OsmC | −2.91 | <0.01 | ||
| PA0081 | fha1 | Fha1 | −2.25 | <0.01 | |||
| PA0084 | tssC1 | TssC1 | −2.01 | <0.01 | |||
| PA0088 | tssF1 | TssF1 | −2.13 | <0.01 | |||
| PA0122 | rahU | X | RahU | −32.8 | 0.05 | −5.50 | <0.01 |
| PA0176 | aer2 | Aerotaxis transducer Aer2 | −3.67 | <0.01 | |||
| PA0180 | cttP | Chemotactic transducer for trichloroethylene (positive chemotaxis), CttP | −2.10 | <0.01 | |||
| PA0523 | norC | Nitric-oxide reductase subunit C | −10.9 | 0.04 | |||
| PA0575 | Conserved hypothetical protein | −2.18 | <0.01 | ||||
| PA0586 | Conserved hypothetical protein | −2.17 | <0.01 | ||||
| PA0587 | Conserved hypothetical protein | −2.38 | <0.01 | ||||
| PA0704 | Probable amidase | −5.21 | <0.01 | ||||
| PA0707 | toxR | Transcriptional regulator ToxR | −5.4 | 0.02 | |||
| PA0852 | cbpD | X | Chitin-binding protein CbpD precursor | −22.0 | 0.04 | −4.92 | <0.01 |
| PA1001 | phnA | X | Anthranilate synthase component I | −7.2 | 0.01 | ||
| PA1027 | Probable aldehyde dehydrogenase | −3.22 | <0.01 | ||||
| PA1127 | Probable oxidoreductase | −2.09 | <0.01 | ||||
| PA1130 | rhlC | X | Rhamnosyltransferase 2 | −11.7 | 0.04 | ||
| PA1131 | X | Probable MFS transporter | −8.9 | 0.05 | |||
| PA1134 | Hypothetical protein | −6.0 | 0.01 | ||||
| PA1214 | Hypothetical protein | −8.2 | 0.03 | ||||
| PA1215 | Hypothetical protein | −7.7 | 0.02 | ||||
| PA1216 | Hypothetical protein | −9.0 | 0.04 | ||||
| PA1220 | Hypothetical protein | −5.4 | 0.03 | ||||
| PA1221 | Hypothetical protein | −6.8 | 0.04 | ||||
| PA1245 | Hypothetical protein PA1245 | −5.46 | <0.01 | ||||
| PA1246 | aprD | X | Alkaline protease secretion protein AprD | −2.43 | <0.01 | ||
| PA1247 | aprE | X | Alkaline protease secretion protein AprE | −2.99 | <0.01 | ||
| PA1248 | aprF | X | Alkaline protease secretion outer membrane protein AprF precursor | −3.84 | <0.01 | ||
| PA1249 | aprA | X | Alkaline metalloproteinase precursor | −3.99 | <0.01 | ||
| PA1300 | Probable sigma-70 factor, ECF subfamily | −5.5 | 0.03 | ||||
| PA1318 | cyoB | Cytochrome o ubiquinol oxidase subunit I | −2.15 | <0.01 | |||
| PA1323 | X | Hypothetical protein | −6.9 | 0.03 | |||
| PA1324 | X | Hypothetical protein | −7.7 | 0.05 | −3.96 | <0.01 | |
| PA1327 | Probable protease | −3.80 | <0.01 | ||||
| PA1408 | Hypothetical protein PA1408 | −2.31 | <0.01 | ||||
| PA1529 | ligA | DNA ligase | −2.03 | <0.01 | |||
| PA1559 | Hypothetical protein | −7.3 | 0.03 | ||||
| PA1560 | Hypothetical protein | −8.9 | 0.02 | ||||
| PA1705 | pcrG | Regulator in type III secretion | −7.3 | 0.03 | |||
| PA1706 | pcrV | Type III secretion protein PcrV | −6.1 | 0.03 | |||
| PA1708 | popB | Translocator protein PopB | −6.7 | 0.03 | |||
| PA1710 | exsC | ExsC, exoenzyme S synthesis protein C precursor. | −6.6 | 0.01 | |||
| PA1730 | Conserved hypothetical protein | −3.06 | <0.01 | ||||
| PA1871 | lasA | X | LasA protease precursor | −25.5 | 0.02 | ||
| PA1875 | X | Probable outer membrane protein precursor | −5.1 | 0.04 | |||
| PA1906 | Hypothetical protein | −20.4 | 0.01 | ||||
| PA1912 | femI | ECF sigma factor, FemI | −5.1 | 0.01 | |||
| PA1914 | Conserved hypothetical protein | −14.0 | 0.02 | ||||
| PA1927 | metE | 5-Methyltetrahydropteroyltriglutamate–homocysteine S-methyltransferase | −3.19 | <0.01 | |||
| PA1930 | Probable chemotaxis transducer | −2.66 | <0.01 | ||||
| PA2067 | Probable hydrolase | −16.4 | 0.04 | ||||
| PA2068 | X | Probable MFS transporter | −37.5 | 0.02 | |||
| PA2069 | X | Probable carbamoyl transferase | −107.8 | 0.02 | −5.97 | <0.01 | |
| PA2072 | Conserved hypothetical protein | −2.48 | <0.01 | ||||
| PA2144 | glgP | Glycogen phosphorylase | −2.55 | <0.01 | |||
| PA2151 | Conserved hypothetical protein | −3.18 | <0.01 | ||||
| PA2152 | Probable trehalose synthase | −4.32 | <0.01 | ||||
| PA2153 | glgB | 1,4-α-Glucan branching enzyme | −3.44 | <0.01 | |||
| PA2159 | Conserved hypothetical protein | −5.4 | 0.04 | ||||
| PA2160 | Probable glycosyl hydrolase | −4.85 | <0.01 | ||||
| PA2162 | Probable glycosyl hydrolase | −3.89 | <0.01 | ||||
| PA2163 | Hypothetical protein PA2163 | −3.32 | 0.03 | ||||
| PA2164 | Probable glycosyl hydrolase | −2.15 | 0.01 | ||||
| PA2167 | Hypothetical protein | −5.3 | 0.02 | ||||
| PA2169 | Hypothetical protein | −6.7 | 0.03 | ||||
| PA2170 | Hypothetical protein | −9.1 | 0.04 | ||||
| PA2177 | Probable sensor/response regulator hybrid | −2.35 | <0.01 | ||||
| PA2193 | hcnA | X | Hydrogen cyanide synthase HcnA | −27.7 | 0.05 | ||
| PA2194 | hcnB | X | Hydrogen cyanide synthase HcnB | −15.9 | 0.01 | ||
| PA2195 | hcnC | X | Hydrogen cyanide synthase HcnC | −12.6 | <0.01 | ||
| PA2261 | Probable 2-ketogluconate kinase | −3.08 | 0.04 | ||||
| PA2300 | chiC | X | Chitinase | −29.9 | 0.03 | −5.71 | <0.01 |
| PA2305 | ambB | X | AmbB | −2.30 | <0.01 | ||
| PA2322 | Gluconate permease | −11.4 | 0.02 | ||||
| PA2345 | Conserved hypothetical protein | −2.60 | <0.01 | ||||
| PA2363 | Hypothetical protein PA2363 | −2.41 | 0.01 | ||||
| PA2371 | Probable ClpA/B-type protease | −2.78 | <0.01 | ||||
| PA2377 | Hypothetical protein | −8.5 | 0.05 | ||||
| PA2384 | Hypothetical protein | −7.4 | <0.01 | ||||
| PA2414 | X | l-Sorbosone dehydrogenase | −10.5 | 0.02 | |||
| PA2415 | X | Hypothetical protein | −8.6 | 0.02 | |||
| PA2416 | treA | Periplasmic trehalase precursor | −2.79 | 0.04 | |||
| PA2427 | Hypothetical protein | −6.9 | 0.01 | ||||
| PA2448 | Hypothetical protein PA2448 | −3.16 | <0.01 | ||||
| PA2566 | X | Conserved hypothetical protein | −5.9 | 0.04 | |||
| PA2570 | lecA | X | LecA | −38.9 | 0.03 | ||
| PA2573 | Probable chemotaxis transducer | −2.94 | <0.01 | ||||
| PA2587 | pqsH | X | Probable FAD-dependent monooxygenase | −2.19 | <0.01 | ||
| PA2588 | X | Probable transcriptional regulator | −8.9 | 0.02 | |||
| PA2717 | cpo | Chloroperoxidase precursor | −2.88 | 0.01 | |||
| PA2788 | Probable chemotaxis transducer | −5.8 | 0.03 | −2.64 | <0.01 | ||
| PA2815 | fadE | Probable acyl-CoA dehydrogenase | −2.51 | <0.01 | |||
| PA2920 | Probable chemotaxis transducer | −2.03 | <0.01 | ||||
| PA3040 | Conserved hypothetical protein | −2.15 | 0.03 | ||||
| PA3118 | leuB | 3-Isopropylmalate dehydrogenase | −2.15 | <0.01 | |||
| PA3155 | wbpE | UDP-2-acetamido-2-dideoxy-d-ribo-hex-3-uluronic acid transaminase, WbpE | −2.03 | <0.01 | |||
| PA3305.1 | phrS | PhrS | −6.5 | 0.05 | |||
| PA3326 | clpP2 | X | ClpP2 | −12.7 | 0.04 | −4.97 | 0.01 |
| PA3327 | fabH2 | X | Probable nonribosomal peptide synthetase | −8.1 | 0.02 | −4.69 | <0.01 |
| PA3328 | fabH2 | X | Probable FAD-dependent monooxygenase | −27.0 | 0.01 | −4.93 | <0.01 |
| PA3329 | fabH2 | X | Hypothetical protein | −11.4 | 0.04 | −5.28 | <0.01 |
| PA3330 | fabH2 | X | Probable short-chain dehydrogenase | −31.5 | 0.03 | ||
| PA3331 | fabH2 | X | Cytochrome P450 | −15.9 | 0.03 | −4.61 | <0.01 |
| PA3332 | fabH2 | X | Conserved hypothetical protein | −19.5 | 0.03 | −3.07 | <0.01 |
| PA3333 | fabH2 | X | 3-Oxoacyl-[acyl-carrier-protein] synthase III | −19.0 | 0.03 | ||
| PA3334 | X | Probable acyl carrier protein | −14.0 | 0.02 | |||
| PA3346 | Probable two-component response regulator | −2.35 | <0.01 | ||||
| PA3361 | lecB | X | Fucose-binding lectin PA-IIL | −35.3 | 0.02 | ||
| PA3408 | hasR | Heme uptake outer membrane receptor HasR precursor | −2.71 | <0.01 | |||
| PA3418 | ldh | Leucine dehydrogenase | −2.42 | <0.01 | |||
| PA3431 | Conserved hypothetical protein | −6.1 | 0.05 | ||||
| PA3460 | Probable acetyltransferase | −3.61 | <0.01 | ||||
| PA3461 | Conserved hypothetical protein | −3.91 | <0.01 | ||||
| PA3477 | rhlR | X | Transcriptional regulator RhlR | −5.7 | <0.01 | ||
| PA3478 | rhlB | X | Rhamnosyltransferase chain B | −27.6 | <0.01 | −5.47 | <0.01 |
| PA3479 | rhlA | X | Rhamnosyltransferase chain A | −110.4 | <0.01 | ||
| PA3520 | X | Hypothetical protein | |||||
| PA3554 | arnA | ArnA | −2.56 | <0.01 | |||
| PA3613 | Hypothetical protein PA3613 | −2.14 | <0.01 | ||||
| PA3621.1 | rsmZ | Regulatory RNA RsmZ | −4.0 | 0.04 | |||
| PA3691 | X | Hypothetical protein PA3691 | −3.43 | <0.01 | |||
| PA3692 | lptF | X | Lipotoxon F, LptF | −2.23 | <0.01 | ||
| PA3724 | lasB | X | Elastase LasB | −124.4 | 0.02 | ||
| PA3734 | Hypothetical protein | −5.6 | 0.05 | ||||
| PA3923 | Hypothetical protein | −2.07 | <0.01 | ||||
| PA3974 | ladS | Lost adherence sensor, LadS | −2.03 | 0.01 | |||
| PA4078 | Probable nonribosomal peptide synthetase | −3.60 | <0.01 | ||||
| PA4112 | Probable sensor/response regulator hybrid | −2.82 | <0.01 | ||||
| PA4141 | X | Hypothetical protein | −144.7 | 0.02 | |||
| PA4142 | X | Probable secretion protein | −43.3 | 0.01 | −2.15 | 0.03 | |
| PA4143 | Probable toxin transporter | −20.8 | 0.02 | −5.39 | <0.01 | ||
| PA4144 | Probable outer membrane protein precursor | −16.2 | <0.01 | ||||
| PA4156 | Probable TonB-dependent receptor | −11.5 | <0.01 | ||||
| PA4159 | fepB | Ferrienterobactin-binding periplasmic protein precursor FepB | −9.7 | 0.02 | |||
| PA4160 | fepD | Ferric enterobactin transport protein FepD | −5.0 | 0.03 | |||
| PA4175 | piv | X | Protease IV | −24.3 | <0.01 | −2.56 | <0.01 |
| PA4207 | mexI | Probable RND efflux transporter | −2.94 | <0.01 | |||
| PA4209 | phzM | X | Probable phenazine-specific methyltransferase | −25.1 | 0.02 | ||
| PA4213 | phzD1 | Phenazine biosynthesis protein PhzD | −71.8 | 0.05 | |||
| PA4217 | phzS | X | Flavin-containing monooxygenase | −4.69 | 0.02 | ||
| PA4228 | pchD | Pyochelin biosynthesis protein PchD | −2.92 | <0.01 | |||
| PA4229 | pchC | Pyochelin biosynthetic protein PchC | |||||
| PA4230 | pchB | Salicylate biosynthesis protein PchB | −3.15 | <0.01 | |||
| PA4362 | Hypothetical protein PA4362 | −2.37 | <0.01 | ||||
| PA4471 | Hypothetical protein | −16.0 | 0.04 | ||||
| PA4570 | Hypothetical protein | −5.7 | 0.01 | ||||
| PA4624 | Hypothetical protein PA4624 | −2.62 | <0.01 | ||||
| PA4785 | Probable acyl-CoA thiolase | −2.21 | <0.01 | ||||
| PA4835 | Hypothetical protein PA4835 | −2.58 | <0.01 | ||||
| PA4836 | Hypothetical protein PA4836 | −2.33 | <0.01 | ||||
| PA5058 | phaC2 | X | Poly(3-hydroxyalkanoic acid) synthase 2 | −4.42 | <0.01 | ||
| PA5061 | Conserved hypothetical protein | −4.40 | 0.01 | ||||
| PA5113 | Hypothetical protein | −4.40 | 0.01 | ||||
| PA5171 | arcA | Arginine deiminase | −2.23 | <0.01 | |||
| PA5213 | gcvP1 | Glycine cleavage system protein P1 | −3.27 | <0.01 | |||
| PA5220 | X | Hypothetical protein | −8.5 | 0.01 | |||
Downregulation was defined as a >5-fold decrease in the RNA-Seq result and a >2-fold decrease in the iTRAQ proteomics result. Only genes/proteins showing a significant difference in expression from the control (i.e., P value < 0.05) were selected. Empty lines within fold change columns indicate no significant change in expression levels.
An “X” indicates that the gene/protein is regulated by quorum sensing (4), in reference to the QS-regulated genes/proteins, as determined previously.
MFS, major facilitator superfamily; ECF, extracytoplasmic function; FAD, flavin adenine dinucleotide; CoA, coenzyme A; RND, resistance-nodulation-cell division.
Empty lines indicate no significant change in expression levels.
Biosensor assay.
The growth and gfp expression of the P. aeruginosa strains containing the lasB-gfp, rsmY-gfp, or rsmZ-gfp biosensors in the presence of iberin were monitored using a Victor X multilabel plate reader (PerkinElmer). These strains were cultivated in 96-well microtiter plates with ABTGC medium with different concentrations of iberin at 34°C without shaking. The OD450 (or OD600, in the case of the strain with lasB-gfp) and green fluorescent protein (GFP) fluorescence (in relative fluorescence units [RFU], with excitation and emission wavelengths of 485 and 535 nm, respectively) were measured every 30 min until the culture reached stationary growth phase.
The growth, gfp expression, and pyoverdine production of the P. aeruginosa strain containing the pvdA-gfp biosensor in the presence of iberin were measured using an Infinite 200 Pro Series plate reader (Tecan). These strains were cultivated in 96-well microtiter plates with ABTGC medium with different concentrations of iberin at 37°C without shaking. OD600 readings, GFP fluorescence (in relative fluorescence units [RFU], with excitation and emission wavelengths of 485 and 535 nm, respectively) and pyoverdine fluorescence (in RFU, with excitation and emission wavelengths of 398 and 460 nm, respectively [21]) were measured every 30 min until the culture reached stationary growth phase.
Biofilm assay.
Overnight cultures of PAO1 were diluted 100-fold in 50-ml BD Falcon tubes containing 10 ml of ABTGC medium with 500 μM iberin. No iberin was added in the controls. Sterilized coverslips were placed into the medium and incubated at 37°C under static conditions for the cultivation of surface-attached biofilm at the air-liquid interface. Images of 1-day-old biofilms were captured using confocal microscopy at ×63 magnification (Zeiss LSM 780 confocal system) and analyzed using the IMARIS software package (Bitplane, Zurich, Switzerland).
Nucleotide sequence accession numbers.
The RNA-Seq data sets are available in the NCBI GEO Sequence Read Archives under accession no. SRP028308 and SRR950389.
RESULTS AND DISCUSSION
Comparative analysis of the mode of QS inhibition in iberin.
In order to study the mechanisms behind the inhibition of QS in iberin, we first wanted to find out the changes in gene expression and protein abundance of P. aeruginosa PAO1 in the presence or absence of medium containing 500 μM iberin. RNA-Seq and iTRAQ proteomics were used to examine the changes in gene expression and protein abundance, respectively.
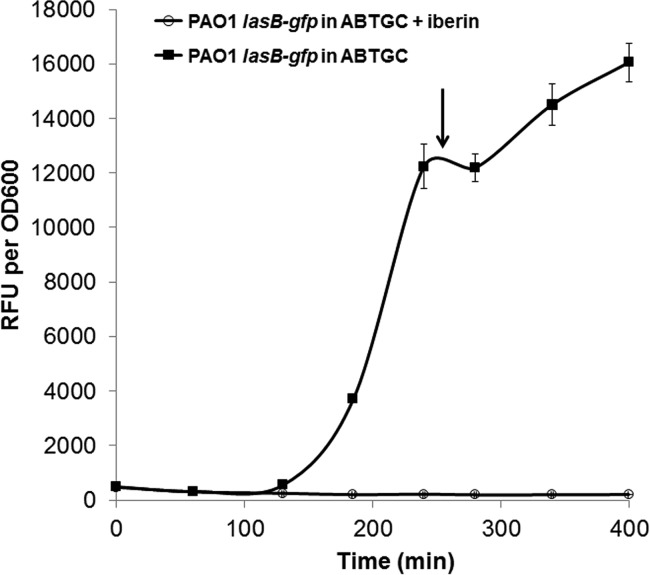
Prior to the extraction of samples for either proteomics or RNA-Seq analysis, we optimized the appropriate time point for harvesting the cells by using P. aeruginosa PAO1 containing the lasB-gfp reporter, which can monitor the onset of QS induction of gene expression (Fig. 2). Based on this assay, we chose the sampling time point to be in the late-log phase of growth. Hence, for the actual RNA-Seq and iTRAQ proteomics experiments, we cultured P. aeruginosa PAO1 in the presence or absence of 500 μM iberin and harvested the samples for subsequent analyses when the cultures reached late-log phase.
FIG 2.
Growth and normalized gfp gene expression (RFU/OD600) of the P. aeruginosa PAO1 biosensor strains carrying lasB-gfp in ABTGC medium with 500 μM iberin. Every 60 min, growth and gfp fluorescence were measured using OD600 values and relative fluorescence units (RFU), respectively. The experiments were performed in triplicate; only representative ones are shown. The arrow indicates the sampling time point for RNA sequencing, while error bars are the averages of triplicate readings.
The genes and proteins whose expression or abundance was significantly increased or decreased in the presence of 500 μM iberin are summarized in Table 2 (upregulated genes/proteins) and Table 3 (downregulated genes/proteins), respectively. For the RNA-Seq results, 62 genes had increased expression (>5-fold) and 79 genes had decreased expression (>5-fold) in the presence of iberin. In the iTRAQ proteomics results, 27 proteins had increased abundance (>2-fold) and 92 proteins had decreased abundance (>2-fold). For these results in Tables 2 and 3, we had determined an arbitrary cutoff of ±5-fold change for upregulation and downregulation of gene expression, respectively, in the RNA-Seq results. Nevertheless, we have included in the supplemental material all the genes in the RNA-Seq results with more than a ±2.0-fold change. The complete lists of the 145 upregulated and 258 downregulated genes in the RNA-Seq results are presented in Tables S1 and S2 in the supplemental material, respectively.
Iberin was able to affect the expression of genes regulated by QS, with reference to the list of QS-regulated genes in Hentzer et al. (4); these are marked with an “X” in Tables 2 and 3. Among the genes/proteins that had significantly increased expression/abundance in the presence of iberin, only 3% of the upregulated genes (2 of 62) in the RNA-Seq results were QS regulated, which supported the previous view of iberin acting as a QSI and not a QS activator. None of the 27 upregulated proteins in the iTRAQ proteomics results was related to QS control. Among the genes/proteins that had significantly decreased expression/abundance in the presence of iberin, 49% of the downregulated genes (39 of 79) in the RNA-Seq results and 27% of the downregulated proteins (25 of 92) in the iTRAQ proteomics results were QS regulated.
Surprisingly, the groups of genes and proteins up- or downregulated by iberin, as identified by either RNA-Seq or iTRAQ proteomics analysis, do not share much in common. Only 9 of the 81 unique significantly upregulated genes/proteins (11%) and 16 of the 157 unique significantly downregulated genes/proteins (10%) were detected by both methods. Among the 16 downregulated genes/proteins, 14 (i.e., 88%) were subject to QS control, with important examples being chitinase (chiC), protease IV (piv), and rhamnosyltransferase chain B (rhlB). This finding suggests the strong inhibition of these particular QS-regulated virulence factors by iberin on both the transcriptional and posttranscriptional/translational levels.
The suppression of many known QS-regulated genes (4) was observed only by the RNA-Seq and not the iTRAQ proteomics analysis, and vice versa (Table 3). For example, the decreased expression of genes encoding hydrogen cyanide synthase (hcnA [PA2193], hcnB [PA2194], and hcnC [PA2195]), lectin (lecA and lecB [PA2570/PA3361]), rhamnosyltransferase chain A (rhlA [PA3479]), and elastase (lasB [PA3724]) was mainly reflected in the RNA-Seq but not the proteomics results (Table 3). This indicates that the expression of these virulence factors was strongly inhibited by iberin on the transcriptional level, which would also be expected to manifest as a reduction in the protein content. However, the missing correlation to the proteomic analysis suggests that these proteins were below the detection limit of the iTRAQ method. In fact, our iTRAQ protocol allowed for the detection (with 99% confidence) of only a total of 1,948 proteins. Elastase (LasB) was detected in only the RNA-Seq (−124.4-fold decrease) and not the proteomics analysis, which agrees with our previous paper in which iTRAQ proteomics was used to study the effects of the QSI 5-imino-4,6-dihydro-3H-1,2,3-triazolo[5,4-d]pyrimidin-7-one on P. aeruginosa (22). In that study, elastase was also not detected through proteomics. Since only the cells and not the surrounding medium were subjected to iTRAQ analysis, this extracellular enzyme was likely not to be present intracellularly in large enough amounts to be detected. We put forward that this explanation is also valid for lectin (LecA and LecB).
Our results also show that iberin was able to inhibit the expression of multiple components of the type III secretion system (T3SS) of P. aeruginosa, which is an important system in pathogenesis (23). Gene expression of the effector protein ExoT and ExoS toxins known to impair phagocytosis by host immune cells (24, 25) was reduced by −2.0- and −3.0-fold in the RNA-Seq results, respectively (see Table S2 in the supplemental material). Also, the expression levels of the genes for the T3SS regulatory proteins ExsB, ExsD, ExsC, and ExsE (26) were reduced by −3.5-, −6.6-, −3.2-, and −4.4-fold, respectively. The expression levels of the genes for the needle components PscC and PscG were reduced −2.1- and −2.6-fold, respectively. Lastly, gene expression of the translocator protein PopB (27) was decreased −6.7-fold in the RNA-Seq results.
In the previous study by Jakobsen et al. (15), it was shown that iberin was able to compete effectively with C4-HSL for binding to RhlR, with the expression levels of rhlR, rhlB, and rhlA reduced by −5.3-, −42.1-, and −59.0-fold, respectively, upon the addition of 64 μg/ml iberin to PAO1, as determined by DNA microarray. Also, iberin inhibited rhamnolipid production by PAO1 in a concentration-dependent manner, with complete inhibition occurring at a concentration of 200 μM iberin. Similarly, our results showed that the expression levels of the rhlR, rhlB, and rhlA genes were significantly decreased in the RNA-Seq results by −5.7-, −27.6-, and −110.4-fold, respectively. Our iTRAQ result showed only RhlB being significantly downregulated (−5.47-fold).
In Jakobsen et al. (15), the expression of lasB was found to be decreased by 89.8-fold upon the addition of 64 μg/ml iberin to PAO1, as determined by DNA microarray. However, they also showed that iberin was unable to competitively inhibit N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL)-driven expression of the lasB-gfp fusion when present in an Escherichia coli background (15). In the present study, neither the transcriptomic nor proteomic analysis showed iberin to inhibit the expression of the major QS regulator, LasR. These findings taken together strongly suggest that iberin does not target LasR, and it does not compete with 3-oxo-C12-HSL for binding to LasR. Despite this, the lasB gene, the expression of which is positively regulated by LasR, was found to be reduced by 124.4-fold in the RNA-Seq results. Thus, a comparison of the mRNA and corresponding protein content does not reveal the mechanisms by which iberin inhibits the las QS-controlled gene expression.
Analysis of the inhibition of small regulatory RNAs by iberin.
One valuable advantage of the RNA-Seq method over the DNA microarray method used previously (15) is that RNA-Seq is able to monitor the expression of small regulatory RNAs. Our RNA-Seq results showed that the expression of two small regulatory RNAs, encoded by the phrS and rsmZ genes (PA3305.1 and PA3621.1, respectively), was repressed by iberin treatment by 6.5-fold and 4.0-fold, respectively, compared to the untreated control (Table 3). PhrS and RsmZ have been reported to positively regulate alkyl quinolone-based and AHL-based QS systems, respectively (28, 29). A ΔrsmY ΔrsmZ mutant was shown to have deficient synthesis of the exoproducts hydrogen cyanide, pyocyanin, elastase, chitinase, and chitin-binding protein (29). This phenotype agrees with our RNA-Seq results showing that iberin inhibited the transcription of genes involved in the production of those exoproducts. Hence, we wanted to confirm whether iberin inhibited rsmY/rsmZ expression.
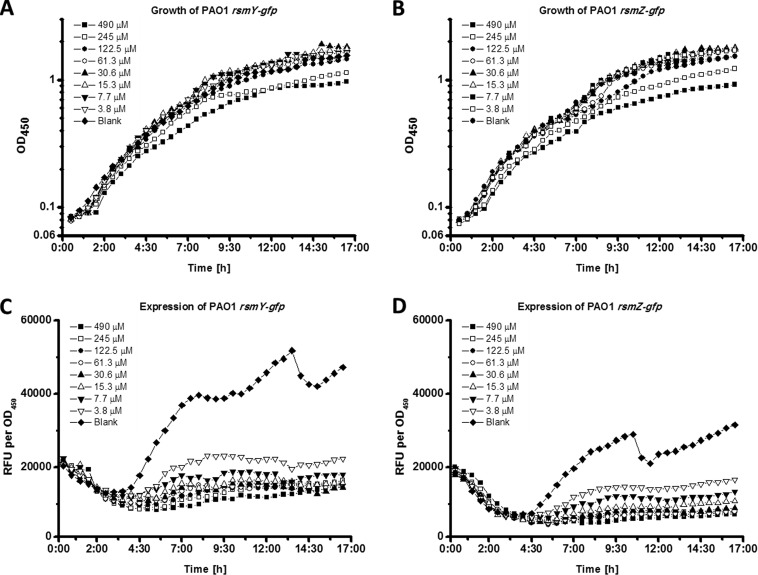
In order to test the inhibitory effects of iberin on rsmY and rsmZ expression, PAO1 strains containing rsmY-gfp or rsmZ-gfp plasmids (30) were cultured in the presence of various concentrations of iberin. Our results show that iberin inhibited the expression of rsmY and rsmZ in a dose-dependent manner (Fig. 3); however, high levels of iberin were growth inhibiting.
FIG 3.
Growth and normalized gfp gene expression (RFU/OD450) of the P. aeruginosa PAO1 biosensor strains carrying rsmY-gfp (A and C) and rsmZ-gfp (B and D) in ABTGC medium containing various concentrations of iberin. Every 30 min, growth and gfp fluorescence were measured using OD450 values and relative fluorescence units (RFU), respectively. The experiments were performed in triplicate; only representative ones are shown.
The expression of the GacA-dependent small RNAs, rsmY and rsmZ, is essential for AHL-mediated QS and virulence in P. aeruginosa (29). Previous works aiming to elucidate QS inhibitory mechanisms have reported that the QS inhibitor azithromycin inhibits the expression of rsmY and rsmZ transcription through the PA0588-PA0584 gene cluster (31). However, our transcriptomic data did not show that iberin affected the expression of the PA0588-PA0584 gene cluster, which suggests that iberin uses a different mechanism from that of azithromycin to inhibit the expression of rsmY and rsmZ.
Our proteomics result shows that iberin led to a 2-fold decrease in the abundance of the lost adherence sensor protein LadS (PA3974). LadS is a sensor kinase that phosphorylates GacS, which in turn is responsible for activating GacA and the expression of rsmY and rsmZ (32). This result suggests that iberin posttranscriptionally inhibits the expression of LadS, which in turn leads to reduced activation of GacS, thus reducing RsmY and RsmZ levels.
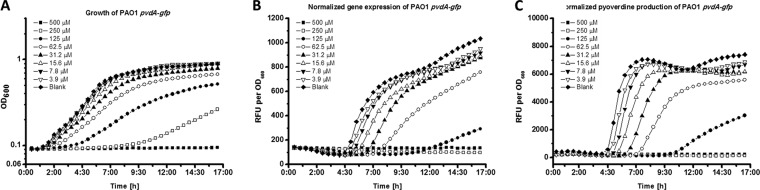
A study by Frangipani et al. (16) showed that RsmY and RsmZ sequester RsmA, a situation that allows for the production of the siderophores pyochelin and pyoverdine. Pyoverdine is important for development of the characteristic mushroom-like structures of P. aeruginosa biofilms (33), and it is also believed to play a role in its virulence, for example, in the production of the virulence factor pyocyanin (34). Hence, we were interested to find out whether iberin might affect pyoverdine production. A P. aeruginosa PAO1 wild-type strain containing the pvdA-gfp reporter was cultured in various concentrations of iberin. Iberin inhibited the expression of the pvdA-gfp genes and the production of pyoverdine (Fig. 4B and C), indicating that the inhibition of pyoverdine synthesis occurs on a transcriptional level, mediated through released RsmA protein, which is usually sequestered by the small regulatory RNA molecules. This is supported by our RNA-Seq results, in which the expression levels of the pyoverdine biosynthesis genes pvdA, pvdP, pvdG, and pvdS were reduced by −3.2-, −3.6-, −3.9-, and −3.6-fold, respectively (see Table S2 in the supplemental material).
FIG 4.
Growth (A), normalized gfp expression (B), and normalized pyoverdine production (C) of the P. aeruginosa PAO1 biosensor strain carrying pvdA-gfp in ABTGC medium containing various concentrations of iberin. Every 30 min, growth, gfp fluorescence, and pyoverdine production were measured using OD600 values and relative fluorescence units (RFU), respectively. Normalization was calculated as RFU divided by OD600. The experiments were performed in triplicate; only representative ones are shown.
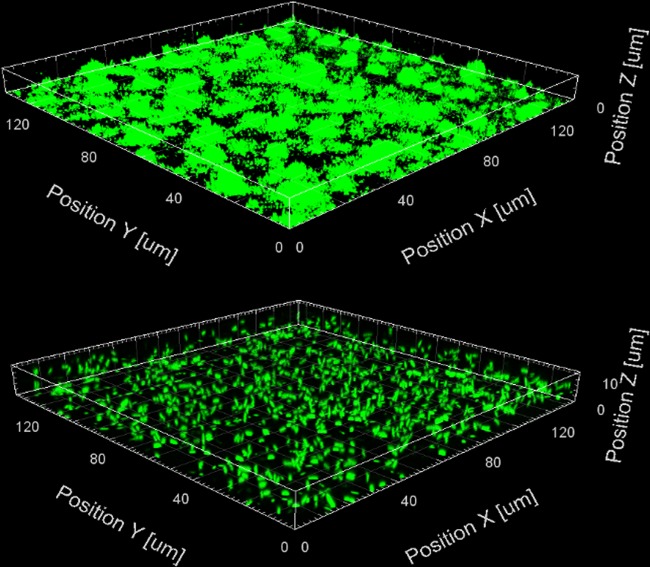
The exopolysaccharides Pel and Psl, along with alginate, are important components of the extracellular matrix of P. aeruginosa biofilms (35). It has been shown that both the pel and psl polysaccharide genes are posttranscriptionally regulated by RsmY and RsmZ (29, 36). As such, we performed a slide biofilm assay in order to determine if iberin was able to reduce biofilm formation by P. aeruginosa through the downregulation of pel and psl. Figure 5 shows that the sample treated with 500 μM iberin had significantly less biofilm formation than the control sample in ABTGC. This suggests that iberin may be used not only as an antipathogenic agent but also as an antibiofilm agent.
FIG 5.
Three-dimensional confocal images of 1-day-old miniTn7-gfp-tagged P. aeruginosa PAO1 slide biofilms with either ABTGC medium (control, top panel) or 500 μM iberin (bottom panel). Images were obtained using confocal microscopy at ×63 magnification (Zeiss LSM 780 confocal system), and analyzed using the Imaris software package (Bitplane AG). Only a representative image of three replicates is shown.
In addition to RsmY and RsmZ, PhrS was also reported to be an important small regulatory RNA that can stimulate the synthesis of the Pseudomonas quinolone signal (PQS) via activating PqsR (28). PQS quorum sensing is well known to regulate the synthesis of the virulence factor pyocyanin and rhamnolipid and the release of extracellular DNA in P. aeruginosa (37, 38). In our RNA-Seq results, we see that iberin reduced the expression of phrS by −6.5-fold. Also, the expression of the pqs operon (PA0996-PA1001) was reduced by 3- to 4-fold and the phenazine biosynthesis genes (phnA and phnB) by 7.2- and 4.8-fold, respectively (see Table S2 in the supplemental material). As such, iberin has an inhibitory effect on the expression of the pqs QS system and phenazine biosynthesis genes.
In summary, our study sheds light on the QS inhibitory mechanisms of a natural food-derived QSI, iberin, by using complementary transcriptomic and proteomic approaches. Iberin is an efficient QSI, not from its interaction with the central transcriptional QS regulators LasR and RhlR but rather through its effect on the expression of the small regulatory RNAs RsmY and RsmZ, as well as PhrS. From our analysis of gene expression and protein content, we believe that iberin modulates the Gac/Rsm network of P. aeruginosa through posttranscriptional inhibition of LadS. Lastly, we also show that iberin effectively reduces biofilm formation, highlighting its potential use as both an antipathogenic and antibiofilm agent. Further studies will be carried out to investigate the possible interaction of iberin with LadS through biochemical and phosphoproteomics methods.
Supplementary Material
ACKNOWLEDGMENTS
This research is supported by the National Research Foundation and Ministry of Education Singapore under its Research Centre of Excellence Programme and a startup grant (M4330002.C70) from Nanyang Technological University, Singapore. This work was also supported by grants from the Danish Council for Strategic Research (to M.G.).
Footnotes
Published ahead of print 25 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02620-13.
REFERENCES
- 1.Miller MB, Bassler BL. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165–199. 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 2.de Kievit TR, Iglewski BH. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839–4849. 10.1128/IAI.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjarnsholt T, Jensen PØ, Burmølle M, Hentzer M, Haagensen JA, Hougen HP, Calum H, Madsen KG, Moser C, Molin S, Høiby N, Givskov M. 2005. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 151:373–383. 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 4.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, Schembri MA, Song Z, Kristoffersen P, Manefield M, Costerton JW, Molin S, Eberl L, Steinberg P, Kjelleberg S, Høiby N, Givskov M. 2003. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 22:3803–3815. 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoang TT, Schweizer HP. 1999. Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J. Bacteriol. 181:5489–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch B, Liljefors T, Persson T, Nielsen J, Kjelleberg S, Givskov M. 2005. The LuxR receptor: the sites of interaction with quorum-sensing signals and inhibitors. Microbiology 151:3589–3602. 10.1099/mic.0.27954-0. [DOI] [PubMed] [Google Scholar]
- 7.Jakobsen TH, Bjarnsholt T, Jensen PØ, Givskov M, Høiby N. 2013. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors. Future Microbiol. 8:901–921. 10.2217/fmb.13.57. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen TB, Givskov M. 2006. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 296:149–161. 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Conaway CC, Yang YM, Chung FL. 2002. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr. Drug Metab. 3:233–255. 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 10.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Tercé F. 2000. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 60:1426–1433. [PubMed] [Google Scholar]
- 11.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. 2003. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis 24:891–897. 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 12.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. 2006. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10:241–252. 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Dufour V, Alazzam B, Ermel G, Thepaut M, Rossero A, Tresse O, Baysse C. 2012. Antimicrobial activities of isothiocyanates against Campylobacter jejuni isolates. Front. Cell. Infect. Microbiol. 2:53. 10.3389/fcimb.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CM, Preston JF, III, Wei CI. 2000. Antibacterial mechanism of allyl isothiocyanate. J. Food Prot. 63:727–734. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsen TH, Bragason SK, Phipps RK, Christensen LD, van Gennip M, Alhede M, Skindersoe M, Larsen TO, Høiby N, Bjarnsholt T, Givskov M. 2012. Food as a source for quorum sensing inhibitors: iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 78:2410–2421. 10.1128/AEM.05992-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangipani E, Visaggio D, Heeb S, Kaever V, Cámara M, Visca P, Imperi F. 2014. The Gac/Rsm and cyclic-di-GMP signalling networks coordinately regulate iron uptake in Pseudomonas aeruginosa. Environ. Microbiol. 16:676–688. 10.1111/1462-2920.12164. [DOI] [PubMed] [Google Scholar]
- 17.Holloway B, Morgan A. 1986. Genome organization in Pseudomonas. Annu. Rev. Microbiol. 40:79–105. 10.1146/annurev.mi.40.100186.000455. [DOI] [PubMed] [Google Scholar]
- 18.Clark DJ, Maaløe O. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99–112. 10.1016/S0022-2836(67)80070-6. [DOI] [Google Scholar]
- 19.Chua SL, Tan SY, Rybtke MT, Chen Y, Rice SA, Kjelleberg S, Tolker-Nielsen T, Yang L, Givskov M. 2013. Bis-(3′-5′)-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 57:2066–2075. 10.1128/AAC.02499-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao P, Guo T, Li X, Adav SS, Yang J, Wei M, Sze SK. 2010. Novel application of electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) in shotgun proteomics: comprehensive profiling of rat kidney proteome. J. Proteome Res. 9:3520–3526. 10.1021/pr100037h. [DOI] [PubMed] [Google Scholar]
- 21.Imperi F, Tiburzi F, Visca P. 2009. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 106:20440–20445. 10.1073/pnas.0908760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan SY, Chua SL, Chen Y, Rice SA, Kjelleberg S, Nielsen TE, Yang L, Givskov M. 2013. Identification of five structurally unrelated quorum-sensing inhibitors of Pseudomonas aeruginosa from a natural-derivative database. Antimicrob. Agents Chemother. 57:5629–5641. 10.1128/AAC.00955-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser AR. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat. Rev. Microbiol. 7:654–665. 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. 1997. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol. 25:1125–1139. 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 25.Garrity-Ryan L, Kazmierczak B, Kowal R, Comolli J, Hauser A, Engel JN. 2000. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68:7100–7113. 10.1128/IAI.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yahr TL, Wolfgang MC. 2006. Transcriptional regulation of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 62:631–640. 10.1111/j.1365-2958.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- 27.Schoehn G, Di Guilmi AM, Lemaire D, Attree I, Weissenhorn W, Dessen A. 2003. Oligomerization of type III secretion proteins PopB and PopD precedes pore formation in Pseudomonas. EMBO J. 22:4957–4967. 10.1093/emboj/cdg499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnleitner E, Gonzalez N, Sorger-Domenigg T, Heeb S, Richter AS, Backofen R, Williams P, Hüttenhofer A, Haas D, Bläsi U. 2011. The small RNA PhrS stimulates synthesis of the Pseudomonas aeruginosa quinolone signal. Mol. Microbiol. 80:868–885. 10.1111/j.1365-2958.2011.07620.x. [DOI] [PubMed] [Google Scholar]
- 29.Kay E, Humair B, Dénervaud V, Riedel K, Spahr S, Eberl L, Valverde C, Haas D. 2006. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J. Bacteriol. 188:6026–6033. 10.1128/JB.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chua SL, Liu Y, Yam JK, Chen Y, Vejborg RM, Tan BG, Kjelleberg S, Tolker-Nielsen T, Givskov M, Yang L. 2014. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat. Commun. 5:4462. 10.1038/ncomms5462. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Martínez I, Haas D. 2011. Azithromycin inhibits expression of the GacA-dependent small RNAs RsmY and RsmZ in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 55:3399–3405. 10.1128/AAC.01801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ventre I, Goodman AL, Vallet-Gely I, Vasseur P, Soscia C, Molin S, Bleves S, Lazdunski A, Lory S, Filloux A. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc. Natl. Acad. Sci. U. S. A. 103:171–176. 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Nilsson M, Gjermansen M, Givskov M, Tolker-Nielsen T. 2009. Pyoverdine and PQS mediated subpopulation interactions involved in Pseudomonas aeruginosa biofilm formation. Mol. Microbiol. 74:1380–1392. 10.1111/j.1365-2958.2009.06934.x. [DOI] [PubMed] [Google Scholar]
- 34.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryder C, Byrd M, Wozniak DJ. 2007. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 10:644–648. 10.1016/j.mib.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman AL, Kulasekara B, Rietsch A, Boyd D, Smith RS, Lory S. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7:745–754. 10.1016/j.devcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Diggle SP, Cornelis P, Williams P, Cámara M. 2006. 4-Quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int. J. Med. Microbiol. 296:83–91. 10.1016/j.ijmm.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Barken KB, Skindersoe ME, Christensen AB, Givskov M, Tolker-Nielsen T. 2007. Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 153:1318–1328. 10.1099/mic.0.2006/004911-0. [DOI] [PubMed] [Google Scholar]
- 39.Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Høiby N, Kjelleberg S, Givskov M. 2002. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148:87–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.