Abstract
Although controversial, it has been suggested that antibiotic treatment of laboratory animals infected with Borrelia burgdorferi often leads to the persistence of residual spirochetes that are claimed to be viable but noncultivable. If viable cells of B. burgdorferi do persist following antibiotic therapy, one possible explanation for the lack of cultivability is that too few organisms persist in any given tissue site that might be sampled and cultured. In this study, we treated SKH (hairless) mice, with B. burgdorferi infection of 3 months' duration, with either ceftriaxone or saline for 5 days and then cultured a suspension extract of nearly the entire mouse using a combined in vivo/in vitro culture method. All of the saline-treated (control) mice were culture positive, compared with none of the antibiotic-treated mice. Our findings further document the effectiveness of antibiotic therapy in eradicating cultivable cells of B. burgdorferi, irrespective of tissue or organ site.
INTRODUCTION
Whether antibiotic therapy can eradicate viable Borrelia burgdorferi from infected laboratory animals is a subject of debate (1–3). The results of certain animal experiments (4–6), but not others (7), have suggested that viable B. burgdorferi may persist based on various definitions of viability other than the gold standard of successfully cultivating the spirochete. The principal explanation put forward to explain the absence of positive cultures is that the organism has been transformed to a viable but noncultivable state that persists as such even in the absence of continued antibiotic therapy (6).
If viable cells of B. burgdorferi do persist following antibiotic therapy, one possible explanation for the lack of cultivability is that too few organisms persist in any given tissue site that might be sampled and cultured. In this study, we treated six mice with single daily doses of the antibiotic ceftriaxone for just 5 days and then cultured a suspension extract of nearly the entire mouse using a combined in vivo/in vitro culture method. No positive cultures were obtained, although B. burgdorferi could be recovered on culture of every mouse in the control group of infected mice treated with saline.
MATERIALS AND METHODS
Animals.
Female and male SKH mice (8 to 12 weeks old) were obtained from Charles River Laboratories (Wilmington, MA). All mice were housed in a filtered-air environment maintained at 20 ± 2°C. Antibiotic-treated and non-antibiotic-treated (control) mice were kept in separate cages for the infectivity experiments. SKH is an uncharacterized/nonpedigreed hairless mouse. It is euthymic and immunocompetent and is equally as susceptible to B. burgdorferi infection as is the more frequently used and well-characterized C3H strain (8). The absence of body hair helps to minimize possible contamination that might occur during processing of extract suspensions of the whole mouse (described below). All animal experiments were approved by our institution's Animal Care and Use Committee, and all animal experimentation guidelines were followed.
Bacteria and bacterial cultures.
A strain of B. burgdorferi designated BL206 was used for these experiments. BL206 was isolated from the blood of a patient who presented with erythema migrans. This organism has been maintained by passage in Barbour-Stoenner-Kelly (BSK) medium and was used for the infectivity experiments (see below) after appropriate dilutions were made in BSK medium. The organism had been passaged between five and eight times before use in these experiments.
Infectivity experiments.
Mice were infected by injection with a tuberculin syringe intradermally in the abdominal area with 100,000 cells of B. burgdorferi in a volume of 0.1 ml of BSK medium. Three months later, the mice were given a single daily dose of either saline (control) or ceftriaxone (Rocephin; Roche Laboratories, Nutley, NJ) at a dose of 50 mg/kg of body weight for 5 consecutive days as previously described (9). The saline and ceftriaxone injections were given in a volume of 0.1 ml intramuscularly (i.m.). Treatment was initiated at a time point at which infection with BL206 is known to be widely disseminated in other mouse species (e.g., C3H mice), on the basis of the results of prior investigations (8, 10).
Seven to 10 days after completion of the saline and ceftriaxone treatment regimens, each mouse was humanely euthanized, exsanguinated, and placed in beakers containing 70% ethanol for 3 min to disinfect the skin surface followed by rinsing in Dulbecco's phosphate-buffered saline. Urinary bladder and ear tissue samples (about 7 by 10 mm each) were removed to ascertain the infection status of each of the infected and treated mice (11). Separate extracts of excised urinary bladders and ears (collected aseptically) were prepared by mincing the tissues finely with scissors and forceps and suspending them in 0.3 to 0.4 ml of BSK medium. After the heavy tissue particles settled out, each of the extract suspensions was added to separate screw-cap tubes (Nunc, Roskilde, Denmark) containing 1.8 ml of BSK medium. Tubes were incubated at 33°C, and the cultures were examined microscopically (by dark-field or phase-contrast microscopy using an Olympus BH-2 microscope equipped with a camera assembly) at 1-, 2-, and 4-week intervals for the presence of live, motile spirochetes as shown in Fig. 1. Positive cultures were then subcultured in BSK medium to ensure that the visualized organisms could be maintained in a replicating state. Whole-body extracts of the euthanized mice were processed similarly but with slight modifications. After the stomach and the entire intestinal tract were excised and discarded (to eliminate possible contamination by the indigenous gastrointestinal flora), the remaining bodies of the mice were processed separately by mincing them finely with scissors and forceps in a volume of 2.0 ml of BSK medium. Included in the whole-body extracts were the following organs/tissue sites: brain, heart, kidneys, liver, lungs, lymph nodes, multiple bones and joints, musculature, pancreas, reproductive organs, spleen, and skin (from abdominal, back, and tail areas). The liquid contents (about 1.0 ml) of the minced body parts were collected and then injected intraperitoneally into separate normal recipient SKH mice. Four weeks later, the recipient mice were euthanized and separate extracts of excised urinary bladders and ears were prepared, cultured, and examined microscopically as described above; positive cultures were then subcultured as described above.
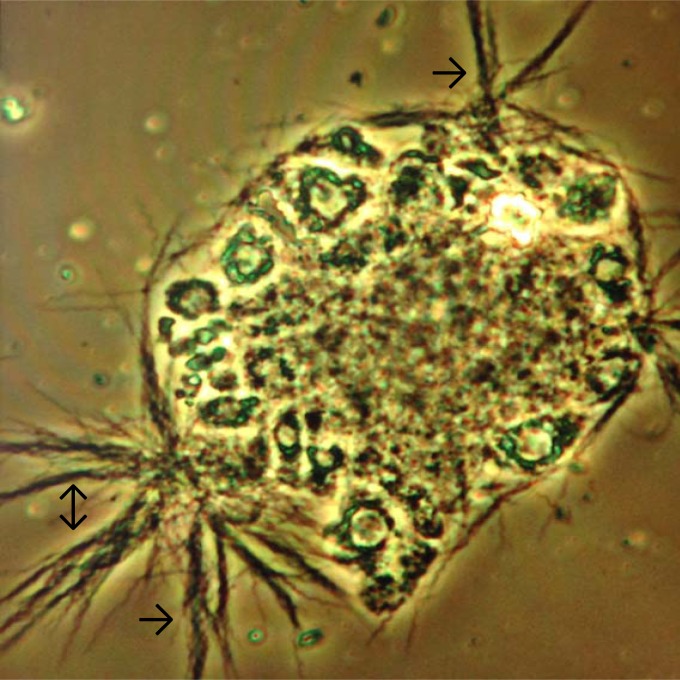
FIG 1.
Photomicrograph of a urinary bladder extract culture derived from a saline-treated mouse. The image depicts small and large clumps (arrows) of microcolonies of Borrelia burgdorferi, along with numerous single spirochetes, that are projecting outward and growing while adhering to a remnant of uroepithelial cells. Phase-contrast microscopy: magnification, ×400.
Inoculation of mouse tissue extracts into other mice as a means of recovering any residual B. burgdorferi bacteria was used because preliminary studies (data not shown) showed that direct in vitro cultivation of whole-body extracts resulted in up to 50% contamination of borrelial cultures with various Gram-positive and Gram-negative bacteria (likely skin flora). However, by first inoculating the extracts into other mice, contamination of borrelial cultures was prevented, presumably because low numbers of nonborrelial bacteria are cleared by the recipient mouse's host defenses.
RESULTS
Twelve mice (6 males and 6 females) were infected with the BL206 strain of B. burgdorferi and 3 months later were treated with i.m. injections of either saline or ceftriaxone given daily for 5 days. B. burgdorferi could be recovered on culture and successfully subcultured from all 6 saline-treated mice (Tables 1 and 2). In addition, cells of B. burgdorferi present in saline-treated mice maintained their infectivity, viability, and cultivability when inoculated into normal recipient mice. None of the 6 mice that received ceftriaxone were culture positive directly or after inoculation of tissue extracts into other mice (Tables 1 and 2). Male and female mice were equally susceptible to infection and curable with antibiotic treatment. None of the cultures from ceftriaxone-treated mice were unevaluable due to contamination with other microorganisms.
TABLE 1.
Efficacy of 5 doses of ceftriaxone given over 5 days to SKH mice infected with Borrelia burgdorferi
| No. of infected mice | Treatment regimen | No. of mice with positive cultures for B. burgdorferi from: |
||
|---|---|---|---|---|
| Urinary bladder | Ear | Whole bodya | ||
| 3 females | Saline | 3 | 2b | 3 |
| 3 females | CTXc | 0 | 0 | 0 |
| 3 males | Saline | 3 | 3 | 3 |
| 3 males | CTX | 0 | 0 | 0 |
Whole-body culture absent the urinary bladder, ears, and entire gastrointestinal tract. The whole body was assessed by a combined intraperitoneal in vivo and in vitro culture technique, while the urinary bladder and ear were assessed by an in vitro culture technique.
One ear culture from one mouse was deemed unevaluable for the presence of Borrelia due to contamination with rapidly growing nonborrelial bacteria.
CTX, ceftriaxone.
TABLE 2.
Evidence for sustained viability and infectivity of cultured isolates from non-antibiotic-treated, B. burgdorferi-infected mice
| No. of infected mice | Treatment regimen | No. of combined B. burgdorferi-positive bladder, ear, and whole-body cultures/no. with positive subcultures (positive infectivity)a |
|---|---|---|
| 3 females | Saline | 9/8 (8)b |
| 3 males | Saline | 9/9 (9) |
Subcultures and infectivity results are from positive cultures of the same groups of saline-treated mice shown in Table 1.
One ear culture from one mouse was deemed unevaluable for Borrelia due to contamination with rapidly growing nonborrelial bacteria.
DISCUSSION
Our findings demonstrate that B. burgdorferi cannot be cultured from experimentally infected mice following just 5 daily doses of ceftriaxone treatment at a dosage of 50 mg/kg administered intramuscularly even when nearly the entire mouse is cultured. Given the short half-life of ceftriaxone in mice (1, 9), residual ceftriaxone would not have been expected at the 7- to 10-day time point posttreatment when the mice were cultured. The ceftriaxone treatment regimen that we chose was based on the uniformly successful results with this dosage and duration of ceftriaxone in a prior study of C3H mice that were also infected with the BL206 strain of B. burgdorferi, in which treatment success was judged based on the absence of a positive culture. Therefore, our findings may not be generalizable to other antibiotic treatment regimens (4, 6).
In fact, it is clear that, after exposure to levels of antibiotics in vitro that are below the minimum bactericidal concentrations, B. burgdorferi can still be cultured in BSK medium (12, 13). It is also clear in several animal systems that recovery of B. burgdorferi in culture is correlated with the dosage of antibiotic administered with a much greater likelihood of positive cultures found in those animals receiving the lowest drug doses (12, 13). However, based primarily on certain mouse studies reported by one group of investigators, it has been concluded that antibiotics, independent of drug class, never fully eradicate viable B. burgdorferi and instead result in the persistence of replication-competent cells that have become noncultivable and remain noncultivable for at least 12 months following completion of antibiotic treatment (6). No mechanism has been discovered to explain this conclusion. These putative residual spirochetes do not appear to cause inflammation even when introduced into SCID mice (i.e., immunodeficient mice which typically develop histologic evidence of inflammation when infected with B. burgdorferi) (4, 5), nor do they apparently elicit a serologic response in the mice which were initially infected despite the reported observation that they may eventually markedly increase in numbers in certain tissue sites, approaching the quantity of borrelial cells in untreated mice (6).
The results of our study indicate that if antibiotic therapy should induce a viable but noncultivable state for B. burgdorferi in mice, the noncultivability is not related to insufficient tissue sampling and is probably unrelated to simply having only a very small number of cultivable cells left in the mouse. Although successful in vitro cultivation of B. burgdorferi can be accomplished with inocula as low as a single bacterial cell (4), a noteworthy limitation of our study is that the minimum number of BL206 B. burgdorferi cells that would successfully infect SKH mice after intraperitoneal inoculation was not determined but is likely to be considerably greater than a single spirochete. For example, the 50% infective dose by this route of inoculation for Syrian hamsters was determined to be 10,000 borrelial cells (14). Accordingly, future experiments are planned to determine the 50% infective dose for BL206 in SKH mice.
Additional studies are warranted to establish or refute the controversial theory that antibiotic treatment of infected mice induces a prolonged phenotypic change in B. burgdorferi leading to viable and replication-competent cells that can no longer be cultured in vitro.
ACKNOWLEDGMENTS
We appreciate the expert technical assistance of Susan Bittker and Dennie Cooper.
This work was supported in part by an NYIT-COM Research Enhancement Award to C.S.P.
Footnotes
Published ahead of print 25 August 2014
REFERENCES
- 1.Wormser GP, Schwartz I. 2009. Antibiotic treatment of animals infected with Borrelia burgdorferi. Clin. Microbiol. Rev. 22:387–395. 10.1128/CMR.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43:1089–1134. 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 3.Wormser GP, O'Connell S. 2011. Treatment of infection caused by Borrelia burgdorferi sensu lato. Expert Rev. Anti Infect. Ther. 9:245–260. 10.1586/eri.10.174. [DOI] [PubMed] [Google Scholar]
- 4.Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. 2008. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 52:1728–1736. 10.1128/AAC.01050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold SW, Hodzic E, Imai D, Feng S, Yang X, Luft BJ. 2010. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob. Agents Chemother. 54:643–651. 10.1128/AAC.00788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodzic E, Imai D, Feng S, Barthold SW. 2014. Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One 9:e86907. 10.1371/journal.pone.0086907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. 2012. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J. Clin. Invest. 122:2652–2660. 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pavia CS, Plummer M. 2013. Intermittent spirochetemia in SKH mice infected with Borrelia burgdorferi, sensu stricto. Transfusion 53:2828–2829. 10.1111/trf.12333. [DOI] [PubMed] [Google Scholar]
- 9.Pavia C, Inchiosa MA, Jr, Wormser GP. 2002. Efficacy of short-course ceftriaxone therapy for Borrelia burgdorferi infection in C3H mice. Antimicrob. Agents Chemother. 46:132–134. 10.1128/AAC.46.1.132-134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G, Ojaimi C, Iyer R, Saksenberg V, McClain SA, Wormser GP, Schwartz I. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect. Immun. 69:4303–4312. 10.1128/IAI.69.7.4303-4312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavia CS, Wormser GP, Nowakowski J, Cacciapuoti A. 2001. Efficacy of an evernimicin (SCH27899) in vitro and in an animal model of Lyme disease. Antimicrob. Agents Chemother. 45:936–937. 10.1128/AAC.45.3.936-937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RC, Kodner C, Russell M. 1987. In vitro and in vivo susceptibility of the Lyme disease spirochete, Borrelia burgdorferi, to four antimicrobial agents. Antimicrob. Agents Chemother. 31:164–167. 10.1128/AAC.31.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson RC, Kodner CB, Jurkovich PJ, Collins JJ. 1990. Comparative in vitro and in vivo susceptibilities of the Lyme disease spirochete Borrelia burgdorferi to cefuroxime and other antimicrobial agents. Antimicrob. Agents Chemother. 34:2133–2136. 10.1128/AAC.34.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson RC, Marek N, Kodner C. 1984. Infection of Syrian hamsters with Lyme disease spirochetes. J. Clin. Microbiol. 20:1099–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]