Abstract
The resistance of multidrug-resistant Acinetobacter baumannii (MDRAB) isolates to most traditional antibiotics results in huge challenges for infection therapy. We investigated the in vitro activities of both l- and d-lycosin-I against MDRAB. These two compounds displayed high antibacterial activities and rapid bactericidal effects against MDRAB. Moreover, the compounds retained their activity even at high salt (Mg2+ or Ca2+) concentrations. These results demonstrate the potential of lycosin-I to be developed as a new antibiotic.
TEXT
Acinetobacter baumannii is one of the predominant pathogens associated with nosocomial infections (1–3). The abuse of antibiotics over the last 2 decades has led to the continuous emergence of multidrug-resistant A. baumannii (MDRAB) isolates (4, 5). The clinical severity of infections with MDRAB isolates with intrinsic and acquired resistance has been exacerbated by the limited number of effective antibiotics. Although polymyxins and, possibly, tigecycline are considered to be the last resort of reliable treatments (6–8), the emergence of MDRAB resistance to these two types of antibiotics has been reported worldwide (9–11), which has created a pressing need to discover effective new alternative agents.
Antimicrobial peptides (AMPs) are recognized as innate immune compounds that can be extracted from insects, bacteria, animals, and plants (12–17). AMPs that possess broad-spectrum activity against bacteria and a low risk of resistance acquisition have brought new hope for overcoming microbial drug resistance (18).
In a previous study, we isolated an AMP named lycosin-I from the venom of the spider Lycosa singoriensis (19). The rapid inhibition of various standard strains of bacteria and fungi by lycosin-I was observed (20), but its effectiveness for the treatment of clinical isolates, particularly multidrug-resistant microorganisms, remains unexplored. In this study, we investigate the in vitro antibacterial properties of two isomers of lycosin-I, namely, wild-type lycosin-I (l-lycosin-I) and an unnatural lycosin-I isomer (d-lycosin-I), against MDRAB clinical isolates.
Unique A. baumannii isolates were collected from the Second Xiangya Hospital during the period of January to July 2013. The identification and analysis of the antibiotic susceptibilities of these strains was performed using a BD Phoenix-100 automated microbiology system (Diagnostic Systems, Sparks, MD) and an API 20 NE system (bioMérieux, Inc.). Without molecular identification, we must acknowledge that some isolates of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex which were not Acinetobacter baumannii could not be excluded in our research. The results were interpreted according to the breakpoints suggested by the Clinical and Laboratory Standards Institute (CLSI) (21). The A. baumannii strains which were resistant to various kinds of agents, especially carbapenem antibiotics, were classified as MDRAB strains (22, 23), and the strains that were sensitive to most of the conventional clinical antibiotics were regarded as drug-susceptible isolates.
The presence of a series of genes was determined using PCR with specific primers (see Table S1 in the supplemental material). All PCR assays were performed using Red Load Taq master (Jena Bioscience, Jena, Germany) in a Techne thermocycler (Techne, United Kingdom). The higher prevalence of these genes in MDRAB isolates (see Table S2) further confirms the multidrug resistance of the MDRAB strains at the genetic level.
Three AMPs were used: the l isomer of lycosin-I (l-lycosin-I), the d isomer of lycosin-I (d-lycosin-I), which consists of l- and d-amino acid residues (RKGWFKAMKSIAKFIAKEKLKEHL), and a scrambled lycosin-I (S-lycosin-I), which was synthesized from the N terminus to the C terminus of lycosin-I (LHEKLKEKAIFKAISKMAKFWGKR). The peptides were synthesized and purified as described in our previous study (19).
MICs of lycosin-I.
The MICs of the three isomers of lycosin-I and several other clinical drugs were determined through the broth microdilution method in accordance with the CLSI protocol (Table 1; see also Table S2 in the supplemental material) (21). With MICs ranging from 8 to 32 μg/ml, l- and d-lycosin-I exhibited more potent inhibitory activities against both drug-susceptible A. baumannii and MDRAB isolates than most of the traditional drugs tested, except polymyxin B, which was reported to be of high toxicity (24, 25). However, no distinct differences were observed between l- and d-lycosin-I. The MICs of S-lycosin-I ranged from 128 to >256 μg/ml, which indicates that this compound exhibits only slight activity against the microorganisms tested. There were no distinct differences between the MIC ranges of l- and d-lycosin-I against MDRAB and drug-susceptible isolates.
TABLE 1.
MICs of three types of lycosin-I and various traditional antibiotics against Acinetobacter baumannii
| Druga | MIC (μg/ml) for: |
|||||
|---|---|---|---|---|---|---|
| MDRAB isolates (n = 18) |
Drug-susceptible A. baumannii isolates (n = 15) |
|||||
| Range | 50% | 90% | Range | 50% | 90% | |
| l-Lycosin-I | 8–32 | 8 | 16 | 8–32 | 8 | 16 |
| d-Lycosin-I | 8–32 | 8 | 16 | 8–32 | 8 | 16 |
| S-Lycosin-I | 128–>256 | 256 | >256 | 128–>256 | 256 | >256 |
| AMK | >256 | >256 | >256 | 4–16 | 8 | 16 |
| SCF | 32–>256 | 64 | >256 | 4–16 | 8 | 16 |
| SAM | 32–>256 | 256 | >256 | 4–16 | 8 | 16 |
| IPM | 32–256 | 64 | 256 | 1–4 | 2 | 4 |
| MEM | 32–>256 | 64 | 128 | 1–4 | 2 | 4 |
| MIN | 4–32 | 4 | 32 | 1–4 | 1 | 4 |
| CIP | >256 | >256 | >256 | 1 | 1 | 1 |
| PMB | 1–8 | 2 | 8 | 0.125–1 | 0.25 | 1 |
| TGC | 4–32 | 4 | 16 | 0.125–1 | 0.25 | 1 |
AMK, amikacin; SCF, cefoperazone-sulbactam; SAM, ampicillin-sulbactam; IPM, imipenem; MEM, meropenem; MIN, minocycline; CIP, ciprofloxacin; PMB, polymyxin B; TGC, tigecycline.
Time-kill kinetics of lycosin-I.
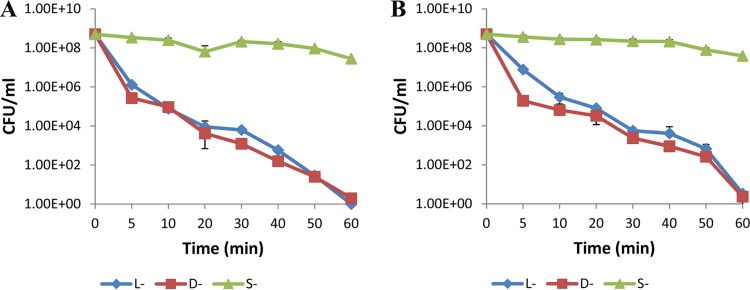
The time-kill curves for two types of lycosin-I (l-and d-) were determined against one representative isolate each of MDRAB and drug-susceptible A. baumannii, respectively, at a concentration equal to 4× MIC. Bacteria from an overnight culture were diluted with LB broth in flasks to a bacterial density of approximately 5 × 108 CFU/ml and cultured to the exponential phase. Viable colonies (CFU/ml) were counted 0, 5, 10, 20, 30, 40, 50, and 60 min after antibiotic addition through serial dilution using sterile saline and plating of 0.01-ml amounts of the serial dilutions onto LB agar. As shown by the results in Fig. 1, both l- and d-lycosin-I displayed rapid bactericidal activity against both multidrug-resistant and drug-susceptible isolates at a concentration equal to 4× MIC. l- and d-lycosin-I reduced the numbers of CFU by approximately 50% during a 30-min exposure period and by 100% within 50 min. In comparison, S-lycosin-I exhibited no obvious bactericidal activity.
FIG 1.
Time-kill curves for representative Acinetobacter baumannii isolates exposed to three types of lycosin-I (4× MIC). (A) Results for MDRAB isolate (isolate 9) exposed to three types of lycosin-I (inoculum, 5 × 108 CFU/ml). (B) Results for drug-susceptible isolate (isolate 25) exposed to three types of lycosin-I (inoculum, 5 × 108 CFU/ml). All of the data are expressed as the means of three independent experiments ± standard errors.
Salt tolerance of lycosin-I.
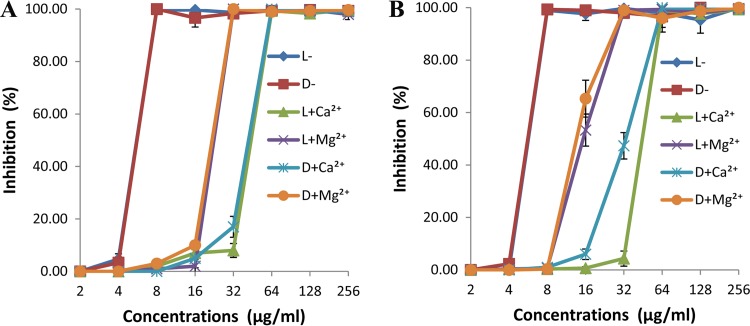
To determine the effects of MgCl2 and CaCl2 on the antibacterial activities of the two types of lycosin-I (l and d), the MICs of lycosin-I against one representative isolate each of MDRAB and drug-susceptible A. baumannii were measured in the presence or absence of 5 mM Mg2+ and Ca2+, and the growth inhibition curves were plotted. The results are displayed in Fig. 2. The MICs of l- and d-lycosin-I against MDRAB and drug-susceptible strains were 4- and 2-fold higher after exposure to 5 mM Ca2+ and Mg2+, respectively. Our results indicate that a high concentration of Ca2+ or Mg2+ reduces the antibacterial activity of these two types of lycosin-I and that Ca2+ exerted a more suppressive effect. However, it is worth noting that, despite their slightly reduced inhibitory activities, l- and d-lycosin-I retained their potent ability to inhibit the growth of the tested isolates in the presence of 5 mM Mg2+ and Ca2+, with MICs of 16 μg/ml and 32 μg/ml, respectively. There were no significant differences in salt sensitivity between l- and d-lycosin-I.
FIG 2.
Effects of Mg2+and Ca2+ on the antimicrobial activities of l- and d-lycosin-I against representative Acinetobacter baumannii isolates. (A) Results for MDRAB isolate (isolate 9). (B) Results for drug-susceptible isolate (isolate 25). All of the data are expressed as the means of three independent experiments ± standard errors.
It is clear that the membrane permeabilization mechanism is the dominant mechanism through which AMPs kill bacteria (18, 20). This mechanism of action is not highly specific toward a protein target, which indicates that it may escape the mechanisms involved in multidrug resistance (26). The higher efficiency of lycosin-I against MDRAB compared to that of traditional drugs makes it a prospective candidate for overcoming multidrug resistance. We compared the in vitro activities of l- and d-lycosin-I against MDRAB but found no significant differences in their MICs and time-kill kinetics, confirming the hypothesis that the antimicrobial activity of AMPs is not mediated by a chirality-dependent interaction with the membrane (27). The fact that lycosin-I exerts its activity against bacteria by acting on a surface target rather than interacting with a chiral center may be one of the reasons for the low risk associated with the acquisition of resistance to this compound. S-Lycosin-I displays very low inhibitory activity and no bactericidal effects on our tested isolates, even at high concentrations, which indicates that the amino acid sequence of the peptide plays a vital role in its inhibitory activity against bacteria, particularly through its binding to the membranes of target cells.
Human body fluids with high salt concentrations can deactivate AMPs. Thus, we must consider the salt sensitivity of these two types of lycosin-I, which may lead to decreases in their activity in vivo (28, 29). Our results demonstrated that l- and d-lycosin-I would retain their activity at high salt concentrations (5 mM Mg2+ or Ca2+), which may indicate that they can be utilized in vivo.
Currently, there are limited choices that are effective in the clinical treatment of MDRAB infections. The in vitro activities of l- and d-lycosin-I against MDRAB strains were found to be higher than those of the traditional antibiotics tested in our study. These compounds were demonstrated to have potential for the development of novel antibiotics, which offers new hope for overcoming microbial drug resistance.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Science and Technology Planning Project of Hunan Province, China (grant no. 2013SK2015), and the Excellent Youth Foundation of Hunan Province, China (grant 14JJ1018).
Footnotes
Published ahead of print 8 September 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03279-14.
REFERENCES
- 1.Spellberg B, Bonomo RA. 2013. “Airborne assault”: a new dimension in Acinetobacter baumannii transmission. Crit. Care Med. 41:2042–2044. 10.1097/CCM.0b013e31829136c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright MS, Haft DH, Harkins DM, Perez F, Hujer KM, Bajaksouzian S, Benard MF, Jacobs MR, Bonomo RA, Adams MD. 2014. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio 5(1):e00963–13. 10.1128/mBio.00963-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee HY, Chen CL, Wu SR, Huang CW, Chiu CH. 2014. Risk factors and outcome analysis of acinetobacter baumannii complex bacteremia in critical patients. Crit. Care Med. 42:1081–1088. 10.1097/CCM.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 4.Baang JH, Axelrod P, Decker BK, Hujer AM, Dash G, Truant AR, Bonomo RA, Fekete T. 2012. Longitudinal epidemiology of multidrug-resistant (MDR) Acinetobacter species in a tertiary care hospital. Am. J. Infect. Control 40:134–137. 10.1016/j.ajic.2011.04.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X-M, Fan Y-F, Feng W-Y, Mi Z-H, Weng X-B. 2014. Antibiotic resistance determinants of a group of multidrug-resistant Acinetobacter baumannii in China. J. Antibiot. (Tokyo) 67:439–444. 10.1038/ja.2014.18. [DOI] [PubMed] [Google Scholar]
- 6.Tas T, Kocoglu E, Mengeloglu Z, Bucak O, Karabörk S. 2013. Investigation of in-vitro susceptibility of multidrug-resistant Acinetobacter baumannii strains isolated from clinical specimens to tigecycline. Bosn. J. Basic Med. Sci. 13:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagihara M, Housman ST, Nicolau DP, Kuti JL. 2014. In vitro pharmacodynamics of polymyxin B and tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 58:874–879. 10.1128/AAC.01624-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Housman ST, Hagihara M, Nicolau DP, Kuti JL. 2013. In vitro pharmacodynamics of human-simulated exposures of ampicillin/sulbactam, doripenem and tigecycline alone and in combination against multidrug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 68:2296–2304. 10.1093/jac/dkt197. [DOI] [PubMed] [Google Scholar]
- 9.Vasilev K, Reshedko G, Orasan R, Sanchez M, Teras J, Babinchak T, Dukart G, Cooper A, Dartois N, Gandjini H, Orrico R, Ellis-Grosse E, 309 Study Group 2008. A phase 3, open-label, non-comparative study of tigecycline in the treatment of patients with selected serious infections due to resistant Gram-negative organisms including Enterobacter species, Acinetobacter baumannii and Klebsiella pneumoniae. J. Antimicrob. Chemother. 62(Suppl 1):i29–i40. 10.1093/jac/dkn249. [DOI] [PubMed] [Google Scholar]
- 10.Poulakou G, Kontopidou FV, Paramythiotou E, Kompoti M, Katsiari M, Mainas E, Nicolaou C, Yphantis D, Antoniadou A, Trikka-Graphakos E, Roussou Z, Clouva P, Maguina N, Kanellakopoulou K, Armaganidis A, Giamarellou H. 2009. Tigecycline in the treatment of infections from multi-drug resistant gram-negative pathogens. J. Infect. 58:273–284. 10.1016/j.jinf.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Barin J, Martins AF, Heineck BL, Barth AL, Zavascki AP. 2013. Hetero- and adaptive resistance to polymyxin B in OXA-23-producing carbapenem-resistant Acinetobacter baumannii isolates. Ann. Clin. Microbiol. Antimicrob. 12:15. 10.1186/1476-0711-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park D, Jung JW, Lee MO, Lee SY, Kim B, Jin HJ, Kim J, Ahn YJ, Lee KW, Song YS, Hong S, Womack JE, Kwon HW. 2014. Functional characterization of naturally occurring melittin peptide isoforms in two honey bee species, Apis mellifera and Apis cerana. Peptides 53:185–193. 10.1016/j.peptides.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Hancock RE. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156–164. 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 14.Pushpanathan M, Gunasekaran P, Rajendhran J. 2013. Antimicrobial peptides: versatile biological properties. Int. J. Pept. 2013:675391. 10.1155/2013/675391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nissen-Meyer J, Nes IF. 1997. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch. Microbiol. 167:67–77. 10.1007/s002030050418. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki K. 1999. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta 1462:1–10. 10.1016/S0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 17.Hancock RE, Brown KL, Mookherjee N. 2006. Host defence peptides from inverbrates—emerging antimicrobial strategies. Immunobiology 211:315–322. 10.1016/j.imbio.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Wimley WC, Hristova K. 2011. Antimicrobial peptides: successes, challenges and unanswered questions. J. Membr. Biol. 239:27–34. 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Deng M, Xiang J, Ma H, Hu W, Zhao Y, Li DW, Liang S. 2012. A novel spider peptide toxin suppresses tumor growth through dual signaling pathways. Curr. Mol. Med. 12:1350–1360. 10.2174/156652412803833643. [DOI] [PubMed] [Google Scholar]
- 20.Tan H, Ding X, Meng S, Liu C, Wang H, Xia L, Liu Z, Liang S. 2013. Antimicrobial potential of lycosin-I, a cationic and amphiphilic peptide from the venom of the spider Lycosa singorensis. Curr. Mol. Med. 13:900–910. 10.2174/15665240113139990045. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24. CLSI, Wayne, PA. [Google Scholar]
- 22.Corbella X, Montero A, Pujol M, Domínguez MA, Ayats J, Argerich MJ, Garrigosa F, Ariza J, Gudiol F. 2000. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J. Clin. Microbiol. 38:4086–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristina ML, Spagnolo AM, Ottria G, Sartini M, Orlando P, Perdelli F, Galliera Hospital Group 2011. Spread of multidrug carbapenem-resistant Acinetobacter baumannii in different wards of an Italian hospital. Am. J. Infect. Control 39:790–794. 10.1016/j.ajic.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Neiva LB, Borges FT, Watanabe M, Pessoa Ede A, Barbosa DA, Vattimo Mde F. 2014. Nephrotoxicity of polymyxin B: experimental study in cells and implications for nursing practice. Rev. Esc. Enferm. USP 48:272–277 (Article in Portuguese.) 10.1590/S0080-6234201400002000011. [DOI] [PubMed] [Google Scholar]
- 25.Tuon FF, Rigatto MH, Lopes CK, Kamei LK, Rocha JL, Zavascki AP. 2014. Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int. J. Antimicrob. Agents 43:349–352. 10.1016/j.ijantimicag.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Rahnamaeian M. 2011. Antimicrobial peptides: modes of mechanism, modulation of defense responses. Plant Signal. Behav. 6:1325–1332. 10.4161/psb.6.9.16319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navon-Venezia S, Feder R, Gaidukov L, Carmeli Y, Mor A. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689–694. 10.1128/AAC.46.3.689-694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aoki W, Ueda M. 2013. Characterization of antimicrobial peptides toward the development of novel antibiotics. Pharmaceuticals (Basel) 6:1055–1081. 10.3390/ph6081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam JP, Lu YA, Yang JL. 2002. Correlations of cationic charges with salt sensitivity and microbial specificity of cystine-stabilized beta-strand antimicrobial peptides. J. Biol. Chem. 277:50450–50456. 10.1074/jbc.M208429200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.