Abstract
In order to determine if triclosan can select for mutants of Acinetobacter baumannii ATCC 17978 that display reduced susceptibilities to antibiotics, we isolated a triclosan-resistant mutant, A. baumannii AB042, by serial passaging of A. baumannii ATCC 17978 in growth medium supplemented with triclosan. The antimicrobial susceptibility of AB042 was analyzed by the 2-fold serial dilution method. Expression of five different resistance-nodulation-division (RND) pump-encoding genes (adeB, adeG, adeJ, A1S_2818, and A1S_3217), two outer membrane porin-encoding genes (carO and oprD), and the MATE family pump-encoding gene abeM was analyzed using quantitative reverse transcriptase (qRT) PCR. A. baumannii AB042 exhibited elevated resistance to multiple antibiotics, including piperacillin-tazobactam, doxycycline, moxifloxacin, ceftriaxone, cefepime, meropenem, doripenem, ertapenem, ciprofloxacin, aztreonam, tigecycline, and trimethoprim-sulfamethoxazole, in addition to triclosan. Genome sequencing of A. baumannii AB042 revealed a 116G→V mutation in fabI, the gene encoding the target enzyme for triclosan. Expression analysis of efflux pumps showed overexpression of the AdeIJK pump, and sequencing of adeN, the gene that encodes the repressor of the adeIJK operon, revealed a 73-bp deletion which would cause a premature termination of translation, resulting in an inactive truncated AdeN protein. This work shows that triclosan can select for mutants of A. baumannii that display reduced susceptibilities to multiple antibiotics from chemically distinct classes in addition to triclosan resistance. This multidrug resistance can be explained by the overexpression of the AdeIJK efflux pump.
INTRODUCTION
Triclosan is a bisphenolic biocide widely used in various domestic cleaning products, such as toothpaste, soaps, and cosmetics, and hospital equipment (1). The increasing use of triclosan in domestic products has raised concerns about its role in selecting for triclosan-resistant bacterial strains that exhibit cross-resistance to clinically relevant antibiotics. Resistance to triclosan can result from target site (fatty acid biosynthetic enzyme, enoyl-[acyl-carrier protein] reductase, fabI) modification, active efflux, or enzymatic degradation (2). Of these mechanisms, active efflux has the most important role in imparting cross-resistance to chemically distinct classes of antibiotics.
In Gram-negative bacteria, efflux pumps belonging to the resistance-nodulation-division (RND) family are considered one of the most important contributors of intrinsic resistance to clinically relevant antibiotics (3). These pumps are known for their broad specificities, and triclosan is a substrate of several of these pumps. In addition, triclosan has been shown to select for RND pump-overexpressing mutants in several organisms, including Escherichia coli (4), Pseudomonas aeruginosa (5, 6), and Salmonella enterica (7).
In this study, we analyzed the ability of triclosan to select for mutants of Acinetobacter baumannii (an organism notorious for causing infections in immunocompromised individuals [8]) that display elevated resistance to chemically unrelated antibiotic classes. Infections caused by A. baumannii are becoming increasingly difficult to treat because of its widespread resistance to almost every class of antibiotic in clinical use (9, 10). Due to the ability of A. baumannii to cause multidrug-resistant infections, the Centers for Disease Control and Prevention (CDC) recently categorized it as an organism that poses a serious threat to human health (http://www.cdc.gov/drugresistance/threat-report-2013/). While the resistance of A. baumannii to various antibiotics can be attributed in part to its impressive ability to acquire various resistance genes (9), it also displays high intrinsic resistance mediated by reduced outer membrane permeability and the activity of energy-dependent efflux proteins (8). To date, three RND pumps in A. baumannii have been characterized, namely, AdeABC (11), AdeFGH (12), and AdeIJK (13). In addition, a multidrug and toxic compound extrusion (MATE) family pump in A. baumannii, AbeM, has been characterized (14). While none of the RND pumps characterized has been shown to efflux triclosan, it is a substrate of the AbeM pump.
Although triclosan reistance has been reported in clinical isolates of A. baumannii as a result of mutations in fabI (15), its role in A. baumannii cross-resistance to antibiotics has never been reported. In this paper, we report that triclosan can indeed select for AdeIJK-overexpressing mutants of A. baumannii with reduced susceptibilities to various classes of chemically unrelated antibiotics.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. A. baumannii ATCC 17978 was used to generate the triclosan-resistant mutant. Lennox broth (LB) (BioShop, Inc., Burlington, ON, Canada) was used as the growth medium. Bacterial cultures were incubated at 37°C with shaking (200 rpm).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Reference or source |
|---|---|---|
| A. baumannii strains | ||
| ATCC 17978 | Wild type | ATCC |
| AB042 | Triclosan-resistant mutant of ATCC 17978 | This study |
| P. aeruginosa strains | ||
| PAO750 | PAO1: ΔmexAB-oprM ΔmexCD-oprJ ΔmexEF-oprN ΔmexJK ΔmexXY ΔopmH ΔpscC | 34 |
| PA005 | PA0750 with chromosomally integrated mini-Tn7-LAC-adeIJK containing the Genr marker | This study |
| PA006 | PA0750 with chromosomally integrated mini-Tn7-LAC-adeIJK with Genr marker removed | This study |
| E. coli strains | ||
| DH5ɑ | F− ϕ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ-thi-1 gyrA96 relA1 | Invitrogen |
| Plasmids | ||
| pGEM-T Easy | Ampr, PCR cloning vector | Promega |
| pPLS001 | Ampr, pGEM-T Easy-adeIJ′ | This study |
| pPLS002 | Ampr, pGEM-T Easy-adeJ′K | This study |
| pPLS007 | Ampr, pGEM-T Easy-adeIJK | This study |
| pUC18T-mini-Tn7T-Gm-LAC | Ampr Genr; mini-Tn7 expression vector containing lacIq and tac promoter | 24 |
| pPLS009 | Ampr Genr, pUC18T-mini-Tn7T-Gm-LAC-adeIJK | This study |
| pPLS097 | Ampr Gmr, E. coli-A. baumannii shuttle vector | This study |
| pPLS093 | Ampr, pGEM-T Easy:adeN | This study |
| pPLS145 | Ampr Gmr, pPLS097:adeN | This study |
| pPLS147 | pGEM-T Easy:fabI | This study |
| pPLS148 | Ampr, pGEM-T Easy:fabI116G→V Ampr | This study |
| pPLS150 | Ampr Gmr, pPLS097:fabI116G→V | This study |
| pPLS151 | Ampr Gmr, pPLS097:fabI | This study |
| pTNS2 | Ampr, helper plasmid encoding the site-specific TnsABCD Tn7 transposition pathway | 24 |
| pFLP2 | Ampr; source of FLP recombinase | 24 |
Isolation of triclosan-resistant mutant.
The triclosan-resistant mutant A. baumannii AB042 was isolated by serial transfer of A. baumannii ATCC 17978 in LB supplemented with increasing concentrations (2-fold for each transfer) of triclosan (KIC Chemicals, Inc., New Paltz, NY, USA), starting at 4 mg/liter. A 1:100 inoculum was transferred to the LB supplemented with increasing concentrations of triclosan. A. baumannii AB042 was isolated by streaking cells from LB supplemented with 128 mg/liter of triclosan on LB agar.
Antimicrobial susceptibility assays.
Susceptibility testing for triclosan and antibiotics was carried out using the broth microdilution method as described by the Clinical and Laboratory Standards Institute (16). Susceptibility assays were performed on three biological replicates.
RNA extraction and cDNA synthesis.
Overnight cultures of bacterial strains were subcultured in LB (1:100 dilution) and allowed to grow to an A600 of 0.6 to 0.8. Then, 1 ml of this culture was centrifuged, and the cell pellet was frozen at −80°C for 1 h to facilitate cell lysis. RNA extraction was performed using the RNeasy kit (Qiagen, Mississauga, ON, Canada) following the manufacturer's instructions. To remove any genomic DNA carryover, the samples were treated with DNase I (Qiagen) for 30 min at 37°C, followed by heat inactivation at 65°C for 5 min. Then, 1 μg of total RNA was used to synthesize cDNA using the Bio-Rad iScript reverse transcriptase kit (Bio-Rad, Mississauga, ON, Canada) following the manufacturer's instructions. The control reaction (with no reverse transcriptase [NRT]) was set up using all components of the reaction mixture but without the reverse transcriptase enzyme.
Reverse transcriptase quantitative PCR (qRT-PCR).
Analysis was carried out on five RND pump-encoding genes (adeB, adeG, adeJ, A1S_2818, and A1S_3217), two outer membrane porins (carO and oprD), the MATE family pump abeM, the triclosan target-encoding gene fabI, and the TetR regulator-encoding gene of the adeIJK operon, adeN. The primers used for qRT-PCR analysis are listed in Table 2. The efficiency of each primer was tested by using a 10-fold serial dilution of cDNA mix, and only primers with an efficiency between 95% and 105% were used for the analysis. The expression of genes was determined by quantitative PCR using SsoFast Evagreen Supermix (Bio-Rad, Mississauga, ON, Canada). Two different control reactions were included in the analysis, a no-template control (NTC) and an NRT control. We used 16S rRNA as the reference gene. Relative expression was determined using the cycle threshold (ΔΔCt) method on the Bio-Rad C1000 CFX96 real-time system (Bio-Rad). Reactions were set up using 300 nM primers and 5 μl of the cDNA template (diluted 1:10). All reactions were carried out in triplicate with at least two biological replicates. Target gene expression was measured using expression relative to that of the 16S reference gene, and A. baumannii ATCC 17978 was used as the control strain. Data analysis was carried out using the Bio-Rad CFX 2.0 software.
TABLE 2.
Primers used in this study
| Primer | Sequence | Target gene and purpose | Reference |
|---|---|---|---|
| 16S_RT_F | ACATCTCACGACACGAGCTG | 16S rRNA, gene expression | 38 |
| 16S_RT_R | CGTAAGGGCCATGATGACTT | ||
| adeB_RT_F | GGATTATGGCGACTGAAGGA | adeB, gene expression | 38 |
| adeB_RT_R | AATACTGCCGCCAATACCAG | ||
| adeG_RT_F | CGTAACTATGCGGTGCTCAA | adeG, gene expression | 38 |
| adeG_RT_R | ATCGCGTAGTCACCAGAACC | ||
| adeJ_RT_F | CATCGGCTGAAACAGTTGAA | adeJ, gene expression | 38 |
| adeJ_RT_R | GCCTGACCATTACCAGCACT | ||
| A1S_3217_RT_F | ACCGCTTTAGAGGTCGAACA | A1S_3217, gene expression | 38 |
| A1S_3217_RT_R | GTGACTTGGGAAAGCCCATA | ||
| A1S_2818_RT_F | AATTGAGCCAAGCTCATGCT | A1S_2818, gene expression | 38 |
| A1S_2818_RT_R | TCCGCGATGAAATTGATACA | ||
| carO_RT_F | AGCAGTTCGTGGTCAAGAGG | carO, gene expression | 38 |
| carO_RT_R | TTGGAGCAAAACCAAAACCT | ||
| oprD_RT_F | CCAGCTCAGTTGCTCAATCA | oprD, gene expression | 38 |
| oprD_RT_R | AACAACGCCTACACCGAAAC | ||
| abeM_RT_F | TGCCAATTGGTTTAGCTGTG | abeM, gene expression | This study |
| abeM_RT_R | TACTTGGTGTGCGGCAATAA | ||
| adeN_RT_F | CAACCTGAACACATTGCCTTT | adeN, gene expression | This study |
| adeN_RT_R | TTTTGGACATCCAGAGCACA | ||
| fabI_RT_F | TTTAGAAGCTGGCGTTCGTT | fabI, gene expression | This study |
| fabI_RT_R | AGCAGCCAAAGTACGGATTG | ||
| fabI_FL_F | GTGAGATCGGCATGACACAA | fabI, cloning and sequencing of fabIa | This study |
| fabI_FL_R | ATAACGGTAGCGGAGTTCAG | ||
| adeN_FL_R | AGTCTACTATACTATAAGCATTTC | adeN, cloning and sequencing of adeNb | This study |
| adeN_ FL_F | GATAAGCAGTGTTAGCCGTCG | ||
| adeIJK_PR_F | CTTCAGAAATTTGATATGCT | adeIJK promoter, sequencingc | This study |
| adeIJK_PR_R | GATTATGTTATGCCATAAGC | ||
| adeI_For_Sp | TTACTAGTTATCTAAACGAGGTGd | adeI, adeJ, cloning of adeIJ′ fragment (4,051 bp) | This study |
| adeJ_Rev_Kp | TCAATACGATTGCACCAATGAC | ||
| adeJ_For_Kp | TATATGAAAGCTGGTCAATTCCG | adeJ, adeK, cloning of adeJ′K fragment (1,983 bp) | This study |
| adeK_Rev_Xh | CCCACCGACTCGAGCTTTTATAAGe | ||
| PaglmS_Dn | GCACATCGGCGACGTGCTCTC | glmS and pUC18T-miniTn7T-Gm-LAC, confirmation of the insertion of mini-Tn7 element in P. aeruginosa | 24 |
| Tn7R | CACAGCATAACTGGACTGATTTC |
Primers bind 233 bp upstream of the start and 11 bp downstream of stop codons, respectively.
Primers bind 32 bp upstream of the start and 38 bp downstream of stop codons, respectively.
Promoter/sequencing of the promoter region 244 bp upstream and 220 bp downstream of the start codon of the adeI gene.
Engineered SpeI site is underlined, and introduced base changes are shown in bold type.
Engineered XhoI site is underlined, and introduced base changes shown are bold.
Sanger sequencing.
Genomic DNA from A. baumannii ATCC 17978 and A. baumannii AB042 was extracted from overnight cultures using a genomic DNA extraction kit (Bio Basic, Markham, ON, Canada). Sequencing of fabI, adeN, and the promoter region of adeIJK was carried out at the McGill University Innovation Centre for sequencing (Montreal, QC, Canada).
Whole-genome sequencing.
Whole-genome sequencing of A. baumannii ATCC 17978 and A. baumannii AB042 was carried out at the next-generation sequencing (NGS) facility of the Manitoba Institute of Child Health (MICH) using the Illumina MiSeq platform. In total, 1,764,672 and 3,669,973 pairs of 150-base sequence reads were generated for AB042 and ATCC 17978, respectively. These sequence runs passed the quality check with 90% passed-filter (PF) reads, and 87% had a Q score of ≥30. Assembly of the genomes was carried out using MIRA (v4.0.2) (17) and Velvet (v1.2.10) (18) software. Comparison of the genomes with those of the published A. baumannii ATCC 17978 (GenBank accession no NC_009085) was carried out using BWA version 0.6.2 (19), with the default three-mismatch penalty setting. Before the sequence variation analysis, the duplicate sequence reads were also removed from the mapped bam files. We used the Genome Analysis Toolkit (GATK) package (20) for sequence variation analysis, including single-nucleotide variants (SNV) and insertions/deletions (indels). The variation calls were ensured by filtering against variant confidence and mapping qualities. We used the ANNOVAR program (21) for sequence variation annotations.
To screen for large deletions/insertions, we developed a program based on the coverage distribution model for detection (our unpublished data). The deletions with a P value of <0.001 were selected. The large deletions/insertions of the two strains and those of the parent strain were compared and visualized by the Integrated Genome Browser (IGV version 2.3.23) (22).
Cloning and single-copy expression for adeIJK operon.
For cloning of the adeIJK operon, primers were designed (Table 2) to amplify 4,051- and 1,983-bp regions of the adeIJK operon, both of which were cloned in the PCR cloning vector pGEM-T Easy (Promega, Madison, WI, USA) separately to construct plasmids pPLS001 and pPLS002, respectively. In order to assemble the entire operon, pPLS001 was digested using AatII and KpnI (New England BioLabs, Pickering, ON, Canada), and a resulting 4,061-bp fragment (containing adeI and the partial adeJ gene) was gel purified (BioBasic, Markham, ON, Canada). This fragment was ligated with pPLS002, which itself was digested with the same enzymes, yielding the plasmid pPLS007. The entire adeIJK operon in pPLS007 was sequenced and subcloned by digesting pPLS007 with SpeI and XhoI (New England BioLabs) and subsequently ligating the gel-purified fragment to pUC18T-mini-Tn7T-Gm-LAC, digested with the same enzymes to yield the recombinant plasmid pPLS009. Therefore, pPLS009 contains the adeIJK operon, the expression of which is driven from the tac promoter and controlled by the lacIq-encoded Lac repressor, and it can be induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) (BioBasic) to the growth medium.
Insertion of the adeIJK operon in the surrogate P. aeruginosa strain PAO750 in a single copy was carried out by a method previously described (23). Briefly, 50 ng each of pPLS009 and the helper plasmid pTNS2 (24) was electroporated in P. aeruginosa PAO750 (25). Transformants were selected on LB agar supplemented with gentamicin (30 mg/liter) (BioBasic, Markham, ON, Canada). The gentamicin resistance marker was subsequently removed using the FLP recombinase to obtain P. aeruginosa PA006. PCR was used to confirm the insertion of the mini-Tn7 element in P. aeruginosa PA006 as described previously (24).
Cloning of adeN, fabI, and fabI116G→V.
A PCR-based strategy was used to clone wild-type (WT) and mutant fabI and the wild-type adeN. Gene-specific primers (listed in Table 2) were used to amplify the respective genes and the PCR products cloned into the cloning vector pGEM-T Easy (Promega, Madison, WI, USA). For subcloning adeN, pPLS093 (pGEM-T Easy:adeN) was digested with PstI and SphI (New England BioLabs, Pickering, ON, Canada), and the gene was gel purified and cloned into pPLS097, which itself was digested with the same enzyme. Subcloning of the wild-type and mutant fabI was achieved by digesting pPLS148 (pGEM-T Easy:fabI) and pPLS147 (pGEM-T Easy:fabI116G→V) with SalI and SphI (New England BioLabs), respectively, gel purifying the respective genes, and cloning in pPLS097, digested with the same enzymes. Recombinant plasmids were sequenced to confirm the presence of target genes.
Plasmids were introduced into A. baumannii by using a method previously described (25), with some modifications. Briefly, an overnight culture of A. baumannii was subcultured in 15 ml of LB, and cells were grown to mid-log phase. The culture was distributed in 1-ml aliquots into microcentrifuge tubes, and cells were harvested by centrifugation (10,000 rpm) at room temperature for 2 min. Cells were then washed twice with ice-cold water, and pellets in each microcentrifuge tube were resuspended in 20 μl of ice-cold double-distilled water (dH2O) and pooled. Then, 500 ng of plasmid was added to 100 μl of cells and incubated on ice for 15 min. Cells were then electroporated at 2,500 V, following which, 1 ml of LB was added immediately and cells were incubated at 37°C for 1 h with shaking for recovery. Cells were plated on LB agar plates supplemented with carbenicillin (200 μg/ml) and incubated at 37°C overnight.
RESULTS AND DISCUSSION
While the benefits of using antibacterial cleaning products in domestic settings continue to be a topic of debate, the exponential growth in their use over the past few decades has led to increasing concerns regarding their ability to select for multidrug-resistant bacteria (2). Indeed, the phenomenon of cross-resistance has been demonstrated in studies on E. coli (4) and P. aeruginosa (26), among other pathogens. In this study, we analyzed the potential of triclosan to select for mutants of A. baumannii that display elevated resistance to antibiotics from chemically distinct classes.
A. baumannii AB042 was isolated from the serial passaging of A. baumannii ATCC 17978 in LB supplemented with triclosan. The MIC of A. baumannii AB042 for triclosan was 256 mg/liter (Table 3). This represents a 32-fold increase in resistance to triclosan compared to that of the parent strain. Next, we analyzed the susceptibility of A. baumannii AB042 to various clinically relevant antibiotics. We observed an ≥8-fold increase in resistance to piperacillin-tazobactam, a ≥4-fold increase in resistance to doxycycline, moxifloxacin, and tigecycline, and a 2-fold increase in resistance to ceftriaxone, cefepime, meropenem, doripenem, ertapenem, ciprofloxacin, aztreonam, and trimethoprim-sulfamethoxazole (Table 3). The MICs of ceftazidime, imipenem, amoxicillin-clavulanic acid, and colistin (polymyxin E) were unchanged.
TABLE 3.
Antimicrobial susceptibilities of A. baumannii isolates
| Strain | MIC (mg/liter)a |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRI | TZP | CRO | FEP | CAZ | IPM | MEM | DOR | ETP | CIP | MXF | AMC | ATM | TGC | DOX | CST | SXT | |
| ATCC 17978 | 8 | 4 | 16 | 4 | 8 | 0.25 | 0.5 | 0.25 | 8 | 0.25 | ≤0.06 | 32 | 16 | 0.12 | ≤0.12 | 2 | 2 |
| AB042 | 256 | 32 | 32 | 8 | 8 | 0.25 | 1 | 0.5 | 16 | 0.5 | 0.25 | 32 | 32 | 0.5 | 0.5 | 2 | 4 |
| AB042/pPLS097 | 256 | ND | ND | ND | ND | ND | ND | ND | ND | 0.5 | 0.125 | ND | ND | ND | ND | ND | ND |
| AB042/pPLS145(adeN) | 256 | ND | ND | ND | ND | ND | ND | ND | ND | 0.25 | ≤0.06 | ND | ND | ND | ND | ND | ND |
| PA006 | 32 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| PA006 plus IPTGs | 64 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| ATCC 17978/pPLS097 | 8 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| ATCC 17978/pPLS151(fabI) | 32 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| ATCC 17978/pPLS150 (fabI116GàV) | >256 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
TRI, triclosan; TZP, piperacillin-tazobactam; CRO, ceftriaxone; FEP, cefepime; CAZ, ceftazidime; IPM, imipenem; MEM, meropenem; DOR, doripenem; ETP, ertapenem; CIP, ciprofloxacin; MXF, moxifloxacin; AMC, amoxicillin-clavulanic acid; ATM, aztreonam; TGC, tigecycline; DOX, doxycycline; CST, colistin; SXT, trimethoprim-sulfamethoxazole; IPTG, isopropyl β-D-1-thiogalactopyranoside; ND, not determined.
In order to understand the mechanism of triclosan resistance in A. baumannii AB042, we analyzed the expression of fabI and also sequenced the gene in this strain. The FabI enzyme is the target for triclosan, and mutations in fabI have been known to result in high triclosan resistance. Although we did not observe any changes in the expression of fabI in A. baumannii AB042 compared to that of the parent strain, we observed that fabI from AB042 contained a single base pair change, leading to a 116G→V mutation. A similar mutation was shown to cause triclosan resistance in other organisms as well, including E. coli (27) and P. aeruginosa (28) (Fig. 1). However, in S. enterica, this mutation was not found to contribute to the high triclosan resistance (7). Therefore, in order to confirm the role of the 116G→V fabI mutation in triclosan resistance of A. baumannii AB042, we introduced fabIWT and fabI116G→V separately into A. baumannii ATCC 17978, and their susceptibilities to triclosan were determined. We found that the introduction of fabI116G→V in A. baumannii ATCC 17978 elevated its resistance to triclosan by 32-fold (Table 3). This shows that the 116G→V mutation in fabI of A. baumannii AB042 is indeed responsible for its reduced susceptibility to triclosan. While we also observed slightly reduced susceptibility of A. baumannii ATCC 17978 upon the introduction of wild-type fabI (4-fold), it is most likely due to the overexpression of the triclosan target gene, as no change in the susceptibility was observed when the empty vector was introduced into the host strain (Table 3).
FIG 1.
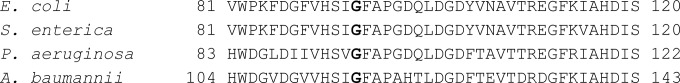
Alignment of fabI region containing the G→V mutation in Acinetobacter baumannii with E. coli, P. aeruginosa, and S. enterica. The 116G residue of A. baumannii and the corresponding glycine residues from E. coli, P. aeruginosa, and S. enterica are shown in bold. Only partial protein sequences are shown.
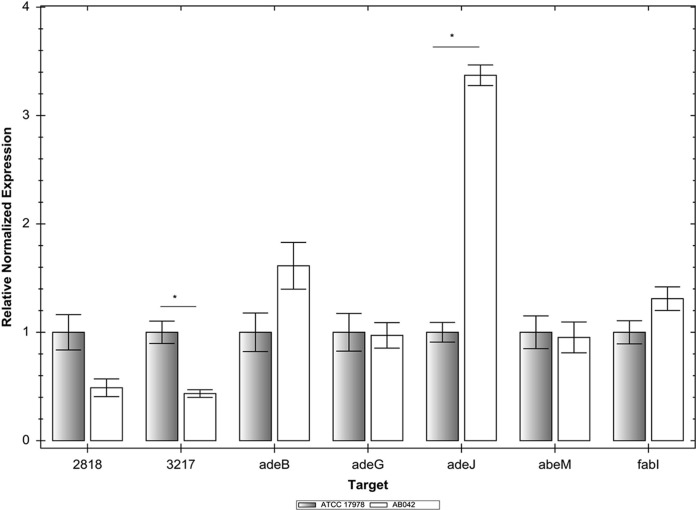
Since A. baumannii AB042 displayed reduced susceptibility to multiple chemically unrelated antibiotics compared to that of the parent A. baumannii ATCC 17978, we analyzed the expression of efflux pumps in this strain (Fig. 2). We analyzed the expression of four different efflux pump-encoding genes characterized to date in A. baumannii, namely, adeB (RND pump-encoding gene of the AdeABC pump), adeG (RND pump-encoding gene of the AdeFGH pump), adeJ (RND pump-encoding gene of the AdeIJK pump), and abeM (the MATE family pump). In addition, we also analyzed the expression of two yet-uncharacterized RND pump-encoding genes, namely, A1S_2818 and A1S_3217, which are a part of the A1S_2817 to A1S_2818 and A1S_3219, A1S_3218, A1S_3217, and A1S_3214 operons, respectively. AdeABC, AdeFGH, and AdeIJK, the three RND family efflux pumps characterized in A. baumannii, have been shown to efflux various clinically relevant antibiotics, including fluoroquinolones, β-lactams, tetracycline, tigecycline, and trimethoprim-sulfamethoxazole (12, 13, 23, 29), and are commonly found to be overexpressed in clinical isolates of A. baumannii (30). To our knowledge, none of these three RND pumps has been shown to efflux triclosan. However, AbeM, a MATE family pump, effluxes triclosan in addition to antibiotics, such as norfloxacin, ofloxacin, ciprofloxacin, gentamicin, kanamycin, erythromycin, chloramphenicol, and trimethoprim (14).
FIG 2.
Expression of efflux pump-encoding genes and fabI in Acinetobacter baumannii AB042. Expression of five RND pump-encoding genes (adeB, adeG, adeJ, A1S_2818, and A1S_3217), MATE pump-encoding abeM, and the enoyl-[acyl-carrier protein] reductase gene fabI as measured by qRT-PCR compared with that in the parent strain, A. baumannii ATCC 17978. We used 16S rRNA as the housekeeping gene control. Error bars represent the standard errors of the mean. Significant change in expression is shown by an asterisk.
We observed that the only efflux pump-encoding gene that showed an increased expression (3.5-fold) in A. baumannii AB042 was adeJ, which is part of the adeIJK operon (Fig. 2). None of the other pumps analyzed (with the exception of A1S_3219, whose expression was reduced in A. baumannii AB042) showed any altered expression in A. baumannii AB042, including the AbeM pump that effluxes triclosan. Antibiotics to which A. baumannii AB042 showed reduced susceptibility—namely, moxifloxacin, cefepime, meropenem, ertapenem, ciprofloxacin, tigecycline, and trimethoprim-sulfamethoxazole (Table 3)—are known substrates of the AdeIJK pump (13, 31). While doxycycline, doripenem, and ceftriaxone have not been recognized as substrates of the AdeIJK pump, their structural similarities to its established substrates (tetracycline, meropenem-ertapenem, and cefepime, respectively) suggest that these three antibiotics are also effluxed by AdeIJK. In addition to the expression of efflux pumps, we also analyzed the expression of two porin-encoding genes, carO (32) and oprD (33), shown to play a role in the carbapenem resistance of A. baumannii. Their expression remained unaltered in AB042 (data not shown). Altogether, the antibiotic susceptibility profile of A. baumannii AB042 and the expression analysis of efflux pumps suggest that the overexpression of AdeIJK is responsible for its reduced susceptibility to various antibiotics.
Since the AdeIJK pump had not been shown previously to efflux triclosan, we tested its specificity for triclosan using the single-copy gene expression system in the surrogate P. aeruginosa PAO750 (34), which lacks five different native RND pumps. This system allows us to achieve biologically relevant levels of gene expression and also prevents any interference from native RND pumps. We previously demonstrated the usefulness of this system in studying RND pumps of A. baumannii (23). We observed a small but reproducible 2-fold increase in the resistance of P. aeruginosa PA006 to triclosan upon the induction of expression of the adeIJK operon (Table 3). It should be noted that we confirmed the expression of the AdeIJK pump and its functionality using RT-PCR and measuring the susceptibility of PA006 (with and without the addition of IPTG) to tetracycline, trimethoprim, SDS, and ciprofloxacin (data not shown). This change in the MICs upon induction of AdeIJK shows that it is indeed capable of effluxing triclosan, although it is most likely responsible for a low level of triclosan resistance (as evident from a 2-fold increase in resistance to triclosan in P. aeruginosa PA006 upon the induction of the AdeIJK operon).
Triclosan has been shown to select for RND pump-overexpressing mutants in different organisms. This overexpression can result from mutations in the regulator protein-encoding gene (e.g., mutation in nfxB [26, 35] or mexL [36]) that lead to the overexpression of the MexCD-OprJ and MexJK pumps, respectively, of P. aeruginosa. The overexpression of pumps can also result from the altered expression of global regulators of efflux pumps, such as marA, soxS, or ramA, as shown in E. coli and S. enterica serovar Typhimurium (4, 7). Since adeIJK was the only efflux pump-encoding operon overexpressed in A. baumannii AB042, we investigated the mechanism of its upregulation. Expression of the AdeIJK efflux pump has been shown to be controlled by AdeN, a regulator protein belonging to the TetR family, the gene for which is located at a distant location from the operon (31). In order to see if the change in the expression of adeIJK was a result of the altered expression of adeN in A. baumannii AB042, we analyzed its expression using qRT-PCR but observed no changes in expression compared to that of the parent strain (data not shown). We then sequenced adeN and the promoter region of the adeIJK operon to look for mutations that may be responsible for the increased expression of adeIJK in A. baumannii AB042. While we did not find any changes in the promoter of adeIJK, the sequence of adeN revealed a large 73-bp deletion at position 224. This would result in a truncated protein due to the appearance of a premature stop codon after the 72nd amino acid residue in the protein (Fig. 3a and b). This truncation would result in the loss of six out of nine predicted α-helices (31) toward the C-terminal end of the protein, rendering it inactive, which would explain the overexpression of the AdeIJK pump.
FIG 3.
Nucleotide deletion in adeN resulting in the premature truncation of the protein in Acinetobacter baumannii AB042. A 73-bp deletion in adeN (a) causes the generation of a stop codon (underlined), leading to the translation of truncated protein (b). Only partial sequences are shown.
We then carried out whole-genome sequencing of AB042 and the parent strain, ATCC 17978, to rule out the presence of any other mutation that may be responsible for the reduced susceptibility of AB042 to triclosan and antibiotics. We found a single base pair change (91G→A) leading to a 31D→N mutation in A1S_0863, a gene that encodes a beta-ketoacyl-acyl carrier protein synthase I or FabB. FabB is involved in the condensation reaction in fatty acid biosynthesis (37) but is not known to play a role in triclosan resistance; therefore, the significance of this mutation in AB042 is not yet clear.
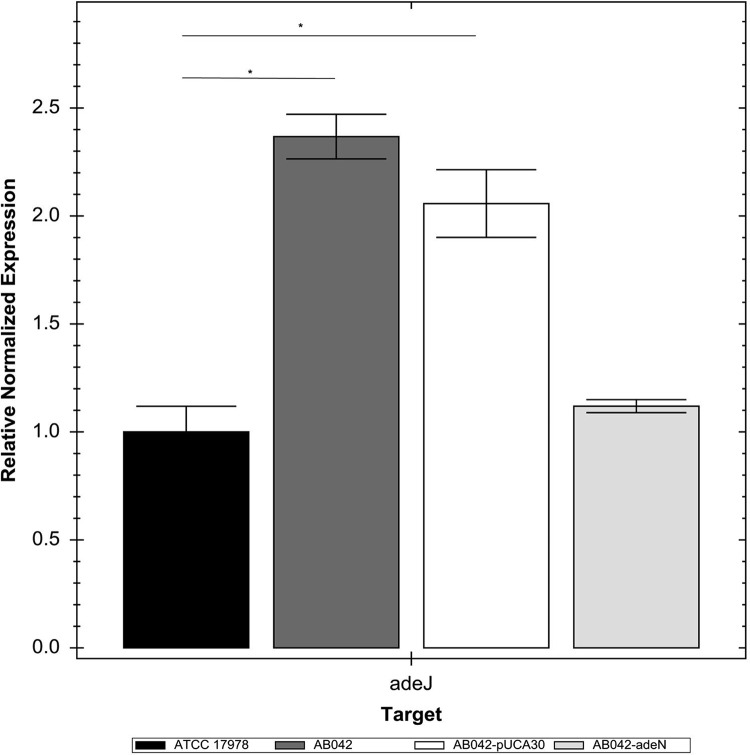
In order to further study the role of the AdeIJK overexpression in the efflux of triclosan, we attempted to create a gene deletion of the adeIJK operon in AB042. However, despite numerous attempts and for reasons that are currently unclear to us, we were unable to isolate the gene deletion. We therefore employed an alternative strategy. We introduced the adeN gene into AB042 to repress the expression of adeIJK, which was confirmed by qRT-PCR (Fig. 4). We did not observe a change in the susceptibility of AB042 (Table 3) to triclosan upon the introduction of adeN, even when expression of the AdeIJK pump was repressed, indicating that this pump is likely to be responsible for a low level of resistance to triclosan in A. baumannii, as shown by data obtained from the experiment with the surrogate P. aeruginosa strain above. We also measured the susceptibility of AB042 complemented with adeN to ciprofloxacin and moxifloxacin as controls. The susceptibility of AB042 to these two antibiotics, which are substrates of the AdeIJK pump, is much lower than that of the parent, ATCC 17978. The introduction of adeN into AB042 increased its susceptibility to ciprofloxacin and moxifloxacin to the same levels as those of the parent strain (Table 3), therefore validating our approach of repressing the expression of adeIJK in lieu of gene knockouts to study its role in triclosan efflux. Altogether, these results suggest that while high resistance of AB042 to triclosan is the result of a mutation in fabI, triclosan exposure can result in the upregulation of AdeIJK, leading to multidrug resistance in A. baumannii.
FIG 4.
Expression of adeJ in Acinetobacter baumannii ATCC 17978 complemented with adeN. Expression of the adeJ was measured by qRT-PCR in Acinetobacter baumannii ATCC 17978 complemented with the wild-type adeN. We used 16S rRNA as the housekeeping gene control. Error bars represent the standard errors of the mean. Significant change in expression is shown by an asterisk.
In summary, it is evident from our work that the triclosan-resistant mutant A. baumannii AB042 exhibits higher resistance to clinically relevant antibiotics as a result of the overexpression of the AdeIJK multidrug resistance efflux pumps, resulting from the deletion in adeN.
This is the first study to show that triclosan can select for mutants of A. baumannii that overexpress the AdeIJK multidrug resistance efflux pump and display increased resistance to various unrelated classes of antibiotics. This study also further supports concerns about the potential of biocides such as triclosan in selecting for antibiotic-resistant mutants of clinically relevant bacterial pathogens.
ACKNOWLEDGMENTS
We thank Yasser Alsaadi for technical help and Deborah Tsuyuki (Manitoba Institute of Child Health) for assistance with the genome sequencing.
P.C.L. is supported by grants from the Natural Science and Engineering Research Council (NSERC) and the Canada Research Chair program. This work was supported by grants from the NSERC and the University of Manitoba University Research Grants Program (to A.K.).
Footnotes
Published ahead of print 18 August 2014
REFERENCES
- 1.Gnanadhas DP, Marathe SA, Chakravortty D. 2013. Biocides: resistance, cross-resistance mechanisms and assessment. Expert Opin. Investig. Drugs 22:191–206. 10.1517/13543784.2013.748035. [DOI] [PubMed] [Google Scholar]
- 2.Schweizer HP. 2001. Triclosan: a widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 202:1–7. 10.1111/j.1574-6968.2001.tb10772.x. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Schweizer HP. 2005. Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv. Drug Deliv. Rev. 57:1486–1513. 10.1016/j.addr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 4.McMurry LM, Oethinger M, Levy SB. 1998. Overexpression of marA, soxS, or acrAB produces resistance to triclosan in laboratory and clinical strains of Escherichia coli. FEMS Microbiol. Lett. 166:305–309. 10.1111/j.1574-6968.1998.tb13905.x. [DOI] [PubMed] [Google Scholar]
- 5.Chuanchuen R, Narasaki CT, Schweizer HP. 2002. The MexJK efflux pump of Pseudomonas aeruginosa requires OprM for antibiotic efflux but not for efflux of triclosan. J. Bacteriol. 184:5036–5044. 10.1128/JB.184.18.5036-5044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mima T, Joshi S, Gomez-Escalada M, Schweizer HP. 2007. Identification and characterization of TriABC-OpmH, a triclosan efflux pump of Pseudomonas aeruginosa requiring two membrane fusion proteins. J. Bacteriol. 189:7600–7609. 10.1128/JB.00850-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webber MA, Randall LP, Cooles S, Woodward MJ, Piddock LJV. 2008. Triclosan resistance in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 62:83–91. 10.1093/jac/dkn137. [DOI] [PubMed] [Google Scholar]
- 8.Gordon NC, Wareham DW. 2010. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int. J. Antimicrob. Agents 35:219–226. 10.1016/j.ijantimicag.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Abbo A, Navon-Venezia S, Hammer-Muntz O, Krichali T, Siegman-Igra Y, Carmeli Y. 2005. Multidrug-resistant Acinetobacter baumannii. Emerg. Infect. Dis. 11:22–29. 10.3201/eid1101.040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlowsky JA, Draghi DC, Jones ME, Thornsberry C, Friedland IR, Sahm DF. 2003. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob. Agents Chemother. 47:1681–1688. 10.1128/AAC.47.5.1681-1688.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchand I, Damier-Piolle L, Courvalin P, Lambert T. 2004. Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob. Agents Chemother. 48:3298–3304. 10.1128/AAC.48.9.3298-3304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyne S, Rosenfeld N, Lambert T, Courvalin P, Perichon B. 2010. Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:4389–4393. 10.1128/AAC.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damier-Piolle L, Magnet S, Bremont S, Lambert T, Courvalin P. 2008. AdeIJK, a resistance-nodulation-cell division pump effluxing multiple antibiotics in Acinetobacter baumannii. Antimicrob. Agents Chemother. 52:557–562. 10.1128/AAC.00732-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su X-Z, Chen J, Mizushima T, Kuroda T, Tsuchiya T. 2005. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob. Agents Chemother. 49:4362–4364. 10.1128/AAC.49.10.4362-4364.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Pi B, Zhou H, Yu Y-S, Li L. 2009. Triclosan resistance in clinical isolates of Acinetobacter baumannii. J. Med. Microbiol. 58:1086–1091. 10.1099/jmm.0.008524-0. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M7-A7 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Chevreux B, Pfisterer T, Drescher B, Driesel AJ, Müller WEG, Wetter T, Suhai S. 2004. Using the miraEST assembler for reliable and automated mRNA transcript assembly and SNP detection in sequenced ESTs. Genome Res. 14:1147–1159. 10.1101/gr.1917404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829. 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43:491–498. 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Li M, Hakonarson H. 2010. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38:e164. 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorvaldsdóttir H, Robinson JT, Mesirov JP. 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14:178–192. 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortez-Cordova J, Kumar A. 2011. Activity of the efflux pump inhibitor phenylalanine-arginine beta-naphthylamide against the AdeFGH pump of Acinetobacter baumannii. Int. J. Antimicrob. Agents 37:420–424. 10.1016/j.ijantimicag.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Choi KH, Gaynor JB, White KG, Lopez C, Bosio CM, Karkhoff-Schweizer RR, Schweizer HP. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods 2:443–448. 10.1038/nmeth765. [DOI] [PubMed] [Google Scholar]
- 25.Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391–397. 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkhoff-Schweizer RR, Schweizer HP. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob. Agents Chemother. 45:428–432. 10.1128/AAC.45.2.428-432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. 1999. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J. Biol. Chem. 274:11110–11114. 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 28.Hoang TT, Schweizer HP. 1999. Characterization of the Pseudomonas aeruginosa enoyl-acyl carrier protein reductase: a target for triclosan and its role in acylated homoserine lactone synthesis. J. Bacteriol. 181:5489–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnet S, Courvalin P, Lambert T. 2001. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45:3375–3380. 10.1128/AAC.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernando D, Zhanel G, Kumar A. 2013. Antibiotic resistance and expression of resistance-nodulation-division pumps and outer membrane porins in Acinetobacter species isolated from Canadian hospitals. Can. J. Infect. Dis. Med. Microbiol. 24:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenfeld N, Bouchier C, Courvalin P, Périchon B. 2012. Expression of the resistance-nodulation-cell division pump AdeIJK in Acinetobacter baumannii is regulated by AdeN, a TetR-type regulator. Antimicrob. Agents Chemother. 56:2504–2510. 10.1128/AAC.06422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mussi MA, Relling VM, Limansky AS, Viale AM. 2007. CarO, an Acinetobacter baumannii outer membrane protein involved in carbapenem resistance, is essential for l-ornithine uptake. FEBS Lett. 581:5573–5578. 10.1016/j.febslet.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 33.Dupont M, Pagès J-M, Lafitte D, Siroy A, Bollet C. 2005. Identification of an OprD homologue in Acinetobacter baumannii. J. Proteome Res. 4:2386–2390. 10.1021/pr050143q. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Chua KL, Schweizer HP. 2006. Method for regulated expression of single-copy efflux pump genes in a surrogate Pseudomonas aeruginosa strain: identification of the BpeEF-OprC chloramphenicol and trimethoprim efflux pump of Burkholderia pseudomallei 1026b. Antimicrob. Agents Chemother. 50:3460–3463. 10.1128/AAC.00440-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okazaki T, Iyobe S, Hashimoto H, Hirai K. 1991. Cloning and characterization of a DNA fragment that complements the nfxB mutation in Pseudomonas aeruginosa PAO. FEMS Microbiol. Lett. 63:31–35. [DOI] [PubMed] [Google Scholar]
- 36.Chuanchuen R, Gaynor JB, Karkhoff-Schweizer R, Schweizer HP. 2005. Molecular characterization of MexL, the transcriptional repressor of the mexJK multidrug efflux operon in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:1844–1851. 10.1128/AAC.49.5.1844-1851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoang TT, Schweizer HP. 1997. Fatty acid biosynthesis in Pseudomonas aeruginosa: cloning and characterization of the fabAB operon encoding β-hydroxydecanoyl-acyl carrier protein dehydratase (FabA) and β-ketoacyl-acyl carrier protein synthase I (FabB). J. Bacteriol. 179:5326–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernando D, Kumar A. 2012. Growth phase-dependent expression of RND efflux pump- and outer membrane porin-encoding genes in Acinetobacter baumannii ATCC 19606. J. Antimicrob. Chemother. 67:569–572. 10.1093/jac/dkr519. [DOI] [PubMed] [Google Scholar]