Abstract
The EUCAST E.DEF9.1 standard recommends standardization of the inoculum concentration by conidium counting using a hemocytometer rather than a spectrophotometer. In this study, we investigated whether the choice of these methods influenced isavuconazole MICs. A blinded collection of 30 molecularly characterized azole-resistant isolates and 10 wild-type Aspergillus fumigatus isolates was shared with four different laboratories. Additionally, each laboratory selected approximately 100 A. fumigatus isolates and 50 isolates each of A. flavus, A. nidulans, A. niger, and A. terreus (1,237 isolates in total). Three laboratories (laboratories 1 to 3) used conidium counting. One laboratory standardized the inoculum using a spectrophotometer (that is, by use of the optical density [OD]) and is referred to as the OD laboratory. Correlation coefficients, intraclass correlation coefficients, and essential agreement were calculated, and 2-log-unit differences were assessed (paired t test). The MIC range for the blinded collection was 0.25 to 16 mg/liter, and a 1-dilution-step difference between the MIC50 and MIC90 across the four laboratories was detected and a 2-dilution-step difference between the modal MICs was detected. Compared to the results for laboratories 1 and 2, a significant correlation was found for the OD laboratory MIC data (correlation coefficients, 0.85 and 0.93, respectively; intraclass correlation coefficients, 0.88 and 0.96, respectively). The number of mutant isolates whose MICs overlapped those of the wild-type isolates was the lowest for the OD laboratory (14/30 [46.7%] mutant isolates), whereas the numbers were 18/30 (60%) isolates for laboratory 1, 17/30 (56.7%) isolates for laboratory 2, and 21/30 (70%) isolates for laboratory 3. For the A. flavus, A. fumigatus, A. nidulans, A. niger, and A. terreus isolates, comparative analysis again defined the MIC distributions from the OD laboratory to be in excellent agreement with those from laboratories 1 and 2 across all five Aspergillus spp. The findings suggest that EUCAST testing using OD determination is an appropriate alternative for standardization of Aspergillus inoculum concentrations.
INTRODUCTION
The first EUCAST reference method for the susceptibility testing of conidium-forming molds was published in 2008 (1). According to this standard, standardization of the inoculum concentration should be performed by conidium counting using a hemocytometer rather than measuring turbidity using a spectrophotometer. The reason for this recommendation was the concern that the conidia of various molds vary in size and color and, therefore, that the optical density (OD) might not predict the cell concentration with adequate precision. Although in their study Espinel-Ingroff and Kerkering (2) showed an acceptable prediction of the inoculum concentration using a spectrophotometer and 4 to 5 isolates of each of six different mold species, later studies using a greater number of isolates and species suggested the opposite (3–5). Indeed, one study showed that the spectrophotometer method ensured an inoculum concentration that varied between 4 × 105 and 5 × 106 CFU at best when various isolates of Aspergillus, Fusarium, and Scedosporium species were investigated (3). Two subsequent studies also evaluating the precision of counting, determination of the numbers of CFU, and hemocytometer standardization of the fungal suspension similarly suggested a significant variation of fungal suspensions prepared using the spectrophotometer method but not when counting was adopted (4, 5).
It is well accepted that inoculum size influences the MIC achieved in any susceptibility test, and therefore, standardization is mandatory. However, the CLSI mold reference standard recommends the use of inoculum standardization by a spectrophotometer, similar to what both the CLSI and EUCAST recommend for the susceptibility testing of yeasts (6–8). It is less time-consuming to standardize the inoculum according to the optical density than by conidium counting, and laboratories running the reference or commercial tests for yeast are already familiar with the use of a spectrophotometer for standardization of the inoculum concentration of yeast (and bacterial) suspensions. Therefore, we investigated whether EUCAST isavuconazole MICs for a collection of wild-type and cyp51A mutant A. fumigatus isolates tested blindly in four laboratories were within acceptable agreement when three laboratories followed the EUCAST standard regarding inoculum preparation and one adopted spectrophotometric standardization of the inoculum. In addition, the distributions of the MICs for the five most common species obtained by the laboratories using counting and spectrophotometer standardization were compared. The MIC testing was done as part of a study generating epidemiological cutoff values (ECOFFs) for isavuconazole and the various Aspergillus species (9).
MATERIALS AND METHODS
Isolates.
A blinded collection of 30 molecularly characterized azole-resistant A. fumigatus clinical isolates and 10 wild-type A. fumigatus clinical isolates was studied (all four laboratories tested the same blinded panel). The mutant strains had alterations at the following common molecular cyp51A azole resistance hot spots: G54 (n = 10), TR34/L98H (n = 10), and M220 (n = 10). Additionally, each laboratory selected approximately 100 A. fumigatus isolates and 50 isolates each of A. flavus, A. nidulans, A. niger, and A. terreus from their collections of wild-type Aspergillus isolates to be tested for susceptibility in parallel with the blinded collection.
Quality control (QC) strains.
Candida krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used as control strains. The MIC50s for C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were as follows for the individual laboratories: OD laboratory, 0.03 mg/liter (range, 0.015 to 0.06 mg/liter) and 0.015 mg/liter (range, 0.015 mg/liter), respectively; laboratory 1, 0.03 mg/liter (range, 0.03 to 0.125 mg/liter) and 0.03 mg/liter (range, 0.03 mg/liter), respectively, laboratory 2, 0.03 mg/liter (range, 0.03 to 0.125 mg/liter) and 0.015 mg/liter (range, 0.015 to 0.03 mg/liter), respectively; and laboratory 3, 0.125 mg/liter (range, 0.06 to 0.25 mg/liter) and 0.03 mg/liter (range, 0.03 to 0.06 mg/liter), respectively.
Susceptibility testing.
Microdilution plates were made in-house at each of the four laboratories following the EUCAST standard and using a single batch of isavuconazole pure substance and local providers of medium and microtiter plates. Three laboratories (laboratories 1 to 3) adhered strictly to the EUCAST EDEF9.1 standard when standardizing the inoculum (1). One laboratory (referred to as the OD laboratory) also followed the EUCAST EDEF9.1 standard, with the exception that the inoculum was standardized using a spectrophotometer, as follows. The conidia were harvested in water with 0.1% Tween 20 and filtered through an 11-μm-pore-size filter (nylon net filters; Merck Millipore, Tullagreen, Carrigtwohill, County Cork, Ireland) to remove hyphal fragments and conidium clumps, generating a homogeneous suspension, and the suspension was adjusted to an OD of 1. This suspension was diluted 1:10 before being used to inoculate the plates, as described in the EUCAST EDEF9.1 standard.
Spectrophotometer.
For the spectrophotometer (Densimat; bioMérieux, France) used in this study, a linear relationship between the OD and the McFarland scale was demonstrated, with an OD of 1 corresponding to a McFarland standard of 0.5.
Statistical calculations.
The correlation between the MIC results obtained when the inoculum was standardized using conidium counting and the optical density was evaluated by using Pearson's correlation coefficient (CC) and the intraclass correlation coefficient (ICC). The ICC was expressed to a maximum value of 1, and the 95% confidence interval (CI) is provided for ICCs. In order to approximate a normal distribution, the MICs were transformed to log2 values. A P value of <0.01 was considered statistically significant. The ICC is a reverse measurement of the variability of the conidium counting values. The ICC was calculated using the formula (group mean square – error mean square)/(group mean square + error mean square) and thus has a maximum value of 1 if there is a perfect correlation and a minimum value of −1 if there is a complete absence of a correlation. The ICC evaluates the correlation between values offering a statistically significant difference since it takes into account the number of cases and the absolute value of the count. The ICC is a scales analysis and exhibits the highest statistical power for correlation studies (10). Moreover, the log2 differences between the OD and the conidium counting method were calculated, and differences were assessed by use of the paired t test. In addition, essential agreement between the OD and the conidium counting method within 1 2-fold dilution was calculated for each lab individually, and the mean MIC data among all three labs were calculated.
RESULTS
Overall, the isavuconazole MICs for the blinded collection of wild-type and mutant strains generated in the four laboratories fell within a range of 0.25 to 16 mg/liter, with a 1-dilution-step difference between the MIC50 and MIC90 and a 2-dilution step difference between the modal MICs being found across the four laboratories (Fig. 1 and Table 1). The mean ± standard deviation (SD) log2 difference between the MICs generated in the OD laboratory and those from laboratories 1 to 3 using conidium counting was 0.3 ± 1.1 and was equal to a mean ± SD log2 difference of −0.3 ± 1.2 when the data from laboratories 1 to 3 (all of which used conidium counting) were compared. The paired t test did not show significant differences (P = 0.10), with the 90th percentile of the absolute differences being 1.7 (which means differences of less than 2-fold). In comparison, the corresponding value for the three laboratories that used the counting method was 2. The overall essential agreement (within ±1 dilution) was 73% across the four data sets (range among labs, 55% to 95%) and was not different from the essential agreement between the data set from laboratories 1 to 3, which used the counting methods (73%). The overall Pearson correlation coefficient was 0.89 (95% confidence interval, 0.80 to 0.94; P < 0.0001).
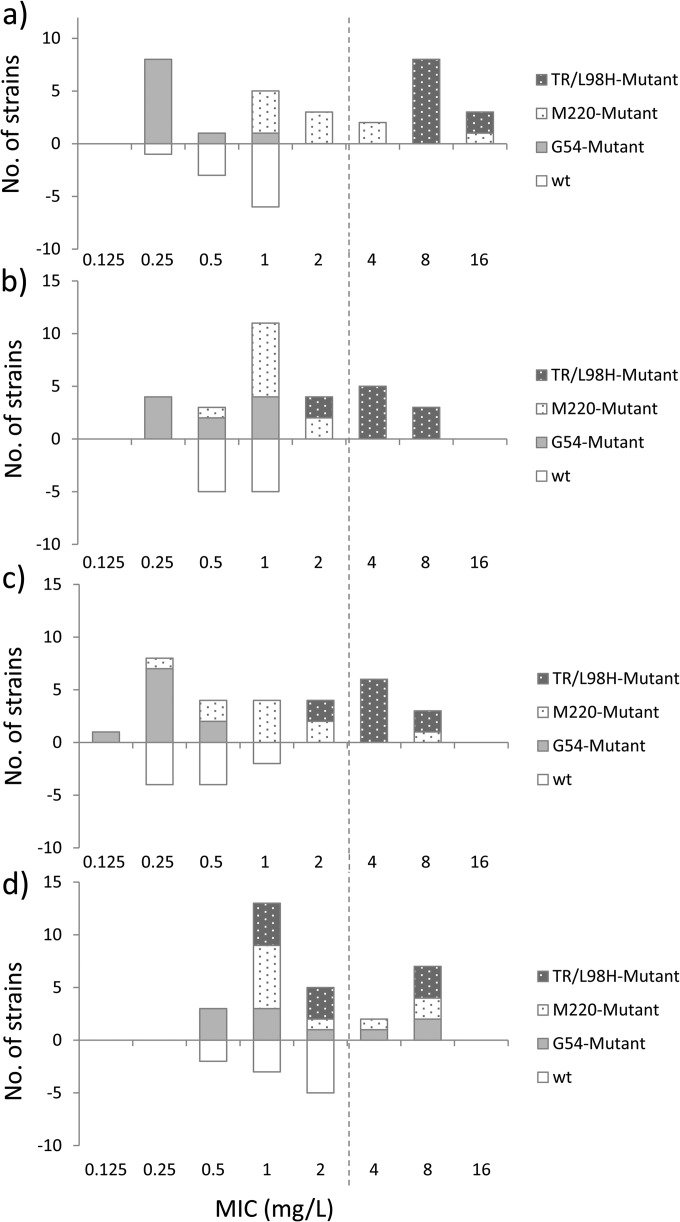
FIG 1.
Number of wild-type (wt; below the x axis) and cyp51A mutant (above the x axis) isolates of A. fumigatus strains with the indicated isavuconazole MICs obtained using OD standardization of the inoculum in the OD laboratory (a) or conidium counting in laboratory 1 (b), laboratory 2 (c), and laboratory 3 (d). The mutant collection included 10 mutant isolates with alterations involving each of the codons G54, M220, and TR34 (TR)/L98H. Dotted line, the recently proposed EUCAST epidemiological cutoff value for isavuconazole and A. fumigatus (9).
TABLE 1.
Descriptive and comparative analysis of the isavuconazole MICs for a blinded collection of wild-type and cyp51A mutant isolates of A. fumigatus generated in the four laboratoriesa
| Laboratory | No. of isolates | MIC (mg/liter) |
CC or ICCb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Modal | Geometric mean | 50% | 90% | Range | OD laboratory | Laboratory 1 | Laboratory 2 | Laboratory 3 | ||
| OD | 40 | 1 | 1.43 | 1 | 8 | 0.25–16.0 | NA | 0.88 (0.78–0.94, <0.01c) | 0.96 (0.92–0.98, <0.01c) | 0.33 (−0.26–0.64, 0.100d) |
| Laboratory 1 | 40 | 1 | 1.12 | 1 | 4 | 0.25–8.0 | 0.85 (<0.01c) | NA | 0.86 (0.74–0.96, <0.01c) | 0.35 (−0.22–0.66, 0.106d) |
| Laboratory 2 | 40 | 0.25 | 0.84 | 0.5 | 4 | 0.25–8.0 | 0.93 (<0.01c) | 0.78 P < 0.01c) | NA | 0.34 (−0.25–0.65, 0.103d) |
| Laboratory 3 | 40 | 1 | 1.68 | 1 | 8 | 0.50–8.0 | 0.22 (0.089d) | 0.21 P = 0.087d ) | 0.21 (0.086d) | NA |
Ten wild-type isolates and 30 cyp51A mutant isolates were tested.
CCs (P values) are shown in the lower left, and ICCs (95% CIs, P values) are shown in the upper right. NA, not applicable.
A statistically significant correlation.
No statistically significant correlation.
The MICs generated in one of the laboratories (laboratory 3) that used conidium counting for standardization of the inoculum were higher, particularly for the wild-type isolates, with MICs being ≥2 mg/liter for 5/10 isolates, whereas MICs were not ≥2 mg/liter for any of the wild-type isolates tested in the other laboratories (Fig. 1). Consequently, the number of mutant strains for which the isavuconazole MIC overlapped the MIC range for the wild-type population was greater for this laboratory, as the MICs for 21/30 (70%) mutant strains overlapped the MIC range for the wild-type population, in contrast to 18/30 (60%) isolates for laboratory 1, 17/30 (56.7%) isolates for laboratory 2, and 14/30 (46.7%) isolates for the laboratory using OD standardization of the inoculum. The correlation coefficient determination also identified laboratory 3 to be an outlier (Table 1). Thus, the correlation coefficient was 0.21 when the results from this laboratory are compared to the results from the two other laboratories that used conidium counting for inoculum standardization, and the intraclass correlation coefficients were 0.34 and 0.35, respectively, when the results from laboratory 3 are compared to the results from laboratories 1 and 2 (Table 1). Similarly, when the MIC values for the data set generated using OD standardization of the inoculum were compared to those from laboratories 1 and 2, which used conidium counting, significant CCs (individually, 0.85 to 0.93) and ICCs of 0.88 to 0.96 were found. However, this was not the case when the data generated in the laboratory using OD standardization were compared to those generated in laboratory 3 (Table 1). Finally, ICCs were significant for laboratories 1 and 2 and the OD laboratory (0.94; 95% CI, 0.89 to 0.96) but not for laboratory 3 but were lower across all four laboratories (0.83; 95% CI, 0.73 to 0.90).
Subsequently, each laboratory tested a panel of routine isolates of A. flavus, A. fumigatus, A. nidulans, A. niger, and A. terreus (Table 2). The comparative analysis again demonstrated a good agreement when the MICs generated in the laboratory using OD for inoculum standardization were comparing with the MICs from laboratories 1 and 2 using conidium counting, but again, the MIC distributions from laboratory 3, which used conidium counting, deviated the most across all five Aspergillus spp. (Table 2).
TABLE 2.
Descriptive analysis of isavuconazole MICs for Aspergillus isolates of the five most common species generated in four laboratories according to the EUCAST EDEF9.1 standarda
| Laboratory |
A. flavus |
A. fumigatus |
A. nidulans |
A. niger |
A. terreus |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | MIC (mg/liter) |
No. of isolates | MIC (mg/liter) |
No. of isolates | MIC (mg/liter) |
No. of isolates | MIC (mg/liter) |
No. of isolates | MIC (mg/liter) |
|||||||||||||||||||||
| Modal | Mean | 50% | 90% | Range | Modal | Mean | 50% | 90% | Range | Modal | Mean | 50% | 90% | Range | Modal | Mean | 50% | 90% | Range | Modal | Mean | 50% | 90% | Range | ||||||
| OD | 51 | 1 | 0.88 | 1 | 1 | 0.125–2.0 | 95 | 1 | 0.89 | 1 | 1 | 0.25–16.0 | 56 | 0.125 | 0.13 | 0.125 | 0.25 | 0.03–0.50 | 54 | 2 | 1.92 | 2 | 4 | 0.50–4.0 | 49 | 1 | 0.76 | 1 | 1 | 0.06–2.0 |
| 1 | 50 | 1 | 1.05 | 1 | 2 | 0.50–2.0 | 100 | 0.5 | 0.6 | 0.5 | 1 | 0.50–2.0 | 50 | 0.125 | 0.12 | 0.125 | 0.125 | 0.06–0.50 | 50 | 1 | 1.19 | 1 | 2 | 0.25–2.0 | 50 | 0.5 | 0.43 | 0.5 | 0.5 | 0.125–1.0 |
| 2 | 52 | 1 | 0.93 | 1 | 2 | 0.25–2.0 | 106 | 1 | 0.68 | 1 | 1 | 0.25–1.0 | 51 | 0.125 | 0.16 | 0.125 | 1 | 0.06–0.50 | 52 | 2 | 2.44 | 2 | 4 | 1.0–8.0 | 53 | 0.12 | 0.45 | 0.5 | 1 | 0.12–2.0 |
| 3 | 62 | 2 | 1.61 | 2 | 4 | 0.50–4.0 | 100 | 1 | 1.16 | 1 | 2 | 0.25–4.0 | 49 | 0.25 | 0.42 | 0.25 | 0.25 | 0.25–8.0 | 53 | 4 | 4.55 | 4 | 8 | 2.0–16.0 | 54 | 4 | 3.3 | 4 | 8 | 0.25–8.0 |
| Total | 215 | 1 | 1.11 | 1 | 2 | 0.125–4.0 | 401 | 1 | 0.81 | 1 | 1 | 0.25–16.0 | 206 | 0.125 | 0.18 | 0.125 | 0.5 | 0.03–8.0 | 209 | 2 | 2.26 | 2 | 4 | 0.25–16.0 | 206 | 0.5 | 0.85 | 0.5 | 4 | 0.06–8.0 |
One laboratory used OD rather that conidium counting for inoculum standardization. The values deviating the most from the total values are underlined.
DISCUSSION
It is well-known that susceptibility testing is associated with some degree of variation, particularly when MIC results from multiple laboratories are compared. In the multicenter study defining QC ranges for the EUCAST method, individual QC strains were tested multiple times. In that study, 3.7 to 8.3% of the EUCAST MIC results for voriconazole were outside a 3-dilution-step concentration range and 0 to 12% of the posaconazole MIC results were outside a 4-dilution-step range for A. fumigatus strains, despite the fact that QC strains are specifically selected because of their stable susceptibility phenotypes to fit the requirements for use as QC strains (11). Despite this, broth dilution methods remain the “gold standard” for mold susceptibility testing.
Since the CLSI and EUCAST methods were established, many MICs have been generated, and both organizations have established epidemiological cutoff values that either are identical or differ by ±1 dilution for most Aspergillus antimold agents available, demonstrating that the methods generate comparable results (9, 12–15). This is despite the differences between methods, including the CLSI recommendation for inoculum standardization using the OD value versus the EUCAST recommendation for inoculum standardization using a hemocytometer. Additionally, a head-to-head comparative study demonstrated a high rate of agreement between the MIC results (16). For example, the essential agreement was excellent when testing the three licensed azoles active against molds and 245 Aspergillus isolates: 100% for itraconazole, 98.4% for posaconazole, and 99.6% for voriconazole when essential agreement was assessed at ±2 dilutions and 99.6% for itraconazole, 87.7% for posaconazole, and 96.3% for voriconazole when essential agreement was assessed at ±1 dilution (16). Although the method for inoculum standardization is only one of the differences between the CLSI and EUCAST methods for the testing of molds, these observations suggest that the time-consuming hemocytometer standardization of the inoculum may not be critical.
The data in this study further suggest that the use of OD standardization of the inoculum suspension is an appropriate approach when testing isavuconazole using the EUCAST methodology. Thus, when the defined strain collection was shared and the isolates were tested blindly in this study, a statistically significant correlation between the data generated in the laboratory using OD determination and those generated in two of the three laboratories using conidium counting was found. In fact, the one laboratory for which the MICs did not correlate significantly with the MICs generated by any of the others used conidium counting, which suggests that other factors are more important contributors to MIC variation. Moreover, when the MIC distributions for the five most common Aspergillus species were compared, the laboratory with the most divergent results was again not the laboratory that used OD for inoculum standardization but one of the three that used conidium counting.
Apart from an acceptable interlaboratory reproducibility, the ability of the method to discriminate between susceptible isolates and those harboring acquired resistance mechanisms that affect clinical efficacy is a crucial parameter when evaluating various methodological procedures for susceptibility testing. When the data for wild-type and mutant strains were compared for the 4 data sets generated in this study, the greatest degree of separation between each molecular genotype (the wild type and the G54, M220, and TR34/L98H mutants) was observed for the data generated in the laboratory using OD for inoculum standardization. The activity of isavuconazole against these mutants has previously been shown to differ, with the MICs being in the range of those for the wild type for isolates with G54 alterations but with MICs for TR34/L98H mutants being notably elevated (9). This pattern was also found with the data generated using OD for inoculum standardization, which again suggests that this is an acceptable method for Aspergillus inoculum preparation.
Our findings suggest that the use of OD standardization results in MIC data in agreement with those obtained by the standard method using the conidium counting chamber and a good separation between wild-type and mutant Aspergillus isolates when testing the in vitro activity of isavuconazole. It is likely that this is applicable to other antimold compounds as well. It is important, however, to ensure proper standardization of the spectrophotometer against the McFarland standard and that the inoculum is prepared with 0.1% Tween and filtered, as the OD value may otherwise not be representative of the inoculum concentration.
ACKNOWLEDGMENTS
This work was sponsored by Astellas Pharma Global Development Inc.
M.C.A. has received research grants and travel grants and has been paid for talks on behalf of Astellas Pharma, Gilead Sciences, Merck Sharpe and Dohme, and Pfizer. S.H. has received research grants from Astellas Pharma; support grants from Gilead Sciences, Pfizer, and the Fungal Research Trust; travel grants from Astellas and Schering-Plough; and equipment grants from the Fungal Research Trust and has been paid for talks on behalf of Pfizer and Astellas Pharma. C.L.-F. has received research grants from and has served as a consultant and/or on the speaker's bureau for Pfizer, Astellas Pharma, Gilead Sciences, and Merck. J.W.M. received research grants from and/or was a consultant for Pfizer, Astellas, Gilead Sciences, and Merck. J.M. has received research grants and travel grants from and has been paid for talks on behalf of Astellas, Gilead Sciences, Schering Plough, and/or Pfizer. M.C.-E. has received grant support from Astellas Pharma, bioMérieux, Gilead Sciences, Merck Sharp and Dohme, Pfizer, Schering Plough, Soria Melguizo SA, Ferrer International, the European Union, the ALBAN program, the Spanish Agency for International Cooperation, the Spanish Ministry of Culture and Education, the Spanish Health Research Fund, the Instituto de Salud Carlos III, the Ramon Areces Foundation, and the Mutua Madrileña Foundation. He has been an advisor/consultant to the Pan American Health Organization, Astellas Pharma, Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp and Dohme, Pfizer, Astellas Pharma, and Schering Plough.
Some isolates tested were kindly provided and are held in the clinical culture collection at the Mycology Reference Centre, Manchester, United Kingdom.
Footnotes
Published ahead of print 18 August 2014
REFERENCES
- 1.Rodriguez-Tudela JL, Arendrup MC, Arikan S, Barchisei F, Bille J, Chryssantou E, Cuenca-Estrella M, Dannaoui E, Denning DW, Donnellly JP, Fegeler W, Lass-Flörl C, Moore C, Richardson M, Gaustad P, Schmalreck A, Velegraki A, Verweij P. 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982–984. 10.1111/j.1469-0691.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 2.Espinel-Ingroff A, Kerkering TM. 1991. Spectrophotometric method of inoculum preparation for the in vitro susceptibility testing of filamentous fungi. J. Clin. Microbiol. 29:393–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrikkou E, Rodriguez-Tudela JL, Cuenca-Estrella M, Gomez A, Molleja A, Mellado E. 2001. Inoculum standardization for antifungal susceptibility testing of filamentous fungi pathogenic for humans. J. Clin. Microbiol. 39:1345–1347. 10.1128/JCM.39.4.1345-1347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aberkane A, Cuenca-Estrella M, Gomez-Lopez A, Petrikkou E, Mellado E, Monzon A, Rodriguez-Tudela JL, the Eurofung Network 2002. Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. J. Antimicrob. Chemother. 50:719–722. 10.1093/jac/dkf187. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Tudela JL, Chryssanthou E, Petrikkou E, Mosquera J, Denning DW, Cuenca-Estrella M. 2003. Interlaboratory evaluation of hematocytometer method of inoculum preparation for testing antifungal susceptibilities of filamentous fungi. J. Clin. Microbiol. 41:5236–5237. 10.1128/JCM.41.11.5236-5237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Tudela JL, Arendrup MC, Barchiesi F, Bille J, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Denning DW, Donnelly JP, Dromer F, Fegeler W, Lass-Florl C, Moore CB, Richardson M, Sandven P, Velegraki A, Verweij PE. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398–405. 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. Document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W. 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 18:E246–E247. 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 9.Howard SJ, Lass-Florl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. 2013. Determination of isavuconazole susceptibility of Aspergillus and Candida species by the EUCAST method. Antimicrob. Agents Chemotherx. 57:5426–5431. 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SPSS Inc. 2009. Statistical analysis and advanced statistical analysis. SPSS Inc., Chicago, IL. [Google Scholar]
- 11.Cuenca-Estrella M, Arendrup MC, Chryssanthou E, Dannaoui E, Lass-Florl C, Sandven P, Velegraki A, Rodriguez-Tudela JL, the AFSTSubcommittee of EUCAST 2007. Multicentre determination of quality control strains and quality control ranges for antifungal susceptibility testing of yeasts and filamentous fungi using the methods of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antimicrobial Susceptibility Testing (AFST-EUCAST). Clin. Microbiol. Infect. 13:1018–1022. 10.1111/j.1469-0691.2007.01790.x. [DOI] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Lass-Florl C, Martin-Mazuelos E, Meis J, Pelaez T, Pfaller MA, Turnidge J. 2013. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for Aspergillus spp. for the CLSI M38-A2 broth microdilution method. Antimicrob. Agents Chemother. 57:3823–3828. 10.1128/AAC.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope WW, Cuenca-Estrella M, Lass-Florl C, Arendrup MC. 2013. EUCAST technical note on voriconazole and Aspergillus spp. Clin. Microbiol. Infect. 19:E278–E280. 10.1111/1469-0691.12148. [DOI] [PubMed] [Google Scholar]
- 14.Espinel-Ingroff A, Diekema DJ, Fothergill A, Johnson E, Pelaez T, Pfaller MA, Rinaldi MG, Canton E, Turnidge J. 2010. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). J. Clin. Microbiol. 48:3251–3257. 10.1128/JCM.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope WW. 2012. EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin. Microbiol. Infect. 18:E248–E250. 10.1111/j.1469-0691.2012.03890.x. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller M, Boyken L, Hollis R, Kroeger J, Messer S, Tendolkar S, Diekema D. 2011. Comparison of the broth microdilution method of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) for testing itraconazole, posaconazole, and voriconazole against aspergillus. J. Clin. Microbiol. 49:1110–1112. 10.1128/JCM.02432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]